Abstract
Motor neurons (MNs) are the principal neurons in the mammalian spinal cord whose activities cause muscles to contract. In addition to their peripheral axons, MNs have central collaterals that contact inhibitory Renshaw cells and other MNs. Since its original discovery >60 years ago, it has been a general notion that acetylcholine is the only transmitter released from MN synapses both peripherally and centrally. Here, we show, using a multidisciplinary approach, that mammalian spinal MNs, in addition to acetylcholine, corelease glutamate to excite Renshaw cells and other MNs but not to excite muscles. Our study demonstrates that glutamate can be released as a functional neurotransmitter from mammalian MNs.
Keywords: synaptic transmission, spinal cord
Motor neurons (MNs) are the output neurons from the central nervous system. Their activity directly leads to muscle contraction. By the 1940s, it was generally accepted that MNs release acetylcholine (ACh) at the neuromuscular junction (1). Shortly thereafter, it was shown that ACh also is released from MNs' central axonal branches contacting Renshaw cells (RCs) (2). As was found at the neuromuscular junction, this transmission was shown to be nicotinic (3, 4). The collaterals contacting other MNs (5) are also thought to be mediated by ACh, although this has not been shown directly (6). Since these initial discoveries and after many later investigations, it has been a general dogma that mammalian MNs contain and release one neurotransmitter, ACh, both centrally and peripherally. It has been suggested recently, based on anatomical data, that MNs might contain glutamate as a neurotransmitter (7, 8). There has been, however, no direct electrophysiological evidence to support this. Here, we examine this question directly by investigating the transmission in central and peripheral MN synapses (Fig. 1a).
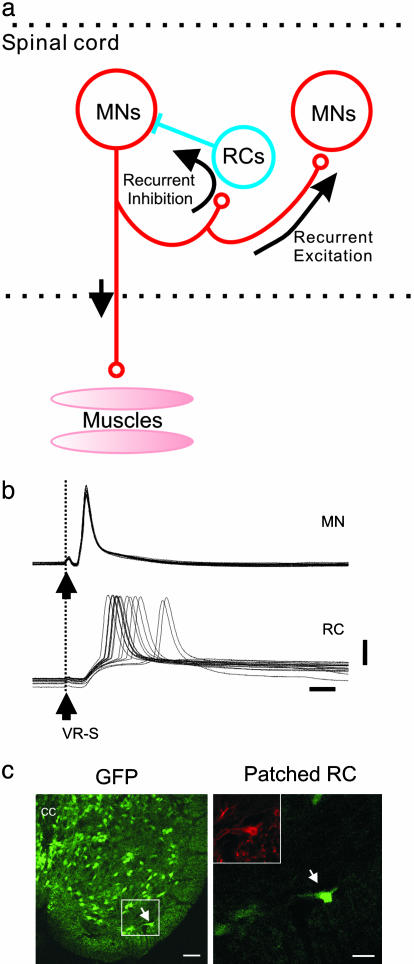
Fig. 1.
Examining MN synapses. (a) Schematic drawing of synapses formed by the MNs. (b) Paired intracellular recording of a MN and a RC. The MN is identified by its antidromic activation from ventral root stimulation (VR-S), and the RC is synaptically activated from VR-S at the same stimulus strength. (Scale bars: 20 mV and 5 ms.) (c) Transverse section of the lumbar spinal cord showing GFP-GAD67-positive cells; Left shows the enlargement of the RC region medial to the motor nucleus showing GFP-GAD67-positive cells (light green) and one GFP-GAD67-positive cell also filled intracellularly with Alexa Fluor 488 (bright green, arrow) that was also labeled with an antibody against calbindin (Inset). (Scale bars: Left, 50 μm; Right, 20 μm.)
Materials and Methods
Recordings from RCs and MNs. All procedures followed Swedish federal guidelines for animal care. Postnatal heterozygote glutamic acid decarboxylase (GAD) 67-GFP mice [postnatal day (P) 0 to P4] were anaesthetized with isoflurane and eviscerated, and spinal cords were removed with ventral laminectomy, as described in ref. 9. The spinal cord was placed in a recording chamber perfused with oxygenated Ringer's solution (128 mM NaCl/4.69 mM KCl/25 mM NaHCO3/1.18 mM KH2PO4/1.25 mM MgSO4/2.5 mM CaCl2/22 mM glucose aerated with 5% CO2 in O2) at room temperature. Whole-cell tight-seal recording of RCs and MNs were performed with patch electrodes pulled from thick-walled borosilicate glass (o.d. of 1.5 mm, i.d. of 1.0 mm; Harvard Instruments) to a final resistance of 5–8 MΩ. The electrode tips were filled with 138 mM K-gluconate, 10 mM Hepes, 0.0001 mM CaCl2, 5 mM ATP-Mg, and 0.3 mM GTP-Li. After filling of the tip, the electrodes were back-filled with the same solution, and to label the recorded cell, Alexa Fluor dye (0.15–0.20%; Molecular Probes) or neurobiotin (1–2%) was diluted into the electrode solution. Cells were filled during recording. Signals were recorded with a Multiclamp 700A amplifier (Axon Instruments). Recorded signals were digitized and recorded to hard disk by using either clampex or axoscope 8.2 (Axon Instruments). Off-line data analysis was performed by using axoscope 8.2 and datapac 2000 2.33 (Run Technologies). Electrical stimulation of the ventral roots was done by a glass suction electrode placed in close proximity to the exit point of the root. RCs were identified based on the criteria given in the text. MNs were identified by the following criteria. (i) They were characterized as being large neurons located in the ventral horn that could be activated antidromically from the ventral root. In accordance with classical criteria, the antidromic spike followed high stimulus frequencies (10–20 Hz) with no jitters in the activation latencies (see Fig. 1b). (ii) MNs had more hyperpolarized membrane potentials and lower input resistance than interneurons and a characteristic depolarizing “hump” on the falling phase of the action potential (10). (iii) MNs were retrogradely labeled by applying crystals of fluorescent dextranamines [3,000 molecular weight (MW) rhodamine dextranamine or 3,000 MW Texas red dextran-amine (Molecular Probes)] to the cut ventral root. The preparations were then incubated in oxygenated Ringer's solution for 2 h, whereafter the MNs could be patched visually.
Electromyogram Recordings. Electromyogram signals were recorded from quadriceps and gastrocnemius muscles in hind-limb-attached preparations (9) by placing coated 25-μm platinium-iridium wires (deinsulated at the tip) on the muscle belly of the muscles. Muscle activity was induced by stimulating the peripheral end of cut ventral lumbar roots. All ventral and dorsal roots were cut before stimulation.
Ventral Root Recordings. To test for recurrent connections between MNs, lumbar ventral root (L) 2 through L4 were placed in suction electrodes (distal to the dorsal root ganglion) for stimulation. Simultaneous recordings were performed from the ventral roots belonging to the stimulated spinal nerve (all dorsal roots were cut).
Drugs. All drugs [d-tubocurarine (d-TC), mecamylamine (MEC), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), d-(–)-2-amino-5-phosphonopentanoic acid (AP5), atropine, and kynurenate (KYN)] were purchased from Sigma or Research Biochemicals. Drugs were dissolved in Ringer's solution and bath-applied to the preparation.
Anatomy. After recording, the spinal cord was fixed (4% paraformaldehyde in 0.1 M PBS) overnight at 4°C. Then, the preparations were cryoprotected in 0.1 M PBS with 15% sucrose.
After freezing, 25-μm-thick transverse sections were obtained from the segment containing the neurobiotin-labeled MNs or RCs. To visualize neurobiotin in MNs, we used streptavidin-conjugated Cy3 (Jackson). To visualize calbindin in RCs, and vesicular glutamate transporter (VGLUT) 1, VGLUT2, or vesicular ACh transporter (VAChT) in MN terminals, the sections were incubated with one or two primary antibodies (calbindin 28, rabbit polyclonal antibody, 1:5,000, Swant; VGLUT1, rabbit polyclonal antibody, 1:1,000, Synaptic Systems; VGLUT2, guinea pig polyclonal, 1:2,500, Chemicon; VGLUT3, rabbit polyclonal, 1:2,500, Synaptic Systems; VAChT, goat polyclonal antibody, 1:5,000, Chemicon). Dilutions were made in 0.1 M PBS with 0.1% Triton X-100 (PBS-T; pH 7.4), and incubations of the primary antibody mixture lasted for 48 h at 4°C. Immunoreactive sites were visualized with appropriate secondary antibodies coupled to Cy2 or Cy5 (dilution of 1:250; Jackson ImmunoResearch) and diluted in PBS-T. The sections were mounted on slides and cover-slipped with PBS:glycerol (1:1). Colocalization was examined with confocal microscope (LSM510, Zeiss) after making optical sections consisting of stacks of 0.6-μm-thick sections. The selected images were then denoised by using wavelet software kindly provided by Jacques Boutet de Monvel (Karolinska Hospital, Stockholm). In some preparations (n = 2), the entire motor L2 population was labeled with neurobiotin applied to the ventral root. These preparations were incubated for 4–5 h before fixation. Neuromuscular junctions were visualized by using fluorescently labeled bungarotoxin (Molecular Probes) in combination with VAChT in mice (P1–P2) that were transcardially perfused with physiological Ringer's solution followed by 4% paraformaldehyde.
Results
The first central synapses we investigated were those from MNs to RCs. To record from RCs, we performed whole-cell recordings from visually identified GABAergic neurons in the lumbar ventral horn using isolated spinal cord preparations taken from newborn (P0–P4) GAD67-GFP [GAD67-GFP(Δneo)] heterozygote knock-in mice (11). At this age, RCs express GABA, like most other inhibitory neurons in the spinal cord. Putative RCs were visually patched in the ventral spinal cord close to the MN pool (see also ref. 12). Neurons recorded in this way were identified as RCs by showing that electrical stimulation of the nearby ventral root evoked short-latency excitatory postsynaptic potentials, at the stimulus threshold that also caused antidromic activation of MNs (Fig. 1b). Recorded cells were filled intracellularly with Alexa Fluor dyes (Fig. 1c; Alexa Fluor 488 or 588), which also allowed us to confirm their identity as RCs because of their expression of calbindin (13) (Fig. 1c). By using these criteria, recorded cells could uniquely be identified as RCs. Motor neurons were identified according to the criteria given in Materials and Methods.
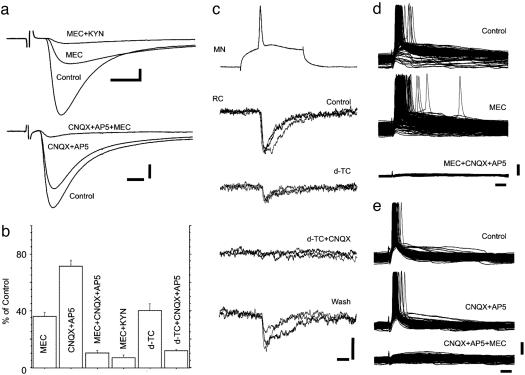
Fig. 2a Upper shows VR-S-evoked excitatory postsynaptic currents (EPSCs) recorded at –60 mV in a RC in control, after blocking nicotinic receptors with MEC (50–100 μM), and ionotropic glutamate receptors (NMDA and non-NMDA) with KYN (2 mM). The EPSCs evoked by VR-S were reduced to ≈35% of control (36.0 ± 3.1% mean ± SEM, n = 5; Fig. 2 a Upper and b) by MEC. KYN further reduced the EPSC to ≈10% of control (10.4% ± 1.7%, n = 4; Fig. 2b). Similar effects were observed when using CNQX (20 μM) and AP5 (20 μM) to block ionotropic glutamate receptors in combination with MEC (Fig. 2 a Lower and b). Comparable results were observed with another nicotinic receptor blocker, d-TC (10 μM) (reduction to 40.2 ± 5.0%, n = 5, Fig. 2b) in combination with CNQX and AP5 (reduction to 11.7 ± 1.1; Fig. 2b). Blocking only ionotropic glutamate receptors with CNQX and AP5 reduced the amplitude of the EPSC to 71.4 ± 4.2% (n = 5; Fig. 2b). There were no significant (Student's t test, P < 0.01) differences between the latency of EPSCs in control (3.5 ± 0.2 ms), in the presence of d-TC (3.6 ± 0.2 ms) and in the presence of CNQX/APV (3.7 ± 0.2 ms). The effects seen in Fig. 2 are not due to lack of potency or specificity or the drugs used. When ACh (100 μM) was bath-applied, MEC used in the same concentrations completely blocked the depolarizing response in RCs, whereas CNQX, AP5, and KYN (n = 3) had no effect on the depolarization induced by ACh. Atropine (n = 4; 10 μM) had no effect on the d-TC/MEC-resistant EPSCs, showing that ACh released from MNs is not acting via muscarinic receptors on other cells to release glutamate, as has been shown, for example, in hippocampus (14).
Fig. 2.
MNs release both glutamate and ACh onto RCs. (a Upper) VR-S-evoked EPSC recorded in a RC in control, after 50 μM MEC, and after adding 2 mM KYN to the perfusate. (a Lower) VR-S-evoked EPSC in a RC cell in control, after 20 μM CNQX plus 20 μM AP5, and after 50 μM MEC. (Scale bars: 20 pA and 5 ms.) (b) Pooled data showing the remaining peak amplitude of the VR-S-evoked EPSCs in RCs after blocking nicotinic receptors alone [MEC or d-TC (10 μM)], ionotropic glutamate receptors alone (CNQX+AP5), or a combination of both (MEC+CNQX+AP5; MEC+KYN; d-TC+CNQX+AP5). (c) Paired recordings from a MN and RC located in L2 segment. Firing the MN evokes EPSCs mediated by nicotinic and glutamatergic receptors in the RC. [Scale bars: 20 mV for MN, 5 pA for RC (vertical), and 10 ms (horizontal).] (d and e) Intracellular recordings from two different RCs showing that only a combined block of nicotinic (MEC) and ionotropic glutamate receptors (CNQX+AP5) block the VR-S-evoked excitatory postsynaptic potentials. (Scale bars: 10 mV and 20 ms.)
Early anatomical studies in the cat have shown that thin C-fibers enter the ventral root in addition to their normal entrance via the dorsal root (15). The glutamate EPSCs could therefore potentially be due to stimulation of such afferents. This seems not to be the case though. First, later studies have shown that C-fibers do not appear to enter the cord (see discussion in ref. 16). Secondly, both the glutamate and ACh component of the VR-S-evoked EPSCs have the same threshold and latency and appear when the antidromic action potential is elicited in the MNs. This finding is incompatible with a simultaneous stimulation of thick MNs axons and small C-fibers. Thirdly, we were able to record directly from 10 MN-RC pairs located close to each other. In nine of these, we could not evoke an EPSC in the RC by stimulating the recorded MN intracellularly. In the remaining pair, MN stimulation clearly evoked a glutamatergic EPSC in the RC (Fig. 2c). The latency of the evoked EPSC after the peak of the spike in the MN was 0.5 ± 0.1 ms, and the peak amplitude of this EPSC was 7.22 ± 0.7 pA. The amplitudes of the EPSCs were reduced to 39.1% (averages from three stimulations) of the control by d-TC (Fig. 2c, third trace from the top) and were completely abolished by adding CNQX to the perfusate (fourth trace from the bottom) in a reversal manner (lowermost trace). Altogether, these data show conclusively that dual glutamatergic and cholinergic EPSCs are generated at MN–RC synapses. The pharmacology shows that ≈65% of the amplitude of the EPSC is generated by ACh, whereas 20–30% is mediated by ionotropic glutamate receptor activation. Although our data show that both glutamate and ACh are released at MN–RC synapses, either one of the transmitters was sufficient to fire RCs. Thus, in current clamp recordings, only the combined blocking of nicotinic and ionotropic glutamate receptors would block the VR-S-evoked response (Fig. 2 d and e).
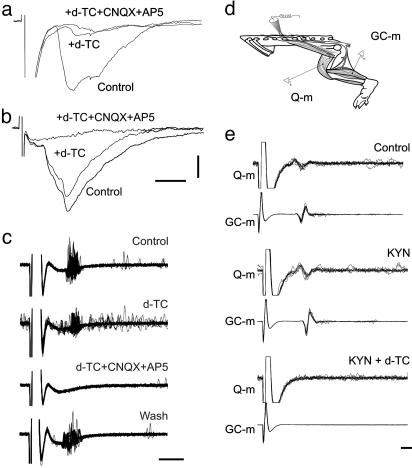
We next tested the synaptic nature of the recurrent connections between MNs. This was done in two ways. First, we recorded intracellularly from MNs while stimulating the VR. Electrical and synaptic coupling between MNs can be visualized by stimulation subthreshold for antidromic activation of the recorded MN (17). This stimulation protocol evoked EPSCs in 9 of 24 MNs tested. Such EPSCs between MNs were either mainly mediated by cholinergic transmission (d-TC reduced the EPSCs to 3.5–12.5; n = 2; Fig. 3a) or mainly mediated by glutamatergic transmission (82.5 ± 6.3 of control with d-TC followed by an almost complete block after adding CNQX+AP5; n = 7; Fig. 3b). A small, short-latency PSC that is probably due to electrical coupling between MNs (18) remained after the combined block. We also tested the pharmacology of ventral root responses that can be recorded extracellularly by stimulating the ventral root by ≈2–10 times the strength that is needed to see the first antidromic volley in the root. This stimulation consistently evoked a long burst of efferent activity that was reduced in amplitude by d-TC and further reduced or fully blocked when ionotropic glutamate receptor blockers were combined with d-TC (n = 6; Fig. 3c). These results show that synapses between MNs can release both glutamate and ACh.
Fig. 3.
Electrophysiological demonstration of glutamate/ACh transmitter release at recurrent connections between MNs. (a) VR-S-evoked EPSCs recorded in MNs in control, after 10 μM d-TC, and after adding 20 μM CNQX and 20 μM AP5. (b) VR-S-evoked EPSCs recorded in MNs in control, after d-TC, and after CNQX/AP5/d-TC. (c) Ventral root responses after stimulation of the corresponding spinal nerve in control, after d-TC (10 μM), and after adding CNQX and AP5. (c was adapted from a figure kindly provided by Makito Iizuka.) (d) Experimental set-up for experiments depicted in e. (e) Muscle responses in quadriceps (Q-m) and gastrocnemius (GC-m) muscles after stimulation of the L3/L4 ventral roots in control, after 2 mM KYN, and after KYN with 10 μM d-TC. (Scale bars: a and b, 10pA and 5 ms; c, 10ms; e, 5 ms.)
In contrast to the transmission at the two types of central MN synapses, we could not demonstrate a glutamate release at peripheral synapses at the muscles. Thus, the stimulus-evoked electromyogram responses (Fig. 3 d and e) were completely blocked by nicotinic receptor blockers (n = 3) and were not reduced by CNQX/AP5 and KYN (n = 2; Fig. 3e). This finding is in agreement with numerous previous studies showing that ACh is responsible for the depolarization at the neuromuscular junction (see, e.g., ref. 19 and references therein).
We also obtained anatomical evidence to support the electrophysiological data. First, we used in situ hybridization for the VGLUT1/2 to visualize mRNA expression in MNs (see Supporting Methods, which is published as supporting information on the PNAS web site). VGLUTs are found in cells that use glutamate as transmitter. We found large cells in the ventral horn, presumably MNs, weakly labeled for VGLUT2 (Fig. 5, which is published as supporting information on the PNAS web site) but not labeled for VGLUT1 (data not shown). This finding is in agreement with previous studies showing that VGLUTs are found in adult rodent MNs (8).
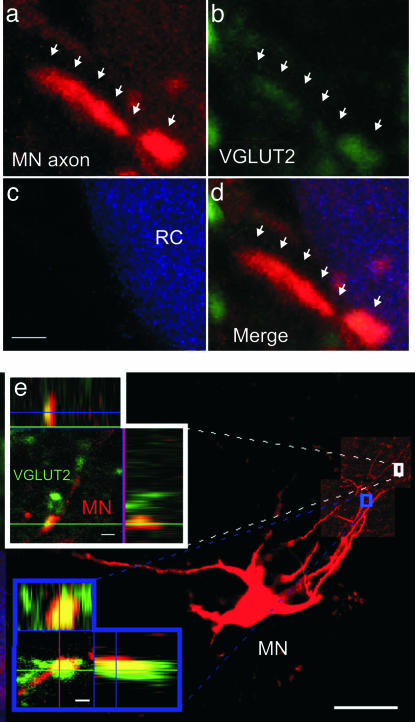
To look for VGLUTs in central MN synapses, we stained the entire ventral root with either neurobiotin (n = 2) or individual MNs (n = 6) and then looked for colocalization of neurobiotin and VGLUT2s. Such colocalizations were indeed found in close apposition to RC somata (Fig. 4 a–d) as well as in axonal branches in the MN region (Fig. 4e). However, when looking for colocalization of VAChT (specific for cholinergic neurons) and VGLUT2 in the RC or MN area, we found very few terminals that coexpress both (Fig. 6a, which is published as supporting information on the PNAS web site); in a volume of 72,500 μm3 (50 × 50 × 30 μm) in the MN region, we found only three double-labeled terminals, whereas the same volume of tissue contained >1,000 VAChT-positive terminals and >3,000 VGLUT2-positive terminals (see also refs. 8 and 20). In addition, VAChT was not colocalized with VGLUT1 or VGLUT3 (data not shown). In agreement with previous studies (8, 21), we were unable to demonstrate the presence of VGLUT1 or VGLUT2 at the neuromuscular junction in hind-limb muscles (Fig. 6 b and c). Our anatomical findings confirm previous anatomical studies (8, 21) and further indicate that although both glutamate and ACh are released at central MN synapses, the two transmitters are only colocalized to a small degree.
Fig. 4.
Morphological evidence of glutamatergic neurotransmission in central MNs synapses. (a) MNs were filled retrogradely with neurobiotin (red). (b) Glutamatergic terminals were visualized with anti-VGLUT2 (green). (c) RCs were labeled with anti-calbindin (blue). (d) Merged image of VGLUT2-postive fiber in close apposition to RC (arrows). (e) MN labeled intracellularly with neurobiotin (red) with its axon projecting into the motor nucleus. (Insets) Enlargements of two MN terminals (red) that colocalized with VGLUT2 (green) in normal and orthogonal X–Y planes, respectively. (Scale bars: 1 μm.)
Discussion
In this article, we have shown by electrophysiological recordings and immunohistochemistry that mammalian MNs contain and release glutamate in addition to ACh. Although it has been suggested from anatomical studies that mammalian MNs contain glutamate (7, 8), cooperative evidence from physiological experiments has not been provided. Our findings thus change a long-held notion that mammalian MNs, the principal neurons in the spinal cord, only use ACh as their transmitter. This conclusion does not seem to be restricted to developing MNs, because anatomical studies from adult mammals provide clear support of our findings (7, 8). Moreover, previous studies in the adult cat have shown that ventral root-evoked activation of RCs is only partly blocked by MEC (22).
Like other examples of cotransmission (23, 24), the functional consequences of having two transmitters at central spinal synapses are not completely clear. Activity in central MN synapses generate negative (MN–RC–MN) and positive (MN–MN) feedback loops that in turn regulate MN activity. Such local recurrent positive and negative feedback loops are found in many areas of the brain including the cortex (25, 26), where they can balance the membrane potential close to firing threshold allowing optimal conditions for persistent firing. The MN feedback loops may play a similar role. Having both transmitters in the loops would stabilize activity, allowing for an optimal integration of activity over the pool of connected neurons. In contrast, at the neuromuscular junction ACh seems sufficient to evoke activity. Although, we cannot completely rule out the possibility that glutamate is released from peripheral axon terminals, our findings suggest that in contrast to the central synapses, glutamate has a less significant role, if any, at the peripheral synapses formed by MNs. This finding is in agreement with previous anatomical studies (8, 21), which could not demonstrate the presence of VGLUT1-3 in the neuromuscular junction. However, a recent study has shown that VGLUT3 is expressed in proximity to the endplates in skeletal muscles (27). At the moment, there is no explanation for the discrepancies between different studies. One possibility is that different types of MNs (e.g., slow or fast) release or do not release glutamate at the neuromuscular junction.
Our study contains another important finding. Not only is there diversity between the overall transmitter release in central (recurrent activation of RCs cells and other MNs) and peripheral (muscle) MN synapses, but our data also suggest that ACh and glutamate are not colocalized at central MN synapses and might not even be released at the neuromuscular junction. To our knowledge, there are few demonstrated examples of such a clear neurotransmitter segregation. Those examples are the Aplysia bag cells (28) and the modulatory proctolin neuron in the crustacean stomatogastric nervous system (29), where two different transmitters are released in different target areas. The experiments also suggest that mammalian MNs are exceptions to the principle first coined by Dale in 1935 (30) that the same transmitter is released from all terminals in a single neuron. Together, the results suggest that a complex intracellular apparatus is able to selectively direct the necessary molecular machinery to synthesize and accumulate transmitters into vesicles on different terminal branches of a neuron. How that internal transmitter sorting takes place is not understood.
Finally, having glutamate in central MN synapses might be a factor for development of motor neurodegenerative diseases such as amyotrophic lateral sclerosis, where glutamate-induced neurotoxic processes are known to play a key role (31, 32).
Supplementary Material
Acknowledgments
The VGLUT probes were kindly provided by Martyn Goulding (The Salk Institute for Biological Studies, San Diego). We are grateful to Drs. Lennart Brodin, Staffan Cullheim, Abdel El Manira, Sten Grillner, Hans Hultborn, and Katharina Quinlan for discussions and for reading a previous version of the manuscript. This work was supported by the Human Frontier Science Program (O.K.), the National Institutes of Health (O.K.), and the Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad (to H.N.). Y.Y. is affiliated with Core Research for Evolutional Science and Technology and Solution Oriented Research for Science and Technology of the Japan Science and Technology Corporation (Kawaguchi, Japan).
Abbreviations: ACh, acetylcholine; AP5, d-(–)-2-amino-5-phosphonopentanoic acid; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; d-TC, d-tubocurarine; EPSC, excitatory postsynaptic current; GAD, glutamic acid decarboxylase; KYN, kynurenate; Ln, lumbar ventral root n; MEC, mecamylamine; MN, motor neuron; Pn, postnatal day n; RC, Renshaw cell; VAChT, vesicular ACh transporter; VGLUT, vesicular glutamate transporter; VR-S, ventral root stimulation.
References
- 1.Bennett, M. (2001) History of the Synapse (Harwood Academic, Chur, Switzerland).
- 2.Eccles, J. C., Fatt, P. & Koketsu, K. (1954) J. Physiol. 126, 524–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis, D. R. & Ryall, R. W. (1964) Nature 203, 652–653. [DOI] [PubMed] [Google Scholar]
- 4.Windhorst, U. (1996) Prog. Neurobiol. 49, 517–587. [DOI] [PubMed] [Google Scholar]
- 5.Cullheim, S., Kellerth, J. O. & Conradi, S. (1977) Brain Res. 132, 1–10. [DOI] [PubMed] [Google Scholar]
- 6.Cullheim, S. & Kellerth, J. O. (1981) J. Physiol. 312, 209–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meister, B., Arvidsson, U., Zhang, X., Jacobsson, G., Villar, M. J. & Hökfelt, T. (1993) NeuroReport 5, 337–340. [DOI] [PubMed] [Google Scholar]
- 8.Herzog, E., Landry, M., Buhler, E., Bouali-Benazzouz, R., Legay, C., Henderson, C. E., Nagy, F., Dreyfus, P., Giros, B. & El Mestikawy, S. (2004) Eur. J. Neurosci. 20, 1752–1760. [DOI] [PubMed] [Google Scholar]
- 9.Kiehn, O. & Kjaerulff, O. (1996) J. Neurophysiol. 75, 1472–1482. [DOI] [PubMed] [Google Scholar]
- 10.Kjaerulff, O. & Kiehn, O. (2001) J. Neurophysiol. 85, 580–593. [DOI] [PubMed] [Google Scholar]
- 11.Tamamaki, N., Yanagawa, Y., Tomioka, R., Miyazaki, J., Obata, K. & Kaneko, T. (2003) J. Comp. Neurol. 467, 60–79. [DOI] [PubMed] [Google Scholar]
- 12.Sapir, T., Geiman, E. J., Wang, Z., Velasquez, T., Mitsui, S., Yoshihara, Y., Frank, E., Alvarez, F. J. & Goulding, M. (2004) J. Neurosci. 24, 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr, P. A., Alvarez, F. J., Leman, E. A. & Fyffe, R. E. (1998) NeuroReport 9, 2657–2661. [DOI] [PubMed] [Google Scholar]
- 14.Araque, A., Martin, E. D., Perea, G., Arellano, J. I. & Buno, W. (2002) J. Neurosci. 22, 2443–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coggeshall, R. E., Coulter, J. D. & Willis, W. D., Jr. (1974) J. Comp. Neurol. 153, 39–58. [DOI] [PubMed] [Google Scholar]
- 16.Hildebrand, C., Karlsson, M. & Risling, M. (1997) Prog. Neurobiol. 51, 89–128. [DOI] [PubMed] [Google Scholar]
- 17.Kiehn, O. & Tresch, M. C. (2002) Trends Neurosci. 25, 108–115. [DOI] [PubMed] [Google Scholar]
- 18.Tresch, M. C. & Kiehn, O. (2000) Nat. Neurosci. 3, 593–599. [DOI] [PubMed] [Google Scholar]
- 19.Misgeld, T., Burgess, R. W., Lewis, R. M., Cunningham, J. M., Lichtman, J. W. & Sanes, J. R. (2002) Neuron 36, 635–648. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira, A. L., Hydling, F., Olsson, E., Shi, T., Edwards, R. H., Fujiyama, F., Kaneko, T., Hökfelt, T., Cullheim, S. & Meister, B. (2003) Synapse 50, 117–129. [DOI] [PubMed] [Google Scholar]
- 21.Kraus, T., Neuhuber, W. L. & Raab, M. (2004) Neurosci. Lett. 360, 53–56. [DOI] [PubMed] [Google Scholar]
- 22.Noga, B. R., Shefchyk, S. J., Jamal, J. & Jordan, L. M. (1987) Exp. Brain Res. 66, 99–105. [DOI] [PubMed] [Google Scholar]
- 23.Jonas, P., Bischofberger, J. & Sandkuhler, J. (1998) Science 281, 419–424. [DOI] [PubMed] [Google Scholar]
- 24.Li, W. C., Soffe, S. R. & Roberts, A. (2004) Proc. Natl. Acad. Sci. USA 101, 15488–15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douglas, R. J., Koch, C., Mahowald, M., Martin, K. A. & Suarez, H. H. (1995) Science 269, 981–985. [DOI] [PubMed] [Google Scholar]
- 26.Shu, Y., Hasenstaub, A. & McCormick, D. A. (2003) Nature 423, 288–293. [DOI] [PubMed] [Google Scholar]
- 27.Boulland, J. L., Qureshi, T., Seal, R. P., Rafiki, A., Gundersen, V., Bergersen, L. H., Fremeau, R. T., Jr., Edwards, R. H., Storm-Mathisen, J. & Chaudhry, F. A. (2004) J. Comp. Neurol. 480, 264–280. [DOI] [PubMed] [Google Scholar]
- 28.Sossin, W. S., Sweet-Cordero, A. & Scheller, R. H. (1990) Proc. Natl. Acad. Sci. USA 87, 4845–4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blitz, D. M. & Nusbaum, M. P. (1999) J. Neurosci. 19, 6774–6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dale, H. (1935) Proc. R. Soc. London 28, 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawahara, Y., Ito, K., Sun, H., Aizawa, H., Kanazawa, I. & Kwak, S. (2004) Nature 427, 801. [DOI] [PubMed] [Google Scholar]
- 32.Lafon-Cazal, M., Pietri, S., Culcasi, M. & Bockaert, J. (1993) Nature 364, 535–537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.