Abstract
A case of Cowden syndrome (CS) is described in a 34-year-old African American female who reported a history of breast and thyroid malignancies. Clinical examination demonstrated multiple soft, white-pink papules across multiple mucosal surfaces of the oral cavity. Microscopy of the lesions revealed hyperkeratotic surface squamous epithelium with papillomatosis and acanthosis along with elongated rete processes. A genomic polymerase chain reaction direct sequencing using the patient’s blood was positive for mutations of the PTEN gene typical of CS.
Keywords: Oral papules, Oral hamartomas, Cowden syndrome
History
A 34-year old African American female was referred for evaluation of multiple asymptomatic oral mucosal lesions. She denied the presence of symptoms and reported first noticing these lesions approximately 10 years prior. She also denied any history of oral surgery, facial trauma, and alcohol, tobacco, or recreational drug use. The patient’s past medical history was significant for left and right mastectomy for bilateral ductal carcinoma and partial thyroidectomy for microcarcinoma. She received blood transfusions at multiple points during her surgical journey but her current medical regimen included only levothyroxine.
Clinical Findings
Intraoral examination (Fig. 1) revealed generalized multiple white-pink round/oval papules on the patient’s lips. She was noted to have a distinct 6 mm × 5 mm × 3 mm papillary lesion on the facial gingiva between teeth #25 and 26 with smaller lesions extending slightly inferiorly to the distal of teeth #27 and 28. Similar small patches of lesions were noted on her contralateral mandibular quadrant. Also noted was a 7 mm × 3 mm white lesion on her right buccal mucosa just superior to the occlusal plane on the distobuccal cusp of tooth #31. In addition, pink papillary growths were found on the palatal gingiva of teeth #1, 2, 3, 6, 7, 9, 10, and 11 (Fig. 2) along with a similar but white papillary lesion on the attached buccal gingiva of tooth #15 measuring 5 mm × 5 mm × 5 mm. Another 3 mm × 7 mm papillary cluster was noted on the lingual mandibular gingiva near teeth #21 and 22. White gingival tissue was noted on the crest of her #14 edentulous ridge site (Fig. 3). None of these lesions were removable with gauze. The tongue was otherwise normal, as was the floor of her mouth. Radiographic imaging of the maxillomandibular region was otherwise unremarkable.
Fig. 1.
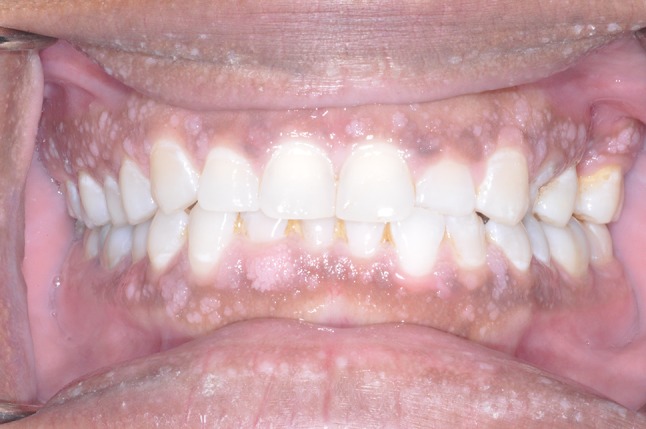
Frontal intraoral photograph demonstrating the presence of multiple bilateral oral papillomatous lesions of the facial surface of the gingiva and mucosal surface of the lips. A 3 mm × 7 mm papillary cluster was noted on the mandibular facial gingiva near teeth #21 and 22, as well as the facial of tooth #26
Fig. 2.
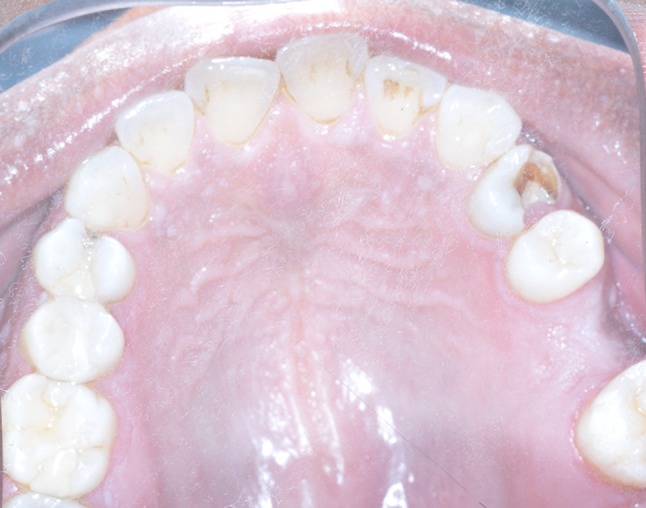
Intraoral maxillary occlusal photograph demonstrating pink papillary growths found on the palatal gingiva
Fig. 3.
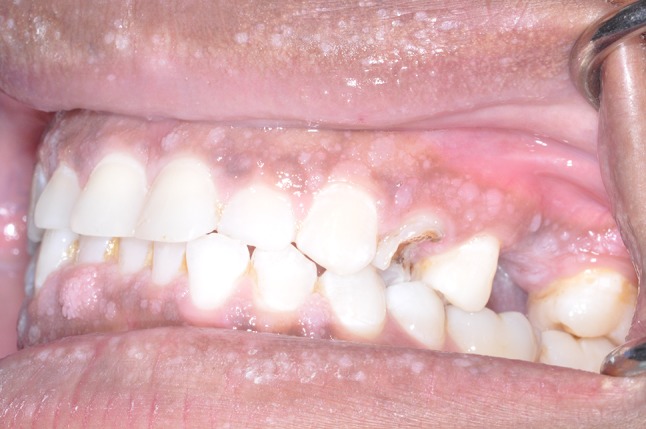
Left oblique intraoral photograph demonstrating a white papillary lesion on the attached buccal gingiva of tooth #15 measuring 5 mm × 5 mm × 5 mm. White gingival tissue was noted on the crest of her #14 edentulous ridge site
Diagnosis
At the time of biopsy, the papillary lesion on the facial gingiva between teeth #25 and 26 (Fig. 1) was removed in its entirety down to bone to reveal a solid and firm mass of tissue, which in turn lacked signs of foreign bodies within or around the lesion. Microscopy revealed a pebbly papulonodular lesion displaying surface stratified squamous epithelium accompanied by hyperparakeratosis, papillomatosis, acanthosis with elongated broad and slender rete processes, and mild suprapapillary epithelial atrophy (Figs. 4, 5, 6). Physiologic pigmentation (melanin pigment) was seen in the cytoplasm of the basal keratinocytes. The clinical and histologic features revealed a diagnosis of benign papillary fibroepithelial hyperplasia. For molecular genetic analysis, genomic DNA was extracted from her peripheral blood; polymerase chain reaction direct sequencing of the phosphatase and tensin homolog (PTEN) gene with primers targeting all nine exons and flanking introns was performed. This testing revealed the presence of a mutation in the PTEN gene characteristic of Cowden syndrome (CS) [1]. The lesion was interpreted to clinically and histologically resemble previously reported oral lesions in CS and no further intraoral surgical intervention was recommended or indicated, unless the lesions were to become symptomatic or presented hygienic challenges. The patient was referred to her primary medical doctor for genetic evaluation as well as gynecologic, gastrointestinal, and dermatologic follow-ups.
Fig. 4.
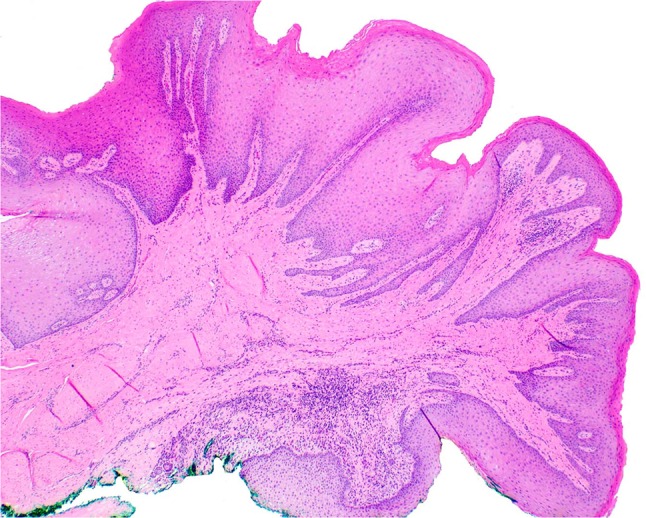
Gingival pebbly papulonodular lesion at low magnification. Surface epithelium demonstrates hyperparakeratosis, irregular acanthosis, papillomatosis, and patchy atrophy. The core of moderately-cellular dense fibrous connective tissue exhibits patchy chronic inflammation
Fig. 5.
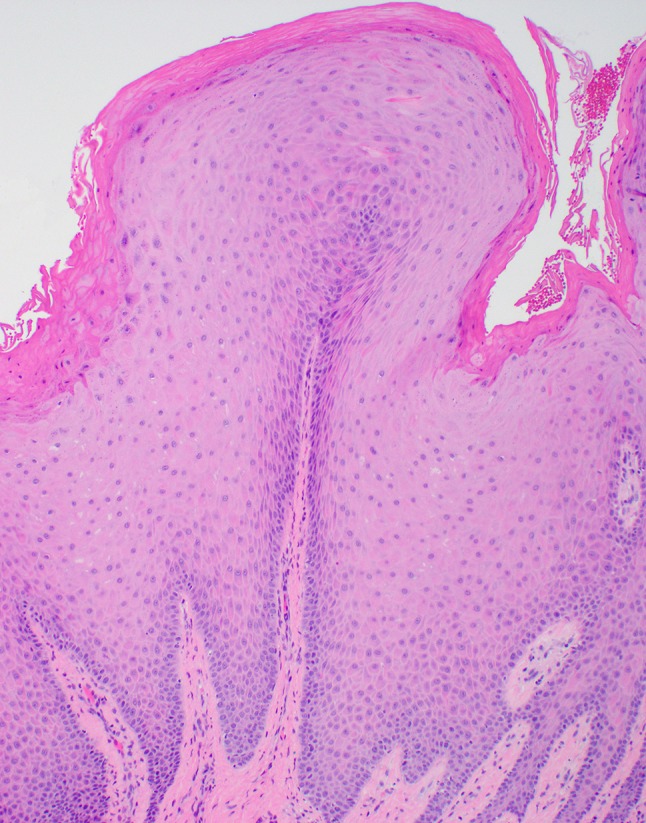
Gingival pebbly fibroepithelial hyperplasia at medium power. Surface epithelium varies in thickness. Fibrotic stroma shows patchy chronic inflammation (lymphocytes, plasma cells)
Fig. 6.
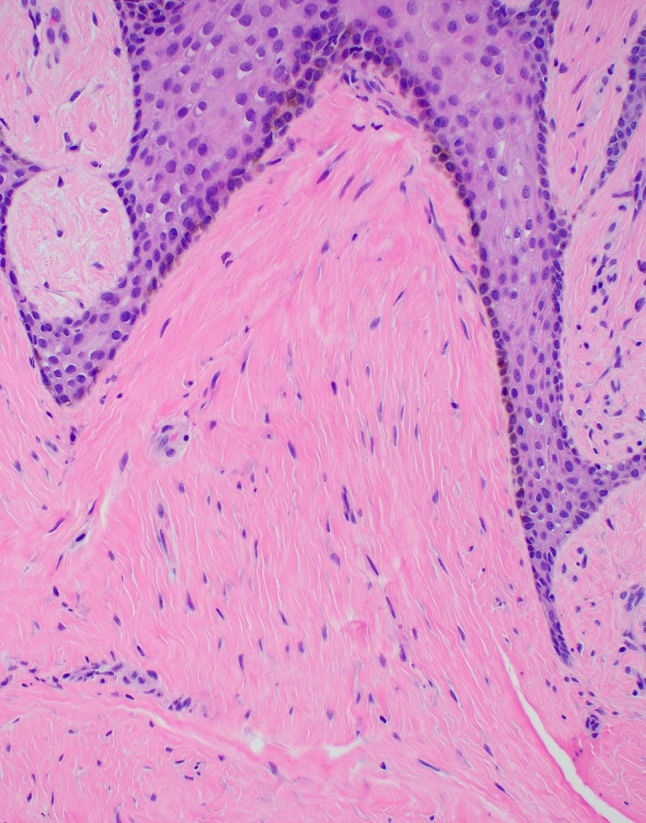
Lesional fibrotic stroma at high power displays parallel collagenous plates with interspersed fibroblasts
Discussion
Cowden syndrome is a rare, autosomal dominant genetic disorder characterized by profound genodermatosis. While the syndrome is both elusive and difficult to diagnose, its prevalence is estimated at approximately 1 in 250,000 people [2]. It was first described by Costello et al. and later named by Lloyds and Dennis who reported their findings in a woman surnamed Cowden [3]. Its significance arises from its association with colorectal, mammary, thyroidal, and genitourinary cancers. Overall, the lifetime risk of cancer in patients with CS was reported to be at approximately 89% with the largest portion of the patients presenting with breast and/or thyroid carcinomas [4]. CS also remains relevant to dental professionals because of the overwhelming prevalence of hamartomatous oral nodules observed in over 99% of patients [1, 3]. As a result, dentists and oral health practitioners could potentially become a first line of discovery against this affliction.
The differential diagnosis of multiple oral papillomatous papules should include Heck’s disease [human papillomavirus (HPV)-associated focal epithelial hyperplasia, or (FEH)], condylomata acuminata [in human immunodeficiency virus (HIV) positive patients], acanthosis nigricans (AN), Darier disease (keratosis follicularis, or KF), oral fibromas in tuberous sclerosis, mucosal neuromas of multiple endocrine neoplasia (MEN), and squamous cell carcinoma (SCC). Limited size, well-defined borders, and the long (10+ years) history and asymptomatic nature of the presentation indicated a benign etiology of the oral lesions, while the incidences of multiple malignancies elsewhere suggested a genetic component to the pathology.
Heck’s disease or FEH is a rare, benign proliferation of mucosal surfaces caused primarily by the HPV subtypes 13 and 32 [5]. It typically presents as multifocal, verrucous papules of the labial, lingual, and buccal mucosa which often lacks a relevant family history [6]. Nodules from FEH are pink and non-inflammatory with no notable symptoms other than their bulk which could theoretically hinder the motion of the various oral structures. However, FEH is unlikely in adults over 18 years of age and is strongly associated in patients of Native American or Latino descent, suggesting a genetic predisposition [5, 7]. Some literature reports have linked the expression of the HLA-DR4 leukocyte antigen as a culprit, noting the possible inability of the HLA-DR4 histocompatibility complex molecules to properly bind to certain HPV-13 or 32 viral proteins [6]. Regardless of this connection, the nodules of FEH are most often self-limited with an average lifespan of 18 months. Our patient’s ethnicity, age, and the years-long presence of her nodules make a diagnosis of FEH implausible [5–7].
Condylomata acuminata (CA) presents as another HPV-related soft tissue lesion(s) of mucosal epithelia. Also known as “genital warts,” CA is described as a reactive growth to infection by HPV subtypes 6 and 11 and may occur as multiple masses that could be nodular or stalky; firm or soft; and pink, brown, or red with ulcerative surfaces. Though they are typically painless, the lesions may be accompanied by itching or discomfort if present near delicate epithelia [8]. Approximately 80–90% of patients with HPV 6 and 11 clear the viruses from their bodies within 2 years, making a consistent, decade-long presentation unlikely except in the setting of extreme immune compromise such as AIDS [9, 10]. Considering the lack of opportunistic infections since the appearance of her nodules many years ago, an underlying infection with HIV is not likely, nor is the presence of persistent, low-risk HPV infection in an otherwise immunocompetent patient.
While AN typically presents as pigmentations of the folded surfaces such as the axilla and the nape, it may also manifest as papillomatous or verrucous patches of oral epithelia [11, 12]. The exact pathophysiology behind AN is debatable—some investigators point to the action of aberrant, tumor-linked growth signaling factors, while others attribute the disease to the increased action of circulating insulin [11]. Interestingly, the condition features strong associations with both diabetes mellitus and cancer—one of the conditions that was experienced by our patient—and was in fact first discovered as a paraneoplastic syndrome in Germany [13]. Epithelial hyperplasia with blunt mucosal projections characteristic of AN also shares significant similarities with the current case, though AN mucosal lesions tend to exhibit flat and velvety architectures rather than a nodular one [14]. In addition, though the patient’s history of adenomatous cancers may lend superficial support for a diagnosis of paraneoplastic AN, it is uncommon for thyroid and breast masses to demonstrate associations with this condition, with intra-abdominal malignancies such as gastric adenocarcinoma instigating a bulk of the signature symptoms [15]. The lack of any over-pigmented surfaces within the flexor surfaces also significantly reduces the possibility of a diabetes-induced case of AN.
Unlike the previously described acquired pathologies, Darier’s disease (KF) is an inherited autosomal dominant condition characterized by warty papules and plaques in a variety of possible areas including palmar/plantar pits, nail beds, and oral mucosa [16]. It occurs as a result of a mutation in the ATP2A2 gene, which disrupts intracellular calcium transportation and may lead to a breakdown in desmosome-keratin complexes. The current condition is somewhat suggestive of KF because both conditions not only demonstrate similar esthetic qualities but because they are both known to show full penetrance later in life, possibly during teenage years or thereafter [17]. Still, the lack of malodorous lesions as well as scalloping or longitudinal ridging of the nails suggests a non-KF etiology of our specific case [18]. Furthermore, KF patients are often subject to various neuropsychiatric conditions that were not evident in this patient [16, 19].
Tuberous sclerosis (TS) is another autosomal dominant condition characterized by a large numbers of tumors (such as neurofibromas and angiofibromas) that could originate from anywhere in the body. Patients commonly exhibit multiple thick, firm masses on their skin, central nervous system, heart, kidneys, and gastrointestinal mucosae including the oral epithelia [20]. Clusters of intraoral fibromas are also common with a reported penetrance of approximately 50–69% and may present with enamel pitting of the permanent dentition [20, 21]. The causes of TS have been mapped to the TSC1 and TSC2 genes, which serve as tumor-suppressors that encode for the proteins hamartin and tuberin, respectively. Interestingly, up to two-thirds of TS cases are thought to occur as a result of sporadic genetic mutations, though a mutation of TSC1 or TSC2 must be accompanied by the mutation of one of a number of proto-oncogenes for the actual symptoms to arise [22]. While this may allow for TS emergence in an adult, it is considered a predominantly neurocutaneous disease with unmistakable psychiatric and extra-oral cutaneous manifestations including epilepsy, cognitive impairment, and angiofibroma [21]. In fact, the diagnostic criterion for TS relies upon the presence of several central nervous tumors of the cortical, subependymal, and retinal regions; and a case without neuropsychiatric symptoms would be considered quite unusual.
Multiple endocrine neoplasia encompasses several syndromes defined by tumors of endocrine and epithelial origin. Presentations of MEN are relevant in that most, if not all, variants are associated with hyperplastic tissues of the parathyroid or thyroid glands that could contribute to a history of surgical intervention [23]. In particular, the MEN2B variant may often be diagnosed within the dental setting because of the pervasive mucosal and digestive neurofibromas [24]. Most cases of the MEN2 varieties are thought to arise from a change in the RET proto-oncogene that occurs in the cells of neural crest origin. This, in turn, strongly predisposes the patient to gain-of-function tumors with common co-presentations of hyperparathyroidism from medullary thyroid carcinoma, sympathetic nervous system hyperactivity from pheochromocytoma, and marfanoid habitus from pituitary adenoma [25]. While the oral neurofibromas of the 2B subset may superficially resemble the presentation of the current case, a large physical stature and body weight is unlikely in the backdrop of pheochromocytoma and hyperparathyroidism. Presentation of MEN with only oral neurofibromas without a distinct endocrine disability is also highly improbable. Furthermore, an association between MEN2 and breast cancer remains questionable at best.
Compared to the previously discussed ranges of pathology, SCC exists as a profoundly invasive, acquired entity that consists of over 90% of all head and neck malignancies. SCC traditionally is most closely associated with a concurrent use of tobacco and alcohol products in middle-aged white males. However, newly discovered cases in younger and female patients indicate a virally-induced variant as well, with certain subtypes such as HPV 16 serving as the primary culprits behind these less aggressive forms of SCC [26]. Regardless of the etiology, their metastatic potential dictates that all variants of SCC are capable of producing multi-locale nodules present across various mucosal surfaces. However, numerous metastatic presentations on both sides of the mouth are unusual, likely because of the relative separation of the bilateral venous and lymphatic structures of the lower skull [27]. Furthermore, metastatic diseases with multiple lesions would be considered extremely unlikely without the presence of other telltale signs such as ulcerations, rolled borders, or regional lymphadenopathy.
Like other similar pathologies, the multi-systemic masses found in CS are often attributed to a mutation which resides in the tumor-suppressing properties of the PTEN gene that occurred at the germline level [3]. The PTEN mutation may also be linked to present traits including macrocephaly and large birthweight, which could help spur an earlier diagnosis during the newborn or infantile stages of life [1]. Regardless, any patient with a history of multiple soft tissue hamartomatous masses should be considered for detailed evaluation. These evaluations may result in significantly reduced mortality and morbidity, and should also extend to immediate family members who are often monitored on a lifelong basis.
Acknowledgements
The authors wish to thank Lee J. Slater, DDS, MS (Staff Pathologist, Scripps Oral Pathology Service, San Diego, CA) for his assistance in the histopathologic evaluation and preparation of the manuscript.
Funding
This study received no funding from any sources.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Celentano A, Adamo D, Leuci S, et al. Oral manifestations of phosphatase and tensin homolog hamartoma tumor syndrome: a report of three cases. J Am Dent Assoc. 2014;145(9):950–954. doi: 10.14219/jada.2014.58. [DOI] [PubMed] [Google Scholar]
- 2.Farooq A, Walker LJ, Bowling J, et al. Cowden syndrome. Cancer Treat Rev. 2010;36(8):577–583. doi: 10.1016/j.ctrv.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Flores IL, Romo SA, Tejeda Nava FJ, et al. Oral presentation of 10 patients with Cowden syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(4):e301–e310. doi: 10.1016/j.oooo.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Riegert-Johnson DL, Gleeson FC, Roberts M, et al. Cancer and Lhermitte-Duclos disease are common in Cowden syndrome patients. Hered Cancer Clin Pract. 2010;8(1):6. doi: 10.1186/1897-4287-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Said AK, Leao JC, Fedele S, et al. Focal epithelial hyperplasia—an update. J Oral Pathol Med. 2013;42(6):435–442. doi: 10.1111/jop.12009. [DOI] [PubMed] [Google Scholar]
- 6.Brehm MA, Gordon K, Firan M, et al. Case report of focal epithelial hyperplasia (Heck’s disease) with polymerase chain reaction detection of human papillomavirus 13. Pediatr Dermatol. 2016;33(3):e224–e225. doi: 10.1111/pde.12862. [DOI] [PubMed] [Google Scholar]
- 7.Gültekin SE, Tokman Yildirim B, Sarisoy S. Oral focal epithelial hyperplasia: report of 3 cases with human papillomavirus DNA sequencing analysis. Pediatr Dent. 2004;33(7):522–524. [PubMed] [Google Scholar]
- 8.Lacey CJN, Woodhall SC, Wikstrom A, et al. European guideline for the management of anogenital warts. J Eur Acad Dermatol Venereol. 2012;27(3):263–270. doi: 10.1111/j.1468-3083.2012.04493.x. [DOI] [PubMed] [Google Scholar]
- 9.Juckett G, Hartman-Adams H. Human papillomavirus: clinical manifestations and prevention. Am Fam Physician. 2010;82(10):1209–1214. [PubMed] [Google Scholar]
- 10.Gormley RH, Kovarik CL. Human papillomavirus-related genital disease in the immunocompromised host: Part I. J Am Acad Dermatol. 2012;66(6):8–14. doi: 10.1016/j.jaad.2010.12.049. [DOI] [PubMed] [Google Scholar]
- 11.Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14(9):2. [PubMed] [Google Scholar]
- 12.Pentenero M, Carrozzo M, Pagano M, et al. Oral acanthosis nigricans, tripe palms and sign of leser-trélat in a patient with gastric adenocarcinoma. Int J Dermatol. 2004;43(7):530–532. doi: 10.1111/j.1365-4632.2004.02159.x. [DOI] [PubMed] [Google Scholar]
- 13.Sinha S, Schwartz RA. Juvenile acanthosis nigricans. J Am Acad Dermatol. 2007;57(3):502–508. doi: 10.1016/j.jaad.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Damm DD, Roddy SC, White DK. Diffuse oral papillomatosis with corrugated lesions of skin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(5):630–636. doi: 10.1016/j.tripleo.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 15.Zhang N, Qian Y, Feng AP. Acanthosis nigricans, tripe palms, and sign of Leser-Trélat in patient with gastric adenocarcinoma: case report and literature review in China. Int J Dermatol. 2015;54(3):338–342. doi: 10.1111/ijd.12034. [DOI] [PubMed] [Google Scholar]
- 16.Frezzini C, Cedro M, Leao JC, et al. Darier disease affecting the gingival and oral mucosal surfaces. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(4):29–33. doi: 10.1016/j.tripleo.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 17.Sakuntabhai A, Burge S, Monk S, et al. Spectrum of novel ATP2A2 mutations in patients with Darier’s disease. Hum Mol Genet. 1999;8(9):1611–1619. doi: 10.1093/hmg/8.9.1611. [DOI] [PubMed] [Google Scholar]
- 18.Sehgal VN, Srivastava G. Darier’s (Darier–White) disease/keratosis follicularis. Int J Dermatol. 2005;44(3):184–192. doi: 10.1111/j.1365-4632.2004.02408.x. [DOI] [PubMed] [Google Scholar]
- 19.Gordon-Smith K, Jones LA, Burge SM, et al. The neuropsychiatric phenotype in Darier disease. Br J Dermatol. 2010;163(3):515–522. doi: 10.1111/j.1365-2133.2010.09834.x. [DOI] [PubMed] [Google Scholar]
- 20.Harutunian K, Figueiredo R, Gay-Escoda C. Tuberous sclerosis complex with oral manifestations: a case report and literature review. Med Oral Patol Oral Cir Bucal. 2011;16(4):478–481. doi: 10.4317/medoral.16.e478. [DOI] [PubMed] [Google Scholar]
- 21.Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372(9639):657–668. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- 22.Dabora SL, Jozwiak S, Franz DN, et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet. 2001;68(1):64–80. doi: 10.1086/316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacheco MC. Multiple endocrine neoplasia: a genetically diverse group of familial tumor syndromes. J Pediatr Genet. 2016;5(2):89–97. doi: 10.1055/s-0036-1579758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moline J, Eng C. Multiple endocrine neoplasia type 2: an overview. Genet Med. 2011;13(9):755–764. doi: 10.1097/GIM.0b013e318216cc6d. [DOI] [PubMed] [Google Scholar]
- 25.Thosani S, Ayala-Ramirez M, Palmer L, et al. The characterization of pheochromocytoma and its impact on overall survival in multiple endocrine neoplasia type 2. J Clin Endocrinol Metab. 2013;98(11):E1813–E1819. doi: 10.1210/jc.2013-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leemans CR, Braakhuis BJM, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 27.Lim YC, Koo BS, Choi EC. Bilateral neck node metastasis: a predictor of isolated distant metastasis in patients with oral and oropharyngeal squamous cell carcinoma after primary curative surgery. Laryngoscope. 2007;117(9):1576–1580. doi: 10.1097/MLG.0b013e318093ee2b. [DOI] [PubMed] [Google Scholar]


