Abstract
Background
The aim of the study is to investigated if there are differences in associations of stromal vs. intratumoral tumor infiltrating lymphocytes (TILs) with pathology complete response (pCR) among breast cancer (BC) subtypes treated with neoadjuvant therapy (NAT).
Methods and Methods
Hematoxylin and Eosin slides of BC core biopsy consecutive cases (n=331) were reviewed from a single institution between 2000 and 2014. TIL-stroma (TIL-str) was scored from 0% to 100%. Intratumoral lymphocytes (iTu-Ly) were scored semi-quantitatively incorporating the infiltrate grade (0 to 3) and the corresponding percentage resulting in a score ranging from 0 to 300. pCR was defined as no residual infiltrating tumor in the tumor bed and the lymph nodes.
Results
pCR was achieved in 29 of 95 (30.9%) triple negative (TN) cases, 25 of 77 (32.5%) HER2+, and 9 of 159 (5.6%) luminal tumors. In univariate analysis, invasive non-lobular carcinoma, higher Nottingham grade, non-luminal subtypes, trastuzumab therapy, non-advanced clinical T-stage (T1&T2), TIL-str, and iTu-Ly predicted pCR. In luminal subtype, iTu-Ly but not TIL-str was an independent predictor for pCR (OR=1.44, 95% CI 1.08 to1.9, p=0.013). In TN subtype, both TIL-str and iTu-Ly were independent predictors for pCR (OR=1.68, 95% CI 1.29 to 2.18, p=0.001; OR=1.31, 95% CI 1.05 to 1.63, p=0.017, respectively). In HER2+ subtype, neither TIL-str nor iTu-Ly predicted pCR.
Conclusions
TILs are variably correlated with better neoadjuvant chemotherapy response depending on their location and clinical subtype of BC. It could indicate that TILs might be functionally heterogeneous with regard to their role in mediating anti-tumor immune response, depending on their location and BC subtypes.
INTRODUCTION
Proliferation and invasion of malignant tumor cells is controlled by a complex system. One of the factors is the interaction between the tumor cells and the host immune cells. It is known that tumor-immune system interaction is critical in development and progression of many solid tumors. There is a wide interest in immunotherapies in breast cancer. Number of clinical studies has shown that the presence of a lymphocytic infiltrate in various types of cancer tissues including breast is associated with improved outcome (1–8) but are limited by methodology and not considering different clinical subtypes of BC in any given study.
There are studies that evaluated tumor infiltrating lymphocytes (TILs) in breast cancer (BC) in both the stromal and intratumoral compartments separately (6). Intratumoral TILs (iTu-Ly) are defined as lymphocytes in direct cell-to-cell contact with the tumor cells with no intervening stroma. Stromal TILs (TIL-str) are located scattered or clustered in the stroma between the carcinoma cells/clusters and do not directly interact with tumor cells (9). Although the original hypothesis was that lymphocytes directly interacting with carcinoma cells are more biologically relevant (9), most current studies have found that TIL-str to be superior to iTu-Ly and a more reproducible parameter in predicting response to therapy (9). That is mainly due to the difficulty recognizing and scoring iTu-Ly when embedded in the tumor compared to TIL-str. When interpreting TILs in histologic tissue sections, they are assumed to be in a static situation which may be artificial, because lymphocytes move in the microenvironment in a living tissue.
Tumor infiltrating lymphocytes have been studied in various clinical trials and institutional cohorts of BC. It was found that there was heterogeneity in the association of TILs with pathologic complete response (pCR): it was more consistent in triple negative (TN) than in HER2+ BC, and the least consistent in luminal/HER2− cases (10–14).
We hypothesized that tumor-immune interactions add another layer of tumor heterogeneity across tumor subtypes. Our hypothesis was built on two observations. First, it is possible that there are biological differences between the lymphocytes close to the tumor cells and those separate from the tumor and embedded in the stroma. Second, there are differences in the relationships between TILs and different subtypes in terms of the response to cytotoxic neoadjuvant therapy (NAT) regimens. To test this hypothesis, we investigated if there were differences in associations of TILs with pCR among BC subtypes and if TILs location differentially predicted pCR in patients treated with NAT. To overcome the issue of accurately scoring iTu-Ly, we developed a semi-quantitative scoring system incorporating the degree of infiltration and the percentage of involved tumor cells.
MATERIAL AND METHODS
Collection of Pretherapeutic Core Biopsies and Exclusion Criteria
Paraffin-embedded pretherapeutic core biopsies from our institution (n=100) and from the referring institutions (n=231) for a total of 331 cases were used in this study. We excluded skin biopsies with ulceration due to the difficulty in interpreting TILs. Also, cases with microinvasive carcinoma, missing biomarkers results, or tumor only represented in a lymph node biopsy with no biopsy of the primary tumor available were excluded.
Patients
Consecutive BC biopsies (n=331) from our institution between 2000 and 2014 were reviewed. Standard clinicopathologic characteristics were abstracted from the BC database including patient age, race, tumor histologic type, Nottingham grade, clinical T-stage (1 to 4), clinical N-stage (0 to 3), chemotherapy regimen, trastuzumab use, and pathology stage. pCR is defined as no residual invasive carcinoma in the tumor bed or lymph node involvement (y-T0/is and N0). All these patients had locally advanced disease and treated with neoadjuvant cytotoxic chemotherapy.
Estrogen and progesterone receptors were scored using Allred scoring system as previously described (15). HER2 status was abstracted from the pathology reports. Using these three markers, three clinical subtypes were classified: luminal/HER2− was defined as BC with ER+ and/or PR+ and HER2−; TN was ER−/PR−/HER2−; and HER2+ was HER2+ regardless of ER or PR status (16). We acknowledge the limitation of using only these three markers in subclassifying BC. However, we adopted this classification based only on the different therapies that these patients receive where patients with HER2+ receive anti-HER2 therapies, and patients with TN BC receive more aggressive therapies than luminal/HER2−.
Tumor Infiltrating Lymphocytes Scoring
Histopathologic scoring of TILs was jointly performed on hematoxylin and eosin-stained sections by two pathologists (TK, VN). Two types of scoring systems were applied to evaluate TIL-str and iTu-Ly. While the first was located in the stroma separate from the tumor, the latter was intimately located next to the tumor cells. TIL-str was scored following the International TILs Working Group 2014 recommendation (figure 1) (9). iTu-Ly was scored using a semi-quantitative scoring system (H-score) that included the grade of lymphocytic infiltration (0 to 3) (figure 2) and the percentage of tumor harboring each grade. The grade of iTu-Ly ranged from 0 to 3, where 0 = virtually no lymphocytes, 1 = sparse intra-tumoral lymphocytes seen only under 40× magnification, 2 = frequent easily recognized lymphocytes seen under 20× magnification, and 3 = tumor is obscured by lymphocytes. The final score was the sum total of the products of multiplying the grade and the corresponding percentage, resulting a score ranging from 0 to 300. For example, for a tumor with 40% grade 0, 20% grade 1, 20% grade 2, and 20% grade 3, the final score would be 40×0 + 20×1 + 20×2 + 20×3=150.
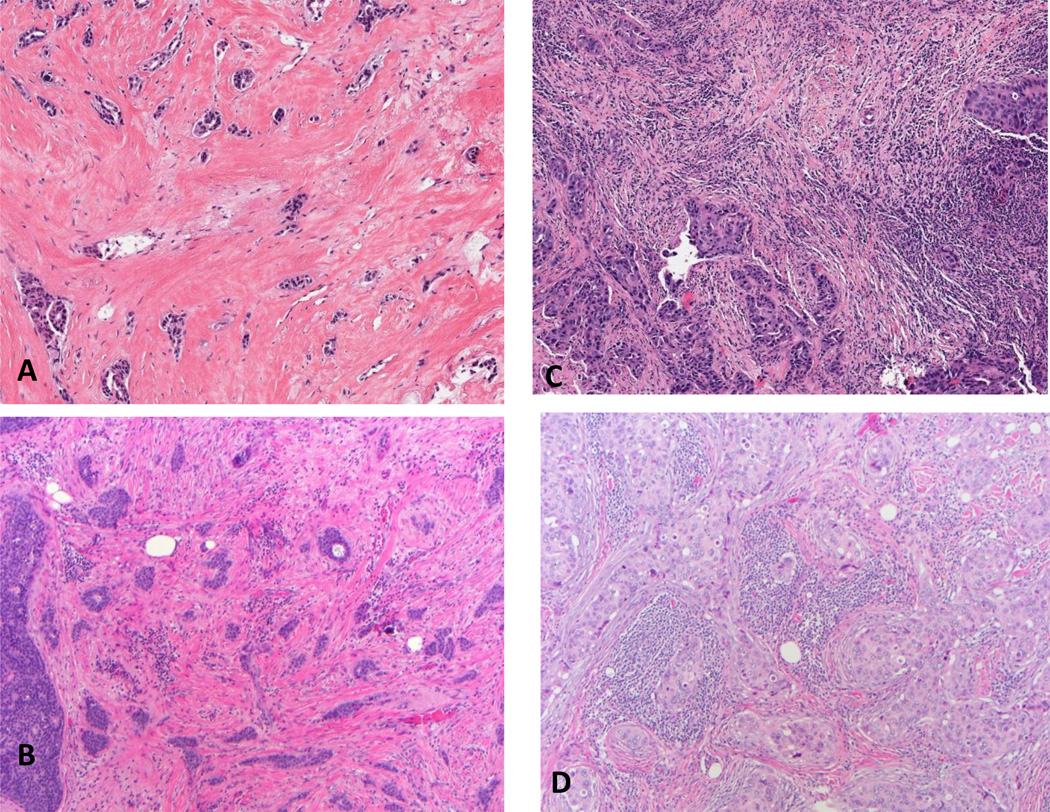
Figure 1.
Scoring TIL-str (hematoxylin and eosin); A, score 0 virtually no lymphocytes in the stroma (20×); B, 20% (20×); C (10×), 50%; D, 80% (20×).
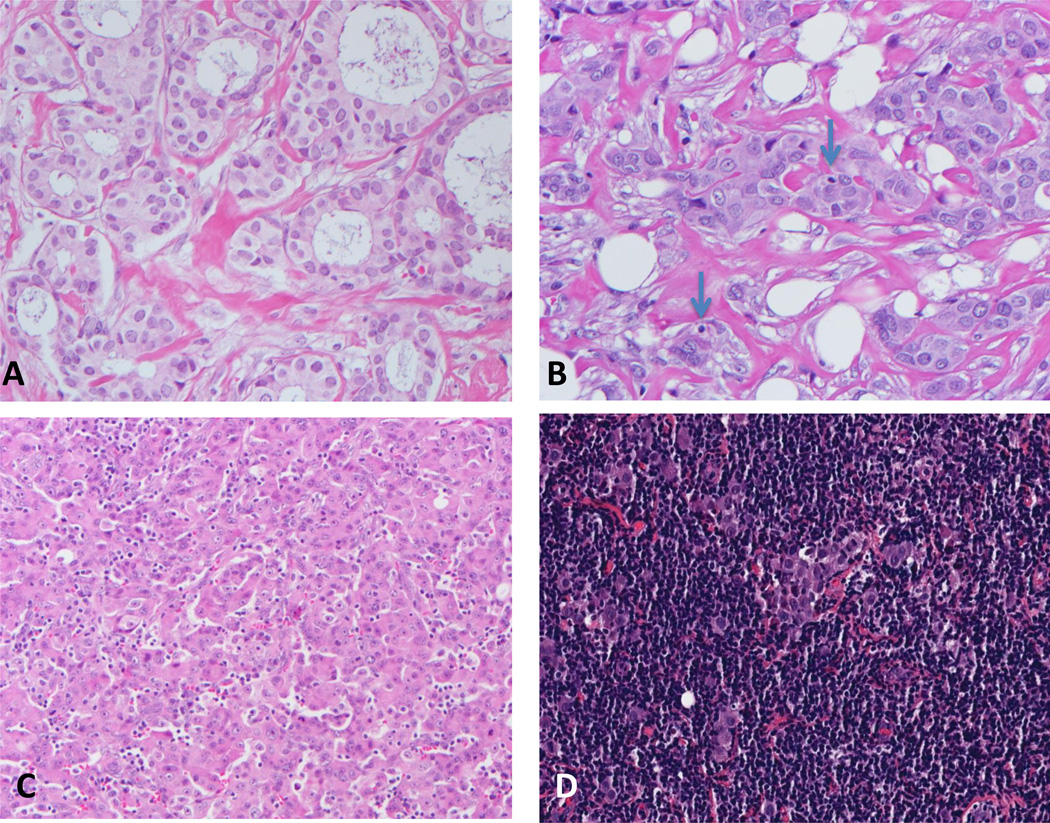
Figure 2.
Scoring iTu-Ly (hematoxylin and eosin); A, grade 0: virtually no identifiable lymphocytes (40×); B, grade 1: rare lymphocytes (arrows) in direct contact with tumor cells (40×); C, frequent lymphocytes (20×); D, grade 3: extensive lymphocytic infiltrate (20×).
Training Set
In order to validate our scoring systems (iTu-Ly and TIL-str), a group of 100 breast carcinoma core biopsies was independently scored by two pathologists (TK and VN). For TIL-str, the percentage of lymphocytes was recorded. For iTu-Ly, the percentage of each grade (0, 1, 2, and 3) was recorded. Then, the scores were compared by calculating the percentage agreement and the weighted kappa values.
Statistical analysis
The clinicopathologic characteristics for a total of 331 patients included age, race, tumor histology type, Nottingham grade, clinical T-stage, clinical N-stage, trastuzumab use, ER, PR status and corresponding Allred scores, HER2 status, BC subtypes, TIL-str and iTu-Ly. Univariate analysis was first performed to evaluate the association between pCR and clinicopathologic variables. Fisher's exact test was used for categorical variables and Wilcoxon non-parametric test for continuous variables. The significant clinicopathologic variables in the univariate analysis were then included in the multivariate analysis by logistic regression. All variables that found to be significant in the univariate analysis for each group were included in the multivariate analysis. The odds ratios (ORs) with their 95% confidence intervals (CIs) and two sided p-values were reported for each variable. Similar approaches were performed in each subgroup of patients (TN, luminal and HER2+). All reported tests were conducted at a nominal significance level of 0.05. Statistical analysis was performed using R version 3.2.2 (http://www.r-project.org).
The degree of agreement between the observers is calculated by kappa statistics. The paired agreement is calculated by squared weighted kappa. The guidelines characterize weighted kappa values as follows: 0-0.2 as poor, 0.21-0.4 as fair, 0.41-0.6 as moderate, 0.61-0.8 as substantial and 0.81-1 as almost perfect agreement.
RESULTS
Interobserver Agreement in The Training Group
In this group (n=100), there were 88 cases of no special type and 12 of lobular type. There were 41 ER+/HER2−, 39 TN, and 20 HER2+. The agreement between the observers was 46% for TIL-str with kappa value of 0.64 (substantial agreement, p<0.0001) and 36% with kappa value of 0.51 (moderate agreement, p<0.0001). The kappa values for iTu-Ly grades 0, 1, 2, and 3 were 0.55, 0.76, 0.77, and 0.87 (p<0.0001 for each). Then the cases were divided into three groups using cutoffs recommended by Issa-Nummer et al (20), as follows, TIL-str (0, >0–<60, ≥60); iTu-Ly (0, >0 to <180, ≥180). The agreement for TIL-str was 91% with kappa 0.69 (substantial, p<0.0001) ad for iTu-Ly 82% with kappa 0.72 (substantial, p<0.0001). However, we acknowledge fact that these cutoffs are not widely used.
Correlation Between TILs and pCR in All Cases
There were a total of 63 (19%) patients who had pCR in all BC subtypes. The overall median and range of TIL-str and iTu-Ly score were 20 (0 to 90) and 80 (0 to 300), respectively. Spearman correlation coefficient between TIL-str and iTu-Ly scores was 0.707 (p<0.001) (figure 3a). Both TIL-str and iTu-Ly scores were highly significant in predicting pCR. Patients with pCR has a higher TIL-str score than those with non-pCR [median range, 30 (0 to 90) vs. 10 (0 to 90), p<0.001]. Similarly, patients with pCR had higher iTu-Ly score than those with non-pCR [130 (0 to 300) vs. 50 (0 to 300), p<0.001]. In addition, invasive non-lobular carcinoma, higher Nottingham grade, non-luminal subtypes, ER−, PR−, HER2+, non-advanced clinical T-stage (T1&T2), and trastuzumab therapy were associated with pCR (table 1).
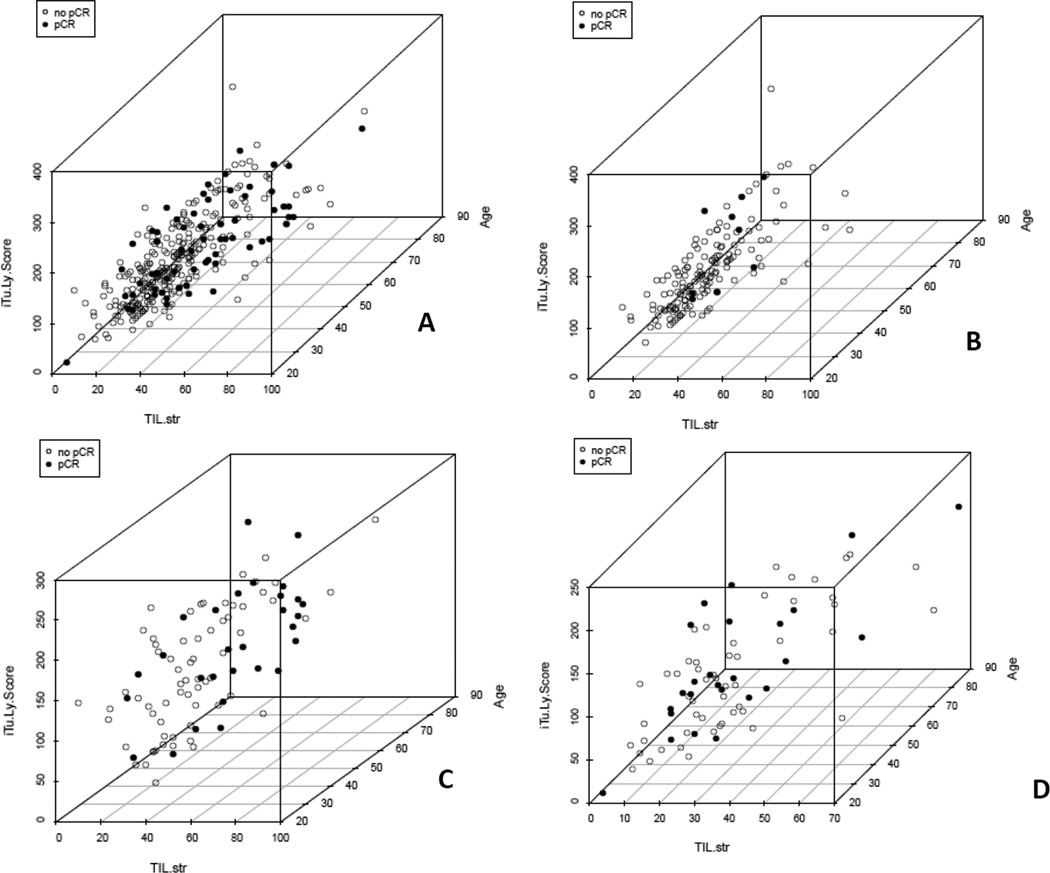
Figure 3.
Correlation between TIL-str and iTu-Ly using 3-dimensional graph including iTu-Ly and TIL-str. Age was used as a third dimension only to better view the clustering of the cases. A, in all cases with Pearson correlation coefficient 0.707; B, luminal subtype with Pearson correlation coefficient 0.711, note clustering of cases with pCR (black spots) in area with high iTu-Ly and low TIL-str; C, TN with Pearson correlation coefficient 0.654, not relatively more even distribution of cases with pCR (black spots) with regard to TIL-str and iTu-Ly scores; and D, HER2+ with Pearson correlation coefficient 0.644. Light spots are tumors without a pCR; the third axis (patient age) is included to reduce overlay of spots.
Table 1.
pCR categories vs. clinical and pathologic variables for all patients
| Variables | Overall No.(%) |
No pCR No.(%) |
pCR No.(%) |
P-value | |
|---|---|---|---|---|---|
| Total | 268(81.0) | 63(19.0) | |||
| Age* | Mean(sd) | 53.12(11.9) | 53.67(12.0) | 50.79(11.3) | 0.064 |
| Median(range) | 53(23,91) | 53.5(23,91) | 49(25,84) | ||
| Age | <50 | 132(39.9) | 100(75.8) | 32(24.2) | 0.063 |
| ≥50 | 199(60.1) | 168(84.4) | 31(15.6) | ||
| Race | African American | 53(16.0) | 42(79.2) | 11(20.8) | 0.568 |
| Caucasian | 271(81.9) | 219(80.8) | 52(19.2) | ||
| Other | 7(2.1) | 7(100.0) | 0(0.0) | ||
| Histology | IC-NST | 284(85.8) | 223(78.5) | 61(21.5) | 0.011 |
| ILC | 33(10.0) | 32(97.0) | 1(3.0) | ||
| Other | 14(4.2) | 13(92.9) | 1(7.1) | ||
| Histology ILC | ILC | 33(10.0) | 32(97.0) | 1(3.0) | 0.009 |
| Non-ILC | 298(90.0) | 236(79.2) | 62(20.8) | ||
| ER | Negative | 142(42.9) | 97(68.3) | 45(31.7) | <0.001 |
| Positive | 189(57.1) | 171(90.5) | 18(9.5) | ||
| PR | Negative | 173(52.3) | 123(71.1) | 50(28.9) | <0.001 |
| Positive | 158(47.7) | 145(91.8) | 13(8.2) | ||
| HER2 | Negative | 254(76.7) | 216(85.0) | 38(15.0) | <0.001 |
| Positive | 77(23.3) | 52(67.5) | 25(32.5) | ||
| Molecular subtypes | TN | 95(28.7) | 66(69.5) | 29(30.9) | <0.001 |
| HER2+ | 77(23.3) | 52(67.5) | 25(32.5) | ||
| Luminal A | 159(48.0) | 150(94.3) | 9(5.6) | ||
| Trastuzumab | No | 259(78.2) | 221(85.3) | 38(14.7) | <0.001 |
| Yes | 72(21.8) | 47(65.3) | 25(34.7) | ||
| Nottingham grade | 1 | 36(10.9) | 36(100.0) | 0(0.0) | <0.001 |
| 2 | 132(39.9) | 114(86.4) | 18(13.6) | ||
| 3 | 163(49.2) | 118(72.4) | 45(27.6) | ||
| Clinical T-Stage | 1–2 | 185(55.9) | 141(76.2) | 44(23.8) | 0.016 |
| 3–4 | 146(44.1) | 127(87.0) | 19(13.0) | ||
| Clinical N-Stage | 0 | 128(38.7) | 103(80.5) | 25(19.5) | 0.886 |
| 1–3 | 203(61.3) | 165(81.3) | 38(18.7) | ||
| ER Score* | Mean(sd) | 3.97(3.5) | 4.49(3.5) | 1.79(2.8) | <0.001 |
| Median(range) | 5(0,8) | 6(0,8) | 0(0,8) | ||
| PR Score* | Mean(sd) | 2.97(3.2) | 3.41(3.3) | 1.10(2.1) | <0.001 |
| Median(range) | 3(0,8) | 4(0,8) | 0(0,8) | ||
| TIL-str* | Mean(sd) | 22.02(19.3) | 19.00(16.9) | 34.87(23.2) | <0.001 |
| Median(range) | 20(0,90) | 10(0,90) | 30(0,90) | ||
| iTu-Ly score* | Mean(sd) | 84.62(70.7) | 75.37(66.4) | 123.97(75.2) | <0.001 |
| Median(range) | 80(0,300) | 50(0,300) | 130(0,300) |
represents continuous variables, otherwise categorical;
IC-NST: invasive cancer of no special type, ILC: Invasive lobular cancer, ER: estrogen receptor, PR: progesterone receptor, HER2: human epidermal growth factor receptor 2, TIL-str: stromal lymphocytes, iTu-Ly: intratumoral lymphocytes, TN: triple negative.
In multivariate analysis using TIL-str as TILs variable, non-advanced clinical T-stage (T1&T2), TIL-str and non-luminal subtypes were independent predictors of pCR. TIL-str score was a significant independent variable (p<0.001), with OR of 1.39 (95% CI, 1.18 to 1.62) per 10% increase in the lymphocyte infiltrate (table 2). In multivariate analysis using iTu-Ly as TILs variable, non-advanced clinical T-stage (T1&T2), trastuzumab therapy, iTu-Ly and non-luminal subtypes were independent predictors of pCR. iTu-Ly score was a significant independent variable (p<0.001), with OR of 1.28 (95% CI, 1.11 to 1.46) per a score of 30 increase in the lymphocyte infiltrate (table 2).
Table 2.
Odds ratio in the univariate and multivariate analysis for all cases (n=331)
| Variables | Univariate analysis | Multivariate analysis with TIL-str |
Multivariate analysis with iTu-Ly |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age <50 vs. ≥50 | 1.73 | 0.99 to 3.01 | 0.051 | 1.77 | 0.94 to 3.31 | 0.076 | 1.75 | 0.94 to 3.25 | 0.078 |
| Histology non ILC vs. ILC | 8.41 | 1.13 to 62.74 | 0.038 | 1.68 | 0.20 to 13.82 | 0.631 | 1.99 | 0.24 to 16.59 | 0.525 |
| non Luminal vs. Luminal | 7.63 | 3.62 to 16.08 | <0.001 | 5.09 | 2.06 to 12.62 | <0.001 | 4.74 | 1.95 to 11.52 | <0.001 |
| Trastuzumab Yes vs. No | 3.09 | 1.71 to 5.61 | <0.001 | 1.87 | 0.92 to 3.81 | 0.086 | 2.07 | 1.00 to 4.27 | 0.049 |
| Nottingham grade 3 vs. 1&2 | 3.18 | 1.75 to 5.78 | <0.001 | 1.08 | 0.51 to 2.27 | 0.846 | 1.11 | 0.54 to 2.27 | 0.770 |
| Clinical T-Stage 1&2 vs. 3&4 | 2.09 | 1.16 to 3.76 | 0.014 | 2.33 | 1.20 to 4.53 | 0.013 | 2.34 | 1.22 to 4.50 | 0.011 |
| TIL-str*(per 10%) | 1.45 | 1.27 to 1.67 | <0.001 | 1.39 | 1.18 to 1.62 | <0.001 | |||
| iTu-Ly score*(per 30) | 1.30 | 1.16 to 1.46 | <0.001 | 1.28 | 1.11 to 1.46 | <0.001 | |||
continuous variable; all others are categorical
Correlation Between TILs and pCR in Luminal Subtype
There were 159 (48%) luminal A cases, 9 (5.7%) of which had pCR. Both iTu-Ly and TIL-str were statistically significant in predicting pCR. The median and range of TIL-str in the cases with pCR vs. non-pCR was 20 (10 to 50) vs. 10 (0 to 90), respectively (p=0.021). The median and range of iTu-Ly in the cases with pCR vs. non-pCR was 120 (10 to 220) vs. 50 (0 to 270), respectively (p=0.008) (supplemental table 1). However, iTu-Ly (p=0.013) but not TIL-str (p=0.174) predicted pCR in multivariate analysis with OR of 1.44 (95% CI, 1.08 to 1.91) per a score of 30 increase in the lymphocyte infiltrate (table 3). It is worth noting that ER−/PR+ phenotype (n=10) was independent predictor for pCR with borderline significance (p=0.061 using TIL-str variable and p=0.056 using iTu-Ly variable) (table 3). Spearman correlation coefficient between TIL-str and iTu-Ly scores was 0.711 (p<0.0001).However, there was clustering for cases with pCR in the region of high iTu-Ly and low TIL-str (figure 3b). Figure 4 shows an example of high iTu-Ly infiltrate in luminal type BC.
Table 3.
Odds ratio in the univariate and multivariate analysis for luminal (n=159)
| Variables | Univariate analysis | Multivariate analysis with TIL-str |
Multivariate analysis with iTu-Ly |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| ER− vs. ER+ | 8.29 | 1.36 to 50.46 | 0.022 | 6.13 | 0.92 to 41.06 | 0.061 | 6.31 | 0.95 to 41.77 | 0.056 |
| TIL-str*(per 10%) | 1.32 | 0.98 to 1.77 | 0.067 | 1.52 | 0.91 to 1.71 | 0.174 | |||
| iTu-Ly score*(per 30) | 1.45 | 1.11 to 1.90 | 0.007 | 1.44 | 1.08 to 1.91 | 0.013 | |||
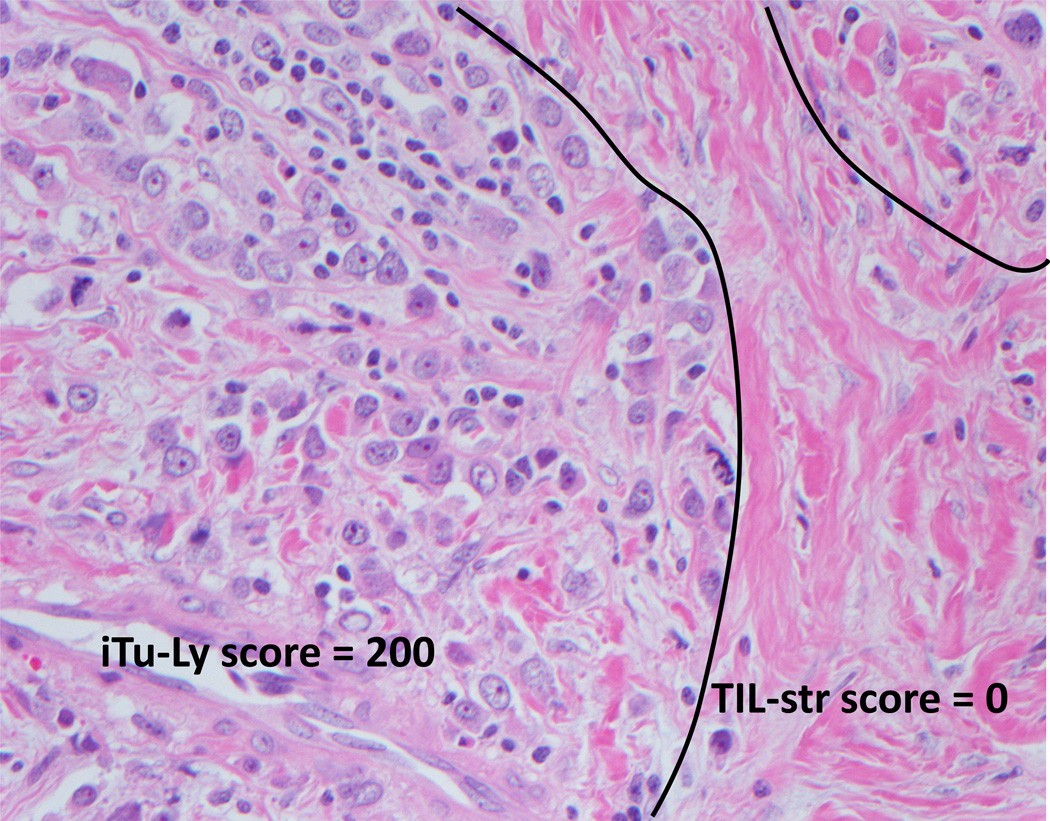
Figure 4.
An example of frequent lymphocytes intimate with the tumor (iTu-Ly grade 2, score 200) with virtually no stromal lymphocytes (score 0%) (H&E, 40×).
Correlation Between TILs and pCR in Triple Negative Subtype
There were 95 (26.8%) TN cases, 29 (30.5%) of which had pCR. Patient’s age, TIL-str and iTu-Ly were statistically significant in predicting pCR. The median and range of TIL-str in the cases with pCR vs. non-pCR was 20 (0 to 80) vs. 50 (0 to 90), respectively (p<0.001). The median and range of iTu-Ly in the cases with pCR vs. non-pCR was 100 (0 to 200) vs. 150 (20 to 300), respectively (p=0.014) (supplemental table 2).
In multivariate analysis, TIL-str score was a significant independent variable (p=0.001), with OR of 1.68 (95% CI, 1.29 to 2.18) per a score of 10 increase in the lymphocyte infiltrate (table 4). iTu-Ly score was also a significant independent variable (p=0.017), with OR of 1.31 (95% CI, 1.05 to 1.63) per a score of 30 increase in the lymphocyte infiltrate (table 4). Spearman correlation coefficient between TIL-str and iTu-Ly scores was 0.654 with more even distribution of cases with pCR in terms of TIL-str and iTu-Ly scores (p<0.0001) (figure 3c).
Table 4.
Odds ratio in the univariate and multivariate analysis for TN cases (n=95)
| Univariate Analysis | Multivariate analysis with TIL-str |
Multivariate analysis with iTu-Ly |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age <50 vs. ≥50 | 2.65 | 1.08 to 6.49 | 0.033 | 2.56 | 0.87 to 7.31 | 0.090 | 2.33 | 0.89 to 6.12 | 0.085 |
|
Nottingham grade 3 vs. 1&2 |
4.32 | 0.92 to 20.21 | 0.063 | 2.28 | 0.43 to 11.99 | 0.332 | 3.23 | 0.65 to 16.05 | 0.152 |
|
Clinical T-Stage 1&2 vs. 3&4 |
2.36 | 0.94 to 5.94 | 0.068 | 2.12 | 0.72 to 6.26 | 0.174 | 2.15 | 0.79 to 5.85 | 0.136 |
| TIL-str*(per 10%) | 1.72 | 1.33 to 2.21 | <0.001 | 1.68 | 1.29 to 2.18 | 0.001 | |||
| iTu-Ly score*(per 30) | 1.31 | 1.07 – 1.61 | 0.011 | 1.31 | 1.05 to 1.63 | 0.017 | |||
Correlation Between TILs and pCR in HER2+ Subtype
There were 77 (23.3%) HER2+ cases, 25 (32.5%) of which had pCR. The majority (88.3%) of the patients received trastuzumab. Trastuzumab was not given to 9 patients for one of three reasons: 1) patients (n=6) had their disease prior to mid-2005 when trastuzumab was first implemented in the neoadjuvant setting; 2) patient (n=1) was on a clinical trial; or 3) patients (n=2) deemed not tolerant to this agent. None of the patients who did not receive trastuzumab (n=9) achieved pCR. Therefore, the rate of pCR to trastuzumab was 25 of 68 (36.8%). Only non-advanced clinical T-stage (T1&T2) predicted pCR (p=0.036). All other variables, including TIL-str and iTu-Ly were not statistically significant (table 5). The overall median and range of TIL-str and iTu-Ly was 20 (0 to 70) and 60 (0 to 250), respectively. The median and range of TIL-str in the cases with pCR vs. non-pCR was 20 (0 to 70) vs. 20 (0 to 70), respectively (p=0.74). The median and range of iTu-Ly in the cases with pCR vs. non-pCR was 90 (0 to 250) vs. 55 (0 to 190), respectively (p=0.38) (supplemental table 3). Multivariate analysis was not performed due to the lack of more than one significant variable in univariate analysis. Spearman correlation coefficient between TIL-str and iTu-Ly scores was 0.644 (p<0.0001) (figure 3d).
Table 5.
Odds ratio in the univariate analysis for HER2+ cases (n=77)
| Variables | Univariate Analysis | ||
|---|---|---|---|
| OR | 95% CI | P-value | |
| Age <50 vs. ≥50 | 1.15 | 0.44 to 3.04 | 0.763 |
| Histology non ILC vs ILC | NA | NA | NA |
| ER− vs. ER+ | 1.27 | 0.49 to 3.32 | 0.622 |
| PR− vs. PR+ | 2.51 | 0.86 to 7.31 | 0.091 |
| ER+ and/or PR+ vs. ER− and PR− | 1.01 | 0.39 to 2.61 | 0.995 |
| Trastuzumab Yes vs. No | NA | NA | NA |
| Nottingham grade 3 vs. 1&2 | 1.20 | 0.45 to 3.23 | 0.712 |
| ER Score* | 0.91 | 0.78 to 1.06 | 0.212 |
| PR Score* | 0.83 | 0.68 to 1.02 | 0.072 |
| Clinical T-Stage 1&2 vs. 3&4 | 3.00 | 1.07 to 8.40 | 0.036 |
| TIL-str*(per 10%) | 1.06 | 0.81 to 1.40 | 0.662 |
| iTu-Ly score*(per 30) | 1.14 | 0.89 to 1.46 | 0.286 |
Then HER2+ cases were further divided into two subgroups: ER+ and/or PR+ and ER−/PR−. In ER+ and/or PR+ group, lower ER or PR scores predicted pCR in univariate analysis (p=0.003 and 0.11, respectively), as well as non-advanced clinical T-stage (T1&T2) (p=0.003) (supplemental table 4). None of these variables was statistically significant in multivariate analysis. In ER−/PR− subgroup, none of the variables was statistically significant (supplemental table 5). When cases without trastuzumab therapy were excluded, none of the variables, including both TILs patterns, was statistically significant.
DISCUSSION
To our knowledge, this is the first study that investigated the role of TILs location in response to NAT in various BC clinical subtypes. We found that both TIL-str and iTu-Ly were independent predictors of pCR when all BC cases were considered together. However, when taking the clinical subtype of BC into considerations the results differed. In luminal subtype lymphocytes that were intimate to tumor cells but not stromal lymphocytes predicted pCR. Regardless of TILs location, they predicted pCR in TN subtype but not in HER2+ subtype independent of hormonal receptor status.
Although some investigators have suggested that the location of TILs was biologically insignificant since these cells were scored as they were artificially static (9), some others suggested otherwise. It is known that stroma but not tumor harbors various components that influence the host immune response, including fibroblasts, macrophage-lineage cells and vascular endothelial cells, with variable amounts of extracellular matrix. These components, in addition to positively regulate tumor growth, can impair host immune responses (17). This theory is supported by the observation that pure tumor cells were rejected more easily by mice when implanted in vivo than tumors containing stromal elements (18). For example, Doug et al generated mice that allowed deletion of fibroblast activating protein-expressing cells in vivo, which could eliminate these fibroblasts from the tumor microenvironment. This led to tumor regression by a mechanism that was dependent on host immunity (19). These observations may explain our findings that the lymphocytes in the tumor predictive of response to cytotoxic chemotherapeutic agents. However, it is unclear why only luminal BC had this property of iTu-Ly-significant/TIL-str-non-significant. We assume that this subtype may have unique biologic factors that interact with tumoral but not stromal lymphocytes; an area could be targeted for future studies. Also, it should be noted that the number of cases that had pCR in the luminal was relatively small (n=9). Therefore, more studies like ours are needed to ascertain our findings.
We found strong correlation coefficient between TIL-str and iTu-Ly in our study. That is consistent with the findings by Denkert et al who found a correlation coefficient of 0.61 in the training cohort and 0.80 in the validation cohort (6). In the training group we found that the agreement between the two observers substantial for TIL-str and moderate for iTu-Ly. The main issue with scoring iTu-Ly was recognizing grade 0 (virtually no lymphocytes). All other grades had substantial to almost perfect agreements between the two observers. That is because some cell may be difficult to recognize (lymphocyte vs. apoptotic body). When the cases were divided into three groups based on cutoffs recommended by Issa-Nummer et al (20) the agreement became substantial for both scoring systems. Although we think iTu-Ly scoring system is valid, we recommend more independent studies in order to test its reproducibility.
In a meta-analysis including 12 studies, there was significant correlation between TILs and NAT response in all BC subtypes including ER−, TN, HER2+, and HER2−, but not ER+ with OR 6.21 (95% CI, 0.86–45.15, p=0.071) (10). However, these studies included all ER+ cases with or without HER2+. In a study conducted by Wang-Lopez et al, 10% of 68 patients with luminal subtype had pCR. They found that TIL-str >15% predicted response to NAT. However, no more details are available regarding the study design and if TIL-str is an independent factor in predicting pCR (14). Issa-Nummer et al studied a subgroup of GeparQuinto clinical trial including HER2− cases only [hormonal receptor (HR)-positive (equivalent to our luminal type) and TN]. In HR+ subtype, 12% of 209 cases had pCR. The pCR rate for lymphocytic predominant BC (defined ≥60%) was 28.2% (11 pCRs of 39 tumors), while it was only 8.2% (14 pCRs of 170 tumors) for non-lymphocytic predominant tumors. However, the investigators did not perform multivariate analysis to evaluate if TILs are independent factor in this tumor subtype. Also, they did not examine if the location of TILs (stromal or intratumoral) differentially predict pCR (20).
Both TIL-str and iTu-Ly were statistically significant as independent factors in predicting pCR in TNBC. Multiple studies have shown that TILs could predict pCR in TNBC patients treated with NAT. In the above meta-analysis of 12 studies, TILs was found to predict pCR with OR 2.49 (95% CI, 1.61–3.83, p=0.000) (10). Denkert et al investigated TILs’ role in achieving pCR in a subset of TN subtype in GeparSixto trial where TNBC patients received cytotoxic chemotherapy plus bevacizumab. They found significant correlation between lymphocytic predominant BC and pCR in both univariate and multivariate analyses. However, in a subgroup treated with non-pegylated liposomal doxorubicin and carboplatin, TILs did not predict pCR. Although the investigators scored both TIL-str and iTu-Ly, they did not evaluate them separately in terms of predicting pCR (13).
The response rate to trastuzumab-based NAT of 36.8% is consistent with the findings of two previous clinical trials [31.7% in GeparQuattro study (21) and 29% in NeoSphere study (22)]. We did not find significant correlation between any of the two types of TILs (TIL-str or iTu-Ly) and the response to trastuzumab based-NAT. Several other studies investigated the predictive role of TILs in HER2 BC with varied results. Similar to the findings in our study, a retrospective analysis of samples from NeoSphere clinical trial did not show any correlation between detection of TILs and pCR. However, they found that the expression of immune genes/metagenes had different associations with pCR (12). On the other hand, other studies have shown correlation between TILs and pCR in this tumor type. In the NeoALTTO trial, they found that TILs >5% were associated with higher pCR rates independent of treatment regimens (11). In a subgroup analysis of GeparSixto trial, Denkert et al investigated the role of TILs in HER2+ patients who received trastuzumab and lapatinib. They found that TIL-str, as well as lymphocytic predominant BC, was significant in predicting pCR in univariate and multivariate analyses. However, when the definition of pCR changed from ypT0 ypN0 to ypT0is ypN0, the interaction between therapy and lymphocytic predominant BC was not significant (13). Interestingly, analysis of patients in N9831 revealed that addition of trastuzumab to therapy of HER2+ patients with TIL-str lead to worse progression free survival (23). Overall this data might suggest that HER2+ disease and its interaction with host immune system might not be clearly reflected just by analyzing TILs and more functional assays might be required to truly determine prognostic and predictive role of infiltrating lymphocytes.
In this study we show that the degree of TILs inform on chances of achieving pCR with use of chemotherapy uniquely to each clinical subtype of BC. In luminal subtype only iTu-Ly were shown to correlate to achieving pCR. In TNBC presence of lymphocytic infiltrate independent of its location strongly correlated with pCR. In HER2+ disease, independent of hormonal receptors status, neither TIL-str nor iTu-Ly correlated with pCR reflecting conflicting results from previous investigations. Therefore, one has to be cautious of using presence of TILs as a prognostic marker without taking into account other tumor characteristics.
We have two limitations to our study; first, the number of luminal cases that achieved pCR was too small to make strong conclusions; and second, it was retrospective study where the patients treated with different chemotherapy regimens. Our results suggest that TILs might be functionally heterogeneous with regard to their role in mediating anti-tumor immune response, depending on their location and BC subtypes. More studies using a similar approach are needed to further examine our hypothesis and validate our findings.
Supplementary Material
Acknowledgments
We thank Dr. Sateesh Satchidanand and his staff at Sister’s hospital for their assistance on retrieving the core biopsies of the patients who had been referred to Roswell Park Cancer Institute.
Footnotes
This data was presented in part as a platform in United States and Canadian Academy of Pathology in Seattle, WA 2016
Disclosures: The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 3.DeNardo DG, Coussens LM. Inflammation and breast cancer: Balancing immune response-Crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aaltomaa S, Lipponen P, Eskelinen M, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992;28A:859–864. doi: 10.1016/0959-8049(92)90134-n. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt M, Bohm D, von Tome C, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008;68:5405–5413. doi: 10.1158/0008-5472.CAN-07-5206. [DOI] [PubMed] [Google Scholar]
- 6.Denkert C1, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(4):708. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 7.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicinbased chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 8.Adams S, Demaria S, Goldstein L, et al. Prognostic value of tumor-infiltrating lymphocytes (TILs) in Triple Negative Breast Cancers (TNBC) from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao Y, Qu Q, Zhang Y, Liu J, Chen X, Shen K. The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. PLoS One. 2014;9(12):1–21. doi: 10.1371/journal.pone.0115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salgado R, Denkert C, Campbell C, et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015;1(4):448–454. doi: 10.1001/jamaoncol.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianchini G, Pusztai L, Pienkowski T, et al. Immune modulation of pathologic complete response after neoadjuvant HER2-directed therapies in the NeoSphere trial. Ann Oncol. 2015;26(12):2429–2436. doi: 10.1093/annonc/mdv395. [DOI] [PubMed] [Google Scholar]
- 13.Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33(9):983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 14.Wang-Lopez Q, Abrial C, Kwiatkowski F. Total (TIL-t) and CD8+ Tumor-Infiltrating Lymphocytes (TIL-CD8) Predict the Extent of Luminal/HER2-Breast Cancer (BC-Lum) Pathological Response To Neoadjuvant Cytotoxic Therapy (NACT) Lab Inv. 2015;95:73A–73A. [Google Scholar]
- 15.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 16.T Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh S, Ross SR, Acena M, et al. Stroma is critical for preventing or permitting immunological destruction of antigenic cancer cells. J Exp Med. 1992;175:139–146. doi: 10.1084/jem.175.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraman M, Bambrough PJ, Arnold JN, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 20.Issa-Nummer Y, Darb-Esfahani S, Loibl S, et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer--a substudy of the neoadjuvant GeparQuinto trial. PLoS One. 2013;8(12):e79775, 1–7. doi: 10.1371/journal.pone.0079775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Untch M, Rezai M, Loibl S, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28(12):2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 22.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomized multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 23.Perez1 EA, Ballman KV, Anderson SK, et al. Stromal tumor-infiltrating lymphocytes (S-TILs): In the alliance N9831 trial S-TILs are associated with chemotherapy benefit but not associated with trastuzumab benefit. Cancer Res. 2014 (abstract). Abstract bS1-06. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.