Abstract
Mucosal melanomas of the head and neck (MMHN) are aggressive tumors with poor prognosis, different opposed to cutaneous melanoma. In this study, we characterized primary mucosal malignant melanoma for the expression of Kallikrein-related peptidase 6 (KLK6), a member of the KLK family with relevance to the malignant phenotype in various cancer types including cutaneous melanoma. Paraffin-embedded MMHN of 22 patients were stained immunohistochemically for KLK6 and results were correlated with clinical and pathological data. In 77.3% (17/22) of MMHN cases, positive KLK6 staining was found. Staining pattern for tumor cells showed a predominant cytoplasmic staining. However, in six cases we also observed a prominent nuclear staining. MMHN with a high KLK6 expression showed significantly better outcome concerning local recurrence-free survival (p = 0.013) and nuclear KLK6 staining was significantly associated with the survival status (p = 0.027). Overexpression of KLK6 was detected in more than 70% of MMHN and approximately 40% of tumors showed a strong expression pattern. Correlation between clinical outcome of MMHN patients and overexpression of KLK6 has not been addressed so far. Our data demonstrate for the first time increased levels of KLK6 in MMHN and strengthen the hypothesis that there might be a context-specific regulation and function of KLK6 in mucosal melanoma.
Keywords: Mucosal melanoma, Head and neck cancer, Kallikrein-related peptidase 6, KLK6
Introduction
Primary mucosal melanoma (MM) is a rare malignant tumor with an incidence of approximately 1:1,000,000 [1]. Its growth pattern is characterized by an infiltrative and local destructive behavior. Like cutaneous melanoma of the skin, MM originates from melanocytes, but represent only 0.7–1% of all melanomas [2]. MM develops in 55% within the head and neck region (MMHN), while most other cases are diagnosed in the gastrointestinal and the urogenital tract. In contrast to other tumors, risk factors such as exposure to tobacco smoke, HPV-infection or UV-exposure could not be identified so far [1, 3–5].
The standard of care remains radical tumor resection with adjuvant radiation [6]. Systemic therapeutic options like interferon therapy or conventional chemotherapies usually fail to significantly improve survival [1, 7–9]. In the presence of a c-Kit mutation, a targeted therapy with Imatinib or Sunitinib is indicated in a palliative setting, although the incidence of this mutation in MMHN—in contrast to skin melanoma—is rather low and varies between 15 and 20% [10, 11]. BRAF-mutations are even less frequent [12].
Current efforts to investigate the biological and genomic characteristics of these tumors have been constrained by the low incidence of MMHN. However, prognostic biomarkers are urgently needed for better identification of patients with a high risk for treatment failure, and to support the establishment of novel targeted therapies [13].
Due to the discovery of Kallikrein-related peptidase 3 (PSA) as a diagnostic marker for prostate cancer, the Kallikrein gene family has gained increasing interest in the past decades [14, 15]. Kallikreins (KLKs) are a group of serine proteases with diverse physiological functions, and several KLKs, including KLK6 have been implicated in neoplastic transformation and malignant progression [16]. Dysregulation of KLK6 expression is a common event in several human malignancies [17] such as glioma, ovarian, breast, uterine, pancreatic, colorectal, gastric, skin [18], urinary bladder, lung and salivary gland cancers. Most tumors were characterized by a strong increase of KLK6 transcript and protein levels as compared to normal tissues [14, 19]. In several publications, KLK6 has also been described as a promising biomarker for early diagnosis and clinical outcome [20]. However, recent studies questioned the general oncogenic role of KLK6 and stressed the importance to consider its context-dependent, tumor-protective function, as exemplified in breast and renal cancer as well as squamous cell carcinoma of the head and neck (HNSCC) [21, 22]. Although KLK6 has been related to neoplastic transformation and malignant progression, the regulation and function of KLK6 under physiological and pathological settings is poorly understood, and is most likely influenced by multiple mechanisms, including gene copy number imbalances [17], exposure to steroid hormones [15, 23], epigenetic events such as gene promoter hyper-methylation, oncogenic signaling [24, 25] and posttranscriptional control by miRNAs [17, 26, 27].
In the past, the expression of KLK6 has been identified in the microenvironment of skin melanoma and correlated with malignant progression [18, 24, 28]. In HNSCC and breast cancer cells, reactivation of KLK6 was not correlated with poor clinical outcome, but associated with prominent down-regulation of Vimentin, a protein that is expressed in mesenchymal cells. These data suggest a common inhibitory function of KLK6 on Vimentin expression in breast and mucosal epithelial cells [22]; however, the molecular mechanism remains to be fully elucidated. Since the role of KLK6 has not been described in MMHN, the major objective of this study was to analyze KLK6 expression in MMHN and to further detect a possible correlation with the clinical outcome.
Materials and Methods
We retrieved paraffin-embedded tissue from 22 patients, who were diagnosed with MMHN. The local institutional review board in Ulm approved the study (vote no. 374/13). Clinical data were gathered and included basic demographic data such as age, gender, date of diagnosis, initial symptoms, time to recurrence, type or combination of therapy, death and last follow-up. Overall-survival (OS) was assessed from the time of initial diagnosis (date of pathologic report) to last follow-up or date of death. The recurrence-free survival (RFS) was assessed from initial effective treatment in number of month.
Biopsies from patients (n = 9) with cutaneous malignant melanoma (control) were obtained from surgical excisions of affected areas at the Department of Dermatology, University of Cologne. The patients signed the informed consent from the Department of Dermatology, University of Cologne, approved by the Institutional Commission of Ethics (Az. 9645/96).
Immunohistochemical Staining
For immunohistochemical analysis, 3 µm sections were cut from the paraffin blocks and stained according to the manufacturer’s instructions. Heat-induced antigen retrieval was carried out by steaming sections (on Superfrost Plus, Menzel, Braunschweig, Germany) for 30 min in 10 mM citrate buffer pH 6 (Multi Gourmet Steamer, Braun Germany). Immunohistochemistry was conducted with an anti-KLK6 antibody (R&D Systems, Germany, polyclonal goat, AF2008, incubated overnight at 4 °C) using the TSA Amplification Kit (Perkin Elmer, Germany). To visualize staining, we used an AEC-System (Peroxidase substrate kit AEC SK-4200, Vector Laboratories, CA, USA). Counterstaining was done with hematoxylin-eosin solution modified according to Gill III (Merck, Germany) to visualize tissue integrity. Stained sections were evaluated by two experienced observers according to the relative amount of positive tumor cells (range 0-100%) and staining intensity (ranging from 0 to 3: score 0 = no staining, score 1 = weak staining, score 2 = moderate staining, and score 3 = strong staining) [29]. Negative controls omitting the application of primary antibodies were used in all staining. Patient subgroups were arranged according to KLK6high or KLK6low expression and to cytoplasmic and nuclear + cytoplasmic staining. KLK6low includes negative staining for KLK6 and score values below 2, while a score of 3 is considered KLK6high. Any staining in the nucleus was scored as positive. The expression of KLK6 was correlated to the clinical data of each patient.
Statistical Analysis
Statistical analysis was done using SPSS (version 21, IBM, USA) statistics software. Differences between groups were assessed using a Chi square test. OS was defined as the time of initial diagnosis by the institute of pathology until the date of cancer-related death within the follow-up interval (events). Survival times of patients who were alive or were dead due to causes other than cancer were censored. RFS was calculated from the initial tumor therapy until the date of the first local recurrence, lymph node or distant metastasis, second primary carcinoma, or date of cancer-related death within the follow-up period (events). Patients without progression (no event) or cancer-unrelated death were censored. Kaplan–Meier curves were calculated to estimate survival distributions, and log-rank tests revealed differences between groups. In all statistical tests, a p value of 0.05 or below was considered as statistically significant.
Results
Tumor specimens from 22 patients with primary MMHN were included in this retrospective study. Among those 22 patients, 13 were female and 9 were male. An overview of the clinical and histopathological features of the cohort is provided in Table 1. The probability of one- and five-year-survival was 87 and 34.8%, respectively. The mean OS was 51 months and the mean RFS was 15 months. Two patients showed distant metastasis and one patient was diagnosed with a positive lymph node of the neck and underwent neck dissection.
Table 1.
Characteristics of MMHN patients
| Characteristics | Cohort (n = 22) |
|---|---|
| Age (years) | |
| Median | 66 |
| Range | 42–88 |
| n (%) | |
|---|---|
| Sex | |
| Female | 13 (59) |
| Male | 9 (41) |
| Location | |
| Nasal cavity | 10 (45) |
| Paranasal sinus | 10 (45) |
| Orbital cavity | 2 (10) |
| Stage | |
| T3 | 14 (64) |
| T4 | 8 (36) |
| N0 | 21 (95) |
| N+ | 1 (5) |
| M0 | 20 (90) |
| M+ | 2 (10) |
| Treatment | |
| Surgery | 6 (27) |
| Surgery + radiotherapy | 13 (59) |
| Surgery + interferon therapy | 3 (14) |
| Pigmentation | |
| Yes | 11 (50) |
| No | 11 (50) |
Prominent KLK6 Expression is a Common Feature of MMHN
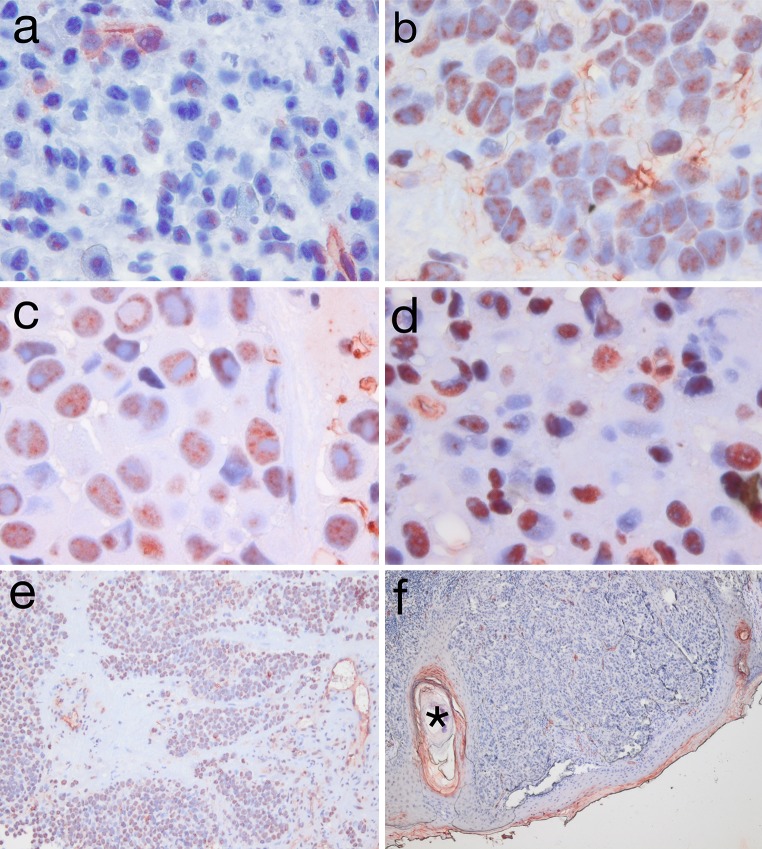
After verification of correct immunolabeling using appropriate positive and negative control tissues (not shown), we stained tissue sections from all 22 cases using the anti-KLK6 antibody to investigate expression of KLK6. Immunohistochemical staining revealed a strong expression of KLK6 in distinct stromal cells, including endothelial cells of blood vessels. This staining served as an internal control and has been described before in cutaneous melanoma [18]. The staining pattern for tumor cells was more heterogeneous with a predominant cytoplasmic staining (Fig. 1c). However, in six cases we also observed a prominent nuclear staining (Fig. 1d), whereas the rest of the cohort did not show any nuclear staining pattern. Positive KLK6 staining in tumor cells was found in 77.3% (17/22) of all MMHN cases with a score 3 (strong staining, Fig. 1b) in 40.9% (9/22), score 2 (moderate) in 27.2% (6/22), and score 1 (weak staining, Fig. 1a) in 9.1% (2/22). In contrast, strong staining in tumor cells was not seen in a cohort of cutaneous melanoma (n = 9), which was used as a reference group (Fig. 1f). The scoring system used is a slightly revised version of the Allred scoring system [30]. Since the nuclear staining pattern was either absent (n = 16) or prominent (n = 6), no scoring supplemental system was applied. Scoring results for all 22 cases for cytoplasmic staining are provided in Table 2.
Fig. 1.
(×60 a–d; ×10 e + f): a + b: mucosal melanoma sample with score 1 (low; a) staining for KLK6 and score 3 (high; b) staining for KLK6; c + d: sample of mucosal melanoma with cytoplasmic (c) and cytoplasmic and nuclear staining for KLK6 (d); e + f: sample of mucosal melanoma with KLK6 in ×10 magnification (e), cutaneous melanoma without KLK6 expression but with internal positive control of a hair follicle with positive keratinocytes* (f); Since MMHN specimens showed a pigmentation in 50% of all cases, a bleaching method (7.5% H2O2 for 1 h) was used to lighten the brown pigmentation of the cells
Table 2.
Scoring results for cytoplasmic KLK6 expression
| Patient | Intensity | Percent | Score |
|---|---|---|---|
| 1 | 2 | 40 | 2 |
| 2 | 2 | 90 | 3 |
| 3 | 3 | 70 | 3 |
| 4 | 3 | 50 | 3 |
| 5 | 2 | 70 | 2 |
| 6 | 3 | 80 | 3 |
| 7 | 3 | 80 | 3 |
| 8 | 3 | 80 | 3 |
| 9 | 1 | 20 | 1 |
| 10 | 3 | 80 | 3 |
| 11 | 3 | 40 | 3 |
| 12 | 0 | 0 | 0 |
| 13 | 0 | 0 | 0 |
| 14 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 |
| 16 | 2 | 70 | 2 |
| 17 | 0 | 0 | 0 |
| 18 | 2 | 40 | 2 |
| 19 | 2 | 50 | 2 |
| 20 | 1 | 20 | 1 |
| 21 | 2 | 80 | 3 |
| 22 | 3 | 50 | 2 |
Low KLK6 Expression Serves as Biomarker for Local Recurrence
MMHN patients were divided in two subgroups according to the KLK6 expression pattern (KLK6low with negative staining for KLK6 and score ≤2. Score 3 is considered KLK6high). There was no significant correlation between subgroups with low or high KLK6 expression and age or gender (Table 3). However, cross-table analysis revealed a significant correlation between a low KLK6 staining pattern and the event local recurrence (p = 0.013) or death (p = 0.041).
Table 3.
Kallikrein-related peptidase 6 expression in mucosal melanoma
| KLK6low | KLK6high | p value | |
|---|---|---|---|
| Age (years) | |||
| <66 | 7 | 3 | |
| ≥66 | 6 | 6 | 0.465 |
| Gender | |||
| Male | 7 | 2 | |
| Female | 6 | 7 | 0.138 |
| Status | |||
| Alive | 3 | 6 | |
| Dead | 10 | 3 | 0.041 |
| Recurrence status | |||
| No recurrence | 1 | 5 | |
| Recurrence | 12 | 4 | 0.013 |
KLK6low includes negative staining for KLK6 and scoring results until score 2. Score 3 is considered KLK6high. Significant p values (<0.05) are indicated in bold. Survival in months
Nuclear KLK6 Expression Serves as Favorable Risk Factor for Survival
In order to evaluate the clinical relevance of intracellular localization, KLK6-positive MMHN cases were divided in subgroups with or without prominent nuclear staining (KLK6cyt and KLK6cyt+nuc). Although intracellular localization was not significantly correlated with age, gender, or recurrence status, nuclear KLK6 staining was significantly associated with the survival status (Table 4, p = 0.027).
Table 4.
Nuclear versus cytoplasmic KLK6 staining in mucosal melanoma
| KLK6nuc+cyt | KLK6cyt | p value | |
|---|---|---|---|
| Age (years) | |||
| <66 | 2 | 4 | |
| ≥66 | 4 | 7 | |
| Gender | |||
| Male | 1 | 6 | |
| Female | 5 | 5 | 0.129 |
| Status | |||
| Alive | 5 | 3 | |
| Dead | 1 | 8 | 0.027 |
| Recurrence status | |||
| No recurrence | 2 | 3 | |
| Recurrence | 4 | 4 | 0.725 |
Significant p values (<0.05) are indicated in bold. Survival times in months
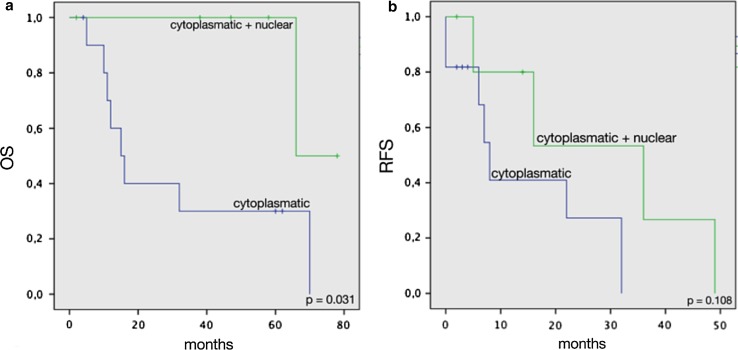
A significant association between nuclear KLK6 staining with prolonged overall survival was further confirmed by Kaplan–Meier analysis (Fig. 2). In contrast, we did not observe any nuclear or cytoplasmic staining in cutaneous melanoma.
Fig. 2.
Overall survival (a) and recurrence-free survival (b) of patients with cytoplasmic and nuclear or solely cytoplasmic stained tumors
Discussion
MMHN is an aggressive tumor, which is characterized by its destructive local growth patterns. The molecular mechanisms of tumor development and progression are poorly understood. Symptoms occur late and tumor stages are often advanced at the time of diagnosis. Satellite metastases and irregular borders with melanosis around the primary tumor are often found, while distant and lymph node metastases occur less frequently than in cutaneous melanoma. The irregular borders in combination with the localization in the head and neck area make a negative margin resection and reconstruction of the defects rather challenging. Local recurrence is found in 40–60% [1] and is often limiting for curative treatment and survival. While 5-year overall survival for cutaneous melanoma is up to 80%, the literature describes a dismal prognosis for MMHN with an OS in the range between 12 and 25% [1, 15]. Most of the primary MMHN are located in the nasal cavity and the nasal sinus, followed by the oral cavity, the larynx and the pharynx [16]. Because of the aggressive and destructive growth pattern of this tumor entity, more efficient systemic therapies, and prognostic markers are urgently needed.
Aberrant expression of KLK6 has been detected in a variety of malignant tumors like pancreas, breast [31], ovarian, skin cancer [32], HNSCC [22] and glioma [20]. An increase in KLK6 expression is a common feature of most human malignancies, but the correlation between KLK6 upregulation and the clinical outcome is poorly understood and even led to controversial findings concerning tumor-regulating properties of KLK6 in tumor recurrences due to treatment failure [21, 31, 32]. Nevertheless, our knowledge about the escape mechanisms of multiple malignant tumors is still incomplete and the molecular mechanism remains to be fully elucidated.
In 2011, Krenzer et al. investigated the KLK6 expression pattern in cutaneous melanoma throughout malignant progression. Interestingly, KLK6 could not be detected in tumor cells but in the adjacent microenvironment of benign nevi, primary melanoma and cutaneous metastatic lesions [18]. Exclusive expression of KLK6 in adjacent keratinocytes and stromal cells but not in tumor cells strongly suggested a paracrine mode of action by extracellular KLK6 to foster malignant progression and tumor cell dissemination, which was confirmed by administration of recombinant KLK6 on melanoma cell lines in vitro.
However, Pampalakis et al. provided compelling evidence that KLK6 could also act as suppressor of malignant progression by promoting mesenchymal-to-epithelial transition [22]. These data are supported by recent findings in head and neck cancer squamous cell carcinoma and question the general oncogenic role of KLK6 and emphasize the importance to consider its context-dependent regulation and function [21, 22, 31].
In our cohort of MMHN, we detected a positive KLK6 staining in tumor cells in more than 70% of cases. Positive tumor cells showed either an exclusive cytoplasmic or a combined cytoplasmic and nuclear staining pattern. Prominent nuclear staining strongly indicates a different regulation and molecular function of KLK6, which differs from its widely excepted mode of action as secreted extracellular protease. So far, the role of nuclear KLK6 remains unclear, but has been observed previously. As an example, Gabril et al. [33] showed diffuse cytoplasmic granular staining of KLK6 in renal cell carcinoma, but also focal moderate nuclear staining in low nuclear grade tumors.
Patients with KLK6-positive tumor cells showed a significantly lower number of local recurrences. Although those results must be interpreted with caution since the total number of patients is rather small, our findings support the hypothesis that the clinical relevance of KLK6 is largely context dependent. The detailed immunohistochemical analyses on human mucosal melanoma tissue sections shown in this study demonstrates that in contrast to cutaneous melanoma, elevated levels of KLK6 are frequently found in mucosal melanoma cells themselves. Our data provides evidence for an important role of KLK6 within tumor cells of mucosal melanoma. The clinical relevance of our results is supported by the fact that nuclear KLK6 levels in MMHN seem to serve as an indicator for better clinical outcome. In this study, we could underline new differences between cutaneous and mucosal melanoma and identify a possible new prognostic marker for mucosal melanoma. Further experiments focusing on underlying mechanisms of tumor development in mucosal melanoma and the potential role of KLK6 will contribute to a better understanding of the molecular nature of this rare tumor entity.
Footnotes
Thomas K. Hoffmann and Jochen Hess have contributed equally to the research project.
References
- 1.Mihajlovic M, Vlajkovic S, Jovanovic P, Stefanovic V. Primary mucosal melanomas: a comprehensive review. Int J Clin Exp Pathol. 2012;5:739–753. [PMC free article] [PubMed] [Google Scholar]
- 2.Prasad ML, et al. Expression and significance of cancer testis antigens in primary mucosal melanoma of the head and neck. Head Neck. 2004;26:1053–1057. doi: 10.1002/hed.20112. [DOI] [PubMed] [Google Scholar]
- 3.Holmstrom M, Lund VJ. Malignant melanomas of the nasal cavity after occupational exposure to formaldehyde. Br J Ind Med. 1991;48:9–11. doi: 10.1136/oem.48.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahlgren L, Schedvins K, Kanter-Lewensohn L, Dalianis T, Ragnarsson-Olding BK. Human papilloma virus (HPV) is rarely detected in malignant melanomas of sun sheltered mucosal membranes. Acta Oncol. 2005;44:694–699. doi: 10.1080/02841860500247461. [DOI] [PubMed] [Google Scholar]
- 5.Lundberg R, et al. Human herpes virus DNA is rarely detected in non-UV light-associated primary malignant melanomas of mucous membranes. Anticancer Res. 2006;26:3627–31. [PubMed] [Google Scholar]
- 6.Thierauf J, et al. Mucosal melanoma of the head and neck. Laryngorhinootologie. 2015;94:812–818. doi: 10.1055/s-0035-1565056. [DOI] [PubMed] [Google Scholar]
- 7.Liao JJ, et al. Fast neutron radiotherapy for primary mucosal melanomas of the head and neck. Head Neck. 2013 doi: 10.1002/hed.23428. [DOI] [PubMed] [Google Scholar]
- 8.Zenda S, et al. Proton beam therapy as a nonsurgical approach to mucosal melanoma of the head and neck: a pilot study. Int J Radiat Oncol Biol Phys. 2011;81:135–139. doi: 10.1016/j.ijrobp.2010.04.071. [DOI] [PubMed] [Google Scholar]
- 9.Yi JH, et al. Dacarbazine-based chemotherapy as first-line treatment in noncutaneous metastatic melanoma: multicenter, retrospective analysis in Asia. Melanoma Res. 2011;21:223–227. doi: 10.1097/CMR.0b013e3283457743. [DOI] [PubMed] [Google Scholar]
- 10.Hodi FS, et al. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol. 2008;26:2046–2051. doi: 10.1200/JCO.2007.14.0707. [DOI] [PubMed] [Google Scholar]
- 11.Minor DR, et al. Sunitinib therapy for melanoma patients with KIT mutations. Clin Cancer Res. 2012;18:1457–1463. doi: 10.1158/1078-0432.CCR-11-1987. [DOI] [PubMed] [Google Scholar]
- 12.Lourenço SV, et al. Head and neck mucosal melanoma: a review. Am J Dermatopathol. 2014 doi: 10.1097/DAD.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 13.Thierauf J, et al. Identification and clinical relevance of PD-L1 expression in primary mucosal malignant melanoma of the head and neck. Melanoma Res. 2015 doi: 10.1097/CMR.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 14.Paliouras M, Borgono C, Diamandis EP. Human tissue kallikreins: the cancer biomarker family. Cancer Lett. 2007;249:61–79. doi: 10.1016/j.canlet.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Yousef GM, et al. Human tissue kallikreins: from gene structure to function and clinical applications. Adv Clin Chem. 2005;39:11–79. doi: 10.1016/S0065-2423(04)39002-5. [DOI] [PubMed] [Google Scholar]
- 16.Prassas I, Eissa A, Poda G, Diamandis EP. Unleashing the therapeutic potential of human kallikrein-related serine proteases. Nat Rev Drug Discov. 2015;14:183–202. doi: 10.1038/nrd4534. [DOI] [PubMed] [Google Scholar]
- 17.Bayani J, Diamandis EP. The physiology and pathobiology of human kallikrein-related peptidase 6 (KLK6) Clin Chem Lab Med. 2012;50:211–233. doi: 10.1515/cclm.2011.750. [DOI] [PubMed] [Google Scholar]
- 18.Krenzer S, et al. Expression and function of the kallikrein-related peptidase 6 in the human melanoma microenvironment. J Investig Dermatol. 2011;131:2281–2288. doi: 10.1038/jid.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oikonomopoulou K, Diamandis EP, Hollenberg MD. Kallikrein-related peptidases: proteolysis and signaling in cancer, the new frontier. Biol Chem. 2010;391:299–310. doi: 10.1515/BC.2010.038. [DOI] [PubMed] [Google Scholar]
- 20.Drucker KL, et al. Clinical significance and novel mechanism of action of kallikrein 6 in glioblastoma. Neuro Oncol. 2013;15:305–318. doi: 10.1093/neuonc/nos313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pampalakis G, et al. A tumor-protective role for human kallikrein-related peptidase 6 in breast cancer mediated by inhibition of epithelial-to-mesenchymal transition. Cancer Res. 2009;69:3779–3787. doi: 10.1158/0008-5472.CAN-08-1976. [DOI] [PubMed] [Google Scholar]
- 22.Schrader CH, et al. Kallikrein-related peptidase 6 regulates epithelial-to-mesenchymal transition and serves as prognostic biomarker for head and neck squamous cell carcinoma patients. Mol Cancer. 2015;14:107. doi: 10.1186/s12943-015-0381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paliouras M, Diamandis EP. Androgens act synergistically to enhance estrogen-induced upregulation of human tissue kallikreins 10, 11, and 14 in breast cancer cells via a membrane bound androgen receptor. Mol Oncol. 2008;1:413–424. doi: 10.1016/j.molonc.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breitenbach U, et al. Keratinocyte-specific onset of serine protease BSSP expression in experimental carcinogenesis. J Investig Dermatol. 2001;117:634–640. doi: 10.1046/j.0022-202x.2001.01437.x. [DOI] [PubMed] [Google Scholar]
- 25.Ignatenko NA, et al. The chemopreventive agent alpha-difluoromethylornithine blocks Ki-ras-dependent tumor formation and specific gene expression in Caco-2 cells. Mol Carcinog. 2004;39:221–233. doi: 10.1002/mc.20008. [DOI] [PubMed] [Google Scholar]
- 26.Kuzmanov U, et al. Separation of kallikrein 6 glycoprotein subpopulations in biological fluids by anion-exchange chromatography coupled to ELISA and identification by mass spectrometry. Proteomics. 2012;12:799–809. doi: 10.1002/pmic.201100371. [DOI] [PubMed] [Google Scholar]
- 27.Chow TF, et al. Kallikreins as microRNA targets: an in silico and experimental-based analysis. Biol Chem. 2008;389:731–738. doi: 10.1515/BC.2008.071. [DOI] [PubMed] [Google Scholar]
- 28.Klucky B, et al. Kallikrein 6 induces E-cadherin shedding and promotes cell proliferation, migration, and invasion. Cancer Res. 2007;67:8198–8206. doi: 10.1158/0008-5472.CAN-07-0607. [DOI] [PubMed] [Google Scholar]
- 29.van Diest PJ, et al. A scoring system for immunohistochemical staining: consensus report of the task force for basic research of the EORTC-GCCG. European Organization for Research and Treatment of Cancer-Gynaecological Cancer Cooperative Group. J Clin Pathol. 1997;50:801–804. doi: 10.1136/jcp.50.10.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qureshi A, Pervez S. Allred scoring for ER reporting and it’s impact in clearly distinguishing ER negative from ER positive breast cancers. J Pak Med Assoc. 2010;60:350–353. [PubMed] [Google Scholar]
- 31.Anisowicz A, Sotiropoulou G, Stenman G, Mok SC, Sager R. A novel protease homolog differentially expressed in breast and ovarian cancer. Mol Med. 1996;2:624–636. [PMC free article] [PubMed] [Google Scholar]
- 32.Seiz L, et al. Stromal cell-associated expression of kallikrein-related peptidase 6 (KLK6) indicates poor prognosis of ovarian cancer patients. Biol Chem. 2012;393:391–401. doi: 10.1515/hsz-2011-0264. [DOI] [PubMed] [Google Scholar]
- 33.Gabril M, et al. Immunohistochemical analysis of kallikrein-related peptidases in the normal kidney and renal tumors: potential clinical implications. Biol Chem. 2010;391:403–409. doi: 10.1515/bc.2010.025. [DOI] [PubMed] [Google Scholar]