Abstract
The processes that govern fracture repair rely on many mechanisms that recapitulate embryonic skeletal development. Hox genes are transcription factors that perform critical patterning functions in regional domains along the axial and limb skeleton during development. Much less is known about roles for these genes in the adult skeleton. We recently reported that Hox11 genes, which function in zeugopod development (radius/ulna and tibia/fibula), are also expressed in the adult zeugopod skeleton exclusively in PDGFRα+/CD51+/LepR+ mesenchymal stem/stromal cells (MSCs). In this study, we use a Hoxa11eGFP reporter allele and loss-of-function Hox11 alleles, and we show that Hox11 expression expands after zeugopod fracture injury, and that loss of Hox11 function results in defects in endochondral ossification and in the bone remodeling phase of repair. In Hox11 compound mutant fractures, early chondrocytes are specified but show defects in differentiation, leading to an overall deficit in the cartilage production. In the later stages of the repair process, the hard callus remains incompletely remodeled in mutants due, at least in part, to abnormal bone matrix organization. Overall, our data supports multiple roles for Hox11 genes following fracture injury in the adult skeleton.
Keywords: Skeletal injury/fracture healing, Mesenchymal stromal/stem cells, Molecular pathways – development, Hox genes, Endochondral ossification - cartilage
Introduction
The mammalian skeleton boasts a remarkable capacity for regeneration following injury. It is one of only a few postnatal processes that are truly regenerative, reestablishing original structure and function without scar formation. Interestingly, many of the mechanisms that govern fracture callus formation and remodeling include those that are also required during embryonic skeletal development(1-5). The expression of several genes required for long bone formation in the embryo (endochondral ossification) are also expressed in the fracture callus, and the patterns of expression and overall histology show similarities to those observed in the growth plate(3-5). A group of transcription factors that have received little attention in this process are the Hox genes.
Hox genes are homeodomain-containing transcription factors that are required for region-specific patterning of the skeleton during development. They are expressed and function in spatially distinct domains along the anterior-posterior body axis and proximal-distal limb axis (6-8). The 39 mammalian Hox genes are subdivided into 13 paralogous groups (Hox1 to Hox13) based on sequence similarity and position within the Hox cluster. Genetic studies show that members of each paralogous group display a remarkable degree of functional redundancy with one another; loss of function of entire paralogous groups results in severe, region-specific skeletal defects along the AP axis(8-15). The posterior Hox genes (Hox9 to Hox13) were also co-opted in limbed vertebrates to function in proximal to distal patterning of the limbs(10,15-20). The Hox11 paralogs instruct proper development of the zeugopod elements of the limb skeleton (radius/ulna and tibia/fibula)(15-17). Loss of Hox11 function results in severe patterning defects of the zeugopod while the remainder of the limb develops normally. Previous studies have reported Hox expression during fracture healing(21-23), however Hox gene function in this process has not been directly tested.
The maintenance of Hox11 expression in the perichondrium/periosteum surrounding the developing skeletal elements of the limbs into late stages of embryonic development(24), led us to question whether Hox genes continue to be expressed and function beyond early patterning events in the embryo. Recently, we reported that Hox11 genes are expressed exclusively in mesenchymal stem/stromal cells (MSCs) in the bone marrow and periosteum at adult stages(25). Using a Hoxa11eGFP insertion allele, we demonstrated that Hoxa11eGFP+ cells are exclusively expressed in PDGFRα+, CD51+ and Leptin Receptor+ (LepR+) cells, markers that have been previously characterized to enrich for mesenchymal progenitor activity in bone marrow non-endothelial stroma and to contribute to fracture healing (26-28). Importantly, we reported that the adult expression and function of Hox11 genes is spatially restricted to same region in which these genes are expressed and function in the embryo (the zeugopod for Hox11 in the limb skeleton), and that other adult Hox gene expression profiles also follow this pattern in other regions of the skeleton(25,29). Together, this work suggests that Hox genes are expressed and function with regional specificity in adult skeletal MSCs.
In this study, we explore the defects associated with the loss of Hox11 function in fracture repair using loss-of-function mouse models. At early stages of repair, we find that Hox11 function is required for chondrocyte differentiation during fracture healing. In addition, we report evidence for bone modeling defects in Hox11 mutants; these mutant fractures display defects in the remodeling phase of repair are unable to remodel to regain normal morphology. Our work is the first to demonstrate adult function(s) for Hox11 genes in skeletal repair and regeneration following fracture injury.
Materials and Methods
Mice
All mice were maintained in a C57BL/6 background. Male and female mice either double or single heterozygous for the Hoxa11 and Hoxd11 null alleles were mated to generate compound mutant animals(16). All animals in the study were maintained on a C57/Bl6 background and group-housed in standard condition, unless separation was required due to fighting. Animals heterozygous for the Hoxa11eGFP allele were generated by traditional breeding strategies as previously described(30). To assess spatial variation in bone fracture repair based on local Hox expression levels, three distinct fracture-healing models were employed: ulnar, tibial and femoral. All animals were anesthetized with isoflurane during each procedure and provided buprenorphine pre- and post-operatively. Carprofen was also given during the recovery period. Post-operative radiographs were taken immediately following fracture (Faxitron X-Ray) to ensure proper fracture location. All animals were fully weight bearing within 1 hour following surgery. Full fracture methods are described in detail in supplemental material. All animal experiments described in this article were reviewed and approved by the University of Michigan's Committee on Use and Care of Animals, Protocol #PRO00006651 (Wellik) and Protocol #PRO00006763 (Goldstein).
Additional materials and methods may be found in supplementary material and includes the following: Fracture methods, X-ray and micro-computed tomography (μCT), Histology, immunohistochemistry, and histomorphometric measurements and Raman spectroscopy.
Results
Hox11 is expressed throughout fracture repair
Fracture healing can be loosely defined by distinct phases of anabolic (callus expansion) and catabolic (callus remodeling) responses to the injury(31,32). Resident MSCs in the skeleton (from the periosteum and bone marrow) provide the major source of progenitors for the repair process(28,33-37). Hoxa11eGFP, at adult stages, is visualized in both the periosteum and bone marrow as a progenitor-enriched, non-endothelial, MSC population(25). To explore a possible role for Hox11 genes in the repair process, the expression of Hoxa11eGFP was examined following fracture injury of both the ulna or the tibia (forelimb or hindlimb zeugopod) using an anti-GFP antibody developed with alkaline phosphatase. Sections were developed without primary antibody to confirm that staining is specific (Supplementary Figure 1D). During hematoma formation (0.5 weeks post-fracture; WPF), Hoxa11eGFP+ cells begin to expand from the periosteum (Figure 1A and Supplemental Figure 1A). During the soft (cartilage) callus (1.5 WPF) and hard (bony) callus (3 WPF) stages, significant expansion of Hoxa11eGFP+ cells throughout the callus is observed. Hoxa11eGFP+ cells are present at the center of the soft callus, in the medullary space at the site of injury, and in the expanded periosteal stromal layer that surrounds the newly formed fracture callus (Figure 1B, and Supplemental Figure 1B). At hard callus stages, Hoxa11eGFP+ cells are observed lining the woven bone surfaces (Figure 1C). Hox11 continues to be expressed well into remodeling stages as evidenced by extensive Hoxa11eGFP expression at 6 WPF (Supplemental Figure 1C). Importantly, our previous work shows that Hox11-expressing cells do not overlap with any markers of differentiated cells throughout the fracture healing process (including osteoblasts, osteoclasts, macrophages, endothelial cells, neurons, and chondrocytes), and flow cytometry demonstrates a cell-surface marker profile that is consistent with expression of Hoxa11eGFP exclusively in mesenchymal stem/stromal cells(25).
Figure 1. Hox11 is expressed throughout fracture injury in the limb zeugopod.
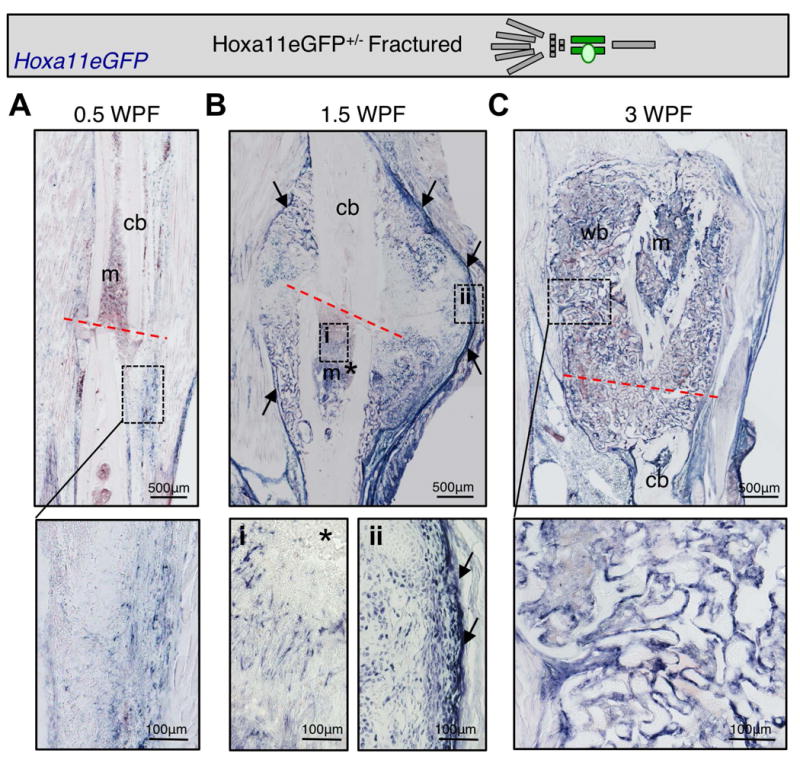
Limb schematic depicts Hoxa11eGFP regional expression (green) and the fracture callus in the zeugopod region (tibia). Hox11 expression is shown using a GFP primary antibody and developing with alkaline phosphatase. (A) Hox11 is expressed at low levels in the hematoma. (B) Hox11 expression expands at 1.5 WPF including the intramedullary space (*) and in the expanded periosteum surround the callus (arrows). (C) Hox11 is expressed near woven bone surfaces in the hard callus at 3 WPF.
Hox11 loss-of-function mice display defects during fracture healing
To assess a role for Hox11 genes in the response to fracture injury, an ulnar fracture model was employed in Hox11 compound mutants (referred to as Hox11 mutant) in which three Hox11 alleles are mutated. Retention of one wild-type allele is sufficient to prevent the developmental skeletal defects observed in four-allele mutant animals(17,24). X-rays and micro-computer tomography (μCT) scans performed at several time points following fracture injury (in accordance with accepted protocols(38)) reveal abnormal fracture healing in Hox11 mutants. During the early response to injury, 1.5 weeks post-fracture (WPF), there are no apparent differences in ossification between mutants and controls (Figure 2A). However, by mid-stage healing (3 WPF) Hox11 mutant animals demonstrate a delay in fracture gap union (Figure 2B).
Figure 2. Loss of Hox11 function results in defects following fracture injury.
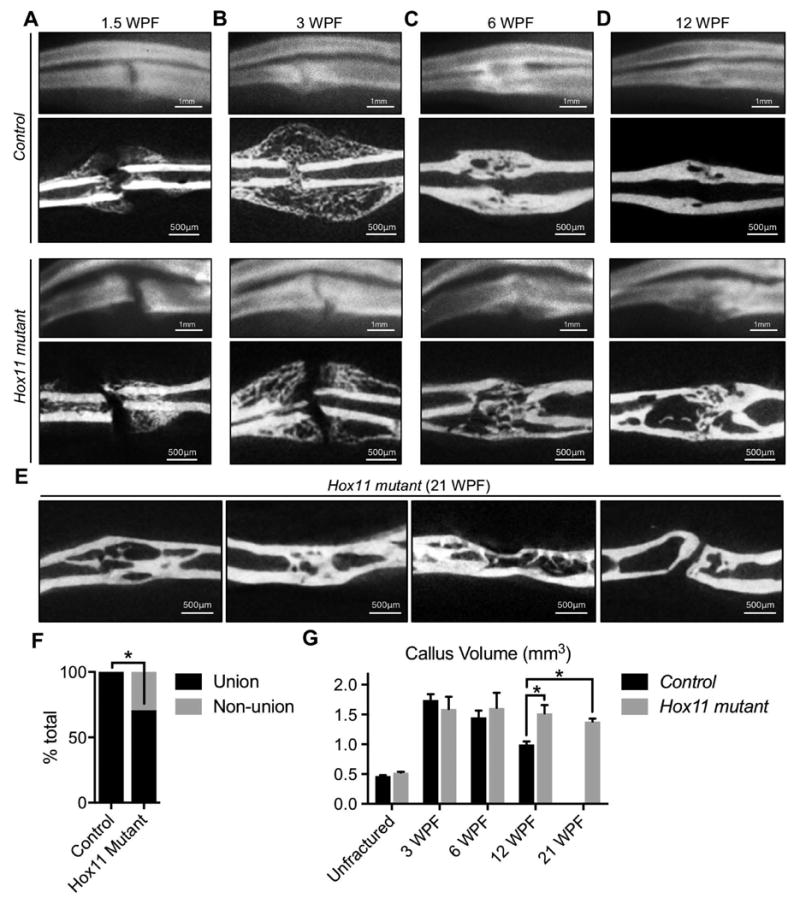
(A-D) X-rays (top panels) and cross-sectional views of mCT (lower panels) in control and in Hox11 mutant animals. (A) At 1.5WPF, x-ray and mCT images are comparable between groups. (B) At 3WPF, controls are bridged and mutants are not. (C-D) At 6WPF and 12WPF, most mutants are now bridged, but exhibit delayed remodeling compared to controls. (E) Cross-sectional views of mCT at 21WPF persistent and incomplete bone remodeling in mutant animals. (F) 100% of control animals demonstrate union fractures compared to 71% of Hox11 mutant animals. Statistical analysis carried out by two-tailed Fisher Exact test and by two-tailed Chi Square test; * = p<0.05. (G) mCT analysis shows statistically significant maintenance of callus volume at late stages in mutants. Statistical analysis carried out by Students' T-test; * = p<0.05. WPF; weeks post-fracture.
During remodeling stages of healing (> 6 WPF), two phenotypes are observed in Hox11 mutant animals. First, a significant number of animals exhibit non-union fractures (Figure 2F). Second, mutant animals that display fracture union demonstrate a dramatic delay in remodeling. Histological analyses reveal a significant amount of woven bone remaining in the bone marrow space at 6 and at 12 WPF compared to wild-type control fracture injuries that have remodeled this space by these time points (Figure 2C-D). Notably, this incomplete remodeling remains at 21 WPF (Figure 2E). μCT analyses support these observations. While the callus volume of control fractures declines with time after injury, indicative of remodeling, the callus volume in Hox11 mutant fracture injuries remains elevated (Figure 2G).
Loss of Hox11 function reduces chondrocyte differentiation in the early callus
Processes required for early callus formation were evaluated in order to understand the cellular mechanism for delayed callus bridging in Hox11 mutant fracture injuries. New bone formation in the callus is accomplished by intramembranous and endochondral ossification, distinct processes that act simultaneously following fracture to promote repair at the site of injury(1,31). Vascularization is critical for this process, and ischemic injuries result in non-union fractures that require medical intervention(39,40).
In both control and mutant fracture injuries, new bone forms at the outer callus, along the periosteal surface, characteristic of intramembranous ossification (Figure 3A-B). In both groups, these regions exhibit high levels of Osterix expression (a marker of osteoblasts) and are highly vascularized (PECAM) (Figure 3B). No defects are observed in the overall vascularization of Hox11 mutant calluses (Figure 3C-D).
Figure 3. Intramembranous ossification and vascularization are unchanged in Hox11 mutant fractures.
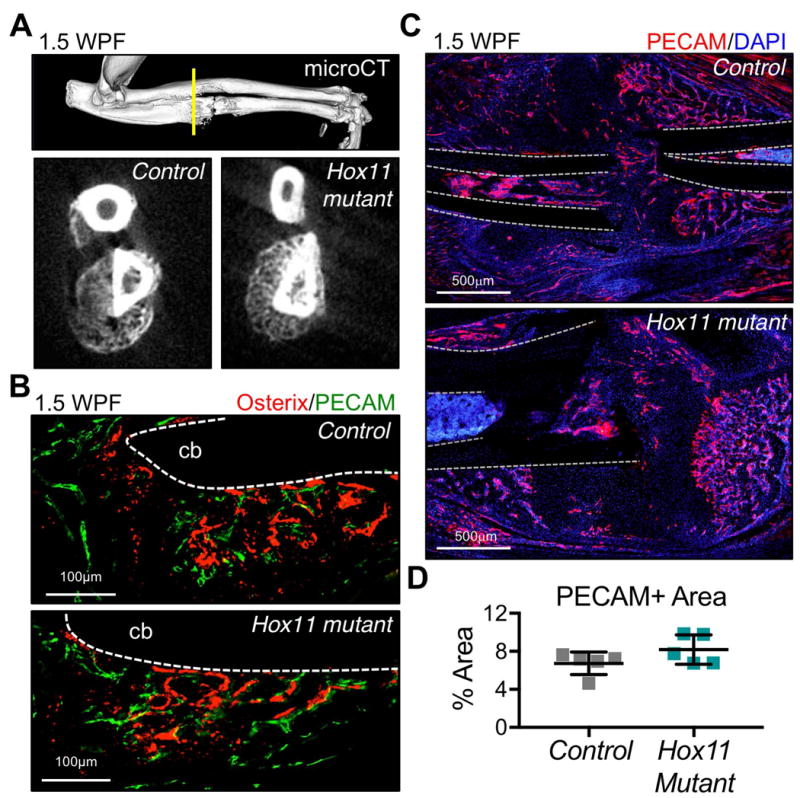
(A) mCT analysis of outer regions of callus show comparable bone formation in regions of intramembranous ossification. (B) Osterix and PECAM-stained sections show bone formation and vascularization in regions of intramembranous ossification. cb; cortical bone. (C-D) PECAM-staining in controls and Hox11 mutants shows comparable vascularization in the early callus (1.5WPF). cb; cortical bone.
To evaluate endochondral ossification, histomorphometry measurements were performed on SafraninO/Fast Green-stained sections of early calluses. At three time points post-fracture (1.5 WPF, 3 WPF, and 6 WPF), the proportion of the callus comprised of mesenchyme, cartilage, or woven bone was measured (Figure 4A-B). Results reveal a decrease in woven bone and an increase in mesenchyme at the earliest stages of Hox11 mutant callus formation compared to controls (Figure 4A-B); a result consistent with delayed union observed by x-ray and μCT analyses (Figure 2). Notably, a significant reduction in cartilage formation (Safranin O) is observed in Hox11 mutant fractures at all stages examined (Figure 4A-B).
Figure 4. Chondroctye differentiation and endochondral ossification is disrupted in the Hox11 mutant callus.
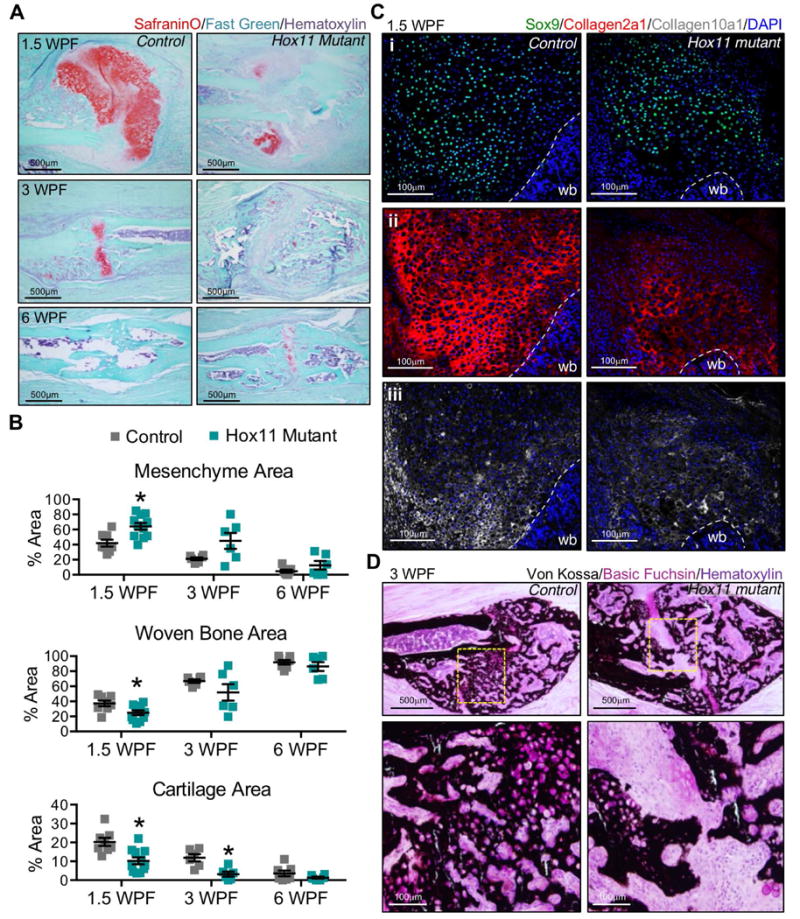
(A-B) Histomorphometric quantifications of the mesenchymal, woven bone and cartilage areas from Safranin O/Fast Green-stained sections at 1.5, 3, and 6 weeks post-fracture (WPF). Cartilage was designated by Safranin O. Woven bone and mesenchyme were designated visually; the latter refers to non-woven bone, non-safranin O-positive area. Abundant cartilage formation is visualized in center regions of control calluses; mesenchyme is maintained in similar regions of mutant calluses. Statistical analysis carried out by Students' T-test; * = p<0.05. (C) Sox9, Collagen2a1 and Collagen 10a1-stained sections at 1.5WPF show chondrocyte differentiation in control and mutant calluses. (D) Von Kossa-stained sections show unbridged callus at 3WPF in mutant fractures and undifferentiated mesenchyme at the center of the callus. wb; woven bone.
To further understand the cartilage defect, immunohistochemistry was performed for specific markers of chondrogenic differentiation. Sox9 and Sox5, the earliest transcription factors expressed during chondrocyte differentiation, are expressed broadly in both control and Hox11 mutant fracture calluses (Figure 4Ci and Supplemental Figure 2A). However, mature cartilage markers, Collagen2a1 (resting, proliferating and pre-hypertrophic chondrocytes) and Collagen10a1 (hypertrophic chondrocytes), are clearly reduced in mutants (Figure 4Cii-iii). The ossification of cartilage as visualized by Von Kossa staining is abundant in control calluses and this is also significantly reduced in the Hox11 mutant callus (Figure 4D). Areas of mesenchyme can also be visualized at the center of Hox11 mutant calluses by this method (Figure 4D). Combined with the callus histomorphometry (increased mesenchyme and decreased Safranin O), these data indicate a defect in chondrocyte differentiation that limits endochondral ossification.
Bone modeling is disrupted to due loss of Hox11 function
By late stages of repair, the majority of Hox11 mutant animals demonstrate successful fracture union (Figure 2F). However, during the remodeling phases of repair, mutant animals display a notable impairment as late as 21 WPF with all mutant animals retaining significant woven bone throughout the central callus region, unlike control animals that have largely re-established pre-fracture morphology by 12 WPF (Figure 2E and G). To explore a possible cause for this phenotype, osteoclasts were examined. Quantification of Tartrate Resistant Acid Phosphatase (TRAP) reveals that osteoclasts are present at a similar density in control and mutant fracture injuries (Figure 5A-B). Cathepsin K, an enzyme that functions in bone-resorbing osteoclasts(41), is similarly expressed in both groups (Figure 5C). However, close examination reveals many osteoclasts in Hox11 mutant fracture injuries are enlarged and detached from the bone surface, which may contribute to incomplete remodeling of Hox11 mutant fracture injuries (Figure 5D). Maintained osteoid at the bone surface can explain such an observation, however Von Kossa and basic fuchsin staining does not reveal differences in osteoid deposition between controls and mutants (Supplemental Figure 3).
Figure 5. Osteoclasts are present, express markers of resorption in the Hox11 mutant callus.
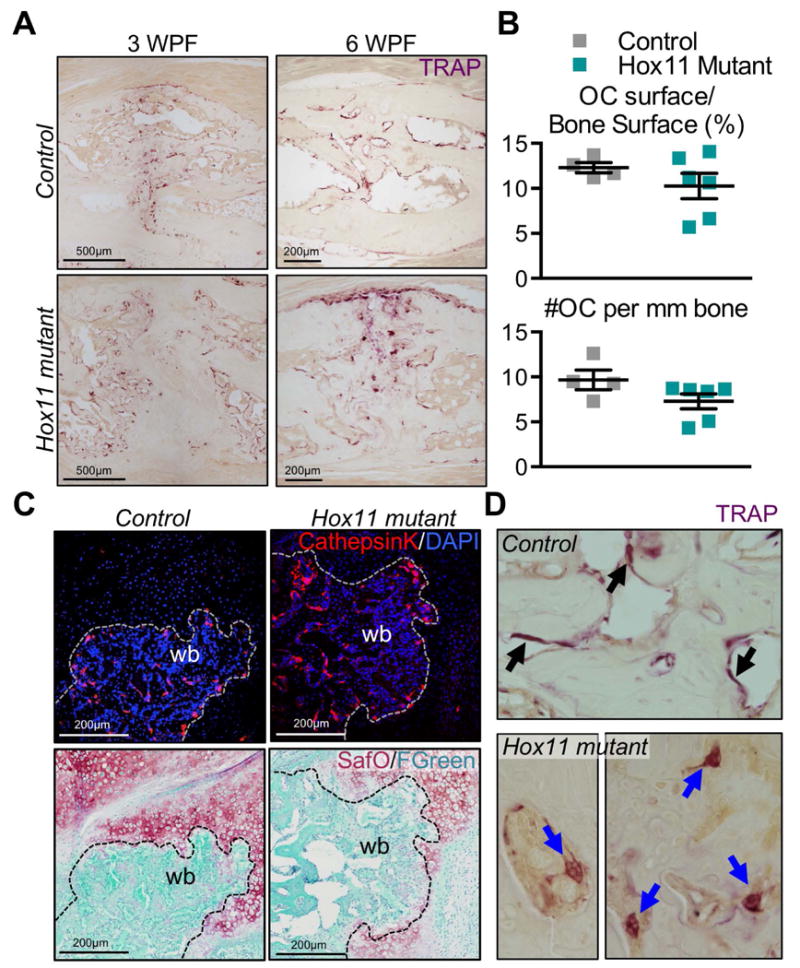
(A) TRAP-stained callus sections from control and Hox11 mutant animals at 3 and 6 WPF show TRAP+ osteoclasts in calluses. (B) Histomorphometric quantification of osteoclasts per bone surface (%) and number of osteoclasts per 1mm of bone surface is comparable in controls and mutants. (C) Cathepsin K-stained callus sections from control and mutant animals show positive staining in controls and mutants. Safranin O/Fast Green staining on the same sections shows the overlap of CathepsinK with woven bone areas. (D) Images of large, detached osteoclasts in the Hox11 mutant callus compared to control osteoclasts that are flat and attached to the bone surface.
Previous work demonstrates that Hox11 genes are not expressed in hematopoietic cells at any stage of development or repair(24,25), therefore, the defects associated with reduced osteoclast attachment are presumably non-cell autonomous, but may be caused by alterations in bony matrix. By μCT, measurements of bone and mineral density (BV/TV, BMD, BMC and TMD) are unchanged between controls and Hox11 mutants at all time points examined (Table 1), and is consistent with a recent study of compound mutants through postnatal stages of development(42). Raman spectroscopy was used to provide a more powerful analysis of possible bone matrix abnormalities in Hox11 mutant animals. This unique and more sensitive tool is capable of assessing bone quality parameters by measuring relative changes in mineral-to-matrix ratios, mineral crystallinity, and collagen crosslink ratios, providing information on tissue-level material properties(43). For this study, the technique was employed on woven bone in the fracture callus, and on cortical bone outside the callus in the fractured limb and on the contralateral limb. Analyses of these fractures show no statistically significant changes in mineral crystallinity, and are consistent with μCT results (Figure 6A and Table 1)(42). However, mineral-to-matrix ratios in the cortical bone of Hox11 mutants are significantly increased compared to controls (Figure 6B). Together, these data are consistent with the absence of changes in mineralization, but abnormalities in the proper organization of bone matrix.
Table 1.
Micro-computed tomography (μCT) parameters measured during fracture injury repair.
| Measured Parameter | Unfractured | 1.5 weeks post fracture | 3 weeks post fracture | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Control (n=6) | Hox11 mutant (n=8) | Control (n=5) | Hox11 mutant (n=8) | Control (n=5) | Hox11 mutant (n=6) | |
| Callus Volume (mm3) | 0.45 (0.03) | 0.52 (0.04)* | 1.81 (0.45) | 2.75 (0.72)* | 1.74 (0.22) | 1.59 (0.50) |
| Bone Volume (mm3) | 0.32 (0.01) | 0.35 (0.03)* | 0.52 (0.13) | 0.78 (0.22)* | 0.57 (0.08) | 0.57 (0.21) |
| BV/TV (%) | .71 (0.02) | 0.67 (0.02) | .32 (0.18) | 0.28 (0.03) | 0.33 (0.05) | 0.35 (0.05) |
| BMD (mg/cc) | 839.6 (40.7) | 740.2 (61.8) | 486.0 (173.2) | 424.1 (101.1) | 511.3 (30.4) | 480.2 (77.5) |
| BMC (mg) | .36 (0.01) | 0.39 (0.05) | 0.82 (0.15) | 1.07 (0.31) | 0.89 (0.08) | 0.78 (0.32) |
| TMD (mg/cc) | 977.0 (32.0) | 905.6 (85.4) | 935.3 (101.8) | 860.7 (101.4) | 838.0 (31.3) | 792.5 (74.3) |
|
| ||||||
| Measured Parameter | 6 weeks post fracture | 12 weeks post fracture | 21 weeks post fracture | |||
|
| ||||||
| Control (n=5) | Hox11 mutant (n=6) | Control (n=5) | Hox11 mutant (n=4) | Control | Hox11 mutant (n=5) | |
|
| ||||||
| Callus Volume (mm3) | 1.45 (0.25) | 1.61 (0.62) | 0.99 (0.12) | 1.52 (0.27)* | ND | 1.49 (0.16)* |
| Bone Volume (mm3) | 0.79 (0.13) | 0.81 (0.28) | 0.63 (0.10) | 0.93 (0.04)* | ND | 0.86 (0.06)* |
| BV/TV (%) | 0.55 (0.07) | 0.51 (0.05) | 0.64 (0.04) | 0.63 (0.09) | ND | 0.58 (0.04) |
| BMD (mg/cc) | 664.3 (83.7) | 606.4 (60.2) | 796.2 (49.3) | 817.7 (104.3) | ND | 751.2 (24.3) |
| BMC (mg) | 0.96 (0.17) | 0.97 (0.34) | 0.79 (0.13) | 1.22 (0.07)* | ND | 1.09 (0.12)* |
| TMD (mg/cc) | 896.8 (48.6) | 866.9 (37.1) | 1036.2 (26.4) | 1054.0 (72.6) | ND | 983.3 (57.1) |
ND, no data collected. Parameters measured include callus volume, total bone volume, bone volume fraction (BV/TV), bone mineral density (BMD), bone mineral content (BMC), and tissue mineral density (TMD). Statistical analyses carried out by Students' T-test;
= p<0.05.
Figure 6. Bone matrix organization is disrupted due to the loss of Hox11 function.
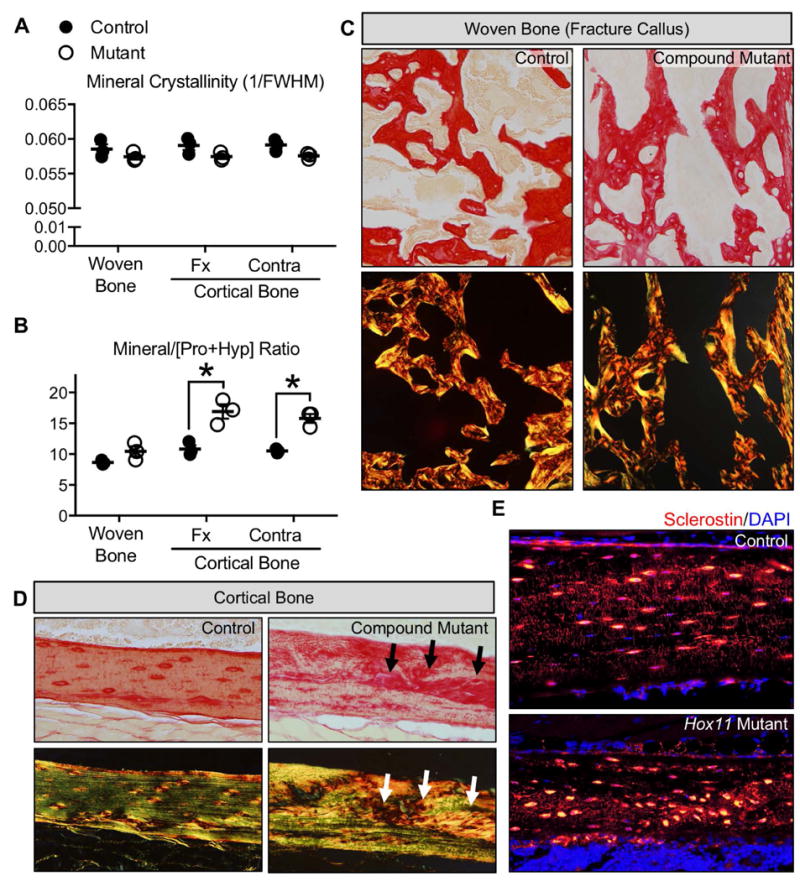
(A-B) Raman spectroscopy of woven bone callus and cortical bone outside the callus from controls and Hox11 mutants at 3 WPF. Parameters measured include mineral crystalinity (A) and mineral to matrix [Pro+Hyp] ratio (B). (C-D) Picrosirius red-stained sections with brightfield (top panels) or polarized light microscopy (bottom panels) for woven bone in fracture callus (C) and cortical bone out the callus (D). (E) Sclerostin-stained cortical bone shows disorganized osteocytes in Hox11 mutants.
Specific matrix bands related to the collagen crosslinks ratio could not be analyzed by Raman spectroscopy in this study due to polymer interference of the PMMA-embedded specimens (data not shown). To further evaluate defects in matrix organization, Picrosirius Red staining combined with polarized light microscopy was performed(44). Using this technique, an organized or directional matrix appears linear (green) under polarized light, while disorganized or multidirectional matrix (red/orange) yields a basket-weave appearance. New woven bone generated in the fracture callus is highly disorganized in both control and Hox11 mutant calluses as is expected for this rapid bone deposition (Figure 6C). However, Hox11 mutant animals also display significant disorganization in their cortical bone matrix (Figure 6D and Supplemental Figure 4). In controls, Sclerostin staining osteocytes are regularly spaced and well organized in the bone matrix (Figure 6E). In Hox11 mutants, however, osteocytes embedded in cortical bone exhibit irregular spacing and areas of aggregation within the bone matrix (Figure 6E).
Discussion
Our understanding of Hox transcription factors in the skeleton is largely limited to embryonic development, however, recent studies have shown that Hox genes are re-expressed during repair, consistent with possible functions during fracture repair(21-23,45). Transcriptome analyses have shown broad increases in the expression of various Hox genes throughout fracture repair of the femur(21,22). Additionally, differential expression (and function) of Hox genes has been suggested as a cause for scar formation in a transplant study where periosteal progenitor cells from different anatomical locations were swapped in fracture injuries(23). When Hox+ cells were transplanted into a mandibular injury (a site normally negative for Hox expressing cells), chondrocytes formed and differentiated to cartilage at this injury site. This is a skeletal region that would have normally healed by intramembranous ossification. This supports a possible function for Hox genes in adult skeletal regeneration, but genetic loss-of-function analyses at endogenous sites of potential Hox activity have been lacking.
We report clear, regional expansion of Hox11-expressing cells in response to fracture injury of the zeugopod, the region of the skeleton that expresses Hox11 genes during development. Complete loss of Hox11 paralogous group function results in severe malformations during embryonic development and neonatal lethality that preclude adult studies. Using a sensitized background of compound Hox11 loss-of-function animals, we show that loss of Hox11 function results in defects in endochondral ossification during early callus formation and in impairment of the bone remodeling phase of repair.
We provide evidence that Hox11 genes function in cartilage differentiation following fracture injury. The differentiation of Hox-expressing mesenchymal stem/stromal cells (MSCs) to chondrocytes during soft callus formation is disrupted. Mesenchyme is abundant in the Hox11 mutant callus, and Sox9 and Sox5 (expressed in mesenchymal progenitors) are expressed broadly, however downstream differentiation markers Collagen2a1, Collagen10a1, and Safranin O are markedly reduced. These combined data support that loss of Hox11 function results in the failure of mesenchyme to differentiate to mature chondrocytes. Notably, these results are consistent with defects reported during embryonic limb formation with loss of Hox11 function and with our more recent work that demonstrates loss of chondrogenic differentiation in vitro from MSCs that carry these same mutations(25,46). The decrease, but not complete absence, of cartilage in this fracture model is likely the result of incomplete loss of Hox11 gene function (one wild-type remains in Hox11 compound mutants).
We also show loss of Hox11 leads to defects during the remodeling phase after fracture injury. Osteoclasts are present, but many are detached from the bony matrix. Our observation of detached osteoclasts could be a result of changes in bone matrix disorganization in Hox11 compound mutant animals, but this will require further study.
The restriction of paralogous group expression and function in skeletal patterning at specific anatomical locations during embryonic development is a key feature of Hox genes. The work described here supports a continued function for Hox genes in the vertebrate skeleton during adult fracture repair. Our previous worked showed that Hox11 expression remains regionally restricted throughout postnatal growth, is maintained in the adult skeleton and expands in fracture injuries only in the zeugopod. Regional expression in the adult animal mirrors the pattern of expression and function during embryonic skeletal development. Importantly, we also reported that femur (stylopod) fracture injuries performed on Hox11 loss-of-function animals display no defects in the repair process(25). We would predict that different Hox paralogs perform similar activities at different anatomical locations. For example, Hox10 paralogous group genes are required for patterning of the femur (stylopod) during embryonic development(10,15) and, given our work on Hox11 genes, would likely be required for repair of these structures, but this has yet to be tested. Perhaps the most interesting question is whether different paralog contribution in unique ways to the bone healing and remodeling process. Certainly, different Hox paralogs contribute differential patterning information during embryonic development. This will be an important area for future study. Overall, our results suggest that regionally specific Hox function is an important and previously unappreciated mechanism required for successful fracture healing.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 AR061402 (DMW), T32 DE007057 (DRR, JYS, KMP), and T32 HD007505 (DRR, KMP), P30 AR069620 (Karl Jepsen, PI; David H. Kohn, Core Director), the 2016 Endowment for the Development of Graduate Education (EDGE, EBS-Michigan, DRR), the Bradley M Patten Fellowship (CDB-Michigan, DRR, KMP) and the University of Michigan Summer Biomedical and Life Sciences Fellowship (AJS). We thank Kathy Sweet, Bonnie Nolan, Charles Roehm, John Baker, Carol Whitinger, Basma Khoury and Joseph Perosky of the Michigan Orthopedic Research Labs for technical support during fracture surgeries, plastic embedding and μCT analyses. We thank Yuji Mishina for guidance on Picrosirius Red staining. We also thank Drs. Benjamin Allen, Renny Franceschi, Karl Jepsen, Ernestina Schipani, Laurie McCauley, Daniel Lucas and Michael Morris and numerous Wellik lab members for their advice and critique of the work.
Footnotes
Disclosures: We have no disclosures, MTAs, patents or patent applications to report.
Authors' roles: Study concept and design: DRR, DMW. Provision of study materials: DMW. Fracture model design: SAG, KMK. Data collection and experimentation: DRR, JYS, KMP, GSM, ILT, AJS, KNG. ILT and AJS conducted many of the preliminary studies leading to this work. Data interpretation: DRR, ILT, GSM, SAG, KMK, DMW. Manuscript writing: DRR and DMW. Revised manuscript content: AJS, GSM, SAG, KMK, DMW.
References
- 1.Bolander ME. Regulation of fracture repair by growth factors. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1992;200(2):165–170. doi: 10.3181/00379727-200-43410a. [DOI] [PubMed] [Google Scholar]
- 2.Einhorn TA. The cell and molecular biology of fracture healing. Clinical orthopaedics and related research. 1998;355(Suppl):S7–21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson C, Alpern E, Miclau T, Helms JA. Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev. 1999;87(1-2):57–66. doi: 10.1016/s0925-4773(99)00142-2. [DOI] [PubMed] [Google Scholar]
- 4.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. Journal of cellular biochemistry. 2003;88(5):873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 5.Vortkamp A, Pathi S, Peretti GM, Caruso EM, Zaleske DJ, Tabin CJ. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech Dev. 1998;71(1-2):65–76. doi: 10.1016/s0925-4773(97)00203-7. [DOI] [PubMed] [Google Scholar]
- 6.Duboule D. Temporal colinearity and the phylotypic progression: a basis for the stability of a vertebrate Bauplan and the evolution of morphologies through heterochrony. Dev Suppl. 1994:135–142. [PubMed] [Google Scholar]
- 7.Iimura T, Pourquie O. Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature. 2006;442(7102):568–571. doi: 10.1038/nature04838. [DOI] [PubMed] [Google Scholar]
- 8.Mallo M, Wellik DM, Deschamps J. Hox genes and regional patterning of the vertebrate body plan. Dev Biol. 2010;344(1):7–15. doi: 10.1016/j.ydbio.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Condie BG, Capecchi MR. Mice with targeted disruptions in the paralogous genes hoxa-3 and hoxd-3 reveal synergistic interactions. Nature. 1994;370(6487):304–307. doi: 10.1038/370304a0. [DOI] [PubMed] [Google Scholar]
- 10.Fromental-Ramain C, Warot X, Lakkaraju S, et al. Specific and redundant functions of the paralogous Hoxa-9 and Hoxd-9 genes in forelimb and axial skeleton patterning. Development. 1996;122(2):461–472. doi: 10.1242/dev.122.2.461. [DOI] [PubMed] [Google Scholar]
- 11.Horan GS, Ramirez-Solis R, Featherstone MS, Wolgemuth DJ, Bradley A, Behringer RR. Compound mutants for the paralogous hoxa-4, hoxb-4, and hoxd-4 genes show more complete homeotic transformations and a dose-dependent increase in the number of vertebrae transformed. Genes Dev. 1995;9(13):1667–1677. doi: 10.1101/gad.9.13.1667. [DOI] [PubMed] [Google Scholar]
- 12.Kostic D, Capecchi MR. Targeted disruptions of the murine Hoxa-4 and Hoxa-6 genes result in homeotic transformations of components of the vertebral column. Mech Dev. 1994;46(3):231–247. doi: 10.1016/0925-4773(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 13.van den Akker E, Fromental-Ramain C, de Graaff W, et al. Axial skeletal patterning in mice lacking all paralogous group 8 Hox genes. Development. 2001;128(10):1911–1921. doi: 10.1242/dev.128.10.1911. [DOI] [PubMed] [Google Scholar]
- 14.Wellik DM. Hox genes and vertebrate axial pattern. Current topics in developmental biology. 2009;88:257–278. doi: 10.1016/S0070-2153(09)88009-5. [DOI] [PubMed] [Google Scholar]
- 15.Wellik DM, Capecchi MR. Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science. 2003;301(5631):363–367. doi: 10.1126/science.1085672. [DOI] [PubMed] [Google Scholar]
- 16.Boulet AM, Capecchi MR. Multiple roles of Hoxa11 and Hoxd11 in the formation of the mammalian forelimb zeugopod. Development. 2004;131(2):299–309. doi: 10.1242/dev.00936. [DOI] [PubMed] [Google Scholar]
- 17.Davis AP, Witte DP, Hsieh-Li HM, Potter SS, Capecchi MR. Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature. 1995;375(6534):791–795. doi: 10.1038/375791a0. [DOI] [PubMed] [Google Scholar]
- 18.Fromental-Ramain C, Warot X, Messadecq N, LeMeur M, Dolle P, Chambon P. Hoxa-13 and Hoxd-13 play a crucial role in the patterning of the limb autopod. Development. 1996;122(10):2997–3011. doi: 10.1242/dev.122.10.2997. [DOI] [PubMed] [Google Scholar]
- 19.Izpisua-Belmonte JC, Duboule D. Homeobox genes and pattern formation in the vertebrate limb. Dev Biol. 1992;152(1):26–36. doi: 10.1016/0012-1606(92)90153-8. [DOI] [PubMed] [Google Scholar]
- 20.Zakany J, Duboule D. The role of Hox genes during vertebrate limb development. Curr Opin Genet Dev. 2007;17(4):359–366. doi: 10.1016/j.gde.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Bais M, McLean J, Sebastiani P, et al. Transcriptional analysis of fracture healing and the induction of embryonic stem cell-related genes. PloS one. 2009;4(5):e5393. doi: 10.1371/journal.pone.0005393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gersch RP, Lombardo F, McGovern SC, Hadjiargyrou M. Reactivation of Hox gene expression during bone regeneration. J Orthop Res. 2005;23(4):882–890. doi: 10.1016/j.orthres.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Leucht P, Kim JB, Amasha R, James AW, Girod S, Helms JA. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development. 2008;135(17):2845–2854. doi: 10.1242/dev.023788. [DOI] [PubMed] [Google Scholar]
- 24.Swinehart IT, Schlientz AJ, Quintanilla CA, Mortlock DP, Wellik DM. Hox11 genes are required for regional patterning and integration of muscle, tendon and bone. Development. 2013;140(22):4574–4582. doi: 10.1242/dev.096693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rux DR, Song JY, Swinehart IT, et al. Regionally Restricted Hox Function in Adult Bone Marrow Multipotent Mesenchymal Stem/Stromal Cells. Dev Cell. 2016;39(6):653–666. doi: 10.1016/j.devcel.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinho S, Lacombe J, Hanoun M, et al. PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. The Journal of experimental medicine. 2013;210(7):1351–1367. doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15(2):154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rux DR, Wellik DM. Hox genes in the adult skeleton: Novel functions beyond embryonic development. Dev Dyn. 2016 doi: 10.1002/dvdy.24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson LT, Rakshit S, Sun H, Wellik DM. Generation and expression of a Hoxa11eGFP targeted allele in mice. Dev Dyn. 2008;237(11):3410–3416. doi: 10.1002/dvdy.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008;19(5):459–466. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nature reviews Rheumatology. 2015;11(1):45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24(2):274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizoguchi T, Pinho S, Ahmed J, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29(3):340–349. doi: 10.1016/j.devcel.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakahara H, Bruder SP, Goldberg VM, Caplan AI. In vivo osteochondrogenic potential of cultured cells derived from the periosteum. Clinical orthopaedics and related research. 1990;259:223–232. [PubMed] [Google Scholar]
- 36.Park D, Spencer JA, Koh BI, et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10(3):259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Worthley DL, Churchill M, Compton JT, et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160(1-2):269–284. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 39.Colnot C, Lu C, Hu D, Helms JA. Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Dev Biol. 2004;269(1):55–69. doi: 10.1016/j.ydbio.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Hausman MR, Schaffler MB, Majeska RJ. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 2001;29(6):560–564. doi: 10.1016/s8756-3282(01)00608-1. [DOI] [PubMed] [Google Scholar]
- 41.Saftig P, Hunziker E, Wehmeyer O, et al. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci U S A. 1998;95(23):13453–13458. doi: 10.1073/pnas.95.23.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pineault KM, Swinehart IT, Garthus KN, et al. Hox11 genes regulate postnatal longitudinal bone growth and growth plate proliferation. Biology open. 2015;4(11):1538–1548. doi: 10.1242/bio.012500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandair GS, Morris MD. Contributions of Raman spectroscopy to the understanding of bone strength. BoneKEy reports. 2015;4(620) doi: 10.1038/bonekey.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11(4):447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 45.Wang KC, Helms JA, Chang HY. Regeneration, repair and remembering identity: the three Rs of Hox gene expression. Trends in cell biology. 2009;19(6):268–275. doi: 10.1016/j.tcb.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gross S, Krause Y, Wuelling M, Vortkamp A. Hoxa11 and Hoxd11 regulate chondrocyte differentiation upstream of Runx2 and Shox2 in mice. PloS one. 2012;7(8):e43553. doi: 10.1371/journal.pone.0043553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


