Abstract
The aim of this paper is to provide a brief review of mitochondrial structure as it relates to function and then present abnormalities in mitochondria in postmortem schizophrenia with a focus on ultrastructure. Function, morphology, fusion, fission, motility, ΔΨmem, ATP production, mitochondrial derived vesicles, and mitochondria-associated ER membranes will be briefly covered. Pathology in mitochondria has long been implicated in schizophrenia, as shown by genetic, proteomic, enzymatic and anatomical abnormalities. The cortex and basal ganglia will be reviewed. In the anterior cingulate cortex, the number of mitochondria per neuronal somata in layers 5/6 in schizophrenia is decreased by 43%. There are also fewer mitochondria in terminals forming axospinous synapses. In the caudate and putamen the number of mitochondria is abnormal in both glial cells and neurons in schizophrenia subjects, the extent of which depends on treatment, response and predominant lifetime symptoms. Treatment-responsive schizophrenia subjects had about a 40% decrease in the number of mitochondria per synapse in the caudate nucleus and putamen, while treatment resistant cases had normal values. A decrease in mitochondrial density in the neuropil distinguishes paranoid from undifferentiated schizophrenia. The appearance, size and density of mitochondria were normal in the nucleus accumbens. In the substantia nigra, COX subunits were affected in rostral regions. Mitochondrial hyperplasia occurs within axon terminals that synapse onto dopamine neurons, but mitochondria in dopamine neuronal somata are similar in size and number. In schizophrenia, mitochondria are differentially affected depending on the brain region, cell type, subcellular location, treatment status, treatment response and symptoms.
Keywords: postmortem, psychosis, electron microscopy, neuropathology, cytochrome oxidase
1. Introduction
The aim of this paper is to provide a brief review of mitochondrial structure as it relates to function and then present abnormalities in mitochondria in schizophrenia with a focus on ultrastructure.
1.1. Normal mitochondrial function
Mitochondria are famous for producing 95% of cellular energy using the electron transport chain (Wong-Riley, 1989). In addition, they are necessary for other cellular functions including intracellular calcium buffering (Babcock and Hille, 1998; Duchen et al., 2008; Gunter et al., 1994), production of reactive oxygen species (Chang and Reynolds, 2006), regulation of apoptosis (Susin et al., 1999) and modulation of synaptic activity (Duchen et al., 2008; Li et al., 2004; Miller and Sheetz, 2004; Sheng and Cai, 2012). Mitochondria are plastic and dynamic organelles that can change shape, location, size and number in response to energy demands (Isaacs et al., 1992; Ligon and Steward, 2000; Mjaatvedt and Wong-Riley, 1988; Prince et al., 1999).
1.2. The afterlife of mitochondria
Neuronal function in the brain requires energy in the form of ATP. To assess mitochondrial activity in human brain, investigators have previously utilized frozen postmortem brain tissue to analyze mitochondrial enzymatic activities (Devi et al., 2008), protein levels (Park et al., 2001), and DNA (Alam et al., 1997; Vila et al., 2008). However, there are several other vital indices that provide insight into brain mitochondrial activity which cannot be accomplished in frozen tissue samples. These include measurements of the mitochondrial membrane potential (ΔΨmem), ATP production, calcium buffering capacity, and respiration, which together give an overall assessment of mitochondrial health and activity. For example, the ΔΨmem, which is the electrochemical gradient across the inner mitochondrial membrane, serves as an important overall indicator of mitochondrial activity. It is also a fundamental component of respiring mitochondria. The ΔΨmem is linked to many crucial mitochondrial functions including ATP synthesis, calcium homeostasis, mitochondrial protein import, and mitochondrial metabolite transport (Huttemann et al., 2008), all of which are typically analyzed in real-time measurements.
Although metabolic activity in the brain ceases at death, we have been able to measure the ΔΨmem and ATP production in mitochondria isolated from human postmortem brains with postmortem intervals of up to 8.5 hours (Barksdale et al. 2010). Furthermore, postmortem brain mitochondria retain their ΔΨmem and ATP production capacities following cryopreservation, indicating that functional isolated mitochondria can be archived for future studies. The findings that ΔΨmem and ATP generation can be reinitiated in brain mitochondria hours after death indicates that postmortem brains can be an abundant source of viable mitochondria for the study of metabolic processes in health and disease, and it is also possible to archive these mitochondria for future studies (Barksdale et al. 2010).
1.3. Morphology
Mitochondria have different shapes; they can be round, elongated, blob-shaped, donut-shaped or have an elaborate configuration (Picard and McEwen, 2014). In most instances the overall shape of the mitochondrion has functional implications (Youle and van der Bliek, 2012; Ahmad et al., 2013). Take for example the relationship between the shape of mitochondria and the production of reactive oxygen species. In cell culture, mitochondrial stressors can induce the conversion of straight (i.e. rod-shaped) mitochondria to donut-shaped mitochondria, to blob-shaped mitochondria (Liu and Hajnóczky, 2011; Ahmad et al., 2013). Blob-shaped mitochondria generate the highest levels of reactive oxygen species, followed by donut shaped compared to straight mitochondria (Liu and Hajnóczky, 2011; Ahmad et al., 2013). While donut-shaped mitochondria can revert back to the straight configuration, blob-shaped mitochondria are unable to revert back to healthier configurations. In axon terminals, donut-shaped mitochondria are associated with shorter synapses and fewer docked vesicles; in the dorsolateral prefrontal cortex donut-shaped mitochondria are correlated with poor delayed response memory (Hara et al., 2014).
In addition to shape, the morphology of the cristae, matrix and inner mitochondrial membrane correspond to the activity of the electron transport chain (Hackenbrock, 1968). At the ultrastructural level, mitochondria have an orthodox or condensed configuration, which corresponds to high or low energy producing states, respectively (Hackenbrock, 1968). In the condensed configuration, the matrix is smaller and denser, the inner membrane is irregularly organized and forms few cristae, and the space between inner and outer membranes is increased. The orthodox configuration is what is usually illustrated in electron micrographs (Figures 1,2).
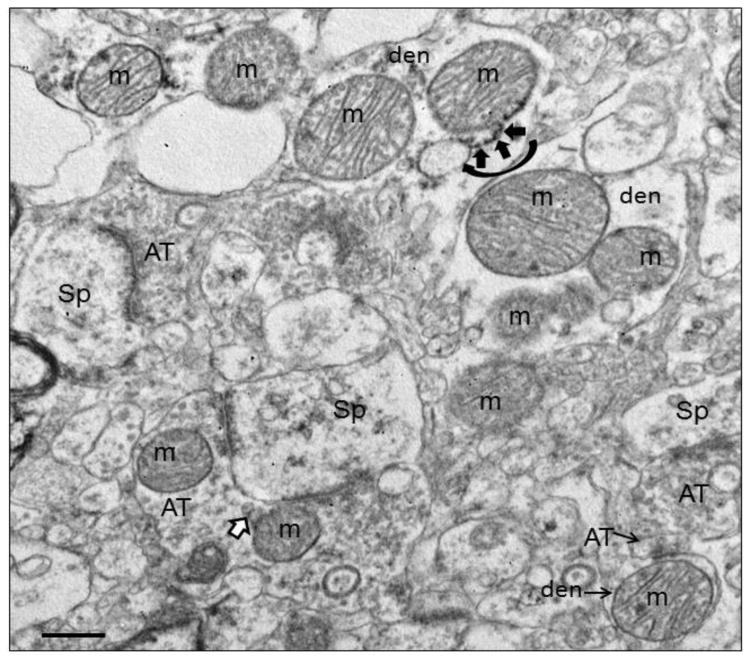
Figure 1.
Electron micrograph of human striatum. Mitochondria (m) are shown in various subcellular locations. In the dendrite (den) at the top of the field, mitochondrial associated ER (MAM) is shown (curved black arrow) with ER (short black arrows) connecting to the adjacent mitochondrion. In the terminal (AT) synapsing on a spine (Sp) in the lower part of the field, mitochondrial derived vesicles (MDVs) are shown (white arrow with black outline) budding off of a mitochondrion. Scale bars = 0.5 μm. Figure is modified from Figure 2a in Somerville et al., 2011b.
In the aging nervous system, there are reports of fewer mitochondria, but they are larger in size (Shigenaga et al., 1994; Soghomonian et al., 2010; Martinelli et al., 2006). Perhaps enlargement of the mitochondria is a compensatory mechanism to deal with the decreased number. However, fewer bigger mitochondria are able to meet short energy demands, but sustained energy demands are not met (Shigenaga et. al., 1994; Soghomonian et al., 2010; Martinelli et al., 2006). Thus, the examination of size and shape of mitochondria will reveal important information about their functionality.
1.4. Fission and Fusion
Mitochondria function as a dynamic network constantly undergoing fission and fusion, the balance of which is important in maintaining their structural integrity and function (Legros et al., 2002). Proteins that cause mitochondrial fusion include mitofusin-1, mitofusion-2 and Opa1 (Koshiba et al., 2004). Although they are critical for mitochondrial fusion, they are necessary for other functions as well. For example, besides its role in mitochondrial fusion, mitofusin-2 contributes to the maintenance and operation of the mitochondrial network (Bach et al., 2003). Mitofusin 2 is also necessary for transporting mitochondria and proper localization in neuronal processes (Misko et al., 2010; Sheng and Cai, 2012). Mitochondria undergo fragmentation in response to various stimuli, including electron transport chain toxins and mitochondrial DNA-linked mutations. Dynamin related protein 1 (Drp1) (Smirnova et al., 2001) interacts with other proteins--mitochondrial fission protein 1 (Fis1) (James et al., 2003), mitochondrial fission factor (Mff) (Otera, et al., 2010), and mitochondrial dynamics proteins MiD49 and MiD51 (recently reviewed by Bertholet et al., 2016)—to cause division of mitochondria. Mitochondrial division occurs at ER contact sites on mitochondrial constrictions (de Brito and Scorrano, 2010; Friedman et al., 2011). While both Fis1 and Mff have roles in mitochondrial fission, either MiD49 or MiD51 can mediate Drp1 recruitment and mitochondrial fission in the absence of Fis1 and Mff (Losón et al., 2013). Therefore, there are multiple proteins that are sufficient, but not necessary, to recruit Drp1 to mediate mitochondrial fission.
1.5. Mitochondria derived vesicles
Mitochondria derived vesicles (MDVs) are structures that bud off of mitochondria and transport damaged cargo to peroxisomes or lysosomes (Figure 1). MDVs have either single or double membranes, unique densities and uniform diameter (Soubannier et al., 2012). MDVs are stimulated upon various forms of mitochondrial stress, and the vesicles incorporate cargo, whose composition depends upon the type of stress. Stress-induced MDVs are selectively enriched for oxidized proteins, suggesting that conformational changes induced by oxidation may initiate their incorporation into the vesicles.
1.6. Mitochondria-associated endoplasmic reticulum membranes
Mitochondria are connected to the endoplasmic reticulum via mitochondria-associated endoplasmic reticulum membranes (MAMs; Hayashi et al., 2009) (Figure 1). MAMs are subregions of the endoplasmic reticulum that have a unique lipid composition, are enriched in cholesterol and anionic phospholipids, and have characteristics of lipid rafts (Hayashi and Fujimoto, 2010). MAMs are involved in a number of key metabolic functions, including phospholipid and cholesterol metabolism (Hayashi et al., 2009). MAMs are also enriched in proteins related to the control of mitochondrial division (Friedman et al., 2011) and dynamics (Schon and Area-Gomez, 2013). Mitofusin 2 tethers endoplasmic reticulum to mitochondria (de Brito and Scorrano, 2008). Defects in MAM-localized proteins and/or disturbances in MAM function play a role in neurodegenerative diseases (Area-Gomez et al., 2012; Schon and Przedborski, 2011; Ottolini et al., 2013).
1.7. Motility
In neurons, biogenesis of mitochondria occurs in the cell soma (Davis and Clayton, 1996) and they have a half-life of functionality of about one month (Menzies and Gold, 1971). Healthy mitochondria have relatively high membrane potentials and 90% of them move anterogradely, suggesting that healthy mitochondria are trafficked away from their point of origin in the cell soma (Miller and Sheetz, 2004). As mitochondria move down a neuronal process, they stop and start; at least half of mitochondria are stationary, whereas the rest move at variable speeds (Allen et al., 1982; Hollenbeck, 1996; Hollenbeck and Saxton, 2005; Ligon and Steward, 2000). Mitochondria are more stationary at synaptic sites, where the energy demand is especially high (Li et al., 2004; Chang et al., 2006). Mitochondria move along microtubules via kinesin and adaptors for anterograde transport and via dynein and adaptors for retrograde transport; they also can be anchored via actin and neurofilaments (reviewed by Lin and Sheng, 2015).
Axonal transport happens at different speeds depending on the particular substance being transported, its functional role, and the direction of transport (Niescier et al., 2016). In axons, a large range of average velocities for mitochondria have been reported for both anterograde and retrograde movement, probably due to sampling different lengths of axon, and/or different distances from the cell body (MacAskill and Kittler, 2010). It has been classically thought that at the end of their functional life span mitochondria are degraded by autophagy, or retrogradely transported back to the cell soma and recycled there (reviewed by Ploumi et al., 2016). New evidence challenges the notion that mitochondria go back to the cell body to die (Niescier et al., 2016). Only a few studies to date have examined transport of mitochondria in the entire axon and over a long period of time (O’Toole et al., 2008; Niescier et al., 2016). Niescier et al., (2016) showed that mitochondria originating from axon terminals moving retrogradely never actually make it all the way back to the cell body, and only a few mitochondria moving anterogradely out of the cell soma ever arrive at the axon terminals. Importantly, the velocity of mitochondria varies depending on the location in the axon, so examining the entire axon becomes very important to obtain average mitochondrial speeds. While it was classically thought that the rate of anterograde transport in the axon was independent of electrical activity (reviewed by Oztas, 2003), new evidence indicates otherwise (Sajic et al., 2013).
2. Mitochondrial abnormalities in schizophrenia
Among the many abnormalities in schizophrenia are those related to mitochondrial function (for reviews see Ben-Shachar, 2002; Ben-Shachar and Laifenfeld, 2004; Clay et al., 2011). Evidence of mitochondrial dysfunction in schizophrenia includes genetic (Marchbanks et al., 2003; Kvajo et al., 2008; Verge et al., 2011), metabolic (Prabakaran et al., 2004), enzymatic (Prince et al., 1999, 2000; Maurer et al., 2001) and anatomical abnormalities (reviewed herein). The production of ATP and calcium buffering are essential in maintaining synaptic strength and abnormalities in these processes could lead to decreased metabolism and defective synaptic activity (Ben-Shachar and Laifenfeld, 2004; Chang and Reynolds, 2006; Duchen et al., 2008). Many of our electron microscopic studies and those of Uranova and colleagues (Kolomeets and Uranova, 2009; Uranova et al., 1996, 2001, 2007; Vikhreva et al., 2016) have examined mitochondrial size, number, location, structural integrity and markers of viability in various brain regions implicated in schizophrenia. Ultrastructural studies are possible with well-preserved tissue with short postmortem intervals (Figure 2). Here mitochondrial abnormalities in structure and function in schizophrenia are reviewed in several, but not all, brain regions (see Figure 3 for a summary).
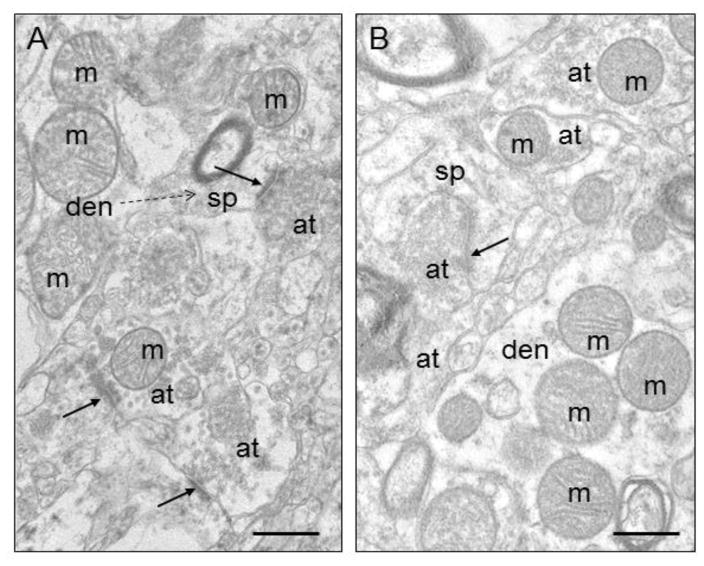
Figure 2.
Electron micrographs of human striatum. A) Control. B) Schizophrenia. Mitochondria (m) are shown in various subcellular locations. Abbreviations: s, spine; at, axon terminal; den, dendrite; ma, myelinated axon; solid arrows, synapses; dotted arrow, spine emerging from dendrite. Scale bars = 0.5 μm. Figure is modified from Figure 1 in Somerville et al., 2011b.
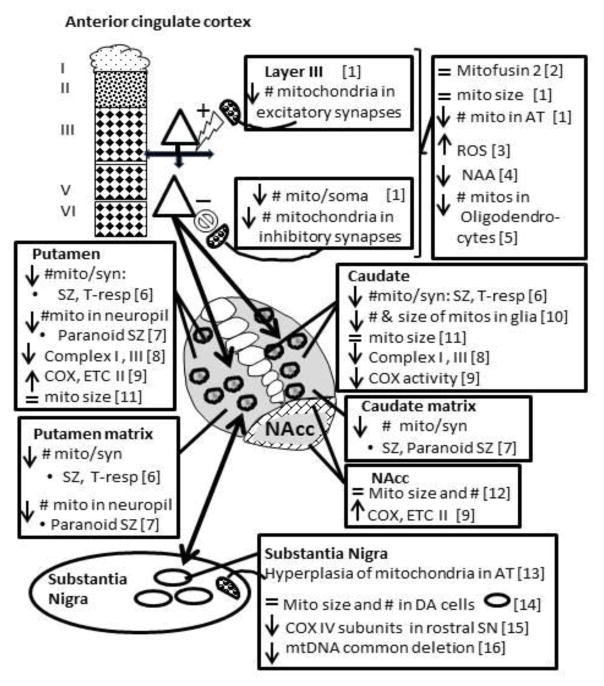
Figure 3.
Schematic drawing of the cortical striatal nigral pathway showing mitochondrial abnormalities in each region in schizophrenia. Depending on the study, the entire structure or subdivisions within, were studied. The cortex is divided in layers for layer specific changes. Patch (circles) and matrix compartments are shown for the caudate and putamen when abnormalities are striosome or matrix specific. The core and shell (hatched) of the nucleus accumbens are depicted. Mito, mitochondria. ROS, reactive oxygen species. NAA, N-acetylaspartate. COX, cytochrome oxidase. ETC, electron transport chain. Mito/synapse, mitochondria per terminal forming a synapse. T-resp, treatment responsive schizophrenia. AT, axon terminals. DA, dopamine. mtDNA, mitochondrial DNA. 1) Roberts et al., 2015; 2) Barksdale, et al., 2014; 3) Wang et al., 1999; 4) Reid et al., 2010; 5) Uranova et al., 2007, Vikhreva et al., 2016; 6) Mamdani et al., 2014; 7) Somerville et al., 2011; 8) Somerville et al., 2012; 9) Maurer et al., 2001; Ben-Shakar & Karry, 2008; 10) Cavalier et al., 1995; Prince et al., 1999, 2000; 11) Uranova et al., 1996, 2001; 12) Kung & Roberts, 1999; 13) McCollum et al., 2015; 14) Kolomeets & Uranova, 1999; 15) Walker & Roberts, 2016; 16) Rice et al., 2014.
2.1. Anterior Cingulate Cortex
The anterior cingulate cortex (ACC), a structurally and functionally diverse region, is one of several brain regions that are abnormal in schizophrenia (Fornito et al., 2009). The ACC serves to regulate several cognitive functions including reward anticipation, emotion, empathy, and decision making. Previous imaging and electrophysiological studies have revealed changes in the ACC related to psychosis and antipsychotic drug response (Lahti et al., 2006, 2009). N-acetylaspartate (NAA), a marker of neuronal integrity, is decreased in the ACC in schizophrenia (Reid et al., 2010). NAA and Glx (a measure of glutamate) are not correlated in schizophrenia, but are in controls (Kraguljac et al., 2012). In vivo imaging has shown decreased BOLD during memory retrieval (Hutcheson et al., 2012). Oxidative stress, a result of mitochondrial production of reactive oxygen species, is increased (Wang et al., 2009). In a recent study markers of synapses and mitochondria were examined in the ACC in a schizophrenia cohort divided by treatment response (Barksdale et al., 2014). Protein levels of the mitochondrial marker, mitofusin-2, were normal in schizophrenia cases; moreover, there were no effects of treatment response. Normal protein levels suggest that mitochondrial fusion and maintenance and operation of the mitochondrial network may be intact. However, while mitofusin 2 may be normal, there are many other mitochondrial proteins involved in fusion that might be abnormal.
In an ultrastructural study, the number of mitochondria per neuronal somata in schizophrenia was decreased by 43% of that of controls (Roberts et al., 2015). This was due to a selective loss in layers 5/6. The density of mitochondria in the neuropil (connections in between cell bodies) was similar between controls and the schizophrenia cases in the combined ACC (layers 3,5,6) as well as the separate analysis of layer 3 and layers 5/6. There were no differences in size of mitochondria in the neuronal somata or in the neuropil. Moreover, there were no obvious blob or donut shaped mitochondria. This is somewhat surprising considering the observed increase in reactive oxygen species (Wang et al., 2009), which are produced at a higher rates in blob and donut shaped mitochondria (Liu and Hajnóczky, 2011; Ahmad et al., 2013), yet only round or rod shaped mitochondria were observed.
The number of mitochondria in axon terminals in the combined ACC was lower in schizophrenia as compared to controls. There were fewer mitochondria in certain subtypes of terminals, especially in terminals forming axospinous synapses. Interestingly, in Layer 3, mitochondria were less frequent in asymmetric axospinous synapses, while in layers 5/6 there were fewer mitochondria in symmetric axospinous synapses. Asymmetric synapses (also called Gray Type I) are excitatory, while symmetric synapses (also called Gray Type II) are inhibitory (Gray, 1959). In layer 3 of non-human primates, asymmetric axospinous synapses are formed by afferents from the medial dorsal thalamus, contralateral ACC and other intracortical connections (see Hoftman et al., 2016). Symmetric axospinous synapses in deep cortical layers originate in part from dopamine connections (Kubota et al., 2016). In other ultrastructural studies, Uranova and Aganova (1989) have shown layer specific abnormalities such as fewer synapses in the ACC, and fewer mitochondria in oligodendrocytes in both gray and white matter (Uranova et al., 2007; Vikhreva et al., 2016). The latter observation suggests that oligodendrocytes need or have less energy, which may have an impact on proper myelination.
Fewer mitochondria in axon terminals suggest a decrease in efficacy of synaptic transmission (Brodin et al., 1999; Verstreken et al., 2005; Hall et al., 2012), and in the case of the ACC this would affect both excitatory and inhibitory connections. Pyramidal neurons in layers 5/6 project to the striatum or brainstem, or thalamus (Goldman and Nauta, 1977). The reduction of mitochondria per soma in the deep layers suggests that neurons that project to these subcortical regions may have compromised metabolism. The layer specific location suggests abnormalities in multiple connections that may have effects within the cortex as well as in several downstream pathways. The decrease in NAA and increase in oxidative stress may be reflected by decreased numbers of mitochondria, rather than smaller size or irregular shapes.
2.2. Dorsal Striatum
2.2.1. Treatment Status
Several studies have shown mitochondrial abnormalities in the striatum in subjects with schizophrenia such as decreases in complex I and III activity, protein and/or mRNA levels (Ben-Shachar and Karry, 2008; Maurer et al., 2001), and changes in complex IV (Cavelier et al., 1995; Prince et al., 1999, 2000). The complexes do not necessarily change in the same direction in all nuclei. For instance, there is a decrease in COX activity in the caudate and an increase in COX and succinate dehydrogenase in the putamen in SZ postmortem tissue (Prince, et al., 1999). Most of these changes appear to be caused by antipsychotic drugs (APDs) (Burkhardt et. al., 1993; Prince et al., 1997).
Previous ultrastructural studies in the striatum in schizophrenia have shown similar numbers (Somerville et al., 2011a) and size (Kung and Roberts, 1999) of mitochondria. However, decreases in the number of mitochondria per synapse were detected in both the caudate and putamen in schizophrenia. Since the majority of mitochondria are in dendrites, fewer mitochondria in axon terminals may be missed in overall neuropil counts. Further analysis showed that subjects divided by treatment status into off drug, atypical APD or typical APD, all showed significant decreases in the putamen (Somerville et al., 2011a). Since the schizophrenia subgroups on APD had similar decreases in mitochondrial number compared to the off-drug subjects, this result may not be an APD effect. However, haloperidol, a typical APD, does reduce the number of mitochondria in the striatum of chronically treated rats (Roberts et al., 1995).
When examining patch matrix compartments, striatal compartments associated with different circuitry and function (Graybiel and Ragsdale, 1978), only the matrix exhibited changes. The matrix compartment preferentially receives inputs from the dorsolateral prefrontal cortex (Eblen and Graybiel, 1995), which processes higher cognitive functions, such as working memory (Goldman-Rakic, 1999). Motor and somatosensory cortices also project to the matrix in a topographical fashion (Flaherty and Graybiel, 1993). Thus, it would be expected that mitochondrial abnormalities in the striatal matrix could impact cognition, working memory, motor, and somatosensory functions.
Mitochondria are also affected in glial cells. In the caudate, they are decreased in number in astrocytes (Uranova et. al. 1996) and are smaller in oligodendrogliocytes (Uranova et al., 2001). Either of these results could compromise the function of these cell types.
2.2.2. Symptoms
Symptoms of schizophrenia can vary markedly between patients, and similar symptoms may be related to shared brain pathophysiology. In addition, a relationship between symptoms and mitochondrial pathology is evident in blood. Lymphocytes analyzed from paranoid schizophrenia patients and controls showed less mitochondrial volume in patients in certain types of lymphocytes (Uranova et al., 2007). Moreover, the severity of the mitochondrial deficit was positively correlated with symptom severity, linking paranoid symptoms with mitochondrial impairment, albeit in blood (Uranova et al., 2007). Also, COX and complex II have been shown to correlate with the severity of symptoms in the putamen (Prince et al., 2000), again linking symptoms with mitochondrial dysfunction.
In an ultrastructural study of the caudate and putamen, a decrease in the density of mitochondria in the neuropil was observed in the paranoid group compared to both the controls and the undifferentiated group (Somerville et al., 2012). In addition, the putamen of the paranoid group had fewer mitochondria in axon terminals compared to the NCs and the undifferentiated group. However, in neither the caudate nor the putamen was the number of mitochondria per synapse differentially decreased in either subgroup. When examining patch matrix compartments, only the matrix exhibited changes. These results could be associated with the symptoms of paranoia and/or could represent a protective mechanism against some of the symptoms that are less pronounced in this subtype than in the undifferentiated subgroup, such as cognitive and emotional deficits.
2.2.3. Treatment Response
One third of patients with schizophrenia do not respond to medication and remain psychotic (Meltzer, 1997). The patients that do respond, do so on a continuum, but only respond to positive symptoms. Cognitive and negative symptoms are poorly treated in all people with schizophrenia. Although treatment response and resistance have a biological basis (Sheitman and Lieberman, 1998), all studies conducted outside of our own work have been imaging live people. In a cohort of subjects rated for treatment response or resistance, treatment-responsive schizophrenia subjects had about a 40% decrease in the number of mitochondria per synapse in the caudate nucleus and putamen. In the putamen, treatment-responsive subjects also had decreases in this measure compared to treatment-resistant subjects (34%) (Somerville et al., 2011b). These results provide further support for a biological distinction between treatment response and treatment resistance in schizophrenia. Because treatment-resistant subjects had normal levels of mitochondria per synapse, but treatment responders had fewer mitochondria per synapse than controls, fewer mitochondria per synapse may be related to treatment response.
2.2.4. Summary of Dorsal Striatum
Fewer mitochondria per synapse were observed in a combined cohort of subjects and does not appear to be caused by APDs. This change was confined to treatment responders, and was not observed in treatment resistant subjects. A decrease in mitochondrial density in the neuropil distinguishes the paranoid from the undifferentiated schizophrenia subgroup. Fewer mitochondria may contribute to the pathophysiology of the illness, may be a medication effect, or an adaptive response to normalize overactive neurotransmission that may occur from the higher than normal number of excitatory striatal synapses previously found (Roberts et al., 2005a,b, 2008, 2012).
2.3. Nucleus Accumbens
Increases in COX and succinate dehydrogenase have been reported in the nucleus accumbens in schizophrenia postmortem tissue (Prince, et al., 1999). However, the structural integrity and general appearance of mitochondria were normal in the schizophrenia group (McCollum et al., 2015). The density of mitochondria in the neuropil, the average diameter, and the number of calcium deposits per mitochondrion were similar between controls and schizophrenia in both the core and shell. Taken together, alterations in mitochondrial function may not be detected with morphology.
2.4. Substantia Nigra
In spite of the fact that the substantia nigra (SN) and ventral tegmental area (VTA) house the largest proportion of dopamine neurons in the brain and that antipsychotic medication works by blocking dopamine receptors, there have been very few studies of the SN/VTA in schizophrenia.
2.4.1 The mitochondrial common deletion
Decrease in the global brain mtDNA common deletion levels are seen in several brain regions in schizophrenia, with the largest abnormalities in dopaminergic regions including the ventral midbrain (Mamdani et al., 2014). The common deletion contains genes encoding subunits of cytochrome oxidase, NADH dehydrogenase and ATP synthase (Samuels et al., 2004; Verge et al., 2011). This abnormality suggests that mitochondrial function is impaired in dopaminergic nuclei including the SN.
2.4.2 Cytochrome oxidase
In a recent study COX activity and the protein expression of key subunits for its assembly and activity were measured in postmortem SN/VTA (Rice et al., 2014). While overall COX activity was similar between schizophrenia and NCs, there were decreases in the expression of subunits II and IV-I of COX in schizophrenia in samples containing rostral regions of the SN/VTA. Rats chronically treated with antipsychotic drugs did not show any changes in COX subunit expression, suggesting that the changes seen in the schizophrenia group were not caused by medication. Interestingly, anomalies in COX subunit II mRNA expression have been previously reported in the frontal cortex in schizophrenia without significant changes in COX activity (Clark et al., 1999).
Subunits II and IV of the COX enzyme are crucial for the proper functioning of the COX complex as a whole (Nijtmans et al., 1998; Clark et al., 1999; Rahman et al., 1999). COX subunit II is responsible for the binding of cytochrome c and the subsequent electron transfer to subunit I of the COX enzyme (Taanman, 1997). Suppression of subunit IV has been linked to a reduced function in overall COX activity and an increased susceptibility to apoptosis (Huttemann et al., 2001; Li et al., 2006). These findings suggest that COX subunit expression may be compromised in specific sub-regions of the SN/VTA (i.e. rostral regions), which may lead to a faulty assembly of the enzyme and a greater vulnerability to metabolic insult.
2.4.3 Ultrastructure
At the ultrastructural level, mitochondrial hyperplasia has been observed within axon terminals that synapse onto dopamine neurons in the SN (Kolomeets and Uranova, 1999). However, mitochondria in dopamine neuronal somata were similar in size and structural integrity between SZ and NCs (Walker and Roberts, 2016).
3. Antipsychotic drugs and mitochondria
The general consensus is that antipsychotic drugs can alter mitochondrial function, number and size. Antipsychotic drugs have differential effects on mitochondrial structure and function depending on brain location, type of antipsychotic drug, length of use, length of withdrawal period, dose and route of administration (for some examples see Takeichi and Sato, 1987; Uranova et al., 1991; Roberts et al., 1995; Prince et al., 1999; Streck et al., 2007). For instance, there are more striatal mitochondria after 3 weeks of haloperidol treatment (Uranova et al., 1991), but less after 6 months (Roberts et al., 1995).
Complexes I, II/III and IV of the electron transport chain can be measured to assess mitochondrial function (Wong-Riley, 1989). Results on the functionality of the various complexes of the electron transport chain in SZ have been conflicting and are beyond the scope of this review. The majority of evidence is in agreement that complex I and succinate dehydrogenase appear to be adversely affected by antipsychotic drugs (Burkhardt, et al., 1993; Balijepalli et al., 1999, 2001; Karry et al., 2004; Rosenfeld et al., 2011; Maurer and Moller, 1997; Prince et al., 1997; Streck et al., 2007).
4. Summary
In the brains of subjects with schizophrenia, mitochondria are differentially affected depending on the brain region, cell type, and subcellular location in which they are located (Fig. 3). Moreover, mitochondrial anomalies differ depending on treatment status, treatment response and symptoms. While certain morphological configurations definitely correspond to energy capacity and other functions, it appears that mitochondria can appear intact, while being functionally compromised. Decreases in functional measures may be reflected by decreased number of mitochondria rather than decreased size or structural configuration.
Fewer mitochondria may be a primary deficit of the disease, or mitochondria may die as an epiphenomenon of the disease. Alternatively, mitochondria may be sequestered in neuronal somata located either extrinsic or intrinsic to the striatum. An inability of mitochondria to move into axon terminals or dendrites could account for a decreased number of mitochondria in these structures. Since mitochondria move around the neuron along microtubules between the soma and processes, damage to cytoskeletal elements may lead to a failure of proper mitochondrial movement. In the cingulum bundle, our preliminary work shows abnormal levels of αtubulin, a component of microtubules, in off drug schizophrenia, which is normalized by APD treatment (Roberts and Schoonover, 2016). This suggests cytoskeletal abnormalities.
Numerous studies have reported the occurrence of altered or decreased mitochondrial function in the aging brain, in neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s disease, in neurometabolic disorders such as Leigh syndrome (Dahl, 1998) and in psychiatric disorders other than schizophrenia (Orth and Schapira, 2001; Vila et al., 2008; Rollins et al., 2009; Anglin et al., 2012; Manji et al., 2012; Ottolini et al., 2013). Thus, it is clear that mitochondria play a fundamental role in many brain disease processes.
Acknowledgments
I would like to thank the past and present members of my lab whose work I cite in this review, especially Keri A. Barksdale, Miguel Melendez-Ferro, Lesley A. McCollum, Emma Perez-Costas, Matthew W. Rice, Joy K. Roche, Shahza M. Somerville, Kirsten E. Schoonover, and Courtney K. Walker.
Role of funding sources
This research was supported by the RO1MH66123 and R21MH108867-01. The funding source had no role in study design, data collection, analysis and interpretation of data, writing the manuscript, or in the decision to submit the paper for publication.
Footnotes
Conflict of interest
The author has no conflicts of interest to declare.
Contributors
Dr. Roberts wrote this review based in part on previously published work generated in her laboratory.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad T, Aggarwal K, Pattnaik B, Mukherjee S, Sethi T, Tiwari BK, Kumar M, Micheal A, Mabalirajan U, Ghosh B, Sinha Roy S, Agrawal A. Computational classification of mitochondrial shapes reflects stress and redox state. Cell Death Dis. 2013;4:e461. doi: 10.1038/cddis.2012.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997;(69):1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- Allen RD, Metuzals J, Tasaki I, Brady ST, Gilbert SP. Fast axonal transport in squid giant axon. Science. 1982;218:1127–1129. doi: 10.1126/science.6183744. [DOI] [PubMed] [Google Scholar]
- Anglin RE, Mazurek MF, Tarnopolsky MA, Rosebush PI. The mitochondrial genome and psychiatric illness. Am J Med Genetics Part B. 2012;159B(7):749–759. doi: 10.1002/ajmg.b.32086. [DOI] [PubMed] [Google Scholar]
- Area-Gomez E, Del Carmen Lara Castillo M, Tambinj MD, Guardia-Laguarta C, de Groof AJ, Madra M, Ikenouchi J, Umeda M, Bird TD, Sturley SL, Schon EA. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 2012;31(21):4106–4123. doi: 10.1038/emboj.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock DF, Hille B. Mitochondrial oversight of cellular Ca2+ signaling. Curr Opin Neurobiol. 1998;8:398–404. doi: 10.1016/s0959-4388(98)80067-6. [DOI] [PubMed] [Google Scholar]
- Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, Rabasa-Lhoret R, Wallberg-Henriksson H, Laville M, Palacin M, Vidal H, Rivera F, Brand M, Zorzano A. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism, a novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- Balijepalli S, Boyd MR, Ravindranath V. Inhibition of mitochondrial complex I by haloperidol: the role of thiol oxidation. Neuropharm. 1999;38:567–577. doi: 10.1016/s0028-3908(98)00215-9. [DOI] [PubMed] [Google Scholar]
- Balijepalli S, Kenchappa RS, Boyd MR, Ravindranath V. Protein thiol oxidation by haloperidol results in inhibition of mitochondrial complex I in brain regions: comparison with atypical antipsychotics. Neurochem Int. 2001;38:425–435. doi: 10.1016/s0197-0186(00)00108-x. [DOI] [PubMed] [Google Scholar]
- Barksdale KA, Perez-Costas E, Gandy JC, Melendez-Ferro M, Roberts RC, Bijir GU. Mitochondrial viability in mouse and human postmortem brain. FASEB J. 2010;24:3590–3599. doi: 10.1096/fj.09-152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barksdale K, Lahti AC, Roberts RC. Synaptic proteins in the postmortem anterior cingulate cortex in schizophrenia: Relationship to treatment and treatment response. Neuropsychopharmacology. 2014;39(9):2095–2103. doi: 10.1038/npp.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar D. Mitochondrial dysfunction in schizophrenia: a possible linkage to dopamine. J Neurochem. 2002;83(6):1241–1251. doi: 10.1046/j.1471-4159.2002.01263.x. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar D, Laifenfeld D. Mitochondria, synaptic plasticity, and schizophrenia. Int Rev Neurobiol. 2004;59:273–296. doi: 10.1016/S0074-7742(04)59011-6. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar D, Karry R. Neuroanatomical pattern of mitochondrial complex I pathology varies between schizophrenia, bipolar disorder and major depression. PLoS One. 2008;3(11):e3676. doi: 10.1371/journal.pone.0003676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholet AM, Delerue T, Millet AM, Moulis MF, David C, Daloyau M, Arnauné-Pelloquin L, Davezac N, Mils V, Miquel MC, Rojo M, Belenguer P. Mitochondrial fusion/fission dynamics in neurodegeneration and neuronal plasticity. Neurobiol Dis. 2016;90:3–19. doi: 10.1016/j.nbd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Brodin L, Bakeeva L, Shupliakov O. Presynaptic mitochondria and the temporal pattern of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci. 1999;354:365–372. doi: 10.1098/rstb.1999.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt C, Kelly JP, Lim Y-H, Filley CM, Parker WD., Jr Neuroleptic medications inhibit complex I of the electron transport chain. Ann Neurol. 1993;33:512–517. doi: 10.1002/ana.410330516. [DOI] [PubMed] [Google Scholar]
- Cavelier L, Jazin EE, Eriksson I, Prince J, Bave U, Oreland L, Gyllensten U. Decreased cytochrome-c oxidase activity and lack of age-related accumulation of mitochondrial DNA deletions in the brains of schizophrenics. Genomics. 1995;29:217–224. doi: 10.1006/geno.1995.1234. [DOI] [PubMed] [Google Scholar]
- Chang DT, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci. 2006;26(26):7035–7045. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DTW, Reynolds IJ. Mitochondrial trafficking and morphology in healthy and injured neurons. Progress in Neurobiol. 2006;80:241–268. doi: 10.1016/j.pneurobio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Clark KM, Taylor RW, Johnson MA, Chinnery PF, Chrzanowska-Lightowlers ZM, Andrews RM, Nelson IP, Wood NW, Lamont PJ, Hanna MG, Lightowlers RN, Turnbull DM. An mtDNA mutation in the initiation codon of the cytochrome C oxidase subunit II gene results in lower levels of the protein and a mitochondrial encephalomyopathy. Am J Hum Genet. 1999;64:1330–1339. doi: 10.1086/302361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay H, Sillivan S, Konradi C. Mitochrondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311–324. doi: 10.1016/j.ijdevneu.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AF, Clayton DA. In situ localization of mitochondrial DNA replication in intact mammalian cells. J Cell Biol. 1996;135(4):883–893. doi: 10.1083/jcb.135.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. An intimate liaison: spatial organization of the endoplasmic reticulum mitochondria relationship. EMBO J. 2010;29:2715. doi: 10.1038/emboj.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl HH. Getting to the nucleus of mitochondrial disorders: identification of respiratory chain-enzyme genes causing Leigh syndrome. Am J Hum Genet. 1998;63(6):1594–1597. doi: 10.1086/302169. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of α-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR, Verkhratsky A, Muallem S. Mitochondria and calcium in health and disease. Cell Calcium. 2008;44(1):1–5. doi: 10.1016/j.ceca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Eblen F, Graybiel AM. Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. J Neurosci. 1995;15(9):5999–6013. doi: 10.1523/JNEUROSCI.15-09-05999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. Two input systems for body representations in the primate striatal matrix: experimental evidence in the squirrel monkey. J Neurosci. 1993;13(3):1120–1137. doi: 10.1523/JNEUROSCI.13-03-01120.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yücel M, Dean B, Wood SJ, Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophr Bull. 2009;35(5):973–993. doi: 10.1093/schbul/sbn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334(6054):358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry. 1999;46(5):650–661. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Nauta WJ. An intricately patterned prefronto-caudate projection in the rhesus monkey. J Comp Neurol. 1977;72(3):369–386. doi: 10.1002/cne.901710305. [DOI] [PubMed] [Google Scholar]
- Gray EG. Electron microscopy of synaptic contacts on dendrite spines of the cerebral cortex. Nature. 1959;183:1592–1593. doi: 10.1038/1831592a0. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267(2 Pt 1):C313–339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Ragsdale CW., Jr Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci. 1978;75(11):5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock CR. Ultrastructural bases for metabolically linked mechanical activity in mitochondria; II Electron transport-linked ultrastructural transformations in mitochondria. J Cell Biol. 1968;37(2):345–369. doi: 10.1083/jcb.37.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CN, Klein-Flugge MC, Howarth C, Attwell D. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci. 2012;32:8940–8951. doi: 10.1523/JNEUROSCI.0026-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Yuk F, Puri R, Janssen WG, Rapp PR, Morrison JH. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci. 2014;111(1):486–91. doi: 10.1073/pnas.1311310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Fujimoto M. Detergent-resistant microdomains determine the localization of sigma-1 receptors to the endoplasmic reticulum-mitochondria junction. Mol Pharmocol. 2010;77(4):517–528. doi: 10.1124/mol.109.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoftman GD, Datta D, Lewis DA. Layer 3 Excitatory and Inhibitory Circuitry in the Prefrontal Cortex: Developmental Trajectories and Alterations in Schizophrenia. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.05.022. pii: S0006-3223(16) 32427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ. The pattern and mechanism of mitochondrial transport in axons. Front Biosci. 1996;1:d91–102. doi: 10.2741/a118. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J of Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson NL, Reid MA, White DM, Kraguljac NV, Avsar KB, Bolding MS, Knowlton RC, den Hollander JA, Lahti AC. Multimodal analysis of the hippocampus in schizophrenia using proton magnetic resonance spectroscopy and functional magnetic resonance imaging. Schizophr Res. 2012;140(1–3):136–142. doi: 10.1016/j.schres.2012.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttemann M, Lee I, Pecinova A, Pecina P, Przyklenk K, Doan JW. Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J Bioenerg Biomembr. 2008;40:445–456. doi: 10.1007/s10863-008-9169-3. [DOI] [PubMed] [Google Scholar]
- Huttemann M, Kadenbach B, Grossman LI. Mammalian subunit IV isoforms of cytochrome c oxidase. Gene. 2001;267:111–123. doi: 10.1016/s0378-1119(01)00385-7. [DOI] [PubMed] [Google Scholar]
- Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12(1):110–119. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- Karry R, Klein E, Ben Shachar D. Mitochondrial complex I subunits expression is altered in schizophrenia: a postmortem study. Biol Psychiatry. 2004;55(7):676–684. doi: 10.1016/j.biopsych.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Kolomeets NS, Uranova NA. Synaptic contacts in schizophrenia: studies using immunocytochemical identification of dopaminergic neurons. Neurosci Behav Physiol. 1999;2:217–221. doi: 10.1007/BF02465329. [DOI] [PubMed] [Google Scholar]
- Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305(5685):858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D, Lahti AC. Neurometabolites in schizophrenia and bipolar disorder - a systematic review and meta-analysis. Psychiatry Res. 2012;203(2–3):111–25. doi: 10.1016/j.pscychresns.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Karube F, Nomura M, Kawaguchi Y. The Diversity of Cortical Inhibitory Synapses. Front Neural Circuits. 2016;25(10):27. doi: 10.3389/fncir.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung L, Roberts RC. Mitochondrial pathology in human schizophrenic striatum: a postmortem ultrastructural study. Synapse. 1999;31:67–75. doi: 10.1002/(SICI)1098-2396(199901)31:1<67::AID-SYN9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kvajo M, Dhilla A, Swor DE, Karayiorgou M, Gogos JA. Evidence implicating the candidate schizophrenia/bipolar disorder susceptibility gene G72 in mitochondrial function. Mol Psychiatry. 2008;13:685–696. doi: 10.1038/sj.mp.4002052. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31(1):221–30. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Cropsey KL. Modulation of limbic circuitry predicts treatment response to antipsychotic medication: a functional imaging study in schizophrenia. Neuropsychopharmacology. 2009;34(13):2675–2690. doi: 10.1038/npp.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros F, Lombès A, Frachon P, Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusions. Mol Biol Cell. 2002;13(12):4343–4354. doi: 10.1091/mbc.E02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Park JS, Deng JH, Bai Y. Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J Bioenerg Biomembr. 2006;38:283–291. doi: 10.1007/s10863-006-9052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto KI, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Ligon LA, Steward O. Role of microtubules and actin filaments in the movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol. 2000;427(3):351–361. doi: 10.1002/1096-9861(20001120)427:3<351::aid-cne3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Lin MY, Sheng ZH. Regulation of mitochondrial transport in neurons. Exp Cell Res. 2015;334(1):35–44. doi: 10.1016/j.yexcr.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hajnóczky G. Altered fusion dynamics underlie unique morphological changes in mitochondria during hypoxia-reoxygenation stress. Cell Death Differ. 2011;18(10):1561–1572. doi: 10.1038/cdd.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losón OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24(5):659–67. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAskill AF, Kittle JT. Control of mitochondrial transport and localization in neurons. Trends Cell Biol. 2010;20(2):102–112. doi: 10.1016/j.tcb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Mamdani F, Rollins B, Morgan L, Sequeira PA, Vawter MP. The somatic common deletion in mitochondrial DNA is decreased in schizophrenia. Schizophr Res. 2014;159(2–3):370–375. doi: 10.1016/j.schres.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, Chen G. Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci. 2012;13:293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- Marchbanks RM, Ryan M, Day IN, Owen M, McGuffin P, Whatley SA. A mitochondrial DNA sequence variant associated with schizophrenia and oxidative stress. Schizophr Res. 2003;65:33–38. doi: 10.1016/s0920-9964(03)00011-2. [DOI] [PubMed] [Google Scholar]
- Martinelli C, Sartori P, Ledda M, Pannese E. A study of mitochondria in spinal ganglion neurons during life: quantitative changes from youth to extremely advanced age. Tissue Cell. 2006;38(2):93–8. doi: 10.1016/j.tice.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Maurer I, Moller HJ. Inhibition of complex I by neuroleptics in normal human brain cortex parallels the extrapyramidal toxicity of neuroleptics. Mol Cell Biochem. 1997;174:255–259. [PubMed] [Google Scholar]
- Maurer I, Zierz S, Moller H. Evidence for a mitochondrial oxidative phosphorylation defect in brains from patients with schizophrenia. Schizophr Res. 2001;48:125–136. doi: 10.1016/s0920-9964(00)00075-x. [DOI] [PubMed] [Google Scholar]
- McCollum LA, Walker CK, Roche JK, Roberts RC. Elevated excitatory input to the nucleus accumbens in schizophrenia: a postmortem ultrastructural study. Schizophr Bull. 2015;41(5):1123–1132. doi: 10.1093/schbul/sbv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY. Treatment-resistant schizophrenia--the role of clozapine. Curr Med Res Opin. 1997;14(1):1–20. doi: 10.1185/03007999709113338. [DOI] [PubMed] [Google Scholar]
- Menzies RA, Gold PH. The turnover of mitochondria in a variety of tissues of young adult and aged rats. J Biol Chem. 1971;246(8):2425–2429. [PubMed] [Google Scholar]
- Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J Cell Sci. 2004;117(13):2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci. 2010;30(12):4232–4240. doi: 10.1523/JNEUROSCI.6248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjaatvedt AE, Wong-Riley MT. Relationship between synaptogenesis and cytochrome oxidase activity in Purkinje cells of the developing rat cerebellum. J Comp Neurol. 1988;277(2):155–182. doi: 10.1002/cne.902770202. [DOI] [PubMed] [Google Scholar]
- Niescier RF, Kwak SK, Joo SH, Chang KT, Min KT. Dynamics of Mitochondrial Transport in Axons. Front Cell Neurosci. 2016;(10):123. doi: 10.3389/fncel.2016.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijtmans LG, Taanman JW, Muijsers AO, Speijer D, Van den Bogert C. Assembly of cytochrome-c oxidase in cultured human cells. Eur J Biochem. 1998;254:389–394. doi: 10.1046/j.1432-1327.1998.2540389.x. [DOI] [PubMed] [Google Scholar]
- O’Toole M, Latham R, Baqri RM, Miller KE. Modeling mitochondrial dynamics during in vivo axonal elongation. J Theor Biol. 2008;255:369–377. doi: 10.1016/j.jtbi.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Orth M, Schapira AH. Mitochondria and degenerative disorders. Am J Med Genet. 2001;106:27–36. doi: 10.1002/ajmg.1425. [DOI] [PubMed] [Google Scholar]
- Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, Mihara K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 2010;191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolini D, Calì T, Negro A, Brinin M. The Parkinson disease-related protein DJ-1 counteracts mitochondrial impairment induced by the tumour suppressor protein p53 by enhancing endoplasmic reticulum-mitochondria tethering. Hum Mol Genet. 2013;22(11):2152–2168. doi: 10.1093/hmg/ddt068. [DOI] [PubMed] [Google Scholar]
- Oztas E. Neuronal tracing, a brief review. Neuroanatomy. 2003;2:2–5. [Google Scholar]
- Park LCH, Albers DS, Xu H, Lindsay JG, Beal MF, Gibson GE. Mitochondrial impairment in the cerebellum of the patients with progressive supranuclear palsy. J Neurosci Res. 2001;66:1028–1034. doi: 10.1002/jnr.10062. [DOI] [PubMed] [Google Scholar]
- Picard M, McEwen BS. Mitochondria impact brain function and cognition. Proc Natl Acad Sci USA. 2014;111(1):7–8. doi: 10.1073/pnas.1321881111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploumi C, Daskalaki I, Tavernarakis N. Mitochondrial biogenesis and clearance: a balancing act. FEBS J. 2016 Jul 27; doi: 10.1111/febs.13820. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, Wayland M, Freeman T, Dudbridge F, Lilley KS, Karp NA, Hester S, Tkachev D, Mimmack ML, Yolken RH, Webster MJ, Torrey EF, Bahn S. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684–97. doi: 10.1038/sj.mp.4001511. [DOI] [PubMed] [Google Scholar]
- Prince JA, Blennow K, Gottfries CG, Karlsson I, Oreland L. Mitochondrial function is differentially altered in the basal ganglia of chronic schizophrenics. Neuropsychopharmacology. 1999;21:372–379. doi: 10.1016/S0893-133X(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Prince JA, Harro J, Blennow K, Gottfries CG, Oreland L. Putamen mitochondrial energy metabolism is highly correlated to emotional and intellectual impairment in schizophrenics. Neuropsychopharmacology. 2000;22(3):284–292. doi: 10.1016/S0893-133X(99)00111-6. [DOI] [PubMed] [Google Scholar]
- Prince JA, Yassin MS, Oreland L. Neuroleptic-induced mitochondrial enzyme alterations in the rat brain. J Pharmacol Exp Ther. 1997;280:261–267. [PubMed] [Google Scholar]
- Rahman S, Taanman JW, Cooper JM, Nelson I, Hargreaves I, Meunier B, Hanna MG, García JJ, Capaldi RA, Lake BD, Leonard JV, Schapira AH. A missense mutation of cytochrome oxidase subunit II causes defective assembly and myopathy. Am J Hum Genet. 1999;65:1030–1039. doi: 10.1086/302590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MA, Stoeckel LE, White DM, Avsar KB, Bolding MS, Akella NS, Knowlton RC, den Hollander JA, Lahti AC. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol Psychiatry. 2010;68(7):625–33. doi: 10.1016/j.biopsych.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice MW, Smith KL, Roberts RC, Perez-Costas E, Melendez-Ferro M. Assessment of cytochrome C oxidase dysfunction in the substantia nigra/ventral tegmental area in schizophrenia. PLOS1. 2014;9(6):e100054. doi: 10.1371/journal.pone.0100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RC, Barksdale KA, Roche JK, Lahti AC. Decreased synaptic and mitochondrial density in the postmortem anterior cingulate cortex in schizophrenia. Schizophr Res. 2015;168(1–2):543–53. doi: 10.1016/j.schres.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RC, Gaither LA, Gao XM, Kashyap SM, Tamminga CA. Ultrastructural correlates of haloperidol-induced oral dyskinesias in rat striatum. Synapse. 1995;20(3):234–243. doi: 10.1002/syn.890200307. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Roche JK, Conley R. Synaptic differences in the postmortem striatum of subjects with schizophrenia: a stereological ultrastructural analysis. Synapse. 2005a;56(4):185–197. doi: 10.1002/syn.20144. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Roche JK, Conley R. Synaptic differences in the patch matrix compartments of the striatum of subjects with schizophrenia: a postmortem ultrastructural analysis. Neurobiol Dis. 2005b;20:324–335. doi: 10.1016/j.nbd.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Roche JK, Conley RR. Differential synaptic changes in the striatum of subjects with undifferentiated versus paranoid schizophrenia. Synapse. 2008;62(8):616–627. doi: 10.1002/syn.20534. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Roche JK, Somerville SM, Conley RR. Ultrastructural Distinctions Between Treatment Responders and Non-Responders in Schizophrenia: Postmortem Studies of the Striatum. In: Labate L, editor. Mental Illnesses - Evaluation, Treatments and Implications. InTech; Croatia: 2012. pp. 261–286. [Google Scholar]
- Roberts RC, Schoonover KE. Dysregulation of markers of white matter integrity in the postmortem cingulum bundle in schizophrenia: relation to treatment status. Annual meeting for the American College of Neuropsychopharmacology; December.2016. [Google Scholar]
- Rollins B, Martin MV, Sequeira PA, Moon EA, Morgan LZ, Watson SJ, Schatzberg A, Akil H, Myers RM, Jones EG, Wallace DC, Bunney WE, Vawter MP. Mitochondrial variants in schizophrenia, bipolar disorder, and major depressive disorder. PLoS One. 2009;4:e4913. doi: 10.1371/journal.pone.0004913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M, Brenner-Lavie H, Ari SG-B, Kavushansky A, Ben-Shachar D. Perturbation in mitochondrial network dynamics and in complex I dependent cellular respiration in schizophrenia. Biol Psychiatry. 2011;69:980–988. doi: 10.1016/j.biopsych.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Sajic M, Matrolia V, Lee CY, Trigo D, Sadeghian M, Mosley AJ, Gregson NA, Duchen MR, Smith KJ. Impulse conduction increases mitochondrial transport in adult mammalian peripheral nerves in vivo. Plos Biol. 2013;11(12):e1001754. doi: 10.1371/journal.pbio.1001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DC, Schon EA, Chinnery PF. Two direct repeats cause most human mtDNA deletions. Trends Genet. 2004;20(9):393–398. doi: 10.1016/j.tig.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Schon EA, Area-Gomez E. Mitochondria-associated ER membranes in Alzheimer disease. Mol Cell Neurosci. 2013;55:26–36. doi: 10.1016/j.mcn.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Schon EA, Przedborski S. Mitochondria: the next (neurode) generation. Neuron. 2011;70(6):1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheitman BB, Lieberman JA. The natural history and pathophysiology of treatment resistant schizophrenia. J Psychiatry Res. 1998;32:143–150. doi: 10.1016/s0022-3956(97)00052-6. [DOI] [PubMed] [Google Scholar]
- Sheng Z-H, Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci. 2012;13(2):77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. PNAS. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoubridge EA. Cytochrome c oxidase deficiency. Am J Ned Genet. 2001;106:46–52. doi: 10.1002/ajmg.1378. [DOI] [PubMed] [Google Scholar]
- Smirnova L, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghomonian JJ, Sethares C, Peters A. Effects of age on axon terminals forming axosomatic and axodendritic inhibitory synapses in prefrontal cortex. Neuroscience. 2010;168(1):74–81. doi: 10.1016/j.neuroscience.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville SM, Conley RR, Roberts RC. Mitochondria in the striatum of subjects with schizophrenia. World J Biol Psychiatry. 2011;12(1):48–56. doi: 10.3109/15622975.2010.505662. [DOI] [PubMed] [Google Scholar]
- Somerville SM, Conley RR, Roberts RC. Striatal mitochondria in subjects with chronic undifferentiated vs. chronic paranoid schizophrenia. Synapse. 2012;66:29–41. doi: 10.1002/syn.20981. [DOI] [PubMed] [Google Scholar]
- Somerville SM, Lahti AC, Conley RR, Roberts RC. Mitochondria in the striatum of subjects with schizophrenia: relationship to treatment response. Synapse. 2011;65:215–224. doi: 10.1002/syn.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubannier V, Rippstein P, Kaufman BA, Shoubridge EA, McBride HM. Reconstitution of mitochondira derived vesicle formation demonstrates selective enrichment of oxidized cargo. PLoS One. 2012;7(12):e52830. doi: 10.1371/journal.pone.0052830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streck EL, Rezin GT, Barbosa LM, Assis LC, Grandi E, Quevedo J. Effect of antipsychotics on succinate dehydrogenase and cytochrome oxidase activities in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 2007;376(1–2):127–33. doi: 10.1007/s00210-007-0178-2. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397(6718):441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Taanman JW. Human cytochrome c oxidase: structure, function, and deficiency. J Bioenerg Biomembr. 1997;29:151–163. doi: 10.1023/a:1022638013825. [DOI] [PubMed] [Google Scholar]
- Takeichi M, Sato T. Quantitative electron microscopic investigation on changes of mitochondria in long-term CPZ administration in rat brain, liver and heart. The Japanese J Psych and Neurol. 1987;41:749–753. doi: 10.1111/j.1440-1819.1987.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Aganova EA. Ultrastructure of the synapses of the anterior limbic cortex in schizophrenia. Zh Nevropatol Psikhiatr Im S S Korsakova. 1989;89:56–59. [PubMed] [Google Scholar]
- Uranova NA, Casanova MF, DeVaughn NM, Orlovskaya DD, Denisov DV. Ultrastructural alterations of synaptic contacts and astrocytes in postmortem caudate nucleus of schizophrenic patients. Schizophr Res. 1996;22(1):81–3. doi: 10.1016/0920-9964(96)00059-x. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, Rachmanova V. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Orlovskaya DD, Apel K, Klintsova AJ, Haselhorst U, Schenk H. Morphometric study of synaptic patterns in the rat caudate nucleus and hippocampus under haloperidol treatment. Synapse. 1991;1991(4):253–9. doi: 10.1002/syn.890070402. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Vikhreva OV, Zimina IS, Kolomeets NS, Orlovskaya DD. The role of oligodendrocyte pathology in schizophrenia. Int J Neuropsychopharmacol. 2007;10(4):537–545. doi: 10.1017/S1461145707007626. [DOI] [PubMed] [Google Scholar]
- Verge B, Alonso Y, Valero J, Miralles C, Vilella E, Martorell L. Mitochondrial DNA (mtDNA) and schizophrenia. Eur Psychiatry. 2011;26(1):45–56. doi: 10.1016/j.eurpsy.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJT, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Vikhreva OV, Rakhmanova VI, Orlovskaya DD, Uranova NA. Ultrastructural alterations of oligodendrocytes in prefrontal white matter in schizophrenia: A post-mortem morphometric study. Schizophr Res. 2016 doi: 10.1016/j.schres.2016.04.023. S0920-9964 (16) 30173–30176. [DOI] [PubMed] [Google Scholar]
- Vila M, Ramonet D, Perier C. Mitochondrial alterations in Parkinson’s disease: new clues. J Neurochem. 2008;(107):317–328. doi: 10.1111/j.1471-4159.2008.05604.x. [DOI] [PubMed] [Google Scholar]
- Walker CK, Roberts RC. Ultrastructural study of treatment response in the substantia nigra in schizophrenia. Annual meeting for the National Conference on Undergraduate Research; Asheville, NC. 2016. [Google Scholar]
- Wang JF, Shao L, Sun X, Young LT. Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar Disorders. 2009;11(5):523–529. doi: 10.1111/j.1399-5618.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989;12(3):94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]