Abstract
Sinonasal (Schneiderian) papillomas are benign neoplasms that arise in the sinonasal tract. Since their initial descriptions, sinonasal papillomas have triggered debate regarding their classification, etiology, rate or predictors of malignant transformation, and other issues. While significant strides have been made in recent years, there are still aspects of sinonasal papillomas that remain unclear even now. This review will serve to update the practicing pathologist on the current understanding of sinonasal papillomas.
Keywords: Schneiderian papilloma, Sinonasal papilloma, Inverted papilloma, Fungiform papilloma, Oncocytic papilloma, Cylindrical cell papilloma
Introduction
Papillomas, defined simply as finger-like epithelial proliferations with fibrovascular cores, can arise essentially anywhere in the body there is epithelium. Sinonasal papillomas, also known as Schneiderian papillomas, are uncommon, benign epithelial neoplasms that arise almost exclusively from the ectodermally-derived pseudostratified ciliated (or Schneiderian) epithelium that lines the nasal cavity and sinonasal tract (Fig. 1). From their initial descriptions in the 1800s, there has been debate about nearly all aspects of these tumors, from whether or not they are even neoplastic to their etiology to their histologic classification and many others [1]. This manuscript will review some of the history of these lesions in addition to describing the current knowledge of the nature of sinonasal papillomas.
Fig. 1.
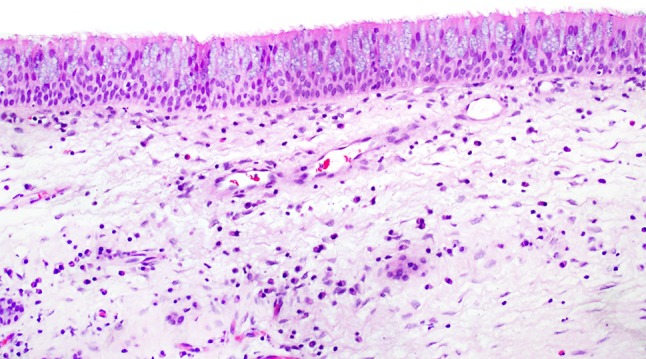
Normal sinonasal (Schneiderian) mucosa. When discussing sinonasal papillomas, it is useful to keep the appearance (particularly the thickness) of the normal sinonasal epithelium in mind
Terminology
The lesion now regarded as sinonasal papilloma was probably first described by Ward in 1854 then by Bilroth in 1855 [1–3]. Subsequent reports and small series followed in the late 1800s, with most authors emphasizing the potential of these sinonasal papillomas to recur and some recognizing cases of carcinomatous transformation [3, 4].
In the first Armed Forces Institute of Pathology (AFIP) Fascicle on Tumors of the Upper Respiratory Tract and Ear published in 1964, the lesion was referred to (somewhat redundantly) as “epithelial papilloma,” with those showing an inverted growth pattern regarded as a subtype but not a separate entity [3]. In 1971, Hyams provided much-needed clarity with his exhaustive review of 315 cases from the AFIP archives [2]. Up to that point, sinonasal papillomas had been variably referred to by many names including papilloma durum, inverted papilloma, Schneiderian papilloma, transitional cell papilloma, papillary sinusitis, Ewing’s papilloma, Ringertz papilloma, papillary adenoma, and cylindrical cell papilloma. In this landmark paper, Hyams classified the papillomas histologically as inverted, fungiform, and cylindrical cell, carefully describing the histologic criteria of each that are still used today, while excluding cutaneous papillomas of the nasal vestibule, inflammatory polyps, and all other polypoid nasal masses [2]. Barnes and Bedetti expanded on Hyams’ work by providing additional descriptions of cylindrical cell papilloma. The authors determined that this subtype was truly oncocytic (i.e., possessed abundant mitochondria resulting in a granular eosinophilic cytoplasm) and thus introduced oncocytic papilloma as a synonym of cylindrical cell papilloma [5].
Interestingly, the second edition of the AFIP Fascicle by Hyams, Batsakis, and Michaels from 1986 reverted back to referring to all subtypes simply as sinonasal papilloma [6]. By the third edition, however, the inverted, fungiform, and oncocytic subtypes were included [7]. The fourth edition of the AFIP fascicle and the 2005 World Health Organization (WHO) classification utilized the terms inverted, oncocytic, and “exophytic” (instead of fungiform) Schneiderian papilloma [8, 9]. Finally, the upcoming 2017 WHO classification will omit the eponymous “Schneiderian” designation in favor of simply sinonasal papilloma, with the inverted, oncocytic, and exophytic subtypes included [10–12]. This is the terminology that will be used in this review. Despite some similarities, the three subtypes of sinonasal papilloma are distinct in many ways and a precise diagnosis should be given when possible.
Discussion
Clinical Features
Inverted sinonasal papilloma (ISP) is the most common form of sinonasal papilloma, followed by exophytic sinonasal papillomas (ESP) and, finally, oncocytic sinonasal papilloma (OSP), the rarest type [1, 10–12]. ISPs and OSPs most commonly arise in patients in their 5th or 6th decades, while ESPs arise in patients who are slightly younger (3rd to 5th decades) [10–12]. However, all three types can affect patients of any age. ISP and ESP are seen predominantly in men (2–3:1 male to female radio for ISP, 10:1 for ESP), while OSP has no sex predilection [10–12]. ISPs and OSPs characteristically arise in the lateral nasal wall and/or paranasal sinuses, while ESPs are almost always seen in the nasal septum (especially lower anterior) [1, 10–12]. Bilateral papillomas of any type are uncommon. ISPs rarely can involve non-nasal sites such as ear, nasopharynx, lacrimal sac, and/or oropharynx, but these sites are usually involved secondarily from sinonasal tract disease [10]. All sinonasal papillomas typically present non-specifically as nasal obstruction, epistaxis, or nasal discharge with or without an evident mass. Radiologically, all sinonasal papillomas appear as a soft-tissue density, with a “septate, striated” appearance classically seen on magnetic resonance imaging [10–12].
Pathologic Findings (Table 1)
Table 1.
Summary of the distinguishing features of the three types of sinonasal papilloma
| Location | Histologic features | Rate of malignant transformation | Molecular alterations | Association with HPV | |
|---|---|---|---|---|---|
| Inverted sinonasal papilloma (ISP) | Lateral nasal wall and paranasal sinuses | Thickened epithelium with scattered mucous cells or cysts, and intraepithelial neutrophils with microabscesses Epithelium is variably squamous, squamoid/transitional, or ciliated columnar Prominent downward growth of rounded, elongated, and anastomosing epithelial nests from the surface epithelium |
5–15% | EGFR mutations | Unclear association. A minority have been reported to harbor low-risk or high risk HPV types, with a possible increase in those with dysplasia or carcinomatous transformation |
| Exophytic sinonasal papilloma (ESP) | Nasal septum | Thickened epithelium with scattered mucous cells or cysts, and intraepithelial neutrophils with microabscesses Epithelium is usually squamous Broad, branching fronds of epithelium surrounding fibrovascular cores Koilocytic cells may be seen |
Close to 0 | Unknown | Most are caused by low-risk HPV types |
| Oncocytic sinonasal papilloma (OSP) | Lateral nasal wall and paranasal sinuses | Thickened epithelium with scattered mucous cells or cysts, and intraepithelial neutrophils with microabscesses Both inverted and exophytic growth Epithelium is pseudostratified columnar epithelial cells, which contain abundant, granular eosinophilic cytoplasm and small, hyperchromatic nuclei |
4–17% | KRAS mutations | No association |
Grossly, ESPs have a verrucoid, cauliflower-like appearance similar to papillomas of other organs. In contrast, ISPs and OSPs appear as firm, tan or reddish polyps that, unlike inflammatory polyps, do not transilluminate. ISP and OSP may demonstrate a “cerebriform” gross appearance [10–13] (Fig. 2).
Fig. 2.
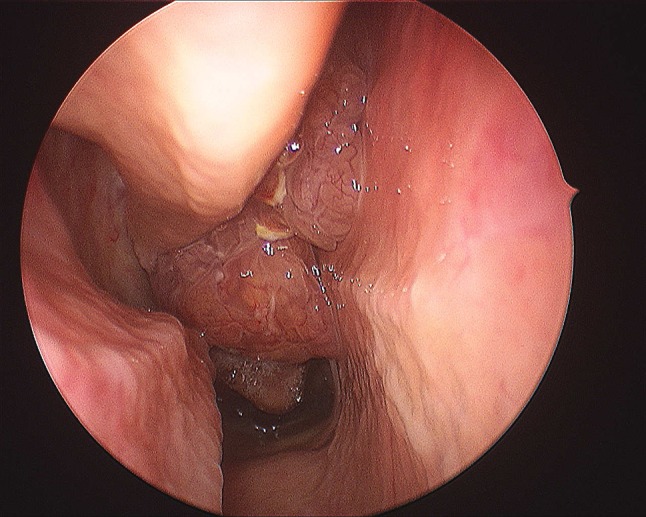
Inverted sinonasal papillomas appear clinically as a lobulated mucosal mass with a cerebriform appearance and prominent vasculature.
(Courtesy of Dr. Andrew Lane)
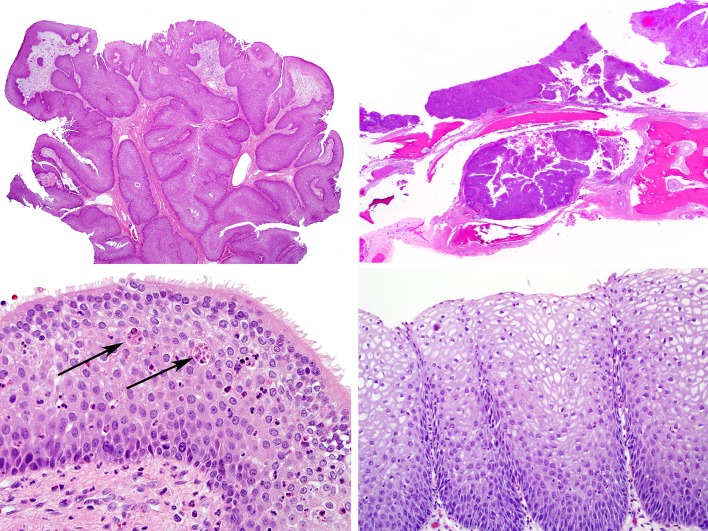
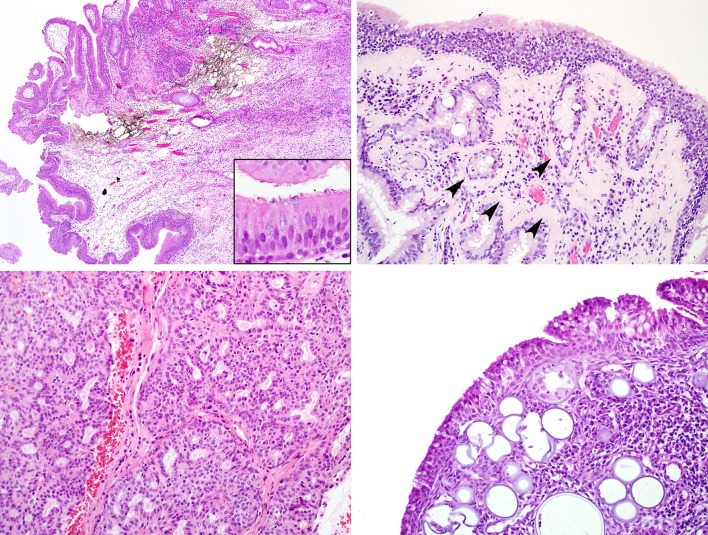
At the histologic level, all three types of sinonasal papilloma share the features of epithelial thickening, scattered mucous cells or cysts, and intraepithelial neutrophils with microabscesses. In addition to these features, ISP is characterized by prominent downward growth of rounded, elongated, and anastomosing epithelial nests from the surface epithelium, surrounded by an intact basement membrane.(Fig. 3a) This downward growth can occasionally appear locally aggressive (e.g., eroding bone histologically or radiographically), a finding that should not be regarded as malignant transformation by itself (Fig. 3b). The ISP epithelium may be variably squamous, squamoid/transitional, or ciliated columnar (Fig. 3c–d). ISP is commonly accompanied by background stromal changes of inflammatory polyp such as submucosal edema and chronic inflammation. Typically, there are few nearby submucosal seromucinous glands. Occasionally, ISPs may be seen in association with sinonasal hamartomas. ISP occasionally (in approximately 5–15%) undergoes malignant transformation, which is synchronous in approximately 70% of cases [1, 14–16]. There are no known histologic features that are predictive of subsequent malignant transformation. Most cases of carcinoma ex-sinonasal papilloma are squamous cell carcinomas, but rarely other tumor types such as sinonasal undifferentiated carcinoma, verrucous carcinoma, or mucoepidermoid carcinoma have been described [1, 14–16] (Fig. 4a). Carcinomas are histologically recognized by their malignant cellular features, with nuclear pleomorphism or anaplasia, increased mitotic rates, atypical mitoses, and necrosis typical of sinonasal carcinomas. Typically, the transition between the ISP and carcinoma is very abrupt [2] (Fig. 4b). Given its inverted nature, it can be difficult to determine whether a carcinoma arising within an ISP is truly invasive. Irregular nests with a desmoplastic stromal reaction point to true invasion, while lymphovascular or perineural invasion are diagnostic. Though strict criteria are lacking, atypical cellular changes that fall short of overt carcinoma are often regarded as dysplasia.
Fig. 3.
Inverted sinonasal papilloma. At low power, the inverted growth pattern is apparent, with broad interconnecting ribbons of epithelium in the submucosa. Seromucinous glands are not seen. a Inverted sinonasal papillomas can exhibit locally aggressive behavior, here eroding into bone. This, by itself, should not be interpreted as carcinomatous transformation. b The classic epithelium of inverted sinonasal papilloma is thickened and permeated by neutrophils, often with microabscesses (arrows). In this case, the epithelium is transitional with a row of retained ciliated columnar epithelium superficially (c). This example of inverted sinonasal papilloma has more mature, glycogenated squamous epithelium with fewer transmigrating neutrophils (d)
Fig. 4.
Carcinoma ex-inverted sinonasal papilloma. In this unusual example, an invasive mucin-producing adenosquamous carcinoma (bottom) is arising in association with an inverted sinonasal papilloma (top). This figure underscores that while squamous cell carcinomas are most common, unusual carcinomas can arise from inverted sinonasal papillomas. a Squamous cell carcinoma ex-inverted sinonasal papilloma (top) exhibits increased nuclear pleomorphism, prominent nucleoli and increased mitotic activity when compared to the inverted sinonasal papilloma (bottom). The papilloma component exhibits increased cellular atypia in the basal layer, a change that may be regarded as squamous dysplasia (b)
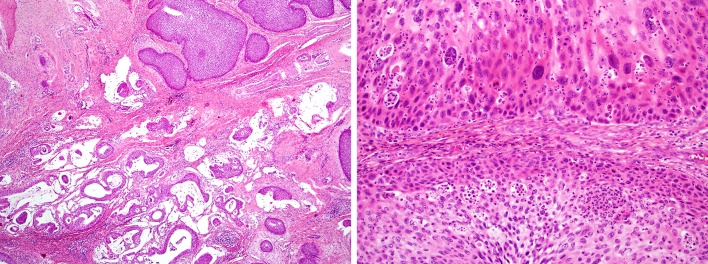
ESP histologically resembles squamous papillomas of other organs such as larynx, with broad, branching fronds of epithelium surrounding fibrovascular cores.(Fig. 5a) Like ISP, the epithelium of ESP is variably squamous, transitional, and columnar, but it is most often squamous (Fig. 5b). ESP may also exhibit viral (koilocytic) changes of the squamous epithelium, including nuclear enlargement, hyperchromasia, irregular, “crinkly” nuclear membranes, multinucleation, and perinuclear “haloes.”(Fig. 5b) ESP only very rarely undergoes malignant transformation [12].
Fig. 5.
Exophytic sinonasal papilloma consists of finger-like growths of squamous epithelium lining delicate fibrovascular cores (a). The lining epithelium of exophytic sinonasal papilloma resembles that of inverted sinonasal papilloma (i.e., squamous with intraepithelial neutrophils) but also demonstrates koilocytic changes superficially. These cells are typically positive for low-risk HPV types by in situ hybridization (inset) (b)
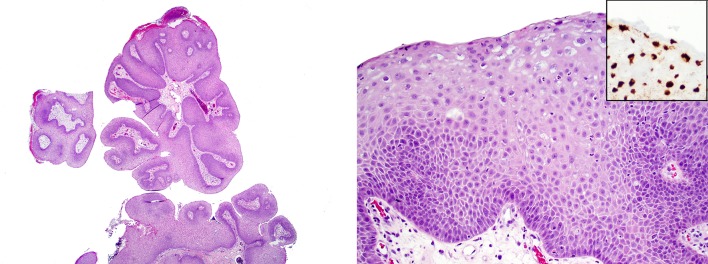
At low power, OSP typically demonstrates both inverted and exophytic growth (Fig. 6a). It is distinguished from the other subtypes on the basis of its pseudostratified columnar epithelial cells, which contain abundant, granular eosinophilic cytoplasm and small, hyperchromatic nuclei (Fig. 6b). Another characteristic feature is the frequent presence of numerous intraepithelial microcysts (Fig. 6c). OSP may on occasion (4–17%) undergo malignant transformation, typically squamous cell carcinoma but rarely other tumor types such as sinonasal undifferentiated carcinoma or small cell carcinoma [11] (Fig. 6d).
Fig. 6.
Oncocytic sinonasal papilloma at low power demonstrates both inverted and exophytic growth (a). The lining epithelium is oncocytic, with cells that have abundant granular eosinophilic cytoplasm and small round nuclei. Like other types of sinonasal papilloma, transmigrating neutrophils and microabscesses are commonly seen (b). Oncocytic sinonasal papillomas frequently exhibit numerous pink microcysts in the oncocytic epithelium. (c) This example of carcinoma ex-oncocytic sinonasal papilloma has a very abrupt transition (arrow) between benign (right) and malignant (left) epithelium (d)
Although each subtype of sinonasal papilloma is typically seen in its “pure” form, on occasion a sinonasal papilloma may exhibit mixed features, a phenomenon that is not extensively reported in the literature. In my experience, this is most commonly encountered with a sinonasal papilloma that demonstrates both inverted and exophytic features. In this scenario, it is my practice to diagnose this tumor as an inverted sinonasal papilloma, given the increased risk of subsequent malignant transformation of the inverted subtype and to avoid confusing the clinician. Much less common is a mixed inverted-oncocytic sinonasal papilloma, a tumor that has been reported but that I have not encountered [17, 18]. Given the similar behavior and rates of malignant transformation between ISP and OSP, it is reasonable to diagnose such a tumor either as a “sinonasal papilloma with features of both inverted and oncocytic subtypes” or diagnose it on the basis of its predominant histology. Given what is now known about the different molecular alterations that characterize ISP and OSP (see below) it would be fascinating to investigate these purported mixed papillomas at a molecular level.
There is essentially no diagnostic or prognostic role for the use of special stains or immunohistochemistry in the diagnosis of sinonasal papillomas.
Etiology
Given the frequent association of papillomas with stromal changes reminiscent of inflammatory polyps, it was historically theorized that either polyps represented a precursor lesion to papillomas or that papillomas represented a metaplastic change within a polyp [2–4]. It has since been demonstrated, however, that papillomas represent true clonal neoplasms and any inflammatory polyp-like changes are likely secondary to obstruction caused by the papilloma [19].
The role of human papillomavirus (HPV) merits special mention. From its earliest descriptions, a viral etiology has been hypothesized for sinonasal papillomas [2–4]. Specifically, given its association to other papillomas throughout the body, it is reasonable to wonder whether HPV plays an etiologic role in the development of sinonasal papillomas. Using modern HPV detection techniques it is now clear that most ESPs—the papillomas that most closely resemble squamous papillomas of other organs—are indeed usually driven by low-risk HPV types, especially 6 and 11 [12, 20, 21] (Fig. 5b). High-risk HPV is not encountered in ESPs. At the other end of the spectrum, OSPs almost never harbor high-risk or low-risk HPV [11, 22].
The role of HPV in ISP, however, remains ambiguous, and the literature on this issue remains to this day confusing and contradictory, with reported HPV detection rates ranging from 0 to 100%! [1, 10, 16, 23–26] A large meta-analysis by Lawson, et al. found that 22–26% of ISPs harbored low-risk or high-risk HPV, with an increased rate of high-risk HPV detection in ISPs with dysplasia (56%) or frank squamous cell carcinoma (55%) [23]. These findings seem to suggest that high-risk types of HPV play a role as a co-factor in the development of carcinoma ex-ISP [23]. Many previous studies included in this analysis, however, utilized suboptimal detection methods that were unable to distinguish biologically active HPV from inactive, “passenger” virus. The recent development of RNA in situ hybridization probes for E6/E7 mRNA now allows for direct visualization of transcriptionally active specifically with high sensitivity [27–33]. Utilizing this technique, Rooper et al. found benign and dysplastic ISPs, as well as carcinomas ex-ISP, to be uniformly HPV-negative [34]. On the other hand, using the same RNA in situ hybridization method, Stoddard, et al. found high-risk HPV in 100% of ISPs, though their signals were often very focal [35]. As a result, the true role of HPV in the development of ISP remains frustratingly unresolved.
The etiologic factors in the development of HPV-unrelated sinonasal papillomas (e.g., almost all OSPs) are as yet unknown. The exposure to organic solvents may be a risk factor for ISP, but there is no clear association to smoking or ethanol exposure [10, 36].
Molecular Diagnostics
Recent studies have uncovered key molecular alterations in sinonasal papillomas. Specifically, Udager, et al. reported activating EGFR mutations in most ISPs (88%) and carcinomas arising from ISPs (77%) [37]. More recently, the same group reported that OSPs and carcinomas ex-OSP consistently (56 of 56 in their series) harbored activating KRAS mutations while ISPs and ESPs were negative [38]. These molecular findings serve to underscore the distinctness of each of the three subtypes of sinonasal papilloma.
Differential Diagnosis
ESP is most likely to be confused with a cutaneous papillomas which can occasionally involve the nasal vestibule. Unlike ESP, squamous papillomas of the skin lack mucocytes and ciliated or transitional epithelium [12]. In addition, the presence of skin adnexae (and absence of seromucinous glands) should inform the pathologist that it is a cutaneous lesion.
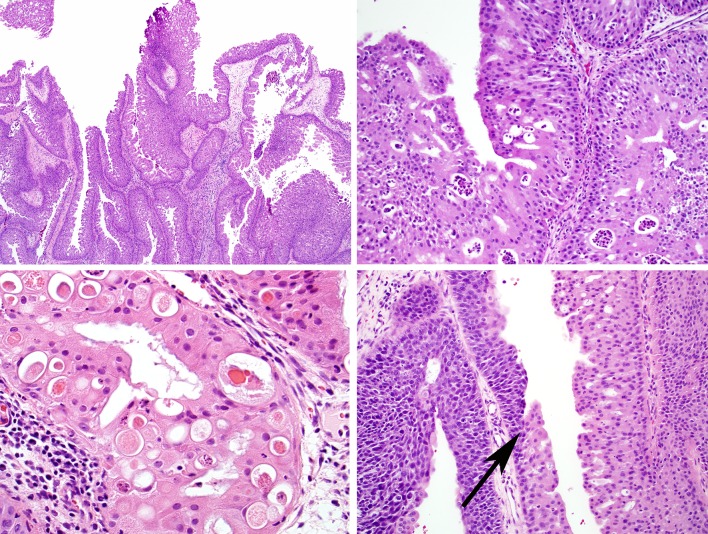
ISP can be mistaken for several lesions. Occasionally, rhinosinusitis can demonstrate papillary hyperplasia (“papillary rhinosinusitis”) or squamous metaplasia of the surface epithelium but in these reactive changes, the epithelium does not show downward growth, is not thickened and lacks the mucous cells, microcysts, neutrophils and microabscesses of ISP (Fig. 7a). ISP can be mistaken for respiratory epithelial adenomatoid hamartoma (REAH), a situation complicated by the fact that these lesions can be seen synchronously. REAH is also characterized by downward epithelial growth from the ciliated columnar surface epithelium, but it again lacks the epithelial thickening, mucous cells, microcysts, neutrophils and microabscesses of ISP (Fig. 7b). In addition, the epithelial nests of REAH are classically surrounded by a thickened basement membrane and are often associated with an increase in seromucinous glands, two features not often seen in ISP. Finally, ISP can be confused with a non-keratinizing squamous cell carcinoma, a tumor that often demonstrates inverted growth in the sinonasal tract. This distinction is made primarily on the basis of cytomorphology: squamous cell carcinoma exhibits much more cellular atypia, mitotic activity, and necrosis than ISP. As a side note, an inverted, ribbon-like growth pattern is common in non-keratinizing squamous cell carcinomas of the sinonasal tract and these carcinomas should not be presumed to have arisen from an ISP without evidence of preceding or concurrent benign ISP component.
Fig. 7.
In papillary rhinosinusitis, the surface is vaguely papillary, but the lining epithelium (inset) is not thickened and does not exhibit the changes of sinonasal papilloma (a). Respiratory epithelial adenomatoid hamartoma, like inverted sinonasal papilloma, exhibits downward epithelial growth. However, the lesional epithelium lacks the characteristic features of sinonasal papillomas, and the nests are often surrounded by a rim of hyalinized basement membrane (arrowheads) (b). Low-grade sinonasal adenocarcinoma grows as a complex proliferation of fused glands of only one cell layer. This appearance is dissimilar to that of oncocytic sinonasal papilloma (see Fig. 6a–c). (c) Rhinosporidiosis is characterized by numerous spores within the sinonasal submucosa. This is in contrast to oncocytic sinonasal papilloma where microcysts are seen in the epithelium only
OSP is most often mistaken for a low-grade sinonasal adenocarcinoma. Both lesions are typically quite bland cytologically, and sinonasal adenocarcinoma can be oncocytic. Unlike OSP with stratified columnar epithelium, however, a low-grade sinonasal adenocarcinoma grows as confluent, back-to-back glands with a single layer of cuboidal cells [5] (Fig. 7c). Historically, rhinosporidiosis has been included in the differential diagnosis of an OSP with abundant microcysts, though it is unclear how often this rare zoonotic disease is a realistic consideration. The distinction is straightforward, as the spores of rhinosporidiosis are variably sized and located primarily in the submucosal stroma in contrast to the uniformly sized epithelial microcysts of OSP [2, 5] (Fig. 7d). Rhinosporidiosis also does not induce oncocytic epithelial alterations.
Treatment and Prognosis
All types of sinonasal papilloma are treated with complete surgical excision, and all are prone to recur (20–30%) following incomplete removal of the tumor [10–12]. ISP and OSP of the frontal sinus is particularly prone to recurrence given the difficulties in reaching this sinus [39]. Rates of malignant transformation are discussed above.
Summary
Nearly 50 years ago, Hyams and others carefully detailed the clinical and histologic characteristics of three distinct forms of sinonasal papilloma. It is fascinating to see that in the past few years, molecular insights have essentially confirmed the uniqueness of these histologic subtypes, a testament to the power of clinicopathologic studies. ESP is a tumor driven by low-risk HPV that arises in the nasal septum and does not undergo malignant transformation. OSP is a rare tumor that is not HPV related, arises in the lateral nasal wall and sinuses, harbors activating KRAS mutations, and undergoes malignant transformation in 4–17% of cases. ISP shares clinical features with OSP (location, rate of malignancy) but harbors a different activating mutation (EGFR) and a still-uncertain relationship to HPV.
Compliance with Ethical Standards
Conflict of interest
Justin Bishop declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Proceedings of the 2017 North American Society of Head and Neck Pathology Companion Meeting (San Antonio, TX).
References
- 1.Barnes L. Schneiderian papillomas and nonsalivary glandular neoplasms of the head and neck. Mod Pathol. 2002;15:279–297. doi: 10.1038/modpathol.3880524. [DOI] [PubMed] [Google Scholar]
- 2.Hyams VJ. Papillomas of the nasal cavity and paranasal sinuses. A clinicopathological study of 315 cases. Ann Otol Rhinol Laryngol. 1971;80:192–206. doi: 10.1177/000348947108000205. [DOI] [PubMed] [Google Scholar]
- 3.Ash JE, Beck MR, Wilkes JD. Tumors of the upper respiratory tract and ear. Washington, D.C.: Armed Forces Institute of Pathology; 1964. Epithelial papilloma; pp. 32–34. [Google Scholar]
- 4.Lampertico P, Russell WO, Maccomb WS. Squamous papilloma of upper respiratory epithelium. Arch Pathol. 1963;75:293–302. [PubMed] [Google Scholar]
- 5.Barnes L, Bedetti C. Oncocytic Schneiderian papilloma: a reappraisal of cylindrical cell papilloma of the sinonasal tract. Hum Pathol. 1984;15:344–351. doi: 10.1016/S0046-8177(84)80033-7. [DOI] [PubMed] [Google Scholar]
- 6.Hyams VJ, Batsakis JG, Michaels L. Tumors of the upper respiratory tract and ear. Washington, D.C.: Armed Forces Institute of Pathology; 1988. Papilloma of the sinonasal tract; pp. 34–44. [Google Scholar]
- 7.Mills SE, Gaffey MJ, Frierson HF Jr. Schneiderian papillomas. Tumors of the upper respiratory tract and ear. Washington, D.C.: Armed Forces Institute of Pathology; 2000. p. 21–9.
- 8.Mills SE, Stelow EB, Hunt JL. Tumors of the upper aerodigestive tract and ear. Washington, D.C.: American Registry of Pahtology; 2012. Schneiderian papillomas; pp. 22–30. [Google Scholar]
- 9.Barnes L, Tse LLY, Hunt JL, et al. Schneiderian papillomas. In: Barnes L, Eveson JW, Reichart P, et al., editors. World health classification of tumors: head and neck. Lyon: IARC Press; 2005. pp. 28–32. [Google Scholar]
- 10.Hunt JL, Bell D, Sarioglu S, et al. Sinonasal papilloma, inverted type. In: el-Naggar A, Slootweg PJ, Chan JKC, et al., editors. World health classification of tumors: head and neck. Lyon: IARC Press; 2017. pp. 18–19. [Google Scholar]
- 11.Hunt JL, Chiosea S, Sarioglu S, et al. Sinonasal papilloma, oncocytic type. In: el-Naggar A, Slootweg PJ, Chan JKC, et al., editors. World Health Classification of Tumors: Head and Neck. Lyon: IARC Press; 2017. pp. 19–20. [Google Scholar]
- 12.Hunt JL, Lewis JS, Richardson M, et al. et al. Sinonasal papilloma, exophytic type. In: el-Naggar A, Slootweg PJ, Chan JKC, et al.et al., editors. World health classification of tumors: head and neck. Lyon: IARC Press; 2017. pp. 20–21. [Google Scholar]
- 13.Bullock MJ. Low-grade epithelial proliferations of the sinonasal tract. Head Neck Pathol. 2016;10:47–59. doi: 10.1007/s12105-016-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuta Y, Shinohara T, Sano K, et al. Molecular pathologic study of human papillomavirus infection in inverted papilloma and squamous cell carcinoma of the nasal cavities and paranasal sinuses. Laryngoscope. 1991;101:79–85. doi: 10.1288/00005537-199101000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Lesperance MM, Esclamado RM. Squamous cell carcinoma arising in inverted papilloma. Laryngoscope. 1995;105:178–183. doi: 10.1288/00005537-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Nudell J, Chiosea S, Thompson LD. Carcinoma ex-Schneiderian papilloma (malignant transformation): a clinicopathologic and immunophenotypic study of 20 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2014;8:269–286. doi: 10.1007/s12105-014-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufman MR, Brandwein MS, Lawson W. Sinonasal papillomas: clinicopathologic review of 40 patients with inverted and oncocytic schneiderian papillomas. Laryngoscope. 2002;112:1372–1377. doi: 10.1097/00005537-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Christensen WN, Smith RR. Schneiderian papillomas: a clinicopathologic study of 67 cases. Hum Pathol. 1986;17:393–400. doi: 10.1016/S0046-8177(86)80463-4. [DOI] [PubMed] [Google Scholar]
- 19.Califano J, Koch W, Sidransky D, et al. Inverted sinonasal papilloma : a molecular genetic appraisal of its putative status as a Precursor to squamous cell carcinoma. Am J Pathol. 2000;156:333–337. doi: 10.1016/S0002-9440(10)64734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchwald C, Lindeberg H, Pedersen BL, et al. Human papilloma virus and p53 expression in carcinomas associated with sinonasal papillomas: a Danish epidemiological study 1980–1998. Laryngoscope. 2001;111:1104–1110. doi: 10.1097/00005537-200106000-00032. [DOI] [PubMed] [Google Scholar]
- 21.Buchwald C, Franzmann MB, Jacobsen GK, et al. Human papillomavirus (HPV) in sinonasal papillomas: a study of 78 cases using in situ hybridization and polymerase chain reaction. Laryngoscope. 1995;105:66–71. doi: 10.1288/00005537-199501000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Vorasubin N, Vira D, Suh JD, et al. Schneiderian papillomas: comparative review of exophytic, oncocytic, and inverted types. Am J Rhinol Allergy. 2013;27:287–292. doi: 10.2500/ajra.2013.27.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawson W, Schlecht NF, Brandwein-Gensler M. The role of the human papillomavirus in the pathogenesis of Schneiderian inverted papillomas: an analytic overview of the evidence. Head Neck Pathol. 2008;2:49–59. doi: 10.1007/s12105-008-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syrjanen KJ. HPV infections in benign and malignant sinonasal lesions. J Clin Pathol. 2003;56:174–181. doi: 10.1136/jcp.56.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syrjanen S, Happonen RP, Virolainen E, et al. Detection of human papillomavirus (HPV) structural antigens and DNA types in inverted papillomas and squamous cell carcinomas of the nasal cavities and paranasal sinuses. Acta Otolaryngol. 1987;104:334–341. doi: 10.3109/00016488709107337. [DOI] [PubMed] [Google Scholar]
- 26.Cheung FM, Lau TW, Cheung LK, et al. Schneiderian papillomas and carcinomas: a retrospective study with special reference to p53 and p16 tumor suppressor gene expression and association with HPV. Ear Nose Throat J. 2010;89:E5–12. doi: 10.1177/014556131008901002. [DOI] [PubMed] [Google Scholar]
- 27.Ukpo OC, Flanagan JJ, Ma XJ, et al. High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–1350. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 28.Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schache AG, Liloglou T, Risk JM, et al. Validation of a novel diagnostic standard in HPV-positive oropharyngeal squamous cell carcinoma. Br J Cancer. 2013;108:1332–1339. doi: 10.1038/bjc.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bishop JA, Ma XJ, Wang H, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36:1874–1882. doi: 10.1097/PAS.0b013e318265fb2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerr DA, Arora KS, Mahadevan KK, et al. Performance of a branch chain RNA in situ hybridization assay for the detection of high-risk human papillomavirus in head and neck squamous cell carcinoma. Am J Surg Pathol. 2015;39:1643–1652. doi: 10.1097/PAS.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 32.Mirghani H, Casiraghi O, Amen F, et al. Diagnosis of HPV-driven head and neck cancer with a single test in routine clinical practice. Mod Pathol. 2015;28:151827. doi: 10.1038/modpathol.2015.113. [DOI] [PubMed] [Google Scholar]
- 33.Rooper LM, Gandhi M, Bishop JA, et al. RNA in-situ hybridization is a practical and effective method for determining HPV status of oropharyngeal squamous cell carcinoma including discordant cases that are p16 positive by immunohistochemistry but HPV negative by DNA in-situ hybridization. Oral Oncol. 2016;55:116. doi: 10.1016/j.oraloncology.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Rooper LM, Bishop JA, Westra WH. Transcriptionally active high-risk human papillomavirus is not a common etiologic agent in the malignant transformation of inverted schneiderian papillomas. Head Neck Pathol. 2017;In press. [DOI] [PMC free article] [PubMed]
- 35.Stoddard DG, Jr, Keeney MG, Gao G, et al. Transcriptional activity of HPV in inverted papilloma demonstrated by in situ hybridization for E6/E7 mRNA. Otolaryngol Head Neck Surg. 2015;152:7528. doi: 10.1177/0194599815571285. [DOI] [PubMed] [Google Scholar]
- 36.d’Errico A, Pasian S, Baratti A, et al. A case-control study on occupational risk factors for sino-nasal cancer. Occup Environ Med. 2009;66:44855. doi: 10.1136/oem.2008.041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Udager AM, Rolland DC, McHugh JB, et al. High-frequency targetable EGFR mutations in sinonasal squamous cell carcinomas arising from inverted sinonasal papilloma. Cancer Res. 2015;75:26006. doi: 10.1158/0008-5472.CAN-15-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Udager AM, McHugh JB, Betz BL, et al. Activating KRAS mutations are characteristic of oncocytic sinonasal papilloma and associated sinonasal squamous cell carcinoma. J Pathol. 2016;239:3948. doi: 10.1002/path.4750. [DOI] [PubMed] [Google Scholar]
- 39.Anari S, Carrie S. Sinonasal inverted papilloma: narrative review. J Laryngol Otol. 2010;124:70515. doi: 10.1017/S0022215110000599. [DOI] [PubMed] [Google Scholar]