Abstract
The androgen receptor (AR) is a widely accepted therapeutic target in prostate cancer and multiple studies indicate that the AR and Wnt/β-catenin pathways intersect. Recent genome-wide analysis of prostate cancer metastases illustrate the importance of the Wnt/β-catenin pathway in prostate cancer and compel us to reexamine the interaction of the AR and Wnt/β-catenin signaling pathways. This review includes newer areas of interest such as non-canonical Wnt signaling and the role of Wnts in prostate cancer stem cells. The effort to develop Wnt modulating therapeutics, both biologics and small molecules, is also discussed.
Keywords: Androgen Receptor, β-catenin, Prostate Cancer, Wnt Signaling
1. Introduction
β-catenin is an effector of the Wnt family of proteins, an evolutionarily conserved group of signaling molecules that regulate developmental and biological processes (Miller et al., 1999; Polakis, 2000; Wodarz and Nusse, 1998). In the absence of extracellular Wnt signals, cytoplasmic β-catenin is phosphorylated by glycogen synthase kinase 3 (GSK3) as part of a destruction complex including adenomatous polyposis coli (APC) and axin proteins. The phosphorylated β-catenin is then ubiquitinated and degraded. Wnt ligands bind their associated frizzled receptors in conjunction with cofactor lipoprotein receptor-related protein (LRP). Frizzled then signals to dishevelled (DVL) which inhibits the APC/axin/GSK3 destruction complex, thus stabilizing β-catenin and allowing its translocation to the nucleus. In the nucleus β-catenin binds the T cell factor (TCF) family of transcription factors to regulate expression of target genes. Aberrant Wnt/β-catenin signaling has been linked to a number of human cancers (Miyoshi and Hennighausen, 2003; Moon et al., 2004) including prostate cancer (PCa) (Beildeck et al., 2010; Wang et al., 2008; Yu et al., 2009). β-catenin mutations were identified in approximately 5% of PCa samples (Voeller et al., 1998). In the late 90s and early 2000s Wnt/β-catenin signaling became a focus of prostate cancer studies (Chesire and Isaacs, 2003).
The androgen steroid hormone receptor (AR) is the major therapeutic target in aggressive prostate cancer. In response to ligands such as 5α-dihydrotestosterone (DHT) the AR directs gene transcription. Therapeutics for late stage prostate cancer, such as enzalutamide and abiraterone, target the activity of the androgen receptor by blocking androgen synthesis or androgen/AR binding (Ryan et al., 2013; Tran et al., 2009). The AR and β-catenin were found to interact directly in yeast and mammalian two hybrid assays, and the interaction was localized to the ligand-binding domain of AR and the armadillo repeats of β-catenin (Pawlowski et al., 2002; Song et al., 2003; Yang et al., 2002). More recently, the crystal structure of β-catenin and the nuclear hormone receptor, LRH-1 (liver receptor homolog-1) protein interaction was solved, revealing three important β-catenin residues in the interaction (Y306, K345, and W383). Using pull down assays, it was shown that mutations in these residues could decrease β-catenin binding to LRH-1. These same mutations also inhibited β-catenin binding to the AR, providing further evidence of the AR and β-catenin protein interaction(Yumoto et al., 2012).
β-catenin has been shown in complex with the AR and could enhance AR signaling in PCa cells (Truica et al., 2000). Multiple other interactions have been discovered between the Wnt and AR signaling pathways (Figure 1). GSK-3 phosphorylates the AR with the potential to suppress AR signaling (Mazor et al., 2004; Salas et al., 2004; Wang et al., 2004). Wnt target gene Cyclin D1 can also repress AR signaling (Petre et al., 2002). Further, AR was recruited to the cMyc promoter and shown to interact with TCF4 independent of β-catenin (Amir et al., 2003). Importantly, at least three active LEF1/TCF binding sites exist in the promoter region of the AR gene and Wnt signaling can increase transcription of the AR (Yang et al., 2006).
Figure 1. Crosstalk between Wnt signaling and the Androgen Receptor.
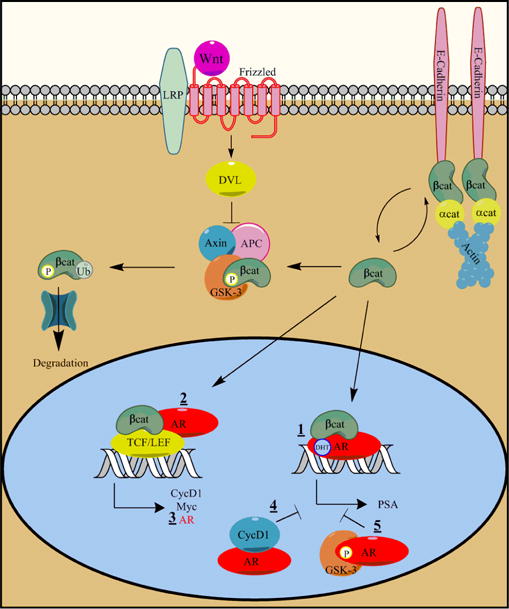
A simplified view of canonical Wnt signaling: Cytoplasmic β-catenin (βcat), when not bound to E-cadherin at the cell membrane, is phosphorylated by GSK-3 in complex with the proteins adenomatous polyposis coli (APC) and Axin. Phosphorylated β-catenin is then ubiquitinated and degraded by the proteasome. In the presence of a Wnt extracellular signal, through Frizzled receptors in complex with LRP, disheveled (DVL) inhibits the β-catenin phosphorylation complex. This stabilizes β-catenin which translocates to the nucleus activating transcription factors of the TCF/LEF family. Multiple intersections of the Wnt pathway have been shown in PCa: 1- β-catenin binds AR directly and can enhance its transcriptional activity; 2- the AR can be recruited to the promoter of TCF/LEF target genes like CycD1 and Myc; 3- β-catenin/TCF bind to the promoter of AR itself, activating AR mRNA transcription; 4- β-catenin/TCF target gene CycD1 can inhibit AR mediated transcription; and 5- GSK-3 can phosphorylate AR leading to a decrease in AR mediated transcription (Amir et al., 2003; Kypta and Waxman, 2012; Mazor et al., 2004; Pawlowski et al., 2002; Petre et al., 2002; Salas et al., 2004; Song et al., 2003; Wang et al., 2004; Yang et al., 2002; Yang et al., 2006).
In addition to tissue culture models, multiple genetically engineered mouse models indicate that β-catenin plays a role in PCa progression. Overexpression of active β-catenin causes high-grade intraepithelial neoplasia and resistance to castration (Yu et al., 2009). β-catenin also cooperates with PTEN loss to promote invasive carcinoma (Francis et al., 2013). The transcription factor SOX9 has been shown to upregulate multiple components of the Wnt pathway suggesting a potential mechanism for the reactivation of Wnt signaling in PCa (Ma et al., 2016).
Taken together, all of the crosstalk between Wnt and AR signaling has led to many different proposals of what role the two pathways play in progression and maintenance of castration resistant prostate cancer (CRPC). Whether AR and Wnt/β-catenin act synergistically or in an opposing manner, is an important question in PCa biology. The role of Wnt signaling in PCa has been previously reviewed, most recently by Kypta and Waxman (2012); and Yokoyama et al (2014) (Beildeck et al., 2010; Chesire and Isaacs, 2003; Kypta and Waxman, 2012; Mulholland et al., 2005; Terry et al., 2006; Verras and Sun, 2006; Yardy and Brewster, 2005; Yokoyama et al., 2014). We do not comprehensively examine the information in these reviews here, but rather discuss the field in light of exciting new developments.
2. Genomic alterations in the Wnt/β-catenin pathway in prostate cancer
Advances in sequencing technology have recently enabled whole exome and whole genome sequencing of localized as well as metastatic PCa. (Barbieri et al., 2012; Grasso et al., 2012; Hieronymus and Sawyers, 2012). Such studies indicated that APC is one of the most significantly mutated genes in primary tumors. Sequencing of 50 lethal and heavily treated tissues obtained at autopsy showed mutations within the Wnt pathway in up to 50% of the samples (Grasso et al., 2012). An additional study included whole exome and transcriptome sequencing of bone or soft tissue biopsy from 150 CRPC affected individuals and demonstrated mutations in the Wnt signaling pathway in 18% of the cases (Robinson et al., 2015). These included activating mutations in beta-catenin as well as mutations and copy number alterations in APC. Mutations or copy number loss was also observed in RNF43 and ZNRF3, E3 ubiquitin ligases thought to negatively regulate the Wnt pathway. Interestingly, alterations of RNF43 and ZNRF3 were mutually exclusive with samples that exhibited alterations in APC (Robinson et al., 2015). The study also identified R-spondin genes fusions involving RSPO2, an activator of the canonical Wnt signaling pathway and important component of media used to generate organoids from patient derived PCa samples (Gao et al., 2014). These studies describing Wnt pathway alterations in the human PCa transcriptome challenge us to determine whether Wnt pathway alterations occur under selective pressure in the context of androgen deprivation or other treatment stresses and to determine whether Wnt/β-catenin and AR act together or separately to promote tumorigenesis.
3. Synergistic interaction of AR and β-catenin
The idea that AR and β-catenin directly interact and that β-catenin is an AR coactivator suggests that these proteins may act synergistically to regulate gene transcription. Importantly, the AR gene itself is a target of nuclear β-catenin action through TCF or the TCF family member, LEF1 that binds to the AR promoter (Li et al., 2009; Yang et al., 2006). The physical and functional interaction of AR and β-catenin has been described in a number of reports (Mulholland et al., 2002; Pawlowski et al., 2002; Song and Gelmann, 2005; Yang et al., 2002), along with characterization of the interaction of β-catenin, TIF2/GRIP1 and AR (Li et al., 2004; Song and Gelmann, 2005; Song et al., 2003; Yang et al., 2002). Crosstalk between the AR and β-catenin pathways was also observed in a hollow fiber model under castrate versus intact conditions (Wang et al., 2008). This study showed AR and β-catenin interaction and localization under castrate, but not non-castrate conditions.
AR was found to activate a Wnt reporter gene and to be recruited to the promoter of the Wnt target genes, myc and cyclin D1 (Schweizer et al., 2008). Further, a transgenic mouse model with overexpression of AR and stabilization of β-catenin, exhibited increased tumor growth (Lee et al., 2016). In actuality however, there is very little evidence beyond reporter gene assays and overexpression studies indicating the synergistic impact of these two proteins on gene transcription in an in vivo setting. Chromatin immunoprecipitation analysis has shown occupancy of both AR and β-catenin on selected endogenous target genes. AR occupancy was demonstrated at a TCF binding site on the Myc gene and occupancy of both β-catenin and AR were observed on the PSA promoter (Amir et al., 2003; Li et al., 2004; Liu et al., 2008; Schweizer et al., 2008; Yang et al., 2006). Despite these studies indicating that AR and β-catenin appear to co-occupy promoters of a limited number of AR and β-catenin target genes the importance of the AR/β-catenin protein:protein interaction in execution of the AR-mediated program of gene transcription is unknown. Understanding the synergistic action of AR and β-catenin is hampered by the challenge of specifically interfering with their interaction and testing the effect on transcription. In particular, the chromatin binding sites of AR and β-catenin along with their overlap and functional significance has not yet been determined on a genome-wide scale.
4. Opposing interaction of the AR and β-catenin signaling pathways
There is a fair amount of evidence that the AR and Wnt/β-catenin signaling pathways may oppose one another. For example, ligand bound AR can inhibit β-catenin target gene expression (Chesire and Isaacs, 2002) and this may occur as a result of competition of AR and TCF for β-catenin binding (Mulholland et al., 2003). It is also possible that a compensatory mechanism can modulate Wnt/β-catenin and AR signaling where inhibition or activation of one can increase or decrease the other. We recently showed that androgen starvation resulted in activation of a Wnt reporter gene and enhanced interaction of β-catenin with TCF4 in PCa cells (Lee et al., 2015). These studies also demonstrated that activation of the Wnt reporter was suppressed by androgen treatment. In vivo, WNT16B is expressed in the prostate tumor microenvironment upon drug-induced damage and may promote resistant disease (Sun et al., 2012). The idea that the Wnt/β-catenin pathway is preferentially upregulated under conditions of androgen ablation is also supported by the finding that a Wnt/β-catenin reporter is activated in the proximal region of the mouse prostate in the castrate environment (Placencio et al., 2008). Consistent with this finding, a gene profiling comparison of the AR antagonist enzalutamide versus agonist DHT- treated LNCaP cells indicated that the Wnt pathway was the most highly overrepresented signaling pathway (Guerrero et al., 2013) in the enzalutamide treated samples suggesting that the Wnt pathway can compensate for loss of AR signaling. In prostate development, investigators have shown that the TCF family member, LEF1, is mutually exclusive with AR during branching morphogenesis and that treatment with an AR antagonist resulted in Wnt/LEF1 positive basal progenitor repopulation of the luminal compartment (Wu et al., 2011).
A reciprocal relationship between activated β-catenin and AR signaling was also demonstrated by compelling studies in mouse hair follicle cells (Kretzschmar et al., 2015; Leiros et al., 2012). Here the investigators use a combination of in vitro and in vivo approaches to show that AR negatively regulates the Wnt pathway (Kretzschmar et al., 2015). They show that AR activation reduced transcription of endogenous β-catenin target genes and conversely, that AR inhibition increased transcription of endogenous Wnt/β-catenin gene targets and promoted hair follicle proliferation and differentiation. The investigators suggest that the reciprocal relationship may be indirect through autocrine factors, proteins or microRNAs that regulate AR or β-catenin function.
5. Wnt/β-catenin driven prostate cancer stem cell growth
Recent studies have shown that small subpopulations of cancer cells, termed “cancer stem cells (CSCs)” or “tumor-initiating cells” based on their ability to self-renew as well as differentiate to a daughter cell type, play a critical role in both initiation and maintenance of tumors. It has been suggested that these cells are resistant to conventional chemotherapy and radiation, making it important to develop therapeutic approaches to selectively target them (Chandler and Lagasse, 2010; Korkaya and Wicha, 2010), perhaps by interfering with cell specific signaling pathways that regulate self-renewal. In PCa, it is possible that CSCs survive after androgen ablation therapy, causing castration-resistant disease (Lawson and Witte, 2007). Growing evidence shows that Wnt/β-catenin signaling is highly active in CSCs, and may have a role in prostate stem cell self-renewal (Bisson and Prowse, 2009; Korkaya et al., 2009). In addition, lineage tracing studies showed that Lgr5, a Wnt target gene, is expressed in an adult prostate stem cell population and that Lgr5 positive stem cells are castration resistant (Wang et al., 2015).
6. Emerging importance of the non-canonical Wnt pathway in prostate cancer
Non-canonical Wnt signaling pathways, which are β-catenin independent, may also promote PCa. Isolation and single cell RNA sequencing of circulating tumor cells from individuals with metastatic PCa showed upregulation of non-canonical Wnt signaling in persons treated with enzalutamide (Miyamoto et al., 2015). Further, the non-canonical Wnt activator WNT5A increased proliferation of LNCaP cells treated with enzalutamide. Multiple other reports have found WNT5A to be important in castration resistant disease. A study of 156 individuals with bone metastasis and treated with androgen deprivation therapy demonstrated expression of bone morphogenetic protein-6 induced by WNT5A, suggesting a potential mechanism for castration resistance (Lee et al., 2014). Another study found that haploinsufficiancy of WNT5A reduced the growth of prostate tumors in a mouse model and also confirmed the increased presence of WNT5A in human prostate tumors relative to benign prostatic hyperplasia (Takahashi et al., 2011). In addition, Frizzled2, Ror2, and protein kinase D were proven important in WNT5A induced cell invasiveness (Yamamoto et al., 2010). WNT5A also induced increased Ca2+ and Ca2+/calmodulin dependent protein kinase activity resulting in actin cytoskeleton remodeling (Wang et al., 2010). Hypomethylation of the WNT5A gene has been suggested as a potential mechanism for its increased activity (Wang et al., 2007). With exciting new technologies, such as the ability to analyze circulating tumor cells, our understanding of the importance of both canonical and non-canonical Wnt signaling in castration resistant disease will only grow, hopefully enabling the development of much needed new treatments.
7. Challenges and successes in targeting nuclear Wnt/β-catenin signaling
While the exact mechanism of Wnt activation in PCa may be uncertain, it is clear that Wnt signaling is an attractive pathway to target in castration resistant disease. Given the role of the Wnt pathway in many disease mechanisms, including many types of cancer, there has been a large effort to develop Wnt modulators as treatments for disease (Kahn, 2014; Zhang and Hao, 2015). Multiple older FDA approved drugs such as Lithium and Celecoxib, were found to modulate Wnt signaling, but the majority of the newly developed potential therapeutics have yet to reach clinical trials (Kahn, 2014).
Several monoclonal antibodies are in trials for diseases where Wnt signaling is important. Romosozumab is one of the closest to the clinic and has proven successful in phase 2 clinical trials in osteoporotic women (McClung et al., 2014). Romosozumab binds sclerostin, an extracellular inhibitor of Wnt signaling secreted by osteocytes, causing an increase in Wnt signaling in osteoblasts resulting in increased bone formation. Biologics that can inhibit Wnt signaling have also been developed for cancer. Vantictumab (OMP18R5) is a monoclonal antibody, targeting Frizzled receptors 1, 2, 5, 7, and 8, that was shown to reduce the growth of breast, pancreatic, colon, and lung tumors in xenograft models (Gurney et al., 2012). Ipafricept (OMP-54F28) is a solubilized Frizzled 8 fusion receptor with a human IgG1 Fc fragment designed to sequester extracellular Wnts that also showed promise in preclinical xenograft models (Fischer et al., 2015; Le et al2015). Vantictumab and Ipafricept, both owned by OncoMed Pharmaceuticals, are currently in phase one clinical trials (Fischer et al., 2015; Zhang et al., 2016; Zhang and Hao, 2015).
Targeting the protein:protein interactions of the central Wnt mediator, β-catenin, and its activating partners is another potential therapeutic approach and has been undertaken by multiple groups. An example is the small molecules ICG-001 and PRI-724 that inhibit the binding of β-catenin to its co-activator CREB-binding protein (CBP), which in a balancing act with binding partner p300, causes stem cells to proliferate and remain potent (Lenz and Kahn, 2014). While ICG-001 has toxicity and stability issues, PRI-724, a second generation CBP/β-Catenin antagonist from Prism and Eisai Pharmaceuticals, showed an acceptable toxicity profile in phase one trials and is currently in trials for refractory colorectal cancer, refractory pancreatic cancer, and for hematologic malignancies (El-Khoueiry et al., 2013; Lenz and Kahn, 2014; Sasaki et al., 2013). It is not known what effect PRI-724 might have in PCa.
Wnt inhibitors that interfere with the β-catenin TCF/LEF interaction were also isolated in a high throughput screen for small molecules that interfered with Wnt reporter gene activity (Gonsalves et al., 2011). In collaboration with the DasGupta laboratory, we found that the small molecule inhibitor of β-catenin responsive transcription-3 (iCRT3), disrupted both β-catenin/TCF and β-catenin/AR interaction (Lee et al., 2013). Treatment with iCRT3 also resulted in decreased occupancy of β-catenin on the AR promoter and diminished AR and β-catenin target gene expression. In addition, iCRT-3 also inhibited xenograft tumor growth and blocked renewal of AR antagonist-resistant sphere-forming cells.
While there has not been extensive testing of Wnt modulators in clinical trials for CRPC, multiple drugs have also been tested in preclinical PCa cell models. A small molecule targeting dishevelled, 3289–8625, inhibited PC-3 cell proliferation (Grandy et al., 2009). Growth of DU145 cells can be inhibited by IWR-3, which stabilizes Axin leading to increased proteosomal β-catenin destruction (Chen et al., 2009). Salinomycin and Niclosamide, LRP6 antagonists, suppress both cell growth of AR negative PC3 and DU145 cells (Lu and Li, 2014; Lu et al., 2011).
While considerable effort has been expended toward the isolation of small molecule inhibitors of the Wnt/β-catenin in cancer biology (Anastas and Moon, 2013), there is still a need for further development. Some compounds were identified in cell-free assays so their in vivo use is uncertain (Lepourcelet et al., 2004). Others, such as ICG-001 and PRI-724, that interfere with β-catenin/CBP interaction (Emami et al., 2004), raise concerns because of the multitude of CBP interacting proteins. Compounds such as XAV939 that affect the stability and expression of β-catenin have also been investigated (Chen et al., 2009; Huang et al., 2009; Thorne et al., 2010) but run the risk of destabilizing β-catenin at the cell membrane, resulting in pleiotropic, non-specific effects. Finally small molecules that influence pathway activity at the level of ligand secretion could modulate activity of non-canonical Wnt pathways in addition to the β-catenin-dependent arm of Wnt signaling (Chen et al., 2009; Dodge et al., 2012).
8. Conclusion
New genome wide studies have highlighted the importance of the interaction between the Wnt/β-catenin and AR signaling in PCa. The interaction has yielded much research over the last 15+ years with many studies detailing its relevance in metastatic disease and the development of castration resistance. Although evidence exists for both synergistic and opposing interactions between β-catenin and AR, their combined role remains unclear. This is particularly true in separating out the importance of the AR/β-catenin protein:protein association from the effects of β-catenin/Wnt control of AR mRNA transcription. Other related research areas are also coming to light as important in PCa, including Wnt effects on cancer stem cells and the role of non-canonical Wnt signaling in castration resistance. Even with some mechanistic uncertainties, continuing progress is being made in the development of Wnt modulating therapeutics. The ongoing refinement of Wnt/β-catenin’s role in PCa and its influence on the AR will only help this effort.
Highlights.
Description of interactions of the Wnt/β-catenin and AR signaling in prostate cancer.
The interaction may be synergistic or opposing depending on context.
Prostate cancer stem cells and non-canonical Wnt signaling in PCa also discussed.
The status of Wnt modulating therapeutics in relation to cancer is detailed.
Acknowledgments
This work was supported by the National Institutes of Health R01 CA112226 (SKL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amir AL, Barua M, McKnight NC, Cheng S, Yuan X, Balk SP. A direct beta-catenin-independent interaction between androgen receptor and T cell factor 4. The Journal of biological chemistry. 2003;278:30828–30834. doi: 10.1074/jbc.M301208200. [DOI] [PubMed] [Google Scholar]
- Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nature reviews. Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, White TA, Stojanov P, Van Allen E, Stransky N, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nature genetics. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beildeck ME, Gelmann EP, Byers SW. Cross-regulation of signaling pathways: an example of nuclear hormone receptors and the canonical Wnt pathway. Experimental cell research. 2010;316:1763–1772. doi: 10.1016/j.yexcr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson I, Prowse DM. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 2009;19:683–697. doi: 10.1038/cr.2009.43. [DOI] [PubMed] [Google Scholar]
- Chandler JM, Lagasse E. Cancerous stem cells: deviant stem cells with cancer-causing misbehavior. Stem Cell Res Ther. 2010;1:13. doi: 10.1186/scrt13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature chemical biology. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesire DR, Isaacs WB. Ligand-dependent inhibition of beta-catenin/TCF signaling by androgen receptor. Oncogene. 2002;21:8453–8469. doi: 10.1038/sj.onc.1206049. [DOI] [PubMed] [Google Scholar]
- Chesire DR, Isaacs WB. Beta-catenin signaling in prostate cancer: an early perspective. Endocrine-related cancer. 2003;10:537–560. doi: 10.1677/erc.0.0100537. [DOI] [PubMed] [Google Scholar]
- Dodge ME, Moon J, Tuladhar R, Lu J, Jacob LS, Zhang LS, Shi H, Wang X, Moro E, Mongera A, et al. Diverse chemical scaffolds support direct inhibition of the membrane-bound O-acyltransferase porcupine. The Journal of biological chemistry. 2012;287:23246–23254. doi: 10.1074/jbc.M112.372029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khoueiry AB, Ning Y, Yang DY, Cole S, Kahn M, Zoghbi M, Berg J, Fujimori M, Inada T, Kouji H, et al. A phase I first-in-human study of PRI-724 in patients (pts) with advanced solid tumors. J Clin Oncol. 2013;31 [Google Scholar]
- Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer MM, Yen WC, Zheng C, Henner R, Cattaruzza F, Tang T, Yeung P, Biswas T, Lewicki J, Gurney A, et al. Abstract 4233: Wnt pathway antagonist ipafricept (FZD8-Fc, OMP-54F28) inhibits tumor growth and reduces tumor-initiating cell frequency in ovarian patient-derived xenograft models. Cancer research. 2015;75:4233–4233. [Google Scholar]
- Francis JC, Thomsen MK, Taketo MM, Swain A. beta-catenin is required for prostate development and cooperates with Pten loss to drive invasive carcinoma. PLoS genetics. 2013;9:e1003180. doi: 10.1371/journal.pgen.1003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, Dowling C, Wanjala JN, Undvall EA, Arora VK, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159:176–187. doi: 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves FC, Klein K, Carson BB, Katz S, Ekas LA, Evans S, Nagourney R, Cardozo T, Brown AM, DasGupta R. An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5954–5963. doi: 10.1073/pnas.1017496108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandy D, Shan J, Zhang X, Rao S, Akunuru S, Li H, Zhang Y, Alpatov I, Zhang XA, Lang RA, et al. Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. The Journal of biological chemistry. 2009;284:16256–16263. doi: 10.1074/jbc.M109.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero J, Alfaro IE, Gomez F, Protter AA, Bernales S. Enzalutamide, an androgen receptor signaling inhibitor, induces tumor regression in a mouse model of castration-resistant prostate cancer. Prostate. 2013;73:1291–1305. doi: 10.1002/pros.22674. [DOI] [PubMed] [Google Scholar]
- Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieronymus H, Sawyers CL. Traversing the genomic landscape of prostate cancer from diagnosis to death. Nature genetics. 2012;44:613–614. doi: 10.1038/ng.2301. [DOI] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Kahn M. Can we safely target the WNT pathway? Nature reviews. Drug discovery. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkaya H, Wicha MS. Cancer stem cells: nature versus nurture. Nat Cell Biol. 2010;12:419–421. doi: 10.1038/ncb0510-419. [DOI] [PubMed] [Google Scholar]
- Kretzschmar K, Cottle DL, Schweiger PJ, Watt FM. The Androgen Receptor Antagonizes Wnt/beta-Catenin Signaling in Epidermal Stem Cells. The Journal of investigative dermatology. 2015;135:2753–2763. doi: 10.1038/jid.2015.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypta RM, Waxman J. Wnt/beta-catenin signalling in prostate cancer. Nature reviews. Urology. 2012;9:418–428. doi: 10.1038/nrurol.2012.116. [DOI] [PubMed] [Google Scholar]
- Lawson DA, Witte ON. Stem cells in prostate cancer initiation and progression. J Clin Invest. 2007;117:2044–2050. doi: 10.1172/JCI32810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le PN, McDermott JD, Jimeno A. Targeting the Wnt pathway in human cancers: therapeutic targeting with a focus on OMP-54F28. Pharmacology & therapeutics. 2015;146:1–11. doi: 10.1016/j.pharmthera.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Ha S, Logan SK. Divergent Androgen Receptor and Beta-Catenin Signaling in Prostate Cancer Cells. PloS one. 2015;10:e0141589. doi: 10.1371/journal.pone.0141589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Madar A, David G, Garabedian MJ, Dasgupta R, Logan SK. Inhibition of androgen receptor and beta-catenin activity in prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15710–15715. doi: 10.1073/pnas.1218168110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GT, Kang DI, Ha YS, Jung YS, Chung J, Min K, Kim TH, Moon KH, Chung JM, Lee DH, et al. Prostate cancer bone metastases acquire resistance to androgen deprivation via WNT5A-mediated BMP-6 induction. British journal of cancer. 2014;110:1634–1644. doi: 10.1038/bjc.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Luong R, Johnson DT, Cunha GR, Rivina L, Gonzalgo ML, Sun Z. Androgen signaling is a confounding factor for β-catenin-mediated prostate tumorigenesis. Oncogene. 2016;35:702–714. doi: 10.1038/onc.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiros GJ, Attorresi AI, Balana ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/beta-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. The British journal of dermatology. 2012;166:1035–1042. doi: 10.1111/j.1365-2133.2012.10856.x. [DOI] [PubMed] [Google Scholar]
- Lenz HJ, Kahn M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer science. 2014;105:1087–1092. doi: 10.1111/cas.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- Li H, Kim JH, Koh SS, Stallcup MR. Synergistic effects of coactivators GRIP1 and beta-catenin on gene activation: cross-talk between androgen receptor and Wnt signaling pathways. The Journal of biological chemistry. 2004;279:4212–4220. doi: 10.1074/jbc.M311374200. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang L, Zhang M, Melamed J, Liu X, Reiter R, Wei J, Peng Y, Zou X, Pellicer A, et al. LEF1 in androgen-independent prostate cancer: regulation of androgen receptor expression, prostate cancer growth, and invasion. Cancer research. 2009;69:3332–3338. doi: 10.1158/0008-5472.CAN-08-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Vinall RL, Tepper C, Shi XB, Xue LR, Ma AH, Wang LY, Fitzgerald LD, Wu Z, Gandour-Edwards R, et al. Inappropriate activation of androgen receptor by relaxin via beta-catenin pathway. Oncogene. 2008;27:499–505. doi: 10.1038/sj.onc.1210671. [DOI] [PubMed] [Google Scholar]
- Lu W, Li Y. Salinomycin suppresses LRP6 expression and inhibits both Wnt/beta-catenin and mTORC1 signaling in breast and prostate cancer cells. Journal of cellular biochemistry. 2014;115:1799–1807. doi: 10.1002/jcb.24850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Lin C, Roberts MJ, Waud WR, Piazza GA, Li Y. Niclosamide suppresses cancer cell growth by inducing Wnt co-receptor LRP6 degradation and inhibiting the Wnt/beta-catenin pathway. PloS one. 2011;6:e29290. doi: 10.1371/journal.pone.0029290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Ye H, He H, Gerrin SJ, Chen S, Tanenbaum BA, Cai C, Sowalsky AG, He L, Wang H, et al. SOX9 drives WNT pathway activation in prostate cancer. Journal of Clinical Investigation. 2016;126:1745–1758. doi: 10.1172/JCI78815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazor M, Kawano Y, Zhu H, Waxman J, Kypta RM. Inhibition of glycogen synthase kinase-3 represses androgen receptor activity and prostate cancer cell growth. Oncogene. 2004;23:7882–7892. doi: 10.1038/sj.onc.1208068. [DOI] [PubMed] [Google Scholar]
- McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, et al. Romosozumab in postmenopausal women with low bone mineral density. The New England journal of medicine. 2014;370:412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene. 1999;18:7860–7872. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- Miyamoto DT, Zheng Y, Wittner BS, Lee RJ, Zhu H, Broderick KT, Desai R, Fox DB, Brannigan BW, Trautwein J, et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science. 2015;349:1351–1356. doi: 10.1126/science.aab0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K, Hennighausen L. Beta-catenin: a transforming actor on many stages. Breast Cancer Res. 2003;5:63–68. doi: 10.1186/bcr566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Mulholland DJ, Cheng H, Reid K, Rennie PS, Nelson CC. The androgen receptor can promote beta-catenin nuclear translocation independently of adenomatous polyposis coli. The Journal of biological chemistry. 2002;277:17933–17943. doi: 10.1074/jbc.M200135200. [DOI] [PubMed] [Google Scholar]
- Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocrine reviews. 2005;26:898–915. doi: 10.1210/er.2003-0034. [DOI] [PubMed] [Google Scholar]
- Mulholland DJ, Read JT, Rennie PS, Cox ME, Nelson CC. Functional localization and competition between the androgen receptor and T-cell factor for nuclear beta-catenin: a means for inhibition of the Tcf signaling axis. Oncogene. 2003;22:5602–5613. doi: 10.1038/sj.onc.1206802. [DOI] [PubMed] [Google Scholar]
- Pawlowski JE, Ertel JR, Allen MP, Xu M, Butler C, Wilson EM, Wierman ME. Liganded androgen receptor interaction with beta-catenin: nuclear co-localization and modulation of transcriptional activity in neuronal cells. The Journal of biological chemistry. 2002;277:20702–20710. doi: 10.1074/jbc.M200545200. [DOI] [PubMed] [Google Scholar]
- Petre CE, Wetherill YB, Danielsen M, Knudsen KE. Cyclin D1: mechanism and consequence of androgen receptor co-repressor activity. The Journal of biological chemistry. 2002;277:2207–2215. doi: 10.1074/jbc.M106399200. [DOI] [PubMed] [Google Scholar]
- Placencio VR, Sharif-Afshar AR, Li X, Huang H, Uwamariya C, Neilson EG, Shen MM, Matusik RJ, Hayward SW, Bhowmick NA. Stromal transforming growth factor-beta signaling mediates prostatic response to androgen ablation by paracrine Wnt activity. Cancer research. 2008;68:4709–4718. doi: 10.1158/0008-5472.CAN-07-6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S, et al. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. The New England Journal of Medicine. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas TR, Kim J, Vakar-Lopez F, Sabichi AL, Troncoso P, Jenster G, Kikuchi A, Chen SY, Shemshedini L, Suraokar M, et al. Glycogen synthase kinase-3 beta is involved in the phosphorylation and suppression of androgen receptor activity. The Journal of biological chemistry. 2004;279:19191–19200. doi: 10.1074/jbc.M309560200. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Hwang H, Nguyen C, Kloner RA, Kahn M. The Small Molecule Wnt Signaling Modulator ICG-001 Improves Contractile Function in Chronically Infarcted Rat Myocardium. PloS one. 2013;8 doi: 10.1371/journal.pone.0075010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer L, Rizzo CA, Spires TE, Platero JS, Wu Q, Lin TA, Gottardis MM, Attar RM. The androgen receptor can signal through Wnt/beta-Catenin in prostate cancer cells as an adaptation mechanism to castration levels of androgens. BMC cell biology. 2008;9:4. doi: 10.1186/1471-2121-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song LN, Gelmann EP. Interaction of beta-catenin and TIF2/GRIP1 in transcriptional activation by the androgen receptor. The Journal of biological chemistry. 2005;280:37853–37867. doi: 10.1074/jbc.M503850200. [DOI] [PubMed] [Google Scholar]
- Song LN, Herrell R, Byers S, Shah S, Wilson EM, Gelmann EP. Beta-catenin binds to the activation function 2 region of the androgen receptor and modulates the effects of the N-terminal domain and TIF2 on ligand-dependent transcription. Molecular and cellular biology. 2003;23:1674–1687. doi: 10.1128/MCB.23.5.1674-1687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, True L, Nelson PS. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nature medicine. 2012;18:1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Watanabe T, Okada M, Inoue K, Ueda T, Takada I, Watabe T, Yamamoto Y, Fukuda T, Nakamura T, et al. Noncanonical Wnt signaling mediates androgen-dependent tumor growth in a mouse model of prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4938–4943. doi: 10.1073/pnas.1014850108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry S, Yang X, Chen MW, Vacherot F, Buttyan R. Multifaceted interaction between the androgen and Wnt signaling pathways and the implication for prostate cancer. Journal of cellular biochemistry. 2006;99:402–410. doi: 10.1002/jcb.20983. [DOI] [PubMed] [Google Scholar]
- Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nature chemical biology. 2010;6:829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, et al. Development of a Second-Generation Antiandrogen for Treatment of Advanced Prostate Cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truica CI, Byers S, Gelmann EP. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer research. 2000;60:4709–4713. [PubMed] [Google Scholar]
- Verras M, Sun Z. Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer letters. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Voeller HJ, Truica CI, Gelmann EP. Beta-catenin mutations in human prostate cancer. Cancer research. 1998;58:2520–2523. [PubMed] [Google Scholar]
- Wang B-e, Wang X, Long JE, Eastham-Anderson J, Firestein R, Junttila MR. Castration-Resistant Lgr5+ Cells Are Long-Lived Stem Cells Required for Prostatic Regeneration. Stem Cell Reports. 2015;4:768–779. doi: 10.1016/j.stemcr.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Wang J, Sadar MD. Crosstalk between the androgen receptor and beta-catenin in castrate-resistant prostate cancer. Cancer research. 2008;68:9918–9927. doi: 10.1158/0008-5472.CAN-08-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Lin HK, Hu YC, Xie S, Yang L, Chang C. Suppression of androgen receptor-mediated transactivation and cell growth by the glycogen synthase kinase 3 beta in prostate cells. The Journal of biological chemistry. 2004;279:32444–32452. doi: 10.1074/jbc.M313963200. [DOI] [PubMed] [Google Scholar]
- Wang Q, Symes AJ, Kane CA, Freeman A, Nariculam J, Munson P, Thrasivoulou C, Masters JR, Ahmed A. A novel role for Wnt/Ca2+ signaling in actin cytoskeleton remodeling and cell motility in prostate cancer. PloS one. 2010;5:e10456. doi: 10.1371/journal.pone.0010456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Williamson M, Bott S, Brookman-Amissah N, Freeman A, Nariculam J, Hubank MJ, Ahmed A, Masters JR. Hypomethylation of WNT5A, CRIP1 and S100P in prostate cancer. Oncogene. 2007;26:6560–6565. doi: 10.1038/sj.onc.1210472. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Wu X, Daniels G, Shapiro E, Xu K, Huang H, Li Y, Logan S, Greco MA, Peng Y, Monaco ME, et al. LEF1 identifies androgen-independent epithelium in the developing prostate. Molecular endocrinology (Baltimore, Md) 2011;25:1018–1026. doi: 10.1210/me.2010-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Oue N, Sato A, Hasegawa Y, Yamamoto H, Matsubara A, Yasui W, Kikuchi A. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene. 2010;29:2036–2046. doi: 10.1038/onc.2009.496. [DOI] [PubMed] [Google Scholar]
- Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, Sun Z. Linking beta-catenin to androgen-signaling pathway. The Journal of biological chemistry. 2002;277:11336–11344. doi: 10.1074/jbc.M111962200. [DOI] [PubMed] [Google Scholar]
- Yang X, Chen MW, Terry S, Vacherot F, Bemis DL, Capodice J, Kitajewski J, de la Taille A, Benson MC, Guo Y, et al. Complex regulation of human androgen receptor expression by Wnt signaling in prostate cancer cells. Oncogene. 2006;25:3436–3444. doi: 10.1038/sj.onc.1209366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardy GW, Brewster SF. Wnt signalling and prostate cancer. Prostate cancer and prostatic diseases. 2005;8:119–126. doi: 10.1038/sj.pcan.4500794. [DOI] [PubMed] [Google Scholar]
- Yokoyama NN, Shao S, Hoang BH, Mercola D, Zi X. Wnt signaling in castration-resistant prostate cancer: implications for therapy. American journal of clinical and experimental urology. 2014;2:27–44. [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wang Y, Jiang M, Bierie B, Roy-Burman P, Shen MM, Taketo MM, Wills M, Matusik RJ. Activation of beta-Catenin in mouse prostate causes HGPIN and continuous prostate growth after castration. Prostate. 2009;69:249–262. doi: 10.1002/pros.20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumoto F, Nguyen P, Sablin EP, Baxter JD, Webb P, Fletterick RJ. Structural basis of coactivation of liver receptor homolog-1 by beta-catenin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:143–148. doi: 10.1073/pnas.1117036108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, O’Young G, Wikstrom K, Davison T, Yeung P, Cattaruzza F, Yen WC, Hoey T, Lewicki J, Rachmann R, et al. Abstract P3-07-57: Development of a 6-gene qPCR RUO-validated assay as a predictive biomarker for response of vantictumab (OMP-18R5; anti-frizzled) in HER2- breast cancer patients. Cancer research. 2016;76:P3-07-57–P03-07-57. [Google Scholar]
- Zhang X, Hao J. Development of anticancer agents targeting the Wnt/-catenin signaling. American journal of cancer research. 2015;5:2344. [PMC free article] [PubMed] [Google Scholar]


