Abstract
The assessment of tumor infiltrating lymphocytes (TILs) has recently emerged as a prognostic biomarker in several solid tumors. Quantification and subtyping of TILs reflects the immune response in the tumor microenvironment, contributing to either tumoral immune attack or escape and thereby affecting outcome. Despite the growing evidence of its value as prognosticator, TILs analysis has not yet found its way to daily clinical practice. The aim of this review is to evaluate whether the current knowledge on TILs in head and neck cancer justifies its clinical implementation. Therefore, we summarize the data on TILs in squamous cell cancer of the head and neck with focus on the most important subsets (T lymphocytes and more specifically CD8+ cytotoxic T cells and FoxP3+ regulatory T cells) and site-specific characteristics such as Human Papilloma Virus infection. In addition, we discuss methodological problems and pitfalls that can account for discordant findings and that may hamper inclusion of TILs assessment in routine practice of pathologists and oncologists.
Keywords: Squamous cell carcinoma of the head and neck, Tumor infiltrating lymphocytes, Prognosis, Biomarker
Introduction
Head and neck cancer is the sixth most common malignancy in the world, accounting for 550,000 new cases every year [1] and is a term used to group together a number of tumors arising from distinct subsites including nasal cavity, oral cavity, pharynx and larynx. Its association with tobacco and alcohol has been investigated and confirmed in various trials [2, 3]. More recent, a growing number of cases of SCCHN has been linked to the Human Papilloma Virus (HPV), as this virus is involved in 30–65% of all SCCHN and up to 80% of oropharyngeal cancers [4–7].
Current treatment regimens for SCCHN rely on a combination of surgery, radiotherapy and systemic therapy, depending on the tumor stage [8, 9].
Outcome for locoregional advanced disease (stage III/IV) remains dismal due to local recurrence and/or distant metastasis. In Europe the relative survival rate for head and neck cancer patients is 72% at one year and 42% at five years [8]. This urges the need for more effective treatments with fewer side effects. In this context, targeting the tumor microenvironment with immunotherapy may present a potential approach for the control of head and neck cancer.
The tumor microenvironment is a unique and complex environment that emerges during the course of tumor progression [10]. It consists of fibroblasts, stroma and immune cells, including those mediating adaptive immunity as well as effectors of innate immunity. T lymphocytes, including CD3+CD4+ and CD3+CD8+ T cells, are the predominant component of the tumor microenvironment in solid tumors [10, 11]. In addition to T cells, macrophages (M1 and M2 macrophages), natural killer (NK) cells, and dendritic cells (DCs) also infiltrate tumor tissue [10–13]. Research has shown that CD4+ T helper cells, CD8+ cytotoxic T cells, NK cells, M1 macrophages and DCs are protective against tumor growth and that a coordinated and balanced interaction of these subsets is needed to protect the host against a developing tumor [14]. Conversely, CD4+ forkhead box P3+ (FoxP3+) regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) promote tumor growth [14].
Tregs, which are characterized by the presence of FoxP3, involved in the development and function of these cells, play a key role in the process of immune-escape by producing the immunosuppressive cytokines IL-10 and TGF-β, and by consuming IL-2. Consequently, Tregs express the negative co-stimulatory checkpoint inhibitors such as CTLA-4 and PD-1, which dampen T-cell activity when bound to their respective ligands [15–17]. The process of immune-escape is further regulated by MDSCs which inhibit lymphocyte function by inducing Treg cells, producing TGF-β, depleting or sequestering the amino acids required for T cell function, or nitrating T cell receptors or chemokine receptors on tumor-specific T cells [18, 19].
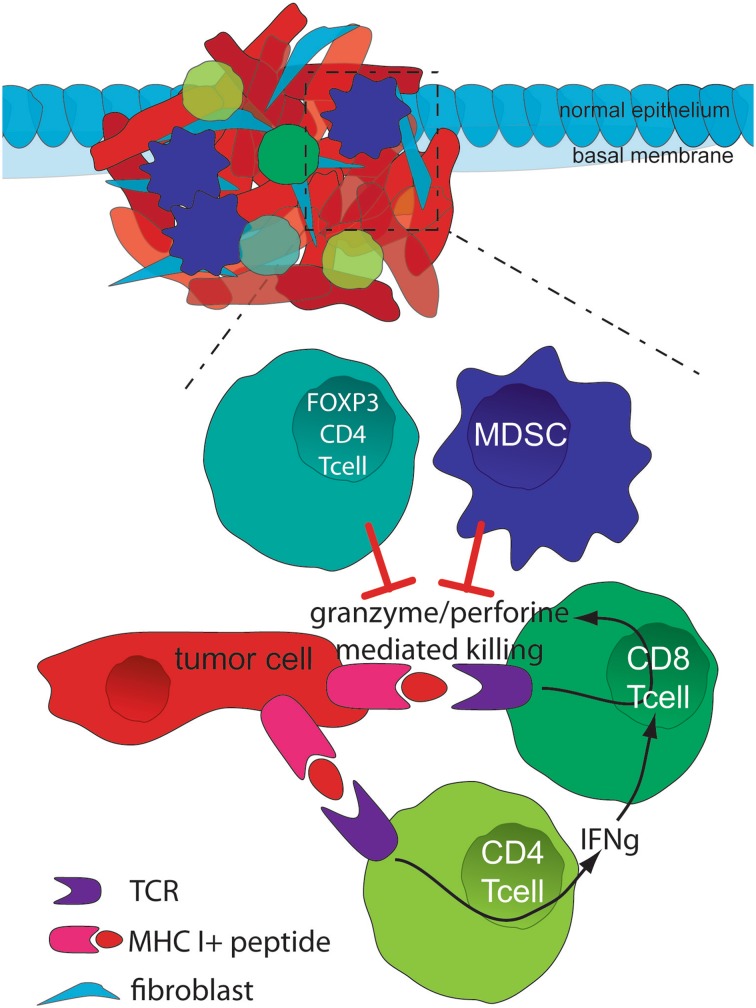
Figure 1 is a simplified schema of the interaction between tumor and immune cells.
Fig. 1.
Simplified schema of the interaction between tumor and immune cells. CD4+ (Th1) T cells produce IFNγ, which in turn mediates the expansion, differentiation, and activation of tumor-specific CD8+ T cells. As a result of the complex interaction between the tumor and cytotoxic T cells (TCR-MHC I/ peptide), high levels of granzymes and perforin are released from these cells, resulting in granule exocytosis and tumor cell death via apoptosis. Both FoxP3+ Treg cells and MDSCs play a critical role during the selection of high-avidity CD8+ T cells, reducing the functionality of CD8+ T cells. IFNγ interferon gamma, MDSC myeloid-derived suppressor cell, MHC I major histocompatibility complex I, TCR T cell receptor
Next, a dynamic interaction takes place between immune cells and the tumor itself, called immunoediting [10]. The process of immunoediting reflects the dual role of the immune system in promoting host protection against cancer and facilitating tumor escape from immune destruction. Those cancer clones that are able to escape the immune system have a survival advantage. Based on the interaction between tumor and immune cells, there are three phases in the process of immunoediting: (1) elimination where cancerous cells are eliminated following immunosurveillance, (2) equilibrium where transformed cells are held in control but are no longer eliminated and (3) escape where tumor cell modifications result in loss of immune response and shape disease progression [10, 11].
In recent years it has become clear that assessment of the immune infiltrate can be of prognostic value in various tumors. In colon cancer, for example, the immunoscore, based on quantification of different types of immune cells, proved to be of greater prognostic significance than conventional TNM staging [20]. Moreover, in the era of immunotherapy, the immune infiltrate of tumors is speculated to be a possible predictive marker of treatment efficacy.
In this review we focus on SCCHN and the available literature on the above mentioned topics. The prognostic role of TILs in SCCHN seems intriguing and will be discussed. Moreover, clinical relevance and the role of TILs in the development of more precise and effective therapies will be highlighted.
Tumor Infiltrating Lymphocytes and Outcome in SCCHN
CD3+ Tumor Infiltrating Lymphocytes
The immune infiltrate of tumors is mainly composed of lymphocytes where T lymphocytes are the predominant cell type. CD3 is a highly specific marker for these T cells. Bound by its antigen, presented in the context of a major histocompatibility complex molecule, the T cell receptor CD3 complex transduces the activating signals to the cytoplasm of the T cell. Given its high specificity and the fact that it is present on T cells in all stages of their development, CD3 is an interesting marker for the detection of T lymphocytes. In SCCHN, high CD3 expression is more common in tumors arising from the oropharynx. Also, patients with early T stage tumors and a lack of smoking history are characterized by high number of CD3+ TILs [21]. Survival analysis demonstrated that SCCHN patients with high CD3 expression, and thus high number of TILs, had a significant superior overall survival (OS), local progression free survival (LPFS) and distant metastasis free survival (DMFS) in univariate analysis. In multivariate analysis, a high number of TILs showed only a benefit for OS [21, 22]. In agreement to these findings, strong tumor infiltration by CD3+ TILs has been correlated with favorable outcome in several tumors [23].
CD8+ Tumor Infiltrating Lymphocytes
CD8 positive T lymphocytes are also called cytotoxic T cells and are able to kill infected, neoplastic, or otherwise targeted cells by direct lysis. They are the most powerful anticancer cells of the immune system. High numbers of tumor infiltrating CD8+ T lymphocytes are associated with oropharyngeal localization, limited tumor growth and lower incidence of smoking [21, 24]. Patients with lower CD8+ T cell count presented a more aggressive disease with higher nodal stage (N2c-3 stage) [22, 24]. Interestingly, strong CD8+ T cell infiltration was also observed in patients with better patient Karnofsky performance score [25]. In general, cytotoxic T cells are positively associated with favorable outcome as described in other cancers such as colorectal and ovarian cancer [20]. Consequently, patients diagnosed with SCCHN whose tumor was highly infiltrated by CD8+ T cells demonstrated a significant better outcome [21, 22, 25–30] compared to minimal or no infiltration. However, in tumors arising from the oral cavity, CD8+ TILs had no significant prognostic value [30–32]. In this matter, Wolf et al. [33] detected that higher CD8 infiltrate counts in oral cavity tumors were associated with tumor recurrence. From these results, we can speculate that tumors arising from the oral cavity could have a different biological behavior from other tumors arising from head and neck. This hypothesis is supported by the fact that tumors arising from the oral cavity are characterized by a high degree of local invasion and a high rate of metastasis to the cervical lymph nodes [34]. Interestingly, the prognostic value of CD8+ TIL levels also seems to vary between tumor compartments [22, 35–38]. In concreto, high CD8 expression in tumor periphery was significantly correlated with improved PFS and local failure free survival whereas high CD8 expression within the tumor cell mass was associated with better OS and DMFS. High CD8 expression in stroma was prognostic for all four clinical end points. In contrast, Balermpas et al. [21] did not report any tumor compartment-dependent differences in the prognostic value of CD8. These opposing results are suggestive for further research to determine the relevance of different subtypes of TILs according to the tumor compartment.
Taken together, the current data on CD8+ TILs and prognosis are not unequivocal and warrant further research. Differences in biological behavior dependent on the anatomic subsite or compartment of the tumor microenvironment may explain the somewhat contradictory findings, as we will discuss below.
CD4+ Tumor Infiltrating Lymphocytes
The benefit of CD4+ T cell infiltration in tumors is somewhat controversial. Firstly, CD4+ T cells were found to be an independent favorable prognostic factor in pancreatic and esophageal squamous cell carcinoma. The same finding was true for SCCHN as high levels of tumor infiltrating CD4+CD69+ T cells in these patients were positively correlated with OS and locoregional control [32]. In contrast, others have described CD4 to be a predictor of poor outcome, especially in oral cavity tumors [39]. These contradictory results could be explained by the heterogeneity of the CD4+ T cell population as besides the classical T helper 1 and T helper 2 cells, other subsets have been identified; including T helper 17, follicular helper T cell, T helper 9, and Tregs; each with its proper characteristic cytokine profile [40]. A further definition of the role and distribution of these subsets in SCCHN is therefore needed.
One of these CD4+ T cell subpopulations, namely FoxP3+ Tregs, is the topic of growing interest and has already been examined closely in SCCHN. As stated before, Tregs play a key role in the process of immune-escape. A high number of FoxP3+ T cells was significantly associated with the primary tumor site: tumors arising from the oral cavity and oropharynx were heavily infiltrated by FoxP3+ T cells [41]. High levels of FoxP3+ T cells also correlated with good pathological differentiation and early T staged SCCHN [33, 42]. Furthermore, patients with high levels of FoxP3+ T cells had better outcome as described already in patients with colorectal cancer [32, 41, 43, 44]. However, Saito et al. [45] recently showed that a low level of FoxP3 expression does relate to a better overall survival in colorectal cancer. These interesting results require further validation and might also be true in SCCHN. On the other hand, in most other tumors the presence of FoxP3+ Tregs was associated with a poor outcome, as was the case in tumors arising from the oral cavity [31, 42, 46–55]. This finding supports the theory that the tumor-infiltrating FoxP3+ Tregs could be an escape mechanism of human cancers to the immune response. The role of Tregs in the control of tumor growth remains a topic of controversy in SCCHN and demands further research on the mechanism(s) by which FoxP3+ Tregs affect outcome.
Influence of HPV Status on TILs and Outcome
Over the past years, the number of cases of SCCHN caused by HPV has increased [4–7]. Although HPV+ tumors are often diagnosed in a more advanced stage, studies report significantly better outcome compared to patients diagnosed with HPV− tumors. The reason for this advantage is however unclear. Hypothetically, the difference in survival between HPV+ and HPV− tumors is probably due to a different immune response directed against the viral antigens. Indeed, HPV+ tumor samples have significantly higher numbers of infiltrating CD4+ and CD8+ T cells [26, 27, 44, 56]. Researchers reported that a high CD8+ T cell infiltrate was positively correlated with a good clinical outcome in both HPV+ and HPV− tumors [21, 26, 27], whereas others failed to reproduce this finding [22, 25, 44, 57, 58]. Furthermore, Ward et al. [44] reported that the prognostic significance for high counts of TILs was only valid in HPV+ tumors, while others reported a prognostic benefit in HPV− tumors only. Hence, the prognostic significance of TILs according to the HPV status of the tumor remains a subject of controversy. More trials, preferably randomized, with a sufficient number of patients are required in order to elucidate the relationship between TILs and the presence of HPV.
Influence of PD-L1 Expression on TILs and Outcome
The programmed death ligand-1/programmed death-1 (PD-L1/PD-1) pathway is known to be involved in T cell activation and differentiation. It has become the focus of attention in oncology, since therapeutic benefits were discovered by blocking either the ligand (PD-L1) or its receptor [59, 60]. To date only little is known on the expression of PD-L1 in SCCHN and most definitely not on its relation with TILs. PD-L1 expression was inversely correlated with the density of intratumoral CD8+ T lymphocytes in patients with oral squamous cell carcinoma [24]. Conversely, there was a positive association between the amount of TILs and PD-L1 expression in laryngeal cancer [61]. In this report, it was also shown that increased PD-L1 levels were associated with better outcome in laryngeal squamous cell cancer.
An overview of the described trials conducted in SCCHN is given in Table 1.
Table 1.
Synoptic overview of the effect of TILs on outcome in patients with SCCHN
| References | Tumor site | Analytic cohort | Tissue | Phenotyping | Scoring | Key finding |
|---|---|---|---|---|---|---|
| Balermpas et al. [21] | SCCHN | 161 | Resection | IHC: CD3, CD8 | Semi-quantitatively | -High CD3+ T cell count positively correlates with better OS, LPFS, DMFS -High CD8+ T cell count positively correlates with better OS, LPFS, DMFS |
| Balermpas et al. [22] | SCCHN | 101 | Biopsy | IHC: CD3, CD4, CD8, FoxP3 | Semi-quantitatively | -High CD3+ T cell count positively correlates with better OS -High CD8+ T cell count positively correlates with better OS, LPFS, DMFS |
| Nordfors et al. [26] | Oropharynx | 275 | Biopsy | IHC: CD4, CD8 | Quantitatively | -High CD8+ T cell count positively correlates with better 3Y OS, 3Y DFS in both HPV+ and HPV− tumors |
| Wansom et al. [25] | Oropharynx | 46 | Biopsy | IHC: CD4, CD8, FoxP3 | Semi-quantitatively | -CD8, FoxP3 and total T cell counts were positively correlated with OS and DSS -T cell infiltrate was not affected by HPV status |
| Näsman et al. [27] | Oropharynx | 83 | Biopsy | IHC: CD8, FoxP3 | Quantitatively | -High CD8+ T cell count positively correlates with better 3Y OS in HPV+ tumors -High number of TILs was positively correlated with HPV+ status |
| Pretscher et al. [28] | Oropharynx Hypopharynx | 33 | Resection Lymph nodes Metastases |
IHC: CD3, CD8, FoxP3 | Quantitatively | -Tumoral CD8 tended towards a favorable DFS |
| Watanabe et al. [29] | Oral cavity | 87 | Resection | IHC: CD4, CD8, CD69, FoxP3 | Quantitatively | -Low CD8+ T cell count was inversely correlated with survival |
| Zancope et al. [30] | Oral cavity | 40 | Resection | IHC: CD8 | Quantitatively | -Peritumoral CD8+ T cells tended towards a higher mean survival |
| Tabachnyk et al. [31] | Oral cavity | 58 | Biopsy Resection |
IHC: CD3,CD4, CD8, FoxP3 | Quantitatively | -Low FoxP3+ T cell count positively correlates with NED survival in the post-RCT samples |
| Badoual et al. [32] | SCCHN | 84 | Biopsy | Immunofluorescence: CD4 subpopulations | Quantitatively | -High CD4+CD69+ T cell count positively correlates with OS and locoregional control -High CD4+FoxP3+ T cell count positively correlates with better locoregional control |
| Wolf et al. [33] | Oral cavity | 39 | Biopsy | IHC: CD4, CD8, FoxP3 | Quantitatively | -High CD8+ T cell count does not correlate with OS or DSS |
| Ward et al. [44] | Oropharynx | 270 | Resection Biopsy |
IHC: CD3, CD4, CD8, FoxP3 | Quantitatively | -High TIL count positively correlates with HPV+ status -High TIL count positively correlates with DSS and PFS in HPV+ tumors |
| Bron et al. [41] | SCCHN | 35 | Resection | IHC: FoxP3 | Quantitatively | -High FoxP3+ T cell count positively correlates with better OS |
| Partlova et al. [56] | Oropharynx | 54 | Resection Lymph nodes Metastases |
Flow cytometry: CD3, CD4, CD8 | Quantitatively | -High number of CD8+ TILs positively correlates with HPV+ status |
| Sun et al. [49] | SCCHN | 83 | Resection | IHC: FoxP3 | Quantitatively | -Intratumoral presence of FoxP3+Tregs is negative prognostic factor |
| Moreira et al. [39] | Oral cavity | 18 | Resection | IHC: CD4, CD8, CD25 | Quantitatively | -Low CD4+ T cell count showed a significant increase in survival |
| Ritta et al. [58] | SCCHN | 71 | Resection | IHC: CD3, CD4, CD8, FoxP3 | Quantitatively | -No difference in the extent of tumor infiltration in HPV+ vs HPV− tumors |
| Liang et al. [42] | Oral cavity | 81 | Resection | Western Blot: FoxP3 IHC: FoxP3 |
Semi-quantitatively | -Low FoxP3+ T cell count positively correlates with OS |
| Cho et al. [24] | Oral cavity | 45 | Resection | IHC: CD4, CD8 | Quantitatively | Intratumoral CD8+ T cell count inversely correlates with tumoral PD-L1 expression |
| Vassilakopoulou et al. [61] | Larynx | 260 | Resection | HE: TILs | Semi-quantitatively | -High number of CD8+ TILs positively correlates with PD-L1 expression -High density of TILs correlates with better DFS and OS |
DMFS distant metastasis free survival, DSS disease specific survival, HPV Human Papilloma Virus, IHC immunohistochemistry, LPFS local progression free survival, NED no evidence for disease, OS overall survival, PFS progression free survival, RCT radiochemotherapy, SCCHN squamous cell carcinoma of the head and neck
Differences in Outcome: What is the Reason?
Studies on the prognostic value of TILs in SCCHN have led to discrepant findings. Several factors might contribute to these differences in prognostic claims and hamper direct comparison of the studies reported in literature.
Biological Factors
Although commonly grouped together, SCCHN comprises malignancies that arise from functionally and anatomically distinct tumor sites with different characteristics e.g. malignancies arising from the oropharynx, which are usually heavily infiltrated by lymphocytes (= pre-existing lymphoid tissue), in contrast to oral cavity tumors. Furthermore, the presence of HPV, which is considered a positive prognosticator, is more often linked to oropharyngeal cancer compared to other tumor sites and as mentioned earlier, TIL infiltration can differ between HPV+ and HPV− tumors. Recent publications also report a heterogeneous molecular and immunological tumor profile of these malignancies on different anatomical localizations [62]. Differences in prognostic value of TILs might reflect these different biologic factors, making it conceivable that TILs exhibit different properties depending on the tumor site, histologic and/or molecular subtype. As discussed earlier it has been suggested that high infiltration of FoxP3+ Tregs may inhibit protumoral effects of inflammatory immune cells and may appear as favorable prognostic markers in one tumor site where in other tumor sites the infiltration is linked to poor prognosis due to their conventional regulatory function [32, 41, 44]. Others believe they exhibit their conventional regulatory function in another tumor site where the infiltration of FoxP3+ Tregs is linked to poor prognosis [31, 42, 46–55].
Additionally, intratumor and intermetastatic heterogeneity complicates this analysis. Table 1 illustrates the use of various samples: biopsies, resection specimens, lymph node metastases. Obviously, a resection specimen of the primary tumor is the ideal study material for a representative assessment of TILs, including their distribution inside the tumor cell mass and in the invasion front of the tumor. However, especially in the field of SCCHN, surgery is not always feasible for different reasons and in these cases, tumor tissue is only available as a small biopsy specimen. It is possible that the immune infiltrate present in the biopsy is not representative for the whole tumor. On the other hand, when limiting a study to resection specimens and excluding cases where only biopsy material can be provided, a selection bias is created towards a group of patients in better general condition and with limited tumor burden, fitted for surgery.
Technical Factors
Different technical aspects of the scoring strategy may have an impact on the quantification of TILs and therefore the outcome of results. Of course, different techniques such as flow cytometry, Western blotting, morphological assessment, or immunohistochemical analysis may yield different results. But for example immunohistochemistry, which is one of the most frequently used methods, may also give various findings depending on the methodology of the test.
Little data have been reported on the different antibodies used for TIL phenotyping. The use of different antibodies can yield different staining patterns because of differences in sensitivity and/ or specificity. Although the effort has been done to compare anti-PDL1 staining antibodies not enough data are available to evaluate the confounding effect of different other antibodies [63, 64].
Next, TILs can reside in tumor cells, stroma or both. Localization may affect the prognostic significance though mixed findings have been reported regarding the impact of the compartment on the prognostic value of TILs. In this context it is also worth mentioning that the tumor and its microenvironment are a dynamic interaction, which questions the relevance of measuring TILs in a somewhat artificial, static manner. On top, it is not clear which region is the most relevant. It has always been assumed that lymphocytes interacting directly with tumor cells are the most important. However most current studies have found stromal TILs to be a more reproducible parameter. Following the recommendations of Denkert et al. [37] TILs should be scored uniquely as a percentage of the stromal area and areas occupied by carcinoma cells should not be included.
Finally, there is no standard cut-off point for TIL quantification studies at this moment. Results are often based on either mean number of TILs or median number of TILs. Before assessing a valid cut-off value, standardization of the technical issues listed above is of the utmost importance.
Statistical Factors
Correct statistical analyses have to be made in order to fully determine the prognostic value of TILs in SCCHN. Several studies made prognostic claims based on univariate analysis. The major advantages of univariate analysis are the ease of application, ease of interpretation, and ease of communication of the results. However, it is conceivable that the prognostic impact of TILs depends on different other variables including primary tumor site, differentiation and TNM stage. Using multivariate analysis may provide interesting results complementary to the univariate analysis, such as the possible influence of confounders. Importantly, results should be interpreted within the statistical framework (uni- or multivariate) with which they have been produced in order to draw more valid conclusions.
Another drawback in the statistical analysis is the small number of patients studied in some reports. A smaller sample will give a result which may not be sufficiently powered to detect a difference between the groups and the study may turn out to be falsely negative.
Considering these factors leading to inconsistent data, there are still many hurdles to overcome in order to determine the correct prognostic and/or predictive value of TILs in SCCHN and to implement its assessment in the daily practice of pathologists and oncologists. It appears obvious that evaluation of TILs in SCCHN has to be performed in a standardized manner. Prospective studies are warranted to evaluate the prognostic and/or predictive relevance of TILs in SCCHN patients by using standardized methodology and validated cut-off values, well defined treatment strategies, strict follow up schemes and multivariate analyses of clinicopathological variables of the patients.
Current Clinical Relevance of TILs
The presence of TILs in SCCHN opens up important questions for patient management. It is likely that patients with high TILs can benefit from strategies designed to enhance the immune response against the tumor. This observation has been a major obstacle in the development of new therapeutic approaches since platinum based regimens and radiotherapy have been considered immunosuppressive. However, recent insights have demonstrated that conventional therapies such as chemotherapy and radiotherapy may result in an immunogenic cell death [65, 66]. This could provide an excellent setting for immunotherapeutic strategies to complement current standard chemoradiation. Like in other tumors, combining chemotherapy, radiotherapy and immunotherapy is topic for investigation with the goal to improve disease control and outcome in patients with SCCHN.
Conclusion
In conclusion, we have demonstrated that the prognostic value of different types of TILs in SCCHN may differ among literature. The original view that FoxP3+ Tregs are associated with poor outcome while CD8+ lymphocytes equal good prognosis is oversimplified. Discrepancies in prognostic impact can be attributed to several factors, both technical and biological in nature. Importantly, all of the aforementioned reports published only on the quantification of TILs in SCCHN, while functionality remains elusive and needs to be studied further.
Further improved understanding of the tumor microenvironment in SCCHN is necessary and could allow the use of immune markers as prognostic markers and enable the development of more precise and effective therapies.
Acknowledgements
This research was supported by the agency for Innovation by Science and Technology (IWT). Sandrine Aspeslagh was sponsored by the Georges Mathé translational research fellowship.
Compliance with Ethical Standards
Conflict of interest
Astrid De Meulenaere declares that she has no conflict of interest. Tijl Vermassen declares that he has no conflict of interest. Sandrine Aspeslagh declares that she has no conflict of interest. Katrien Vandecasteele declares that she has no conflict of interest. Sylvie Rottey declares that she has no conflict of interest. Liesbeth Ferdinande declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Ferlay J, Shin H, Bray F, Forman D, Mathers C, Maxwell Parkin D. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;27(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Wyss A, Hashibe M, Chuang SC, Lee YC, Zhang ZF, Yu GP, et al. Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Am J Epidemiol. 2013;178(5):679–690. doi: 10.1093/aje/kwt029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99(10):777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramqvist T, Dalianis T. Oropharyngeal cancer epidemic and human papillomavirus. Emerg Infect Dis. 2010;16(11):1671–1677. doi: 10.3201/eid1611.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? International journal of cancer Journal international du cancer. 2009;125(2):362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 7.Attner P, Du J, Nasman A, Hammarstedt L, Ramqvist T, Lindholm J, et al. The role of human papillomavirus in the increased incidence of base of tongue cancer. International journal of cancer Journal international du cancer. 2010;126(12):2879–2884. doi: 10.1002/ijc.24994. [DOI] [PubMed] [Google Scholar]
- 8.Gregoire V, Lefebvre JL, Licitra L, Felip E. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2010;21 Suppl 5:v184-6. [DOI] [PubMed]
- 9.http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#head-and-neck.
- 10.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 11.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quezada SA, Peggs KS, Simpson TR, Allison JP. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev. 2011;241(1):104–118. doi: 10.1111/j.1600-065X.2011.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Sullivan T, Saddawi-Konefka R, Vermi W, Koebel CM, Arthur C, White JM, et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med. 2012;209(10):1869–1882. doi: 10.1084/jem.20112738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palucka AK, Coussens LM. The Basis of Oncoimmunology. Cell. 2016;164(6):1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parry RV, Chemnitz JM. Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3 + regulatory T cells. Nature reviews Immunology. 2011;11(2):119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nature reviews Immunology. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monu NR, Frey AB. Myeloid-derived suppressor cells and anti-tumor T cells: a complex relationship. Immunol Invest. 2012;41(6–7):595–613. doi: 10.3109/08820139.2012.673191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016;37(3):208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232(2):199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balermpas P, Rodel F, Rodel C, Krause M, Linge A, Lohaus F, et al. CD8 + tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG) International journal of cancer Journal international du cancer. 2016;138(1):171–181. doi: 10.1002/ijc.29683. [DOI] [PubMed] [Google Scholar]
- 22.Balermpas P, Michel Y, Wagenblast J, Seitz O, Weiss C, Rodel F, et al. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014;110(2):501–509. doi: 10.1038/bjc.2013.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(1):501–509. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho YA, Yoon HJ, Lee JI, Hong SP, Hong SD. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol. 2011;47(12):501–509. doi: 10.1016/j.oraloncology.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Wansom D, Light E, Thomas D, Worden F, Prince M, Urba S, et al. Infiltrating lymphocytes and human papillomavirus-16–associated oropharyngeal cancer. Laryngoscope. 2012;122(1):501–509. doi: 10.1002/lary.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordfors C, Grun N, Tertipis N, Ahrlund-Richter A, Haeggblom L, Sivars L, et al. CD8 + and CD4 + tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. European journal of cancer (Oxford, England : 1990). 2013;49(11):501–9. [DOI] [PubMed]
- 27.Nasman A, Romanitan M, Nordfors C, Grun N, Johansson H, Hammarstedt L, et al. Tumor infiltrating CD8 + and Foxp3 + lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PloS one. 2012;7(6):501–509. doi: 10.1371/journal.pone.0038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, Niedobitek G. Distribution of immune cells in head and neck cancer: CD8 + T-cells and CD20 + B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer. 2009;9:501–509. doi: 10.1186/1471-2407-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe Y, Katou F, Ohtani H, Nakayama T, Yoshie O, Hashimoto K. Tumor-infiltrating lymphocytes, particularly the balance between CD8(+) T cells and CCR4(+) regulatory T cells, affect the survival of patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(5):501–509. doi: 10.1016/j.tripleo.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Zancope E, Costa NL, Junqueira-Kipnis AP, Valadares MC, Silva TA, Leles CR, et al. Differential infiltration of CD8 + and NK cells in lip and oral cavity squamous cell carcinoma. J Oral Pathol Med. 2010;39(2):501–509. doi: 10.1111/j.1600-0714.2009.00792.x. [DOI] [PubMed] [Google Scholar]
- 31.Tabachnyk M, Distel LV, Buttner M, Grabenbauer GG, Nkenke E, Fietkau R, et al. Radiochemotherapy induces a favourable tumour infiltrating inflammatory cell profile in head and neck cancer. Oral Oncol. 2012;48(7):501–509. doi: 10.1016/j.oraloncology.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, et al. Prognostic value of tumor-infiltrating CD4 + T-cell subpopulations in head and neck cancers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(2):501–509. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 33.Wolf GT, Chepeha DB, Bellile E, Nguyen A, Thomas D, McHugh J, et al. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: a preliminary study. Oral Oncol. 2015;51(1):501–509. doi: 10.1016/j.oraloncology.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massano J, Regateiro FS, Januario G, Ferreira A. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(1):501–509. doi: 10.1016/j.tripleo.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 35.Nedergaard BS, Ladekarl M, Thomsen HF, Nyengaard JR, Nielsen K. Low density of CD3+, CD4 + and CD8 + cells is associated with increased risk of relapse in squamous cell cervical cancer. Br J Cancer. 2007;97(8):501–509. doi: 10.1038/sj.bjc.6604001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Distel LV, Fickenscher R, Dietel K, Hung A, Iro H, Zenk J, et al. Tumour infiltrating lymphocytes in squamous cell carcinoma of the oro- and hypopharynx: prognostic impact may depend on type of treatment and stage of disease. Oral Oncol. 2009;45(10):501–509. doi: 10.1016/j.oraloncology.2009.05.640. [DOI] [PubMed] [Google Scholar]
- 37.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(1):501–509. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 38.Dahlin AM, Henriksson ML, Van Guelpen B, Stenling R, Oberg A, Rutegard J, et al. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol. 2011;24(5):501–509. doi: 10.1038/modpathol.2010.234. [DOI] [PubMed] [Google Scholar]
- 39.Moreira G, Fulgencio LB, EF DEM, Leles CR, Batista AC, TA DAS. T regulatory cell markers in oral squamous cell carcinoma: Relationship with survival and tumor aggressiveness. Oncology letters. 2010;1(1):501–509. doi: 10.3892/ol_00000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4(+) T cells in immunity to viruses. Nature reviews Immunology. 2012;12(2):501–509. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bron L, Jandus C, Andrejevic-Blant S, Speiser DE, Monnier P, Romero P, et al. Prognostic value of arginase-II expression and regulatory T-cell infiltration in head and neck squamous cell carcinoma. International journal of cancer Journal international du cancer. 2013;132(3):501–509. doi: 10.1002/ijc.27728. [DOI] [PubMed] [Google Scholar]
- 42.Liang YJ, Liu HC, Su YX, Zhang TH, Chu M, Liang LZ, et al. Foxp3 expressed by tongue squamous cell carcinoma cells correlates with clinicopathologic features and overall survival in tongue squamous cell carcinoma patients. Oral Oncol. 2011;47(7):501–509. doi: 10.1016/j.oraloncology.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 43.Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, et al. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. International journal of cancer Journal international du cancer. 2010;126(11):501–509. doi: 10.1002/ijc.24989. [DOI] [PubMed] [Google Scholar]
- 44.Ward MJ, Thirdborough SM, Mellows T, Riley C, Harris S, Suchak K, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer. 2014;110(2):501–509. doi: 10.1038/bjc.2013.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22(6):501–509. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 46.Yan M, Jene N, Byrne D, Millar EK, O’Toole SA, McNeil CM, et al. Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Res. 2011;13(2):501–509. doi: 10.1186/bcr2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polcher M, Braun M, Friedrichs N, Rudlowski C, Bercht E, Fimmers R, et al. Foxp3(+) cell infiltration and granzyme B(+)/Foxp3(+) cell ratio are associated with outcome in neoadjuvant chemotherapy-treated ovarian carcinoma. Cancer immunology, immunotherapy. CII. 2010;59(6):501–509. doi: 10.1007/s00262-010-0817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. International journal of cancer Journal international du cancer. 2009;125(7):501–509. doi: 10.1002/ijc.24556. [DOI] [PubMed] [Google Scholar]
- 49.Tao H, Mimura Y, Aoe K, Kobayashi S, Yamamoto H, Matsuda E, et al. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer. 2012;75(1):501–509. doi: 10.1016/j.lungcan.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, et al. Higher intratumoral infiltrated Foxp3 + Treg numbers and Foxp3+/CD8 + ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. 2010;136(10):501–509. doi: 10.1007/s00432-010-0816-9. [DOI] [PubMed] [Google Scholar]
- 51.Sheu BC, Hsu SM, Ho HN, Lin RH, Torng PL, Huang SC. Reversed CD4/CD8 ratios of tumor-infiltrating lymphocytes are correlated with the progression of human cervical carcinoma. Cancer. 1999;86(8):501–509. doi: 10.1002/(SICI)1097-0142(19991015)86:8<1537::AID-CNCR21>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 52.Siddiqui SA, Frigola X, Bonne-Annee S, Mercader M, Kuntz SM, Krambeck AE, et al. Tumor-infiltrating Foxp3-CD4 + CD25 + T cells predict poor survival in renal cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(7):501–509. doi: 10.1158/1078-0432.CCR-06-2139. [DOI] [PubMed] [Google Scholar]
- 53.Winerdal ME, Marits P, Winerdal M, Hasan M, Rosenblatt R, Tolf A, et al. FOXP3 and survival in urinary bladder cancer. BJU Int. 2011;108(10):501–509. doi: 10.1111/j.1464-410X.2010.10020.x. [DOI] [PubMed] [Google Scholar]
- 54.Gerber AL, Munst A, Schlapbach C, Shafighi M, Kiermeir D, Husler R, et al. High expression of FOXP3 in primary melanoma is associated with tumour progression. Br J Dermatol. 2014;170(1):501–509. doi: 10.1111/bjd.12641. [DOI] [PubMed] [Google Scholar]
- 55.Sun DS, Zhao MQ, Xia M, Li L, Jiang YH. The correlation between tumor-infiltrating Foxp3 + regulatory T cells and cyclooxygenase-2 expression and their association with recurrence in resected head and neck cancers. Medical oncology (Northwood, London. England) 2012;29(2):501–509. doi: 10.1007/s12032-011-9903-2. [DOI] [PubMed] [Google Scholar]
- 56.Partlova S, Boucek J, Kloudova K, Lukesova E, Zabrodsky M, Grega M, et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology. 2015;4(1):501–509. doi: 10.4161/21624011.2014.965570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kong CS, Narasimhan B, Cao H, Kwok S, Erickson JP, Koong A, et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2009;74(2):501–509. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ritta M, Landolfo V, Mazibrada J, De Andrea M, Dell’Oste V, Caneparo V, et al. Human papillomavirus tumor-infiltrating T-regulatory lymphocytes and P53 codon 72 polymorphisms correlate with clinical staging and prognosis of oropharyngeal cancer. New Microbiol. 2013;36(2):501–509. [PubMed] [Google Scholar]
- 59.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):501–509. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):501–509. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vassilakopoulou M, Avgeris M, Velcheti V, Kotoula V, Rampias T, Chatzopoulos K, et al. Evaluation of PD-L1 Expression and Associated Tumor-Infiltrating Lymphocytes in Laryngeal Squamous Cell Carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(3):501–509. doi: 10.1158/1078-0432.CCR-15-1543. [DOI] [PubMed] [Google Scholar]
- 62.Pai SI, Westra WH. Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Annual review of pathology. 2009;4:501–509. doi: 10.1146/annurev.pathol.4.110807.092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaule P, Smithy JW, Toki M, Rehman J, Patell-Socha F, Cougot D, et al. A Quantitative Comparison of Antibodies to Programmed Cell Death 1 Ligand 1. JAMA Oncol. 2016. [DOI] [PMC free article] [PubMed]
- 64.deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3 + tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(11):501–509. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 65.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):501–509. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 66.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:501–509. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]