Abstract
More than 15 years ago, seminal studies by Dr. E. Leon Barnes and colleagues transformed our understanding of salivary duct carcinoma (SDC) and, in doing so, paved the way for contemporary diagnostic and therapeutic approaches to this aggressive salivary adenocarcinoma. In particular, attention to the apocrine phenotype of SDC and expression of androgen receptor (AR) by immunohistochemistry has improved the diagnostic accuracy and showed how SDC can be reliably distinguished from its morphologic mimics (i.e., other salivary gland carcinomas with high grade transformation, low grade cribriform cystadenocarcinoma, and squamous cell carcinomas involving parotid). Furthermore, the observation that SDC shares AR expression with prostate cancer and apocrine breast cancer foresaw the discovery of common molecular alterations between SDC and these tumor types and draw attention to androgen deprivation therapy for SDC patients.
Keywords: Salivary duct carcinoma, Androgen receptor, Apocrine, High grade transformation
Salivary duct carcinoma (SDC) was first described by Kleinsasser et al. in 1968 [1] and was recognized as a distinct entity by the World Health Organization (WHO) in 1991. In a pair of seminal studies, published in 1998 and 2000, Dr. Barnes and colleagues reported several novel findings regarding the morphology of and androgen receptor (AR) expression in SDC [2, 3]. These reports have led to new diagnostic and therapeutic approaches to SDC. Here we highlight some of the original observations by Dr. Barnes and summarize the progress in our understanding of SDC over the last 15 years.
Observation #1: “Papillary-cribriform areas, necrosis, pleomorphism, apocrine appearance,… and diffuse, strong nuclear immunoreactivity … for androgen receptor… are characteristic of salivary duct carcinoma.”
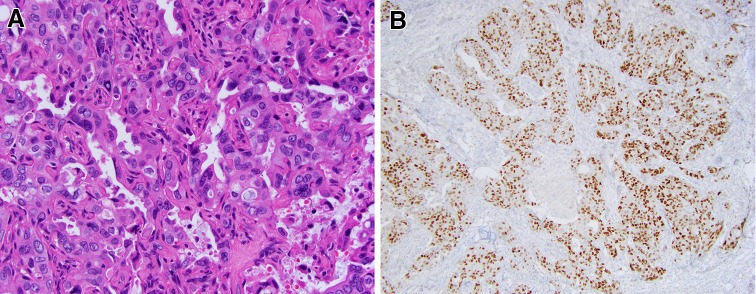
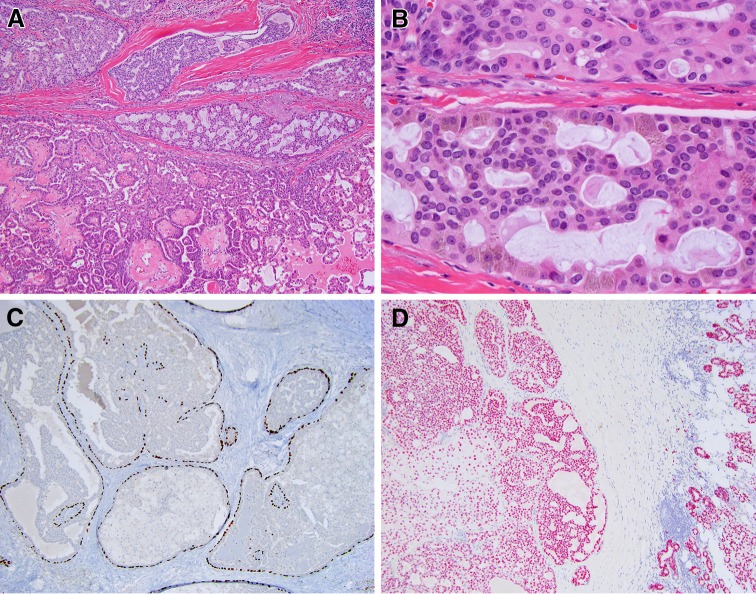
SDC essentially uniformly demonstrates an apocrine phenotype, as indicated by the presence (even if focally) of apical snouts/decapitation secretions (Fig. 1a). Strong diffuse expression of AR in >95% of SDC is a practical tool in workup of high grade salivary carcinomas [4]. The finding of strong AR expression in SDC was an incidental discovery. As mentioned by Fan et al., a pathology resident at the University of Pittsburgh Medical Center was asked to order estrogen receptor (ER) and progesterone receptor (PR) immunostains for a case of SDC. However, in addition to ER and PR, an AR immunostain was inadvertently obtained and turned out to be strongly positive. This discovery led to the initial study of AR expression in SDC by Kapadia and Barnes, which showed that 11 of 12 studied SDC were AR-positive [3]. The follow-up study included additional cases of SDC, all of which were AR-positive [2]. In a recent multi-institutional study, 179 of 183 (97.8%) were AR-positive [4]. Over 85% of SDCs demonstrated easily assessed, unequivocal positive staining with an Allred total score from 6 to 8 (Fig. 1b). Since AR expression correlates with the apocrine phenotype, AR immunoreactivity may serve as one of the measures of diagnostic quality of published cases of SDC. The reported AR positivity varies widely from 56 to 97.8% [4–7]. Stricter diagnostic criteria and attention to technical aspects of AR immunostaining may lead to more consistent AR IHC results in SDC literature [4].
Fig. 1.
Salivary duct carcinoma (SDC) and androgen receptor (AR) expression. a Prototypical SDC with abundant eosinophilic cytoplasm and apocrine type secretion (note apical snout-like projections), H&E, 40x. b Most cases of SDC show strong nuclear AR staining with an Allred score of 6–8. Representative case with Allred score 8, consisting of an intensity score of 3 (strong staining) and a proportion score of 5 (staining in >66% of cells). AR IHC, 100x
The knowledge of nearly uniform AR-positivity in SDC has affected the practice of diagnostic salivary pathology in the following ways.
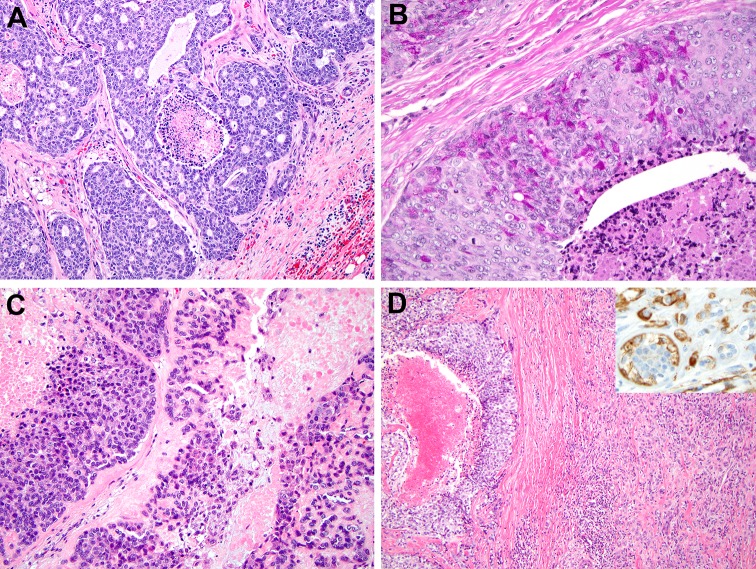
First, it becomes increasingly clear that before a high grade non-apocrine/AR-negative salivary carcinoma is accepted as an SDC, additional sampling and search for a more conventional morphology typical of other types of salivary tumors is warranted. It was recently highlighted that areas of high-grade transformation (HGT) within acinic cell carcinoma, myoepithelial carcinoma, adenoid cystic carcinoma (AdCC), and epithelial-myoepithelial carcinoma (EMCA) commonly show comedonecrosis mimicking “non-apocrine/AR-negative” SDC (Fig. 2) [4]. Importantly, in salivary gland carcinomas with HGT, a component of conventional morphology is typically present and is the key to diagnosis. For example, conventional areas of EMCA or AdCC will show a biphasic cellular population (i.e., inner ductal cells and outer basal/myoepithelial/p63/p40 positive cells).
Fig. 2.
Distinguishing high-grade salivary tumors with comedo-type necrosis from salivary duct carcinoma (SDC). a Adenoid cystic carcinoma (AdCC) with high-grade transformation (HGT). Basophilic appearance, cribriform growth pattern, and pseudo-lumena filled with basement membrane-like material raise the possibility of an AdCC. 14 months after the initial diagnosis of SDC, the patient developed pulmonary metastases with unequivocal, classic AdCC morphology. H&E, 100x. b Acinic cell carcinoma with HGT. A minority of cells surrounding foci of necrosis have numerous cytoplasmic zymogen granules, best appreciated on PASD, 400x. c High grade myoepithelial carcinoma. Solid sheets of neoplastic cells do not form glands and are accompanied by droplets of hyalinized material. Nuclear palisading was absent. H&E, 200x. d Epithelial-myoepithelial carcinoma with HGT: an area with comedo-type necrosis is accompanied by a better-differentiated component with dual cell population: smooth muscle actin immunohistochemistry highlights the outer layer of myoepithelial cells (right upper corner inset, 200x). H&E, 100x.
Modified from [4] with permission
Metastatic squamous cell carcinoma (SCC) is the second most common mimic of “non-apocrine/AR-negative SDC” [4]. While primary SCC of salivary glands are exceedingly rare, cutaneous or mucosal SCC may involve the parotid gland by direct extension or extranodal spread following the initial metastasis to intraparotid lymph nodes. Even when history of prior SCC is not available, SDC can be reliably distinguished from SCC (especially non-keratinizing) with two immunohistochemical stains: AR and p63. Nearly all SDCs are AR-positive, while all SCCs are p63-positive.
As outlined by Dr. Barnes, other rare salivary carcinomas, such as oncocytic carcinoma and cystadenocarcinomas, not otherwise specified, may enter the differential diagnosis of SDC [2, 3]. Oncocytic carcinoma can be distinguished from SDC by its more granular cytoplasm and high content of mitochondria, seen ultrastructurally or on phosphotungstic acid hematoxylin stain. Furthermore, oncocytic carcinoma and cystadenocarcinoma lack the prominent comedonecrosis and apocrine differentiation.
It appears that variant morphologies of SDC represent minimal diagnostic challenges as all histologic variants are accompanied by a conventional apocrine component [4]. Several variant morphologies of SDC have been described: micropapillary, [8] sarcomatoid, [9, 10] mucin-rich, [11] and basal-like [4, 6, 12].
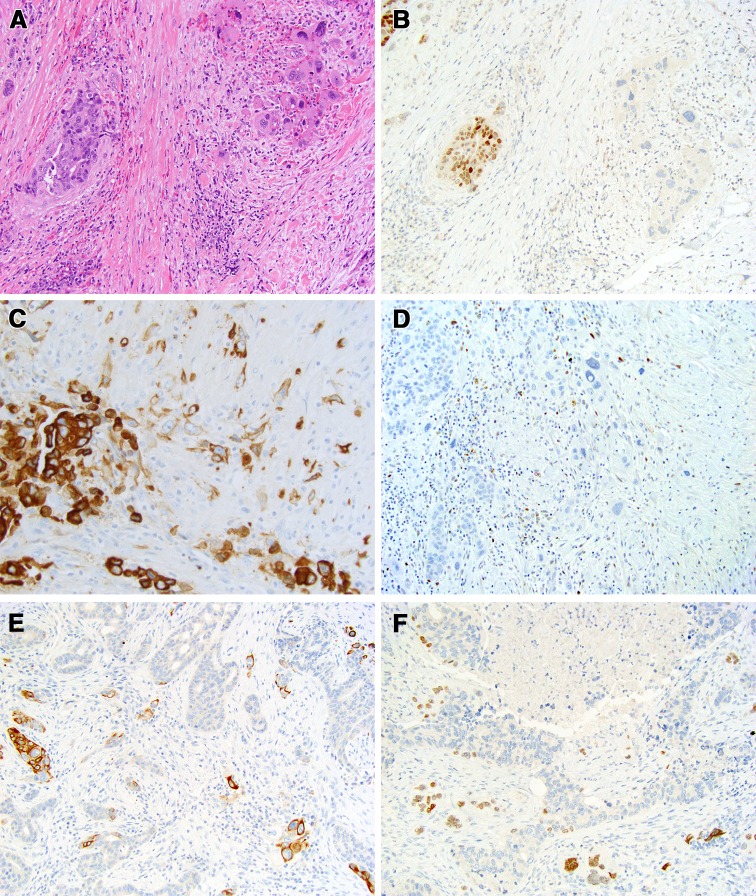
Of these, sarcomatoid/anaplastic (Fig. 3), mucin-rich, and basal-like (Fig. 4) are more likely to have non-apocrine/AR-negative areas. Anaplastic transformation was identified in 3 of 187 cases of SDC [4], one of which has been previously described in greater details [13] and is further illustrated in Fig. 3. SDC with anaplastic change are characterized by enlarged, bizarre, hyperchromatic nuclei, atypical mitotic figures, and spindled cells (Fig. 3). The basal-like variant of SDC remains the most diagnostically challenging and the difficulties of identifying a basal-like phenotype in SDC have been previously recognized [4]. Like all other SDC, basal-like SDC still had a small focus of conventional apocrine AR-positive component. In a basal-like SDC, apocrine morphology may be limited to a small area of pre-existing pleomorphic adenoma (Fig. 4), which may be identified only after extensive sampling. Anecdotally, it was shown that to identify pre-existing pleomorphic adenoma one might have to examine up to a 100 tissue sections [14].
Fig. 3.
Salivary duct carcinoma de novo (with known intact PLAG1 and HMGA2) with anaplastic change and HRAS, PIK3CA, and TP53 deletion/frame shift mutations [26]. a About 90% of salivary duct carcinoma was represented by conventional component with cribriform and solid growth (left half of the image), while the minor component was represented by more bizarre discohesive cells with more prominent nuclear pleomorphism and hyperchromasia (right), H&E, 200x. b Androgen receptor expression is preserved in the conventional component (left) and lost in the anaplastic component (right), immunohistochemistry, 200x. c Cytokeratin 7 is strongly positive in the conventional more cohesive component (left) and is weaker in more discohesive and spindle single cell component (center), immunohistochemistry, 400x. d The presence of TP53 deletion and frame shift mutation was reported previously. [26] p53 immunohistochemistry shows that p53 is lost (extreme negative pattern) in both conventional (left) and anaplastic (center) components, 200x. e Cytokeratin 5/6 highlights small clusters of larger cells and single larger cells, immunohistochemistry, 200x. Occasional smaller cells at the periphery of the lobules of conventional component are basal/myoepithelial cells. f Similarly to cytokeratin 5/6, p63 immunohistochemistry highlights smaller basal cells at the periphery of conventional component and larger bizarre cells of the anaplastic component, 200x
Fig. 4.
Basal-like, predominantly non-apocrine invasive salivary duct carcinoma (SDC) with minor apocrine androgen receptor (AR) positive in situ component in a hyalinized nodule (HN). a Invasive SDC (left), capsule, and hyalinized nodule (right upper corner), H&E, 40x. b A 0.5 cm SDC in situ inside a HN. The upper one-third shows eosinophilic neoplastic cells with apical snouts, H&E, 400x. In the middle, immunohistochemical stain for p63 highlights basal cells, IHC, 400x. AR IHC is positive, lower one-third, IHC, 400x. c The entire invasive component was represented by solid proliferation of basophilic, AR-negative neoplastic cells with comedonecrosis, H&E, 200x. d A randomly distributed subset of neoplastic cells in the invasive component (left half) was highlighted by p63, IHC, 200x. Of note, compared to the basal/myoepithelial cells in the adjacent normal parotid tissue (right half), the p63-positive neoplastic cells (left) have larger and more irregular nuclei.
Modified from [4] with permission
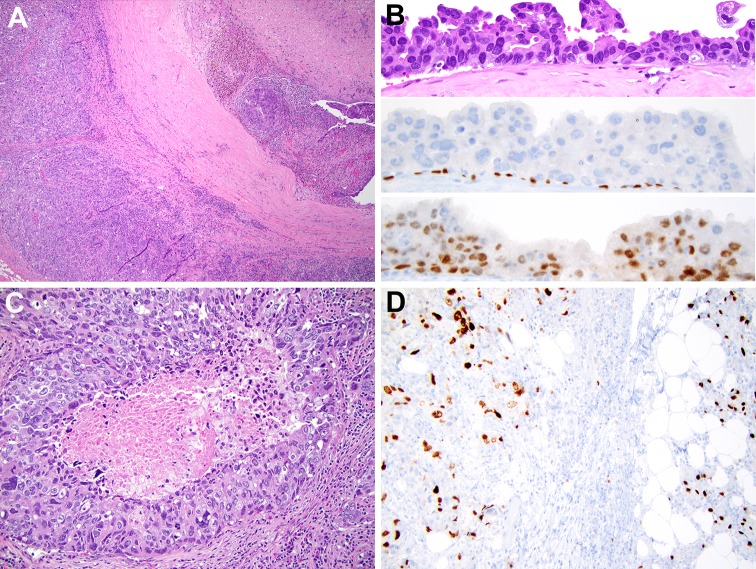
Second, while there may be a morphological overlap between low grade cribriform cystadenocarcinoma (LGCCA) and SDC, when ancillary studies are accounted for (including AR positivity), it becomes clear that there is little, if any, relationship between SDC and LGCCA (Table 1; Fig. 5) [15]. For a long time one of the synonyms for LGCCA was “low grade salivary duct carcinoma” [16, 17], implying a connection between LGCCA and conventional SDC (a high grade tumor by definition). This misleading synonym will be replaced in the upcoming WHO book on head and neck tumors with “intraductal carcinoma”, a descriptor that reflects the predominantly in situ nature of LGCCA.
Table 1.
Comparison of salivary duct carcinoma and low grade cribriform cystadenocarcinoma
| Salivary duct carcinoma | LGCCA | |
|---|---|---|
| Morphology | High grade cytology, invasive growth | Low grade cytology, intraductal/in situ |
| Androgen receptor, IHC | Positive | Negative |
| S100, IHC | Negative | Positive |
| SOX-10, IHC | Negative | Positive |
| p63 or p40, IHC | Negative, highlights small areas of intraductal involvement | Highlights extensive intraductal/in situ component |
| Molecular alterations | PLAG1 or HMGA2 rearrangements, PIK3CA, HRAS, p53, ERBB2 alterations [26] | Occasional RET rearrangement [15] |
LGCCA low grade cribriform cystadenocarcinoma, IHC immunohistochemistry
Fig. 5.
Low grade cribriform cystadenocarcinoma, LGCCA. a Combination of cribriform and micropapillary growth. H&E, 100x. b Low grade cytology and cytoplasmic brown lipofuscin-like pigment, H&E, 600x. c The predominant in situ/intraductal component is highlighted by p63, immunohistochemistry, 10x. Parenthetically, LGCCA recently emerged as one of the more common mimickers of mammary analogue secretory carcinoma (MASC). Extensive in situ component is characteristic of LGCCA and helps to distinguish LGCCA from MASC. d Positive SOX-10 in LGCCA, immunohistochemistry, 10x. The right one-fourth of the image shows SOX-10 expression in normal salivary acini and intercalated ducts
Observation #2: “The scale and magnitude of the androgen receptor expression in salivary duct carcinoma approaches that seen in prostate carcinoma. By contrast, androgen receptor expression in breast carcinoma remains sporadic, except for apocrine breast carcinoma”.
While the discovery of AR expression in SDC was incidental, the idea for androgen deprivation therapy (ADT) was based on the therapeutic utility of hormonal manipulation in breast cancer. One of the earliest reports on AR expression in breast carcinomas was published in 1993 [18].
Extrapolating progress from breast oncology to salivary pathology has been a commonly used approach to advance our knowledge of SDC. More than 15 years ago Dr. Barnes implied that SDC resembles just one type of breast carcinoma (i.e., luminal AR-positive, first described as “molecular apocrine” type) [19–22]. Based on expression of ER, PR, and ERBB2, breast carcinomas are now categorized into several groups, including triple-negative breast carcinomas (TNBC; ER-/PR-/ERBB2-) [20, 22]. TNBC itself is a very diverse group of carcinomas [23]. Within the TNBC category, “luminal AR-positive/molecular apocrine” type [19] represents one of the better-defined subtypes with a high prevalence of TP53, PIK3CA, and PTEN mutations [22–24].
Currently, based on apocrine morphology, ER-/PR-/AR + immunoprofile, prevalence and type of mutations, and gene expression pattern apocrine SDC appears to resemble one subtype of breast carcinoma—“luminal AR-positive/molecular apocrine” [4, 25–27]. The practical value of such similarity is uncertain, as significant differences between the “luminal AR-positive/molecular apocrine” type of breast carcinomas and SDC remain (e.g., HMGA2 or PLAG1 rearrangements in a subset of SDCs ex pleomorphic adenoma) [26].
Observation #3: “This hormonal profile (i.e., ER-/PR-/AR +) suggests that salivary duct carcinoma… is immunophenotypically more related to prostatic carcinoma. The strong, diffuse expression of androgen receptor in salivary duct carcinoma raises the possibility that antiandrogen therapy might have a role in the management of patients with disseminated disease”.
Apparently, Dr. Barnes has identified the first inadvertent attempt to treat a high grade salivary adenocarcinoma with ADT reported in the literature [28, 29]. Although provided photomicrographs and histologic description are most consistent with SDC, the authors did not actually use the term “salivary duct carcinoma”, complicating the literature search. In the early 1990s, a 66-year-old man developed a 5 cm retro-auricular mass believed to be an enlarged lymph node. A 1 cm incisional biopsy revealed an adenocarcinoma positive for prostate specific antigen (PSA) and prostatic acid phosphatase (PAP) by immunohistochemical staining. Before Dr. Barnes showed that PSA and PAP may be positive in a number of SDCs, [2] the PAS+/PAP + immunoprofile was believed to be strongly indicative of prostatic adenocarcinoma. For this reason, the patient was treated with an anti-testosterone (goserelin), despite the fact that there was no clinical evidence of primary prostatic carcinoma (benign prostate biopsies, negative bone scan, and normal PSA serum level). The retro-auricular tumor regressed in response to anti-testosterone therapy.
Currently, ADT in patients with SDC is being actively studied [30] and several similarities and differences between AR pathway activation in SDC and prostate cancer have been delineated [31]. For instance, a subset of hormone therapy-naïve SDCs harbor oncogenic AR splice variants (including AR-V7), which have been associated with resistance to ADT in advanced prostate cancer. In contrast, no activating AR mutations or AR gene amplifications have been identified in hormone therapy-naïve SDC. In the future, SDC patients may be selected for ADT clinical trials based on AR isoforms [31].
In summary, more than 15 years ago, Dr. Barnes and colleagues described novel features of SDC that affect the current practice of diagnostic salivary gland pathology and predicted some of the more recent molecular and therapeutic developments. Recognition of the apocrine nature of SDC and almost uniform AR expression by IHC has improved diagnostic accuracy and “purity” of SDC. Indeed, AR IHC remains one of the most reliable ways to distinguish SDC from its morphologic mimics (i.e., LGCCA, other salivary gland carcinomas with HGT, and metastatic non-keratinizing SCC). By comparing the morphology and immunoprofile of SDC to breast and prostate adenocarcinomas, Dr. Barnes foresaw that SDC resembles a specific subtype of breast cancer (“luminal AR-positive/molecular apocrine”) and may share the challenges and successes of hormonal therapy for prostate adenocarcinoma.
Acknowledgements
The authors wish to thank members of the Developmental Laboratory of the Department of Pathology, University of Pittsburgh, for excellent technical support, and Robyn Roche for outstanding secretarial support.
Compliance with Ethical Standards
Conflict of interest
None.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Footnotes
Proceedings of the 2017 North American Society of Head and Neck Pathology Companion Meeting (San Antonio, TX).
References
- 1.Kleinsasser O, Klein HJ, Hubner G. Salivary duct carcinoma. A group of salivary gland tumors analogous to mammary duct carcinoma. Arch Klin Exp Ohren Nasen Kehlkopfheilkd. 1968;192(1):100–105. doi: 10.1007/BF00301495. [DOI] [PubMed] [Google Scholar]
- 2.Fan CY, Wang J, Barnes EL. Expression of androgen receptor and prostatic specific markers in salivary duct carcinoma: an immunohistochemical analysis of 13 cases and review of the literature. Am J Surg Pathol. 2000;24(4):579–586. doi: 10.1097/00000478-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Kapadia SB, Barnes L. Expression of androgen receptor, gross cystic disease fluid protein, and CD44 in salivary duct carcinoma. Mod Pathol. 1998;11(11):1033–1038. [PubMed] [Google Scholar]
- 4.Williams L, Thompson LD, Seethala RR, Weinreb I, Assaad AM, Tuluc M, et al. Salivary duct carcinoma: the predominance of apocrine morphology, prevalence of histologic variants, and androgen receptor expression. Am J Surg Pathol. 2015;39(5):705–713. doi: 10.1097/PAS.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 5.Williams MD, Roberts D, Blumenschein GR, Jr, Temam S, Kies MS, Rosenthal DI, et al. Differential expression of hormonal and growth factor receptors in salivary duct carcinomas: biologic significance and potential role in therapeutic stratification of patients. Am J Surg Pathol. 2007;31(11):1645–1652. doi: 10.1097/PAS.0b013e3180caa099. [DOI] [PubMed] [Google Scholar]
- 6.Di Palma S, Simpson RH, Marchio C, Skalova A, Ungari M, Sandison A, et al. Salivary duct carcinomas can be classified into luminal androgen receptor-positive, HER2 and basal-like phenotypes*. Histopathology. 2012 doi: 10.1111/j.1365-2559.2012.04252.x. [DOI] [PubMed] [Google Scholar]
- 7.Ko YH, Roh JH, Son YI, Chung MK, Jang JY, Byun H, et al. Expression of mitotic checkpoint proteins BUB1B and MAD2L1 in salivary duct carcinomas. J Oral Pathol Med. 2010;39(4):349–355. doi: 10.1111/j.1600-0714.2009.00835.x. [DOI] [PubMed] [Google Scholar]
- 8.Nagao T, Gaffey TA, Visscher DW, Kay PA, Minato H, Serizawa H, et al. Invasive micropapillary salivary duct carcinoma: a distinct histologic variant with biologic significance. Am J Surg Pathol. 2004;28(3):319–326. doi: 10.1097/00000478-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Henley JD, Seo IS, Dayan D, Gnepp DR. Sarcomatoid salivary duct carcinoma of the parotid gland. Hum Pathol. 2000;31(2):208–213. doi: 10.1016/S0046-8177(00)80221-X. [DOI] [PubMed] [Google Scholar]
- 10.Nagao T, Gaffey TA, Serizawa H, Iwaya K, Watanabe A, Yoshida T, et al. Sarcomatoid variant of salivary duct carcinoma: clinicopathologic and immunohistochemical study of eight cases with review of the literature. Am J Surg Pathol. 2004;122(2):222–231. doi: 10.1309/5J40-08QR-Y1HW-W5W4. [DOI] [PubMed] [Google Scholar]
- 11.Simpson RH, Prasad AR, Lewis JE, Skalova A, David L. Mucin-rich variant of salivary duct carcinoma: a clinicopathologic and immunohistochemical study of four cases. Am J Surg Pathol. 2003;27(8):1070–1079. doi: 10.1097/00000478-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Hungermann D, Roeser K, Buerger H, Jakel T, Loning T, Herbst H. Salivary duct carcinoma. Pathologe. 2005;26(5):353–358. doi: 10.1007/s00292-005-0775-0. [DOI] [PubMed] [Google Scholar]
- 13.Griffith CC, Seethala RR, Luvison A, Miller M, Chiosea SI. PIK3CA mutations and PTEN loss in salivary duct carcinomas. Am J Surg Pathol. 2013;37(8):1201–1207. doi: 10.1097/PAS.0b013e3182880d5a. [DOI] [PubMed] [Google Scholar]
- 14.Foote FW, Jr, Frazell EL. Tumors of the major salivary glands. Cancer. 1953;6(6):1065–1133. doi: 10.1002/1097-0142(195311)6:6<1065::AID-CNCR2820060602>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Weinreb I, Seethala RR, Chiosea SI, Perez-Ordonez B, Hyrcza M, et Zhang L. Molecular analysis of low-grade cribriform cystadenocarcinoma & related in-situ and invasive carcinomas. New York: Nature Publishing Group; 2016. [Google Scholar]
- 16.Brandwein-Gensler M, Hille J, Wang BY, Urken M, Gordon R, Wang LJ, et al. Low-grade salivary duct carcinoma: description of 16 cases. Am J Surg Pathol. 2004;28(8):1040–1044. doi: 10.1097/01.pas.0000128662.66321.be. [DOI] [PubMed] [Google Scholar]
- 17.Delgado R, Klimstra D, Albores-Saavedra J. Low grade salivary duct carcinoma. A distinctive variant with a low grade histology and a predominant intraductal growth pattern. Cancer. 1996;78(5):958–967. doi: 10.1002/(SICI)1097-0142(19960901)78:5<958::AID-CNCR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Isola JJ. Immunohistochemical demonstration of androgen receptor in breast cancer and its relationship to other prognostic factors. J Pathol. 1993;170(1):31–35. doi: 10.1002/path.1711700106. [DOI] [PubMed] [Google Scholar]
- 19.Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, Larsimont D, et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24(29):4660–4671. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- 20.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 21.Hirshfield KM, Ganesan S. Triple-negative breast cancer: molecular subtypes and targeted therapy. Curr Opin Obstet Gynecol. 2014;26(1):34–40. doi: 10.1097/GCO.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner NC, Reis-Filho JS. Tackling the diversity of triple-negative breast cancer. Clin Cancer Res. 2013;19(23):6380–6388. doi: 10.1158/1078-0432.CCR-13-0915. [DOI] [PubMed] [Google Scholar]
- 24.Dumay A, Feugeas JP, Wittmer E, Lehmann-Che J, Bertheau P, Espie M, et al. Distinct tumor protein p53 mutants in breast cancer subgroups. Int J Cancer. 2013;132(5):1227–1231. doi: 10.1002/ijc.27767. [DOI] [PubMed] [Google Scholar]
- 25.Dalin MG, Desrichard A, Katabi N, Makarov V, Walsh LA, Lee KW, et al. Comprehensive Molecular Characterization of Salivary Duct Carcinoma Reveals Actionable Targets and Similarity to Apocrine Breast Cancer. Clin Cancer Res. 2016;22(18):4623–4633. doi: 10.1158/1078-0432.CCR-16-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiosea SI, Thompson LD, Weinreb I, Bauman JE, Mahaffey AM, Miller C, et al. Subsets of salivary duct carcinoma defined by morphologic evidence of pleomorphic adenoma, PLAG1 or HMGA2 rearrangements, and common genetic alterations. Cancer. 2016;122(20):3136–3144. doi: 10.1002/cncr.30179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiosea SI, Williams L, Griffith CC, Thompson LD, Weinreb I, Bauman JE, et al. Molecular characterization of apocrine salivary duct carcinoma. Am J Surg Pathol. 2015;39(6):744–752. doi: 10.1097/PAS.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 28.van der Hulst RW, van Krieken JH, van der Kwast TH, Gerritsen JJ. Baatenburg de Jong RJ, Lycklama a Nijeholt AA et al. Partial remission of parotid gland carcinoma after goserelin. Lancet. 1994;344(8925):817. doi: 10.1016/S0140-6736(94)92372-8. [DOI] [PubMed] [Google Scholar]
- 29.van Krieken JH. Prostate marker immunoreactivity in salivary gland neoplasms. A rare pitfall in immunohistochemistry. Am J Surg Pathol. 1993;17(4):410–414. doi: 10.1097/00000478-199304000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Locati LD, Perrone F, Cortelazzi B, Lo Vullo S, Bossi P, Dagrada G, et al . Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck. 2014 doi: 10.1002/hed.23940. [DOI] [PubMed] [Google Scholar]
- 31.Mitani Y, Rao PH, Maity SN, Lee YC, Ferrarotto R, Post JC, et al. Alterations associated with androgen receptor gene activation in salivary duct carcinoma of both sexes: potential therapeutic ramifications. Clin Cancer Res. 2014;20(24):6570–6581. doi: 10.1158/1078-0432.CCR-14-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]