Abstract
We present an overview of the toxicological profile of the fast acting, lipophilic macrocyclic imine toxins, an emerging family of organic compounds associated with algal blooms, shellfish contamination and neurotoxicity. Worldwide, shellfish contamination incidents are expanding; therefore the significance of these toxins for the shellfish food industry deserves further study. Emphasis is directed to the dinoflagellate species involved in their production, their chemical structures, and their specific mode of interaction with their principal natural molecular targets, the nicotinic acetylcholine receptors, or with the soluble acetylcholine-binding protein, used as a surrogate receptor model. The dinoflagellates Karenia selliformis and Alexandrium ostenfeldii / A. peruvianum have been implicated in the biosynthesis of gymnodimines and spirolides, while Vulcanodinium rugosum is the producer of pinnatoxins and portimine. The cyclic imine toxins are characterized by a macrocyclic skeleton comprising 14 to 27 carbon atoms, flanked by two conserved moieties, the cyclic imine and the spiroketal ring system. These phycotoxins generally display high affinity and broad specificity for the muscle-type and neuronal nicotinic acetylcholine receptors, a feature consistent with their binding site at the receptor subunit interfaces, composed of residues highly conserved among all nAChRs, and explaining the diverse toxicity among animal species.
Keywords: acetylcholine binding protein, dinoflagellates, gymnodimines, marine phycotoxins, muscarinic acetylcholine receptor, nicotinic acetylcholine receptor, pinnatoxins, spirolides
Fast acting, lipophilic macrocyclic imine molecules from dinoflagellates form an emerging family of toxins associated with algal blooms, shellfish contamination and neurotoxicity. This overview covers the species involved in their production, their chemical structures, and their specific modes of interaction with their principal natural molecular targets, the nAChRs, or with the soluble surrogate receptor model, AChBP.
Introduction
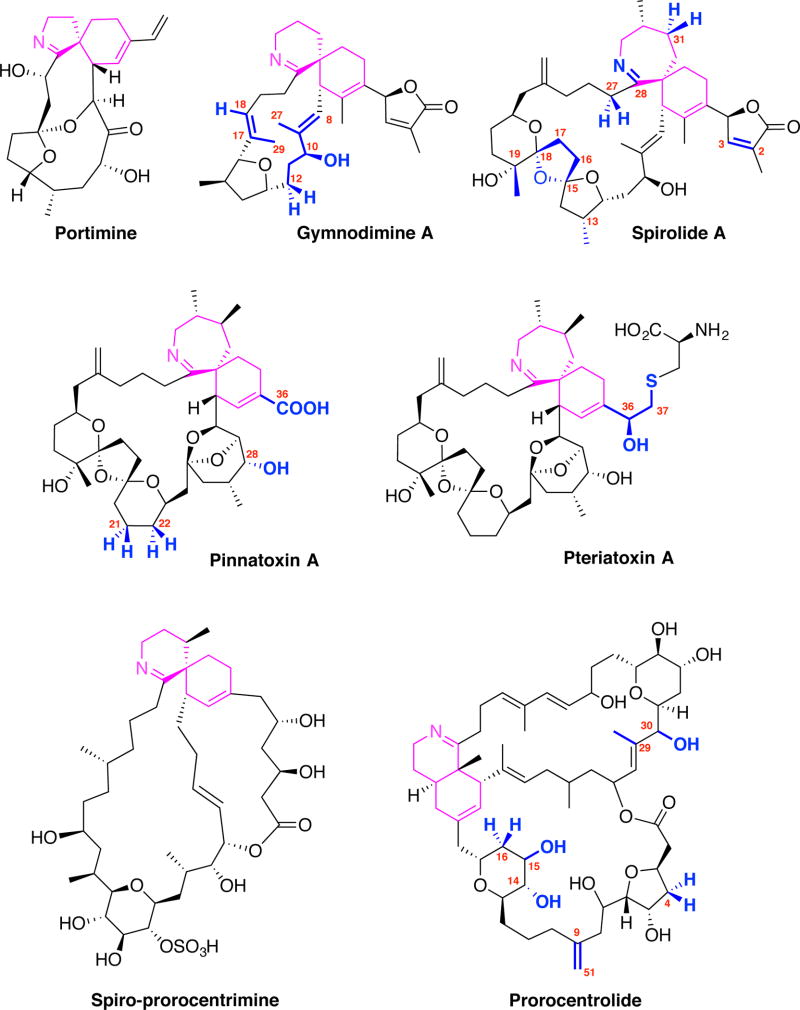
The cyclic imine toxins comprise a growing family of lipophilic organic compounds produced by some species of marine dinoflagellate microorganisms. These neurotoxins have been found in extracts from contaminated shellfish (mainly bivalves), from natural plankton assemblages, from clonal cultures of toxic dinoflagellates, and as resulting products of the shellfish metabolism (fatty acid acyl esters) (for reviews see Molgó et al. 2007; Guéret and Brimble 2010; Stivala et al. 2015). Currently, the cyclic imine family of toxins is exemplified by 40 molecules (not taking into account the acylated esters) and comprises the prorocentrolides, spiro-prorocentrimine, gymnodimines, spirolides, pinnatoxins, pteriatoxins, and portimine (Stivala et al. 2015). Spirolides represent the largest family.
Cyclic imine toxins can accumulate in bivalve mollusks and are considered as emergent toxicants for food safety in the shellfish food industry. Although their potent neurotoxicity led concern on their potential risks to shellfish consumers, currently these toxins are not regulated.
This short review aims at providing an overview of the origin, toxicological profile, chemical structure, and mode of action of the lipophilic cyclic imine toxins, with particular emphasis on their specificity of interaction with muscle-type and neuronal nicotinic acetylcholine (ACh) receptors (nAChR), which are the main molecular targets involved in their toxicity. The nAChRs are prototypical cation-selective, ligand-gated ion channels (LGIC) that mediate fast neurotransmission in the central and peripheral nervous systems (reviewed by Albuquerque et al. 2009). They belong to the Cys-loop superfamily of LGICs and are formed by distinct combinations of five subunits that confer selectivity in pharmacological properties and cellular location (Corringer et al. 2000; Tsetlin et al. 2011).
Dinoflagellate species involved in the production of cyclic imine toxins
Several species of dinoflagellates, distributed worldwide in tropical, temperate and cold marine waters, have been confirmed to be responsible for the production of cyclic imine toxins. Among the Prorocentrum species, P. lima and P. maculosum have been linked to the production of prorocentrolides A and B and spiro-prorocentrimine (Torigoe et al., 1988; Hu et al. 1996b; Lu et al. 2001).
Gymnodimines A, B and C have been shown to be produced by a species of dinoflagellates named Karenia selliformis (Miles et al. 2000; 2003; Haywood et al. 2004). However, other gymnodimine analogs like 12-methyl gymnodimine A and 12-methyl gymnodimine B are produced by the dinoflagellate Alexandrium peruvianum and A. ostenfeldii (Van Wagoner et al. 2011; Van de Waal et al. 2015; Strangman et al. 2016). Also, a new gymnodimine D was found in A. ostenfeldii clonal cultures isolated from the Northern Baltic Sea (Harju et al. 2016). The production of these various gymnodimine analogs indicates that common biosynthetic pathways are shared by distinct species of dinoflagellates.
The toxigenic dinoflagellate A. ostenfeldii and the closely related A. peruvianum have been reported to produce the large family of spirolides (Cembella et al. 1999, 2000; Touzet et al. 2008; Borkman et al. 2012). However, the profile of toxin production seems to differ with environmental conditions, genetic factors, and the location where the dinoflagellates are collected (Otero et al. 2010; Gu, 2011; Suikkanen et al. 2013; Kremp et al. 2014; Almandoz et al. 2014; Salgado et al. 2015). A. ostenfeldii / A. peruvianum appear capable of producing saxitoxin and analogs (Hakanen et al. 2012; Anderson et al. 2012; Van de Waal et al. 2015; Savela et al. 2016; Kremp et al. 2016), that bind and block voltage-gated sodium channels (Catterall, 1980), yet the cyclic imine toxins appear as the primary toxic agent in most of the world regions. During blooms of A. ostenfeldii / A. peruvianum, morphological uniqueness of the dinoflagellates can no longer be used as the indicator of the presumed toxin produced, and should always be accompanied by the chemical characterization of the toxin profile to identify the type(s) of toxins.
Indistinguishable peridinoid dinoflagellate strains were reported to be responsible for the production of pinnatoxins E, F, and G in Australia, pinnatoxin G in Japan, and pinnatoxins E and F in New Zealand (Smith et al. 2011; Rhodes et al. 2010; Rhodes et al. 2011a,b). Then, identification of the dinoflagellate producers of pinnatoxins was associated with the discovery, in coastal waters of France, of a novel dinoflagellate species, Vulcanodinium rugosum (Nézan and Chomérat, 2011), that produced mainly pinnatoxin G (Hess et al. 2013). The smallest cyclic imine toxin so far isolated from V. rugosum was named portimine (Figure 1), which is distinguished by a five-membered cyclic imine ring and a macrocycle of only 14 carbon atoms (Selwood et al. 2013), while pinnatoxins contain a seven-membered cyclic imine ring. In contrast to other cyclic imine toxins, portimine exhibits very low acute toxicity in the mouse, but is extremely toxic to human Jurkat T-lymphoma cells and mouse embryonic fibroblasts cells in culture. Cells treated with portimine display rapid caspase activation and phosphatidylserine exposure, suggestive of apoptotic cell death (Cuddihy et al. 2016). To the best of our knowledge none of the known cyclic imine toxins has been reported to activate caspase-3 and initiate apoptosis, as portimine does. Among toxins produced by the dinoflagellate V. rugosum neither pinnatoxin F nor pinnatoxin G were reported to induce apoptosis (Cuddihy et al. 2016).
Fig. 1.
Chemical structures of cyclic imine toxins. Only the first-described member of each family is represented. The positions where variations in structure were reported are numbered and displayed in blue. The cyclic imine moieties and attached rings are displayed in magenta.
Another pinnatoxin congener (pinnatoxin H) was purified from cultures of V. rugosum collected from the South China Sea (Selwood et al. 2014) and in the marine waters of Qatar, Arabian Gulf (Al Muftah et al. 2016). V. rugosum strains of various origins have been reported to produce not only different pinnatoxins, but also to differ from each other in partial large subunit rDNA, internal transcribed spacer regions, and 5.8S rDNA sequences (Rhodes et al. 2011b), suggesting a complex variation of splicing options. At present, no definitive evidence has been published regarding a dinoflagellate origin of pteriatoxins.
Chemical structure
The general chemical structure of cyclic imine toxins encompasses a macrocyclic ring of 14 to 27 carbon atoms, and two highly conserved moieties: the cyclic imine group (mainly found as a spiroimine) and the spiroketal ring system. The cyclic imines are composed of a 5-membered (portimine), 6-membered (gymnodimines, spiroprorocentrimine, prorocentrolides) or 7-membered rings (spirolides, pinnatoxins, pteriatoxins); all are considered as essential components for bio-activity. Amino-ketone derivatives with an open imine ring, e.g. spirolides E and F (Hu et al. 1996a) or the amino-ketone form of pinnatoxin A (Aráoz et al. 2011; Bourne et al. 2015) are devoid of biological activity. The other features of the ring system can be a simple tetrahydrofuran (in portimine and gymnodimines) or a tetrahydropyran group (prorocentrolides and spiroprorocentrimine), but also more complex 6,5- (spirolides H and I), 6,6,5- (spirolide G), 6,5,5- (spirolides A–F) or 6,5,6-spiroketal moieties (pinnatoxins and pteriatoxins), as shown in Fig. 1.
Several approaches for the synthesis of cyclic imine toxins were reported during the last few years (Beaumont et al. 2010; for reviews see Molgó et al. 2014; Stivala et al. 2015), which were focused mainly on pinnatoxin A (McCauley et al. 1998; Sakamoto et al. 2004; Nakamura et al. 2008; Stivala and Zakarian 2008; Aráoz et al. 2011), pinnatoxins B and C (Matsuura et al. 2006a), pteriatoxins (Matsuura et al. 2006b) and gymnodimine (Kong et al. 2009; Kong et al. 2011). A fragment of gymnodimine A containing the 6,6-spiroimine core induced significant inhibition of ACh-evoked nicotinic currents generated by the nAChRs, a feature revealing the critical role of this moiety for the biological activity of the toxin (Duroure et al. 2011). The unusual stability to hydrolysis of the spiroimine group in pinnatoxin A, which is related to the high oral toxicity of this phycotoxin compared to its congeners (Molgó et al. 2015), was demonstrated by in vitro experiments performed under conditions addressing stability and by computational studies (Jackson et al. 2012).
Mode of action on muscle-type nAChRs
The nAChR from the Torpedo electric organ, a prototype of the vertebrate skeletal muscle nAChR, is a transmembrane heteropentameric molecule composed of four homologous subunits with a α12βγδ stoichiometry. Two binding sites with distinct binding affinities for the nicotinic agonists and competitive antagonists are located at the α-γ and α-δ subunit interfaces. Functional studies revealed that nanomolar concentrations of gymnodimine A (Kharrat et al. 2008), 13-desmethyl spirolide C and 13,19-didesmethyl spirolide C (Aráoz et al. 2015), 20-methyl spirolide G (Couesnon et al. 2016), and pinnatoxins E, F and G (Hellyer et al. 2013) blocked, in a concentration- and time-dependent manner, muscle twitches evoked by nerve stimulation in isolated mouse or rat nerve-muscle preparations without affecting the directly elicited muscle twitches, a feature indicating that these phycotoxins alter neuromuscular transmission without affecting the excitation-contracting coupling process. This interpretation was confirmed by electrophysiological recordings performed at single neuromuscular junctions, which showed that gymnodimine A (Kharrat et al. 2008), 13-desmethyl spirolide C and 13,19-didesmethyl spirolide C (Aráoz et al. 2015), and pinnatoxins E, F and G (Hellyer et al. 2013), either reduced or completely blocked (depending on concentration) the amplitude of spontaneous miniature endplate potentials, an effect suggesting that the toxins impeded the interaction of ACh quanta with endplate nAChRs. In addition, the toxins blocked nerve-evoked endplate potentials without affecting the resting membrane potential of muscle fibers, so that endplate potentials could no longer reach the threshold for opening voltage-gated sodium channels in muscle fibers, and could not trigger a muscle action potential. These in vitro findings are consistent with in vivo studies in anesthetized mice showing that a local injection of toxins caused a dose-dependent block of the maximal compound muscle action potential (CMAP) amplitude, evoked by nerve stimulation. The effective doses needed to block 50% of the maximal CMAP amplitude (ED50) were 1.7 ng/kg for 20-methyl spirolide G (Couesnon et al. 2016) and 6 ng/kg for 13-desmethyl spirolide C, which in turn was about 300 fold more active than gymnodimine A on an equimolar basis (Marrouchi et al. 2013). Overall, these studies indicated that these cyclic imine toxins potently block neuromuscular transmission on junctions expressing the mature muscle-type (α12β1δε) nAChR.
Further studies were carried out using Xenopus skeletal myocytes expressing the embryonic muscle-type α12β1γδ nAChR at their membrane surface, and the patch-clamp technique. Under these conditions, it was disclosed that gymnodimine A blocked nicotinic currents caused by short (5 ms) iontophoretic pulses of ACh, and that the block of ACh-evoked current exhibited no voltage-dependency, and was persistent but reversible (Kharrat et al. 2008). Comparable results were obtained with 13-desmethyl spirolide C (Aráoz et al. 2015).
To gain further insight into the interaction between cyclic imine toxins and nAChRs, studies were performed on Xenopus oocytes microtransplanted with purified electrocyte membranes prepared from the electric organ of the fish Torpedo marmorata that expresses muscle-type α12β1γδ nAChR. Microinjection of the purified electrocyte membranes to the oocyte cytosol allows rapid incorporation of native and functional α12β1γδ nAChR into the oocyte membrane (for reviews see Miledi et al. 2006 and Eusebi et al. 2009; Bourne et al. 2010). Using this approach and the two-microelectrode voltage-clamp technique, the actions of gymnodimine A, 13-desmethyl spirolide C (Aráoz et al. 2009; Bourne et al. 2010) 13,19-didesmethyl spirolide C (Aráoz et al. 2015), 20-methyl spirolide G (Couesnon et al. 2016), and pinnatoxins A and G (Aráoz et al. 2011; Bourne et al. 2015) were studied. None of the cyclic imines analyzed exhibited by themselves an agonist action on the α12β1γδ nAChR incorporated to the Xenopus oocyte membrane, but they decreased the peak amplitudes of the ACh-elicited nicotinic current in a concentration-dependent manner. The IC50 for the various toxins studied are reported in Table 1. The 6,6-spiroimine synthetic analog of gymnodimine A also blocked ACh-evoked currents in Xenopus oocytes having incorporated the α12β1γδ nAChR (Duroure et al. 2011), but it was much less active than the native toxin.
Table 1.
Inhibition constants (IC50, nM) for the action of some cyclic imine dinoflagellate toxins on ACh-evoked nicotinic currents, recorded from oocytes micro-transplanted with the muscle-type α12β1γδ nAChR, or expressing the human neuronal α7 or α4β2 nAChR subtypes (various subunit stoichiometries for the α4β2).
| Cyclic imine toxin | α12β1γδ (Torpedo) |
α7 (human) |
α4β2 (human) |
Reference |
|---|---|---|---|---|
| 20-meSPX-Ga | 0.36 (0.29–0.45)j | 0.48 (0.15–1.4) | 2.1 (1.4–3.1) | Couesnon et al. 2016 |
| 13,19-ddmeSPX-Cb | 0.20 (0.16–0.26) | 0.25 (0.24–0.27) | 6.26 (4.7–8.3) | Aráoz et al. 2015 |
| 13-SPX-Cc | 0.51 (0.4–0.6) | 0.18 (0.16–0.21) | 3.9 (2.9–5.1) | Bourne et al. 2010 |
| GYM-Ad | 2.8 (1.9–4.1) | 0.9 (0.6–1.2) | Kharrat et al. 2008 | |
| PnTX-Ae | 5.53 (4.5–6.8) | 0.107 (0.086–0.132) | 30.4 (19.4–47.5) | Aráoz et al. 2011 |
| PnTX-Gf | 3.82 (2.99–4.88) | 5.06 (3.84–6.67) | 4.90 (3.97–6.06) | Bourne et al. 2015 |
| PnTx-A AKg | 24,760 (9,771–62,750) | 182,500 (2,213–1,505,000) | >1,000,000 | Bourne et al. 2015 |
| PnTx-Fh | 6.8 ± 1.09k | 16 ± 0.44l 24 ± 2.8m | Hellyer et al. 2015 | |
| PnTX-Gi | 10 ± 2.4 k | 230 ± 15l 105 ± 8.7m | Hellyer et al. 2015 |
20-methyl spirolide G;
13,19-didesmethyl spirolide C;
13-desmethyl spirolide C;
gymnodimine A;
synthetic pinnatoxin A;
pinnatoxin G;
synthetic pinnatoxin A amino ketone;
pinnatoxin F;
pinnatoxin F;
Mean values from concentration-response curves recorded from 46–50 oocytes for each experimental condition, 95% confidence intervals are indicated in parentheses;
Mean ± SEM;
Data obtained with (α4)3(β2)2;
Data obtained with (α4)2(β2)3.
Reversibility of antagonism of the nAChR was relatively fast with gymnodimine A and pinnatoxin A, but it was extremely slow with 13-desmethyl spirolide C, 13,19-didesmethyl spirolide C and 20-methyl spirolide G; the latter compounds exhibited much higher potency than the other phycotoxins (see Table 1).
Competition binding studies revealed that the six cyclic imine toxins studied interact with high affinity with the α12β1γδ nAChR, and totally displaced [125I]α-bungarotoxin from its binding site with dissociation constants in the nanomolar or subnanomolar ranges, as reported in Table 2.
Table 2.
Inhibition constants (Ki ± SEM, nM) obtained from competition binding assays at equilibrium for some cyclic imine toxins on various nAChRs, and comparisons with the nicotinic antagonists α-toxin, methyllycaconitine (MLA) and epibatidine.
| Cyclic imine toxin | α12β1γδ (Torpedo) |
α7-5HT3 (chick) |
α3β2 (human) |
α4β2 (human) |
Reference |
|---|---|---|---|---|---|
| 20-meSPX-Ga | 0.028 ± 0.005i | 0.11 ± 0.08 | 0.040 ± 0.001 | 3.6 ± 0.7 | Couesnon et al. 2016 |
| 13,19-ddmeSPX-Cb | 0.017 ± 0.003 | 0.22 ± 0.06 | 0.51± 0.14 | 53 ± 25 | Aráoz et al. 2015 |
| 13-SPX-Cc | 0.080 ± 0.002 | 0.53 ± 0.08 | 0.021 ± 0.005 | 0.58 ± 0.07 | Bourne et al. 2010 |
| GYM-Ad | 0.23 ± 0.08 | 0.33 ± 0.08 | 0.24 ± 0.09 | 0.62 ± 0.07 | Kharrat et al. 2008 |
| PnTX-Ae | 2.80 ± 0.03 | 0.35 ± 0.04 | 9.4 ± 1.9 | 15.6 ± 5.2 | Aráoz et al. 2011 |
| PnTX-Gf | 0.11 ± 0.04 | 0.72 ± 0.03 | 64 ± 2 | 101 ± 30 | Bourne et al. 2015 |
| α-Toxing | 0.011 ± 0.002 | Couesnon et al. 2016 | |||
| MLAh | 0.83 ± 0.12 | Couesnon et al. 2016 | |||
| Epibatidine | 0.034 ± 0.002 | 0.054 ± 0.011 | Couesnon et al. 2016 |
20-methyl spirolide G;
13,19-didesmethyl spirolide C;
13-desmethyl spirolide C;
gymnodimine A;
synthetic pinnatoxin A;
synthetic pinnatoxin G;
synthetic peptidic α-toxin (Naja nigricollis);
methyllycaconitine;
Values expressed as themean± SEM from 3–4 distinct experiments performed in duplicate.
Several methods for detecting cyclic imine toxins have been set up to substitute the conventional mouse bioassay, which has several weaknesses including specificity, sensitivity and ethical concerns (reviewed in Daneshian et al. 2013). The new methods are based on competition assays where the cyclic imine toxins prevent the interaction of fluorescent- or biotin-labeled α-bungarotoxin with the α12β1γδ nAChR contained in purified Torpedo membranes (Vilariño et al. 2009, Fonfría et al. 2010, Otero et al. 2011, Rodriguez et al. 2011, 2013a; Aráoz et al. 2012; Rubio et al. 2014). Also, a receptor-based detection method was developed using Torpedo nAChR, or Lymnaea stagnalis ACh-binding-protein immobilized on the surface of carboxylated microspheres and the competition of cyclic imines toxins with biotin-α-bungarotoxin for binding to these proteins (Rodriguez et al. 2013b).
Available evidence indicates that both mature (α12β1δε) and embryonic (α12β1γδ) muscle-type nAChRs are important targets for cyclic imine toxins, and are certainly responsible for the fast depression of respiratory neuromuscular transmission during acute toxicity assays in rodents (Munday et al. 2004; 2012).
Mode of action on neuronal-types of nAChRs
Cyclic imine toxins are among the few organic compounds produced by dinoflagellates known to interact with the major neuronal nAChRs, as assessed by functional and ligand-binding assays. Vertebrate neuronal nAChRs comprise a varied population of receptors with assorted subunit assemblies of α2 – α10 and β2 – β4 subunits (Millar and Gotti, 2009). The human α3β2 and α4β2 subtypes, which play a predominant role in both pre- and post-synaptic functions in the central and peripheral nervous systems, may have variable stoichiometries of 2 α / 3 β versus 3 α / 2 β subunits, with higher affinity ligand binding at the α/β subunit interfaces and lower affinity ligand binding at the α/α subunit interfaces (Shahsavar et al. 2015).
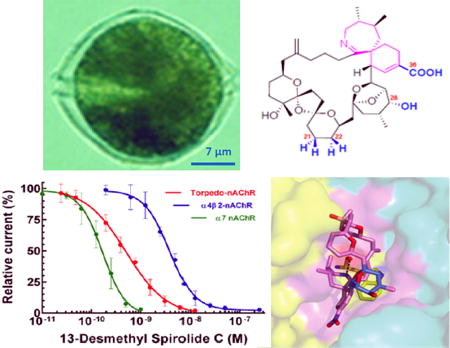
Under voltage-clamp conditions, gymnodimine A, 13-desmethyl spirolide C, 13,19-didesmethyl spirolide C, 20-methyl spirolide G or pinnatoxins A, E, F, and G do not activate the human homomeric α7 or heteromeric α4β2 nAChRs expressed in Xenopus oocytes, and therefore do not exert the agonist action typically observed with ACh or nicotine. In contrast, the toxins reduce the inward peak current elicited by ACh in both human α7 and α4β2 nAChR subtypes (Kharrat et al. 2008; Bourne et al. 2010, 2015; Aráoz et al. 2011, 2015; Hellyer et al. 2015; Couesnon et al. 2016). The IC50 values are reported in Table 1.
The action of cyclic imines was also studied using a fluorescence Ca2+ mobilization assay. In brief, nicotine binding to cell-surface nAChRs caused a release of the intracellular calcium (monitored through its binding to the previously loaded calcium-sensitive dye, FLIPR Calcium 4) and thereby increased the cell fluorescence intensity (measured with a Fluorescence Imaging Plate Reader (FLIPR). Neither gymnodimine A nor 13-desmethyl spirolide C at concentrations up to 10 µM displayed any calcium release from cells expressing the human α4β2 or α4β4 nAChRs (Hauser et al. 2012). The antagonistic blockade effect of gymnodimine A and 13-desmethyl spirolide C was also examined using the calcium flux assay in cells expressing various subtypes of nAChRs by incubating the cells with the toxins for 30 min, and thereafter adding nicotine at an EC80 concentration. Under these conditions, the inhibition of nicotine-induced calcium-flux response, determined at each antagonist concentration, revealed the following rank order of potency for 13-desmethyl spirolide C: α7 > low sensitivity form of α4β2 > human α3β4 > high sensitivity form of α4β2 > human α4β4 > rat α3β4, and for gymnodimine A: low sensitivity form of α4β2 > human α3β4 > α7 > high sensitivity form of α4β2 > human α4β4 > rat α3β4. The antagonism of the nicotine-induced calcium mobilization by both toxins was found to be not surmountable (Hauser et al. 2012). Furthermore, 13-desmethyl spirolide C and gymnodimine A inhibited nicotine-mediated dopamine release from rat striatal synaptosomes with similar high potency (IC50s = 0.2 and 0.3 nM, respectively) (Hauser et al. 2012).
Additionally, none of the toxins studied appeared to modify the desensitization kinetics of the α7 nAChR. The antagonistic activity of gymnodimine A on the human α7 and α4β2 nAChR subtypes was found to be readily reversible, whereas those of 13-desmethyl spirolide C and 20-methyl spirolide G were not abolished after a 30–60 min washout (Bourne et al. 2010; Couesnon et al. 2016). Pinnatoxin A activity on the human α7 nAChR was also reported to be slowly reversible, whereas on α4β2 nAChR it was rapidly reversible (Aráoz et al. 2011; Bourne et al. 2015).
Functional studies were also performed with the voltage-clamp technique on oocytes expressing the α4β2 nAChR in two stoichiometric forms [the low affinity (α4)3(β2)2 and high affinity (α4)2(β2)3 forms] (Nelson et al. 2003). Both pinnatoxins F and G inhibited the ACh-evoked responses, yet with different potencies reflected in their IC50 values. As shown in Table 1, pinnatoxin F was more active on (α4)3(β2)2 and (α4)2(β2)3 nAChRs than pinnatoxin G. However, both pinnatoxins displayed similar potencies on the (α4)3(β2)2 versus (α4)2(β2)3 nAChRs (Hellyer et al. 2015). Thus, the lower sensitivity of the cyclic imine toxins towards the α4β2 nAChR, compared to the homomeric α7 nAChR, does not result from changes in the subunit stoichiometry and creation of an α–α interface, but rather from essential structural properties in the ligand binding sites at the respective subunit interfaces.
The spiroimine fragment seems essential for the functional blocking activity of pinnatoxin A, since the open-ring, amino-ketone derivative of pinnatoxin A has no action on the various neuronal nAChR subtypes (Table 1).
A deeper insight into the interaction between cyclic imine toxins and neuronal nAChRs was obtained from competition binding experiments performed at equilibrium on membranes from cells expressing various nAChR subtypes and an α7-5HT3 chimera, and using radiolabeled probes and standard protocols (Servent et al. 1997). Gymnodimine A, 13-desmethyl spirolide C, 13,19-didesmethyl spirolide C, 20-methyl spirolide G and pinnatoxins A and G totally displaced [125I]α-bungarotoxin from its binding site, thereby confirming that these phycotoxin interact with high affinity with the α7-5HT3 chimera. In addition, their property of displacing [3H]epibatidine binding from human α3β2 and α4β2 neuronal nAChRs, highlights the broad capacity of cyclic imine toxins to interact with either of the homo- and hetero-pentameric forms of neuronal nAChRs.
The rank order of potency for gymnodimine A (from Ki values) was found to be: chicken α7-5HT3 > human α3β2 > human α4β2 nAChR (Kharrat et al. 2008). In another study this pharmacological profile was confirmed and detailed, the order of potency for gymnodimine A being: α7, α6β3β4α5 > rat α3β4 > human α3β4, α4β4 > rat α4β2, human α4β2 (Hauser et al. 2012). Table 2 summarizes the dissociation constant (Ki) values of several cyclic imine toxins relative to distinct neuronal nAChRs subtypes, recorded under the same experimental conditions.
A comparable broad specificity toward the neuronal nAChR subtypes was also observed with 13-desmethyl spirolide C (Bourne et al. 2010). The rank order for inhibition by 13-desmethyl spirolide C was: α7 > α6β3β4α5 ≫ rat α3β4, α4β4, human α3β4 > human α4β2 > rat α4β2 (Hauser et al. 2012). The selectivity profile for pinnatoxin A also exhibited a higher affinity for the human α7 compared to the human α3β2 and α4β2 nAChRs (Aráoz et al. 2011), and this was maintained for other pinnatoxins exhibiting the following order of potency: pinnatoxin F > pinnatoxin G > pinnatoxin E (Hellyer et al. 2015).
Interaction with mAChRs
Muscarinic ACh receptors (mAChRs) have been proposed in early studies to explain part of the acute toxicological mode of action of 13-desmethyl spirolide C in rats (Gill et al. 2003). Indeed, both transcriptional alterations for early injury markers (c-jun and HSP-72) and for mAChRs and nAChRs were revealed. The M1, M4 and M5 mAChR genes, as well as the α2 and β4 nAChR genes were altogether up-regulated, implying that both types of cholinergic receptors could be potential molecular targets for the 13-desmethyl spirolide C. Studies carried out in the human neuroblastoma cell line BE(2)-M17, expressing mAChRs subtypes, reported that 13-desmethyl spirolide C inhibited ACh-induced Ca2+ signals, while the reversible competitive antagonist, atropine, diminished the inhibitory effect of the spirolide. Also, the spirolide at 0.5 µM reduced the [3H]N-methyl scopolamine specific binding to the cells by ca. 53%. Similar inhibition of [3H]quinuclidynyl benzilate binding (59%) was observed under the same experimental conditions. Such data suggested that the spirolide binds to the orthosteric binding site of mAChRs (Wandscheer et al. 2010). However, later competition binding assays, performed on membrane embedded mAChRs from TE671/RD clonal cells and rat cortices using radiolabeled [3H]quinuclidynyl benzilate and both gymnodimine A and 13-desmethyl spirolide C, failed to show any significant interaction with the mAChRs (Hauser et al. 2012).
Further work performed with CHO cells stably expressing each of the five human mAChRs subtypes revealed that pinnatoxin A (1 µM) had no significant action on [3H]N-methyl scopolamine binding to M1, M2, M3, and M4 mAChRs, whereas it displaced radiotracer binding to the M5 mAChR subtype by 35%, a value reflecting interaction in the low micromolar range (Aráoz et al. 2011). This property was not observed using the pinnatoxin A amino-ketone derivative. In a similar assay, 13-desmethyl spirolide C and 13,19-didesmethyl spirolide were found to interact with very low affinity (in the micromolar range) with the five mAChR subtypes yielding affinities 3 to 4 orders of magnitude lower than those for the nAChR subtypes (Aráoz et al. 2015).
Structural studies
The absolute stereochemistry of gymnodimine A was unambiguously assigned from the crystal structure of the p-bromobenzamide derivative of the reduced form of gymnodimine A (Stewart et al. 1997), whereas the relative stereochemistry of 13-desmethyl spirolide C, except for one chiral center, has been determined using the ConGen molecular modeling method, from NOESY and ROESY NMR data (Falk et al. 2001). Later on, the crystal structures of Acetylcholine Binding Protein (AChBP) complexes with gymnodimine A (Bourne et al. 2010), 13-desmethyl spirolide C (Bourne et al. 2010), and pinnatoxins A and G (Bourne et al. 2015) unveiled the molecular determinants of toxin binding selectivity. The soluble AChBPs, with their overall pentameric architecture and their amino acid residues forming a binding pocket for the nicotinic ligands at subunit interfaces, have been extensively used as functional and structural surrogates for the ligand binding domains of the nAChRs and have provided valuable information on how ligands interact with the various nAChR subtypes (Brejc et al. 2001; Celie et al. 2004; for a recent review see Shahsavar et al. 2016).
In addition to the overall structural features of the subunits, the aromatic side chains that form the ligand binding pocket at the subunit interfaces are well conserved in the nAChR family, with greater variability for residues at the complementary (or (−)) face than the principal (or (+)) face of each interface. In fact, the binding pocket of AChBP possesses all the functional residues identified in the nAChR LBD. Hence, the ligand binding pocket encompasses a nest of five electron-rich aromatic side chains provided by residues Tyr93, Trp147, Tyr188, Tyr195 on the (+) face and residue Tyr55 on the (−) face of the interface.
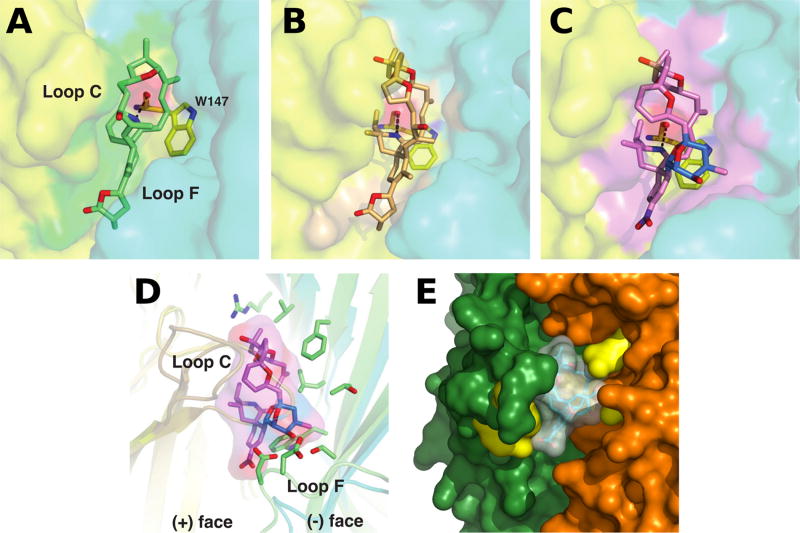
In each complex, the toxin is imbedded within this aromatic nest contributed by loops C and F on opposing faces of the subunit interface and display exquisite shape and chemical complementarity with the ligand binding pocket (Fig. 2). The orientation and conformation of the toxin carbon skeleton, with its long axis roughly aligned parallel with the pentamer five-fold axis, ideally position the protonated cyclic imine donor, similar to the anabaseines (Talley et al. 2006; Hibbs et al. 2009), to be within H-bond distances to the carbonyl oxygen of Trp147 (loop B). At the apical side of the interface, the tetrahydrofuran ring (gymnodimine A) or bulky and more rigid bis-spiroacetal ring system (13-desmethyl spirolide C, pinnatoxin A) abuts against the tip of loop C to localize the binding interface. At the opposing subunit face, the variable terminal γ-butyrolactone (gymnodimine A) or cyclohexene (13-desmethyl spirolide C, pinnatoxin A) rings promote additional interactions including the conserved Tyr93 from the (+) face. In fact, pinnatoxin A contains a bulky bridged 5,6-bicycloketal substructure instead of a smaller allylic alcohol linker found in gymnodimine A and 13-desmethyl spirolide C. This unique substructure in pinnatoxins (also found in pteriatoxins) extends radially from the interfacial binding pocket to interact with the sequence-variable loop F and governs nAChR subtype selectivity (Bourne et al. 2010; Bourne et al. 2015).
Fig. 2.
Close-up views of the binding interfaces of several cyclic imine toxins. Crystal structures of the AChBP subunit interface (the (+) and (−) faces are displayed in yellow and cyan, respectively) with bound gymnodimine A (A), 13-desmethyl spirolide C (B) and pinnatoxin A (C) (Protein Data Bank accession codes 2X00, 2WZY and 4XHE, Bourne et al. 2010, 2015); (D) Overlay of the binding interfaces of AChBP with bound pinnatoxin A and of the human α4β2 receptor (5KXI, Morales-Perez et al. 2016) (the α and β subunits are displayed in light orange and green, respectively). Those side chains from the β2 subunit ((−) face) that could contribute to pinnatoxin A binding are shown in green; (E) Docking complex of pinnatoxin A with the α7 nAChR structure generated by homology modeling (Aráoz et al. 2011). The α7 residues Trp147, Arg186, Tyr188, Tyr195 (in the (+) subunit) and Gln57, Gln116 (in the (−) subunit), which are predicted to establish hydrogen bonds with the toxin, are displayed in yellow.
In addition to the crystal structures of AChBP-toxin complexes, additional complexes between different nAChR subtypes (human α7, α4β2, α3β2, and α12β1γδ) and pinnatoxin A (Aráoz et al. 2011), 13-desmethyl spirolide C (Aráoz et al. 2015) and 13,19-didesmethyl spirolide C (Aráoz et al. 2015) generated by in silico molecular docking, provide complementary information for the identification of key residues responsible for the differences in binding affinities and subtype specificities that were determined experimentally (Fig. 2) (Aráoz et al. 2011; Aráoz et al. 2015).
Conclusion
In conclusion, the globally distributed and well-chemically characterized cyclic imine toxins, from toxic dinoflagellate species, represent a novel source of potent antagonists of muscle- and neuronal-type nAChRs. The distinctive chemical signature of these phycotoxins is related to the presence of a cyclic imine moiety in their structure, and their toxicological profile is predominantly associated to their specific interaction with the nAChRs.
Taking advantage of the competitive binding of cyclic imine toxins to nAChRs, several tests have been developed to detect spirolides, gymnodimines and pinnatoxins in contaminated shellfish with better accuracy than the broad spectrum mouse bioassay. These tests are important in the food safety field, because shellfish represents a rich food resource that may be contaminated by toxins produced by toxic dinoflagellates.
Substantial progress has been obtained on the characterization of the dinoflagellate producing the cyclic imine toxins, but the genes involved in their production, and the pathways leading to the biosynthesis of the various families of toxins remain, at present, elusive. Furthermore, the ecological factors favoring dinoflagellate blooming need to be determined.
Shellfish regularly contain variable amounts of cyclic imine toxins in their edible tissues and can transfer these phycotoxins through the marine food chain. Although cyclic imine levels are not regulated, it has become a matter of concern to assess the risks for human health. Thus, a consensus is emerging that further studies should be conducted to enhance our understanding of the gastrointestinal absorption, tissue disposition, and crossing of the blood brain and placental barriers. Also, more information is needed on the environmental distribution and risks of chronic exposure to these phycotoxins.
Acknowledgments
The works described here were funded by the Agence Nationale de la Recherche (France, grant AQUANEUROTOX ANR-12-ASTR-0037, to J. M. and D. S.), the French Infrastructure for Integrated Structural Biology (FRISBI) ANR-10-INSB-05-01 (to the AFMB laboratory), a CNRS-DRI PICS grant (to Y. B. and P. M.), and the National Institutes of Health (USA, grants NIGMS R01 GM077379, to A. Z and GM18360 to P. T.).
Abbreviations
- ACh
Acetylcholine
- AChBP
acetylcholine binding-protein
- CMAP
compound muscle action potential
- FLIPR
Fluorescence Imaging Plate Reader
- LBD
ligand-binding domain
- LGIC
ligand-gated ion channels
- mAChRs
muscarinic ACh receptors
- nAChRs
nicotinic ACh receptors
Footnotes
conflict of interest disclosure
The authors declare no conflicts of interest.
References
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Muftah A, Selwood AI, Foss AJ, Al-Jabri HM, Potts M, Yilmaz M. Algal toxins and producers in the marine waters of Qatar, Arabian Gulf. Toxicon. 2016;122:54–66. doi: 10.1016/j.toxicon.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Almandoz GO, Montoya NG, Hernando MP, Benavides HR, Carignan MO, Ferrario ME. Toxic strains of the Alexandrium ostenfeldii complex in southern South America (Beagle Channel, Argentina) Harmful Algae. 2014;37:100–109. [Google Scholar]
- Anderson DM, Alpermann TJ, Cembella AD, Collos Y, Masseret E, Montresor M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae. 2012;14:10–35. doi: 10.1016/j.hal.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aráoz R, Servent D, Ouanounou G, Benoit E, Molgó J. The emergent marine dinoflagellate toxins spirolides and gymnodimines target nicotinic acetylcholine receptors. Biol. Res. 2009;42(Suppl. A):R-118. [Google Scholar]
- Aráoz R, Servent D, Molgó J, Iorga BI, Fruchart-Gaillard C, Benoit E, Gu Z, Stivala C, Zakarian A. Total synthesis of pinnatoxins A and G and revision of the mode of action of pinnatoxin A. J. Am. Chem. Soc. 2011;133:10499–10511. doi: 10.1021/ja201254c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aráoz R, Ramos S, Pelissier F, Guérineau V, Benoit E, Vilariño N, Botana LM, Zakarian A, Molgó J. Coupling the Torpedo microplate-receptor binding assay with mass spectrometry to detect cyclic imine neurotoxins. Anal. Chem. 2012;84:10445–10453. doi: 10.1021/ac3027564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aráoz R, Ouanounou G, Iorga BI, Goudet A, Alili D, Amar M, Benoit E, Molgó J, Servent D. The neurotoxic effect of 13,19-didesmethyl and 13-desmethyl spirolide C phycotoxins is mainly mediated by nicotinic rather than muscarinic acetylcholine receptors. Toxicol. Sci. 2015;147:156–167. doi: 10.1093/toxsci/kfv119. [DOI] [PubMed] [Google Scholar]
- Beaumont S, Ilardi EA, Tappin NDC, Zakarian A. Marine toxins with spiroimine rings: Total synthesis of pinnatoxin A. Eur. J. Org. Chem. 2010:5743–5765. doi: 10.1002/ejoc.201000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkman DG, Smayda TJ, Tomas CR, York R, Strangman W, Wright JLC. Toxic Alexandrium peruvianum (Balech and de Mendiola) Balech and Tangen in Narragansett Bay, Rhode Island (USA) Harmful Algae. 2012;19:92–100. [Google Scholar]
- Bourne Y, Radic Z, Aráoz R, Talley TT, Benoit E, Servent D, Taylor P, Molgó J, Marchot P. Structural determinants in phycotoxins and AChBP conferring high affinity binding and nicotinic AChR antagonism. Proc. Natl. Acad. Sci. USA. 2010;107:6076–6081. doi: 10.1073/pnas.0912372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne Y, Sulzenbacher G, Radić Z, Aráoz R, Reynaud M, Benoit E, Zakarian A, Servent D, Molgó J, Taylor P, Marchot P. Marine macrocyclic imines, pinnatoxins A and G: Structural determinants and functional properties to distinguish neuronal α7 from muscle α1(2)βγδ nAChR. Structure. 2015;23:1106–1115. doi: 10.1016/j.str.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Celie PH, van Rossum-Fikkert SE, van Dijk WJ, Brejc K, Smit AB, Sixma TK. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron. 2004;41:907–914. doi: 10.1016/s0896-6273(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Cembella AD, Lewis NI, Quilliam MA. Spirolide composition of micro-extracted pooled cells isolated from natural plankton assemblages and from cultures of the dinoflagellate Alexandrium ostenfeldii. Nat. Toxins. 1999;7:197–206. doi: 10.1002/1522-7189(200009/10)7:5<197::aid-nt62>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Cembella AD, Lewis NI, Quilliam MA. The marine dinoflagellate Alexandrium ostenfeldii (Dinophyceae) as the causative organism of spirolide shellfish toxins. Phycologia. 2000;39:67–74. [Google Scholar]
- Corringer PJ, Le Novère N, Changeux JP. Nicotinic receptors at the amino acid level. Annu. Rev. Pharmacol. Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- Couesnon A, Aráoz R, Iorga BI, Benoit E, Reynaud M, Servent D, Molgó J. The dinoflagellate toxin 20-methyl spirolide-G potently blocks skeletal muscle and neuronal nicotinic acetylcholine receptors. Toxins. 2016;8:E249. doi: 10.3390/toxins8090249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddihy SL, Drake S, Harwood DT, Selwood AI, McNabb PS, Hampton MB. The marine cytotoxin portimine is a potent and selective inducer of apoptosis. Apoptosis. 2016;21:1447–1452. doi: 10.1007/s10495-016-1302-x. [DOI] [PubMed] [Google Scholar]
- Daneshian M, Botana LM, Dechraoui Bottein MY, Buckland G, Campàs M, Dennison N, Dickey RW, Diogène J, Fessard V, Hartung T, Humpage A, Leist M, Molgó J, Quilliam MA, Rovida C, Suarez-Isla BA, Tubaro A, Wagner K, Zoller O, Dietrich D. A roadmap for hazard monitoring and risk assessment of marine biotoxins on the basis of chemical and biological test systems. ALTEX. 2013;30:487–545. doi: 10.14573/altex.2013.4.487. [DOI] [PubMed] [Google Scholar]
- Duroure L, Jousseaume T, Aráoz R, Barre E, Retailleau P, Chabaud L, Molgó J, Guillou C. 6,6-Spiroimine analogs of (−)-gymnodimine A: Synthesis and biological evaluation on nicotinic acetylcholine receptors. Org. Biomol. Chem. 2011;9:8112–8118. doi: 10.1039/c1ob06257c. [DOI] [PubMed] [Google Scholar]
- Eusebi F, Palma E, Amici M, Miledi R. Microtransplantation of ligand-gated receptor-channels from fresh or frozen nervous tissue into Xenopus oocytes: a potent tool for expanding functional information. Prog Neurobiol. 2009;88:32–40. doi: 10.1016/j.pneurobio.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Falk M, Burton IW, Hu T, Walter JA, Wright JLC. Assignment of the relative stereochemistry of the spirolides, macrocyclic toxins isolated from shellfish and from the cultured dinoflagellate Alexandrium ostenfeldii. Tetrahedron. 2001;57:8659–8666. [Google Scholar]
- Fonfría ES, Vilariño N, Molgó J, Aráoz R, Otero P, Espina B, Louzao MC, Alvarez M, Botana LM. Detection of 13,19-didesmethyl C spirolide by fluorescence polarization using Torpedo electrocyte membranes. Anal. Biochem. 2010;403:102–107. doi: 10.1016/j.ab.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Gill S, Murphy M, Clausen J, Richard D, Quilliam M, MacKinnon S, LaBlanc P, Mueller R, Pulido O. Neural injury biomarkers of novel shellfish toxins, spirolides: A pilot study using immunochemical and transcriptional analysis. Neurotoxicology. 2003;24:593–604. doi: 10.1016/S0161-813X(03)00014-7. [DOI] [PubMed] [Google Scholar]
- Gu H. Morphology, phylogenetic position, and ecophysiology of Alexandrium ostenfeldii (Dinophyceae) from the Bohai Sea, China. J. Syst. Evol. 2011;49:609–616. [Google Scholar]
- Guéret SM, Brimble MA. Spiroimine shellfish poisoning (SSP) and the spirolide family of shellfish toxins: Isolation, structure, biological activity and synthesis. Nat. Prod. Rep. 2010;27:1350–1366. doi: 10.1039/c005400n. [DOI] [PubMed] [Google Scholar]
- Hakanen P, Suikkanen S, Franzén J, Franzén H, Kankaanpää H, Kremp A. Bloom and toxin dynamics of Alexandrium ostenfeldii in a shallow embayment at the SW coast of Finland, northern Baltic Sea. Harmful Algae. 2012;15:91–99. [Google Scholar]
- Harju K, Koskela H, Kremp A, Suikkanen S, de la Iglesia P, Miles CO, Krock B, Vanninen P. Identification of gymnodimine D and presence of gymnodimine variants in the dinoflagellate Alexandrium ostenfeldii from the Baltic Sea. Toxicon. 2016;112:68–76. doi: 10.1016/j.toxicon.2016.01.064. [DOI] [PubMed] [Google Scholar]
- Hauser TA, Hepler CD, Kombo DC, Grinevich VP, Kiser MN, Hooker DN, Zhang J, Mountfort D, Selwood A, Akireddy SR, Letchworth SR, Yohannes D. Comparison of acetylcholine receptor interactions of the marine toxins, 13-desmethyl spirolide C and gymnodimine. Neuropharmacology. 2012;62:2239–2250. doi: 10.1016/j.neuropharm.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Haywood AJ, Steidinger KA, Truby EW, Bergquist PR, Bergquist PL, Adamson J, Mackenzie L. Comparative morphology and molecular phylogenetic analysis of three new species of the genus Karenia (Dinophyceae) from New Zealand. J. Phycol. 2004;40:165–179. [Google Scholar]
- Hellyer SD, Selwood AI, Rhodes L, Kerr DS. Neuromuscular blocking activity of pinnatoxins E, F and G. Toxicon. 2013;76:214–220. doi: 10.1016/j.toxicon.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Hellyer SD, Indurthi D, Balle T, Runder-Varga V, Selwood AI, Tyndall JD, Chebib M, Rhodes L, Kerr DS. Pinnatoxins E, F and G target multiple nicotinic receptor subtypes. J. Neurochem. 2015;135:479–491. doi: 10.1111/jnc.13245. [DOI] [PubMed] [Google Scholar]
- Hess P, Abadie E, Herve F, Berteaux T, Séchet V, Aráoz R, Molgó J, Zakarian A, Sibat M, Rundberget T, Miles CO, Amzil Z. Pinnatoxin G is responsible for atypical toxicity in mussels (Mytilus galloprovincialis) and clams (Venerupis decussata) from Ingril, a French Mediterranean lagoon. Toxicon. 2013;75:16–26. doi: 10.1016/j.toxicon.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs RE, Sulzenbacher G, Shi J, Talley TT, Conrod S, Kem WR, Taylor P, Marchot P, Bourne Y. Structural determinants for interaction of partial agonists with acetylcholine binding protein and neuronal α7-nicotinic acetylcholine receptor. EMBO J. 2009;28:3040–3051. doi: 10.1038/emboj.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Curtis JM, Walter JA, Wright JLC. Characterization of biologically inactive spirolides E and F: Identification of the spirolide pharmacophore. Tetrahedron Lett. 1996a;37:7671–7674. [Google Scholar]
- Hu T, deFreitas ASW, Curtis JM, Oshima Y, Walter JA, Wright JLC. Isolation and structure of prorocentrolide B, a fast-acting toxin from Prorocentrum maculosum. J. Nat. Prod. 1996b;59:1010–1014. doi: 10.1021/np960439y. [DOI] [PubMed] [Google Scholar]
- Jackson JJ, Stivala CE, Iorga BI, Molgó J, Zakarian A. Stability of cyclic imine toxins: Interconversion of pinnatoxin amino ketone and pinnatoxin A in aqueous media. J. Org. Chem. 2012;77:10435–10440. doi: 10.1021/jo301632d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharrat R, Servent D, Girard E, Ouanounou G, Amar M, Marrouchi R, Benoit E, Molgó J. The marine phycotoxin gymnodimine targets muscular and neuronal nicotinic acetylcholine receptor subtypes with high affinity. J. Neurochem. 2008;107:952–63. doi: 10.1111/j.1471-4159.2008.05677.x. [DOI] [PubMed] [Google Scholar]
- Kong K, Romo D, Lee C. Enantioselective total synthesis of the marine toxin (−)-gymnodimine employing a Barbier-type macrocyclization. Angew. Chem. Int. Ed. 2009;48:7402–7405. doi: 10.1002/anie.200903432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong K, Moussa Z, Lee C, Romo D. Total synthesis of the spirocyclic imine marine toxin (−)-gymnodimine and an unnatural C4-epimer. J. Am. Chem. Soc. 2011;133:19844–19856. doi: 10.1021/ja207385y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremp A, Tahvanainen P, Litaker W, Krock B, Suikkanen S, Leaw CP, Tomas C. Phylogenetic relationships, morphological variation, and toxin patterns in the Alexandrium ostenfeldii (Dinophyceae) complex: Implications for species boundaries and identities. J. Phycol. 2014;50:81–100. doi: 10.1111/jpy.12134. [DOI] [PubMed] [Google Scholar]
- Kremp A, Oja J, LeTortorec AH, Hakanen P, Tahvanainen P, Tuimala J, Suikkanen S. Diverse seed banks favour adaptation of microalgal populations to future climate conditions. Environ. Microbiol. 2016;18:679–691. doi: 10.1111/1462-2920.13070. [DOI] [PubMed] [Google Scholar]
- Lu C-K, Lee G-H, Huang R, Chou H-N. Spiro-prorocentrimine, a novel macrocyclic lactone from a benthic Prorocentrum sp. of Taiwan. Tetrahedron Lett. 2001;42:1713–1716. [Google Scholar]
- Marrouchi R, Rome G, Kharrat R, Molgó J, Benoit E. Analysis of the action of gymnodimine-A and 13-desmethyl spirolide C on the mouse neuromuscular system in vivo. Toxicon. 2013;75:27–34. doi: 10.1016/j.toxicon.2013.08.050. [DOI] [PubMed] [Google Scholar]
- Matsuura F, Hao J, Reents R, Kishi Y. Total synthesis and stereochemistry of pinnatoxins B and C. Org. Lett. 2006a;8:3327–3330. doi: 10.1021/ol0611548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura F, Peters R, Anada M, Harried SS, Hao J, Kishi Y. Unified total synthesis of pteriatoxins and their diastereomers. J. Am. Chem. Soc. 2006b;128:7463–7465. doi: 10.1021/ja0618954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley JA, Nagasawa K, Lander PA, Mischke SG, Semones MA, Kishi Y. Total synthesis of pinnatoxin A. J. Am. Chem. Soc. 1998;120:7647–7648. [Google Scholar]
- Miledi R, Palma E, Eusebi F. Microtransplantation of neurotransmitter receptors from cells to Xenopus oocyte membranes: new procedure for ion channel studies. Methods Mol Biol. 2006;322:347–55. doi: 10.1007/978-1-59745-000-3_24. [DOI] [PubMed] [Google Scholar]
- Miles CO, Wilkins AL, Stirling DJ, MacKenzie AL. New analogue of gymnodimine from a Gymnodinium species. J. Agric. Food Chem. 2000;48:1373–1376. doi: 10.1021/jf991031k. [DOI] [PubMed] [Google Scholar]
- Miles CO, Wilkins AL, Stirling DJ, MacKenzie AL. Gymnodimine C, an isomer of gymnodimine B, from Karenia selliformis. J. Agric. Food Chem. 2003;51:4838–4840. doi: 10.1021/jf030101r. [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Molgó J, Girard E, Benoit E. Cyclic imines: An insight into this emerging group of bioactive marine toxins. In: Botana LM, editor. Phycotoxins: Chemistry and biochemistry. Blackwell Publishing; Iowa, USA: 2007. pp. 319–335. [Google Scholar]
- Molgó J, Aráoz R, Benoit E, Iorga BI. Cyclic imine toxins: chemistry, origin, metabolism, pharmacology, toxicology, and detection. In: Botana LM, editor. Seafood and freshwater toxins. Pharmacology physiology and detection. 3. CRC Press; 2014. pp. 951–990. [Google Scholar]
- Molgó J, Benoit E, Aráoz R, Zakarian A, Iorga BI. Spirolides and cyclic imines: Toxicological profile. In: Gopalakrishnakone P, Haddad V Jr, Kem WR, Tubaro A, Kim E, editors. Marine and Freshwater Toxins. Springer; Netherlands: 2015. pp. 193–217. [Google Scholar]
- Morales-Perez CL, Noviello CM, Hibbs RE. X-ray structure of the human α4β2 nicotinic receptor. Nature. 2016;538:411–415. doi: 10.1038/nature19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday R, Towers NR, Mackenzie L, Beuzenberg V, Holland PT, Miles CO. Acute toxicity of gymnodimine to mice. Toxicon. 2004;44:173–178. doi: 10.1016/j.toxicon.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Munday R, Selwood AI, Rhodes L. Acute toxicity of pinnatoxins E, F and G to mice. Toxicon. 2012;60:995–999. doi: 10.1016/j.toxicon.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kikuchi F, Hashimoto S. Total synthesis of pinnatoxin A. Angew. Chem. Int. Ed. 2008;47:7091–7094. doi: 10.1002/anie.200802729. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol. Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- Nézan E, Chomérat N. Vulcanodinium rugosum gen. et sp. nov. (Dinophyceae), un nouveau dinoflagellé marin de la côte méditerranéenne française. Cryptogamie, Algologie. 2011;32:3–18. [Google Scholar]
- Otero P, Alfonso A, Vieytes MR, Cabado AG, Vieites JM, Botana LM. Effects of environmental regimens on the toxin profile of Alexandrium ostenfeldii. Environ. Toxicol. Chem. 2010;29:301–310. doi: 10.1002/etc.41. [DOI] [PubMed] [Google Scholar]
- Otero P, Alfonso A, Alfonso C, Aráoz R, Molgó J, Vieytes MR, Botana LM. First direct fluorescence polarization assay for the detection and quantification of spirolides in mussel samples. Anal. Chim. Acta. 2011;701:200–208. doi: 10.1016/j.aca.2011.05.034. [DOI] [PubMed] [Google Scholar]
- Rhodes L, Smith K, Selwood A, McNabb P, van Ginkel R, Holland P, Munday R. Production of pinnatoxins by a peridinoid dinoflagellate isolated from Northland, New Zealand. Harmful Algae. 2010;9:384–389. [Google Scholar]
- Rhodes L, Smith K, Selwood A, McNabb P, Molenaar S, Munday R, Wilkinson C, Hallegraeff G. Production of pinnatoxins E, F and G by scrippsielloid dinoflagellates isolated from Franklin Harbour, South Australia. N. Z. J. Mar. Freshw. Res. 2011a;45:703–709. [Google Scholar]
- Rhodes L, Smith K, Selwood A, McNabb P, Munday R, Suda S, Molenaar S, Hallegraeff G. Dinoflagellate Vulcanodinium rugosum Nézan et Chomérat newly identified as the causative organism of pinnatoxins in Australia, New Zealand and Japan. Phycologia. 2011b;50:624–628. [Google Scholar]
- Rodríguez LP, Vilariño N, Molgó J, Aráoz R, Antelo A, Vieytes MR, Botana LM. Solid-phase receptor-based assay for the detection of cyclic imines by chemiluminescence, fluorescence, or colorimetry. Anal. Chem. 2011;83:5857–5863. doi: 10.1021/ac200423s. [DOI] [PubMed] [Google Scholar]
- Rodríguez LP, Vilariño N, Molgó J, Aráoz R, Botana LM. High-throughput receptor-based assay for the detection of spirolides by chemiluminescence. Toxicon. 2013a;75:35–43. doi: 10.1016/j.toxicon.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Rodríguez LP, Vilariño N, Molgó J, Aráoz R, Louzao MC, Taylor P, Talley T, Botana LM. Development of a solid-phase receptor-based assay for the detection of cyclic imines using a microsphere-flow cytometry system. Anal. Chem. 2013b;85:2340–2347. doi: 10.1021/ac3033432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio F, Kamp L, Carpino J, Faltin E, Loftin K, Molgó J, Aráoz R. Colorimetric microtiter plate receptor-binding assay for the detection of freshwater and marine neurotoxins targeting the nicotinic acetylcholine receptors. Toxicon. 2014;91:45–56. doi: 10.1016/j.toxicon.2014.08.073. [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Sakazaki H, Hagiwara K, Kamada K, Ishii K, Noda T, Inoue M, Hirama M. A formal total synthesis of (+)-pinnatoxin A. Angew. Chem. Int. Ed. 2004;43:6505–6510. doi: 10.1002/anie.200461802. [DOI] [PubMed] [Google Scholar]
- Salgado P, Riobo P, Rodriguez F, Franco JM, Bravo I. Differences in the toxin profiles of Alexandrium ostenfeldii (Dinophyceae) strains isolated from different geographic origins: Evidence of paralytic toxin, spirolide, and gymnodimine. Toxicon. 2015;103:85–98. doi: 10.1016/j.toxicon.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Savela H, Harju K, Spoof L, Lindehoff E, Meriluoto J, Vehniäinen M, Kremp A. Quantity of the dinoflagellate sxtA 4 gene and cell density correlates with paralytic shellfish toxin production in Alexandrium ostenfeldii blooms. Harmful Algae. 2016;52:1–10. doi: 10.1016/j.hal.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Selwood AI, Wilkins AL, Munday R, Shi F, Rhodes LL, Holland PT. Portimine: A bioactive metabolite from the benthic dinoflagellate Vulcanodinium rugosum. Tetrahedron Lett. 2013;54:4705–4707. [Google Scholar]
- Selwood AI, Wilkins AL, Munday R, Gu H, Smith KF, Rhodes LL, Rise F. Pinnatoxin H: A new pinnatoxin analogue from a South China Sea Vulcanodinium rugosum isolate. Tetrahedron Lett. 2014;55:5508–5510. [Google Scholar]
- Shahsavar A, Ahring PK, Olsen JA, Krintel C, Kastrup JS, Balle T, Gajhede M. Acetylcholine-binding protein engineered to mimic the α4-α4 binding pocket in α4β2 nicotinic acetylcholine receptors reveals interface specific interactions important for binding and activity. Mol. Pharmacol. 2015;88:697–707. doi: 10.1124/mol.115.098061. [DOI] [PubMed] [Google Scholar]
- Shahsavar A, Gajhede M, Kastrup JS, Balle T. Structural studies of nicotinic acetylcholine receptors: Using Acetylcholine-Binding Protein as a structural surrogate. Basic Clin. Pharmacol. Toxicol. 2016;118:399–407. doi: 10.1111/bcpt.12528. [DOI] [PubMed] [Google Scholar]
- Smith KF, Rhodes LL, Suda S, Selwood AI. A dinoflagellate producer of pinnatoxin G, isolated from sub-tropical Japanese waters. Harmful Algae. 2011;10:702–705. [Google Scholar]
- Stewart M, Blunt JW, Munro MHG, Robinson WT, Hannah DJ. The absolute stereochemistry of the New Zealand shellfish toxin gymnodimine. Tetrahedron Lett. 1997;38:4889–4890. [Google Scholar]
- Stivala CE, Zakarian A. Total synthesis of (+)-pinnatoxin A. J. Am. Chem. Soc. 2008;130:3774–3776. doi: 10.1021/ja800435j. [DOI] [PubMed] [Google Scholar]
- Stivala CE, Benoit E, Aráoz R, Servent D, Novikov A, Molgó J, Zakarian A. Synthesis and biology of cyclic imine toxins, an emerging class of potent, globally distributed marine toxins. Nat. Prod. Rep. 2015;32:411–435. doi: 10.1039/c4np00089g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strangman W, Anttila M, Tomas C, Jeffrey LC, Wright JLC. (5S)-5-[(4aR,8aS,9E,11S,13R,14S,16R,17R,19S)-11,19-Dihydroxy-8,10,13,16-tetramethyl-18-methylidene-3,4,5,6,8a,11,12,13,14,15,16,17,18,19,20,21-hexadecahydro-2H-14,17-epoxybenzo[2,3]cyclohexadeca[1,2-b]pyridine-7-yl]-3-methylfuran-2(5H)-one (12-Methylgymnodimine B. Molbank. 2016;2:M896. [Google Scholar]
- Suikkanen S, Kremp A, Hautala H, Krock B. Paralytic shellfish toxins or spirolides? The role of environmental and genetic factors in toxin production of the Alexandrium ostenfeldii complex. Harmful Algae. 2013;26:52–59. [Google Scholar]
- Talley TT, Yalda S, Ho KY, Tor Y, Soti FS, Kem WR, Taylor P. Spectroscopic analysis of benzylidene anabaseine complexes with acetylcholine binding proteins as models for ligand-nicotinic receptor interactions. Biochemistry. 2006;45:8894–8902. doi: 10.1021/bi060534y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torigoe K, Murata M, Yasumoto T, Iwashita T. Prorocentrolide, a toxic nitrogenous macrocycle from a marine dinoflagellate, Prorocentrum lima. J. Am. Chem. Soc. 1988;110:7876–7877. [Google Scholar]
- Touzet N, Franco JM, Raine R. Morphogenetic diversity and biotoxin composition of Alexandrium (Dinophyceae) in Irish coastal waters. Harmful Algae. 2008;7:782–797. [Google Scholar]
- Tsetlin V, Kuzmin D, Kasheverov I. Assembly of nicotinic and other Cys-loop receptors. J. Neurochem. 2011;116:734–741. doi: 10.1111/j.1471-4159.2010.07060.x. [DOI] [PubMed] [Google Scholar]
- Van de Waal DB, Tillmann U, Martens H, Krock B, van Scheppingen Y, John U. Characterization of multiple isolates from an Alexandrium ostenfeldii bloom in The Netherlands. Harmful Algae. 2015;49:94–104. [Google Scholar]
- Van Wagoner RM, Misner I, Tomas CR, Wright JLC. Occurrence of 12-methylgymnodimine in a spirolide-producing dinoflagellate Alexandrium peruvianum and the biogenetic implications. Tetrahedron Lett. 2011;52:4243–4246. [Google Scholar]
- Vilariño N, Fonfría ES, Molgó J, Aráoz R, Botana LM. Detection of gymnodimine A and 13-desmethyl C spirolide phycotoxins by fluorescence polarization. Anal. Chem. 2009;81:2708–2714. doi: 10.1021/ac900144r. [DOI] [PubMed] [Google Scholar]
- Wandscheer CB, Vilariño N, Espiña B, Louzao MC, Botana LM. Human muscarinic acetylcholine receptors are a target of the marine toxin 13-desmethyl C spirolide. Chem. Res. Toxicol. 2010;23:1753–1761. doi: 10.1021/tx100210a. [DOI] [PubMed] [Google Scholar]