Abstract
Although cellular prion protein PrPC is well known for its implication in Transmissible Spongiform Encephalopathies, its functions remain elusive. Combining in vitro and in vivo approaches, we here show that PrPC displays the intrinsic capacity to protect neuronal cells from a pro-inflammatory TNFα noxious insult. Mechanistically, PrPC coupling to the NADPH oxidase-TACE α-secretase signaling pathway promotes TACE-mediated cleavage of transmembrane TNFα receptors (TNFRs) and the release of soluble TNFR, which limits the sensitivity of recipient cells to TNFα. We further show that PrPC expression is necessary for TACE α-secretase to stay at the plasma membrane in an active state for TNFR shedding. Such PrPC control of TACE localization depends on PrPC modulation of β1 integrin signaling and downstream activation of ROCK-I and PDK1 kinases. Loss of PrPC provokes TACE internalization, which in turn cancels TACE-mediated cleavage of TNFR and renders PrPC-depleted neuronal cells as well as PrPC knockout mice highly vulnerable to pro-inflammatory TNFα insult. Our work provides the prime evidence that in an inflammatory context PrPC adjusts the response of neuronal cells targeted by TNFα through TACE α-secretase. Our data also support the view that abnormal TACE trafficking and activity in prion diseases originate from a-loss-of-PrPC cytoprotective function.
Introduction
Chronic neuroinflammation is a hallmark of several neurodegenerative disorders such as Alzheimer’s or Parkinson’s diseases that relies on the long-standing activation of microglia and astrocytes in the central nervous system (CNS). These cells produce neurotoxic mediators, such as pro-inflammatory cytokines (TNFα) and interleukins (IL1, IL6) that contribute to dysfunction and degeneration of diseased neurons. The release of pro-inflammatory mediators by microglia also favors the permeabilisation of the blood brain barrier and the subsequent infiltration of peripheral leukocytes, including T cells and macrophages that amplify the disease states (for review see ref. 1).
The cellular prion protein PrPC, which is mainly known for its role in Transmissible Spongiform Encephalopathies (TSEs), was shown to exert protective effect against inflammation. Indeed, PrPC displays the intrinsic capacity to modulate in mice the lipopolysaccharide-induced activation of both microglia in the CNS and macrophages in the periphery2. In immune cells, PrPC was also reported to balance the release of pro-inflammatory factors during the acute phase of bacterial infection with the production of anti-inflammatory cytokines during the later stage of infection3. However, it remains unknown whether PrPC protection against inflammation would also depend on PrPC capacity to adjust the response of cells targeted by pro-inflammatory factors.
PrPC is a ubiquitous protein that is more abundantly expressed in neurons. It is a GlycosylPhosphatidylInositol(GPI)-anchored protein tethered to the outer leaflet of the plasma membrane. The presence of PrPC in detergent-resistant microdomains, i.e. lipid-rafts or caveolae, of the plasma membrane, and interaction with several partners fits in with the notion that PrPC is physiologically associated with signaling events and behaves as receptor or co-receptor or as a scaffolding protein regulating the assembly of diverse interactors and signaling modules4, 5. This includes β1 integrins as loss of PrPC in neural stem cells leads to micro-aggregation of β1 integrins at the plasma membrane and a rise of β1 integrin signaling that promotes overactivation of the rhoA-associated coiled-coil containing kinase (ROCK) and impairs neuritogenesis6. Besides, using an antibody-based approach to mimic an activation signal for PrPC, we provided evidence that PrPC can activate in neuronal cells several signaling effectors such as the Fyn tyrosine kinase7 and NADPH oxidase8. Reactive oxygen species (ROS) produced by NADPH oxidase are not toxic and act as second message signals involved in the activation of TACE α-secretase, a member of a disintegrin and metalloproteinase (ADAM) family9, 10. Via its coupling to NADPH oxidase, PrPC stimulates TACE-dependent cleavage of transmembrane pro-TNFα into soluble TNFα (sTNFα) that behaves in neuronal cells as an autocrine modulator of neurotransmitter-associated functions through binding to plasma membrane TNFα receptors (TNFRs)9. Of note, TACE was shown to also assume the cleavage of TNFRs in fibroblasts, leukocytes or neuronal cells11–13. This raises the possibility that PrPC would exert protection against the pro-inflammatory TNFα cytokine by balancing the TACE-dependent release of sTNFα with the TACE-dependent shedding of TNFRs.
In this study, we show that PrPC positively contributes to the shedding of plasma membrane TNFRs through its coupling to the NADPH oxidase-TACE α-secretase pathway in neuronal stem cells, their neuronal derivatives and primary cerebellar granule neurons. The cytoprotective effect of PrPC against sTNFα also depends on a PrPC control of TACE localization at the plasma membrane. Cell depletion of PrPC (PrPnull-cells) provokes the internalization of TACE, which diverts TACE activity away from TNFR substrate that accumulates at the plasma membrane and renders PrPnull-cells highly sensitive to exogenous sTNFα. We show that TACE internalization in PrPnull-cells relates to a loss of PrPC regulatory function towards plasma membrane β1 integrins and downstream signaling. Finally, we substantiate in PrPC knockout mice that such deregulation of the TACE-TNFR pathway in the brain is at the root of exaggerated sensitivity to sTNFα noxious insult. Our work thus unravels a new role for PrPC signaling related to cytoprotection against sTNFα-mediated inflammation.
Results
PrPC coupling to the NADPH oxidase-TACE signaling pathway promotes TNFR1 shedding
Because the cell sensitivity to sTNFα depends on the amount of TNFRs present at the plasma membrane, we first sought to determine whether PrPC would impact on cell surface TNFR level focusing on TNFR type 1 (TNFR1), a transmembrane trimeric receptor composed of three identical subunits, that mainly relays sTNFα toxicity14.
Antibody mediated-PrPC ligation, used to mimic an activation signal for PrPC 5, 7, elicited the release of soluble TNFR1 (sTNFR1) into the culture media of 1C11 neuroectodermal cells and their serotonergic 1C115-HT neuronal derivatives15 as assessed by ELISA (Fig. 1a). sTNFR1 was detected as soon as 30 min after PrPC ligation with SAF61 antibody (10 µg ml−1). ELISA-based quantification of monomeric TNFR1 subunit in the culture medium revealed that the level of sTNFR1 reached at 120 min was ~4-fold above basal level (Fig. 1a). Concomitantly, immunofluorescence experiments that detect the trimeric form of TNFR1 at the plasma membrane showed that PrPC ligation triggered a time-dependent depletion of TNFR1 at the cell surface with a maximum immunostaining decrease (~1.5-fold) reached by 120 min exposure to SAF61 antibody (Fig. 1b,c). Inhibition of NADPH oxidase (diphenyleneiodonium- DPI, 100 µM) or TACE (TAPI-2, 100 µM) abrogated the shedding of TNFR1 induced by PrPC ligation (Fig. 1a–c), indicating that PrPC coupling to the NADPH oxidase-TACE α-secretase signaling pathway controls cell surface TNFR1 level in 1C11 neuronal stem cells and their serotonergic progenies. Beyond the direct activation of TACE α-secretase by ROS through modification of the valence state of Zn2+ at the catalytic site16, redox activation of guanylate cyclase (GC) and subsequent production of cGMP was also shown to promote TACE-dependent TNFR1 shedding in sepsis conditions17. We thus probed the involvement of GC in PrPC-induced TNFR1 shedding by inhibiting GC with NS-2028 (1 µM). We found that GC inhibition had no impact on sTNFR1 release induced by SAF61 antibody in 1C11 precursor cells (Supplementary Fig. 1a), while TNFR1 shedding was 40% decreased in 1C115-HT cells (Supplementary Fig. 1b). This result suggests that guanylate cyclase is a potential signaling intermediate in PrPC coupling to TACE α-secretase in 1C115-HT neuronal cells only.
Figure 1.
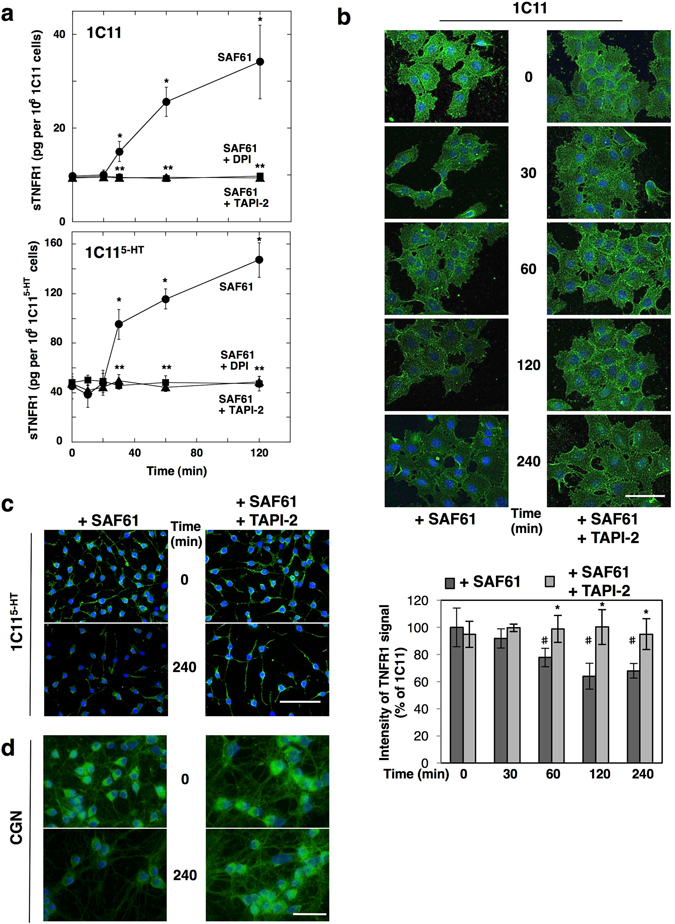
PrPC coupling to the NADPH oxidase-TACE signaling pathway promotes TNFR1 shedding. (a) Time-course accumulation of sTNFR1 in the cell culture medium of 1C11 cells and 1C115-HT neuronal cells upon PrPC ligation with SAF61 PrP antibody (10 μg ml−1). TNFR1 shedding induced by PrP antibodies is abolished upon inhibition of NADPH oxidase with DPI (100 μM) or TACE with TAPI-2 (100 μM). *p < 0.01 vs. nontreated cells. **p < 0.01 vs. cells exposed to SAF61 antibody. (b–d) Immunofluorescence experiments and quantification histograms showing progressive TNFR1 depletion at the cell surface of 1C11 cells (b), 1C115-HT cells (c), and primary CGNs (d) exposed to SAF61 PrP antibody and cancellation upon addition of TAPI-2. Scale bar = 50 μm. # p < 0.05 vs. nontreated cells. *p < 0.05 vs. cells exposed to SAF61 antibody alone. Data shown are the mean ± SEM from three experiments performed in triplicate.
ADAM10 and γ-secretase have also been involved in the shedding of TNFR118, 19. We thus probed the implication of these two proteases in the PrPC-regulated cleavage of TNFR1 using the pharmacological inhibitors GI254023X (50 nM) for ADAM10 and DAPT (10 µM) for γ-secretase. As assessed through the measure of sTNFR1 level in the cell culture medium, the inhibition of ADAM10 or γ-secretase had no effect on the release of TNFR1 ectodomain induced by PrPC ligation with SAF61 antibody in 1C11 and 1C115-HT cells (Supplementary Fig. 1a,b). This indicates that ADAM10 and γ-secretase play no role in PrPC-stimulated TNFR1 shedding.
Finally, we extended our study to other cell systems and found that PrPC-dependent control of TACE-mediated TNFR1 shedding also occurred in primary cultures of cerebellar granule neurons (CGNs). Exposure of CGNs to PrP antibodies (SAF61 10 µg ml−1) triggered a time-dependent decrease in the level of TNFR1 at the neuronal cell surface up to 240 min (Fig. 1d), which was cancelled by inhibiting TACE activity (Fig. 1d).
As a whole, these data indicate the amount of TNFR1 molecules expressed at the plasma membrane of neuronal stem cells and neurons depends on PrPC signaling that stimulates TNFR1 shedding via the NADPH oxidase-TACE α-secretase cascade.
PrPC-dependent regulation of TNFR1 shedding governs cell sensitivity to sTNFα toxicity
As PrPC intrinsically stimulates TACE-mediated TNFR1 shedding, we next assessed whether PrPC confers cell protection against sTNFα toxicity. To address such PrPC function, we exploited siRNA-mediated PrPC silenced cells and primary neurons from PrP0/0 mice and compared the sensitivity to sTNFα of PrPC-depleted cells to that of their corresponding PrPC expressing counterparts.
We first determined the dose of exogenous sTNFα that induces 50% cell death (LD50 TNFα) of 1C11 precursor cells, serotonergic 1C115-HT neural cells and their counterparts silenced for PrPC expression (PrPnull-cells). As shown in Fig. 2a and Table 1, PrPnull-1C11 and 1C115-HT cells were ~5- to 9-fold more sensitive to a 48 h exposure to sTNFα than their corresponding PrPC expressing cells. In primary cultures of CGNs, we also found a PrPC role in the control of cell sensitivity to sTNFα. In this set of experiments, we determined the dose of exogenous sTNFα inducing neuronal dysfunction for 50% of CGNs through dendrite fragmentation13. We monitored that CGNs isolated from PrP0/0-FVB mice were ~20-fold more sensitive to a 48 h exposure to sTNFα than PrPC-expressing CGNs (Fig. 2b and Table 1).
Figure 2.
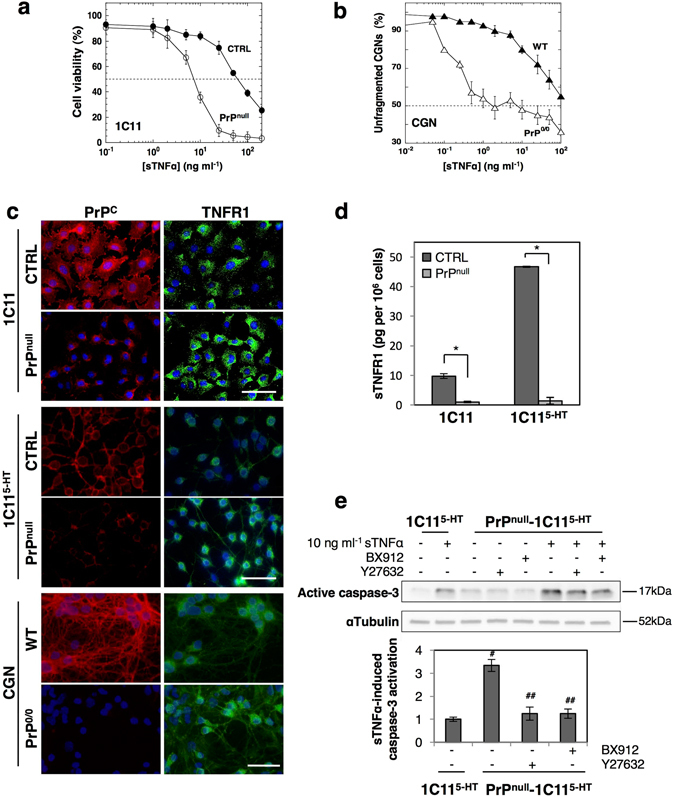
Loss of PrPC exacerbates cell sensitivity to sTNFα by reducing TNFR1 shedding in a ROCK-I- and PDK1-dependent manner. (a) Reduced viability of PrPnull-1C11 cells after exposure to increasing sTNFα concentrations for 72 h as compared to 1C11 cells (CTRL). (b) Increased dendritic fragmentation in PrP0/0-CGNs after exposure to increasing concentrations of sTNFα for 72 h compared to wild-type CGNs (WT). For figures a and b, LD50 TNFα values are indicated in Table 1. (c) Immunofluorescence experiments showing enhanced level of TNFR1 at the cell surface of PrPnull-1C11 and PrPnull-1C115-HT cells as well as PrP0/0 CGNs as compared to their corresponding PrPC expressing cells (CTRL, WT). Scale bar = 50 µm. (d) ELISA-based quantification experiments indicating reduced concentration of sTNFR1 in the culture medium of PrPnull-1C11/1C115-HT cells compared to PrPC expressing cells. *p < 0.01. (e) Western blots showing a stronger activation of caspase-3 in PrPnull-1C115-HT cells exposed to sTNFα (10 ng ml−1) for 120 min than in PrPC expressing cells. Antagonizing either ROCK activity with Y-27632 (100 µM for 1 h) or PDK1 activity with BX912 (1 µM for 1 h) in PrPC-depleted cells reduces toxic action of sTNFα. # p < 0.05 vs. PrPC-expressing cells exposed to sTNFα. ## p < 0.05 vs. PrPnull-cells treated with sTNFα. Data shown are the mean ± SEM from three experiments performed in triplicate.
Table 1.
Impact of PrPC depletion on cell sensitivity to sTNFα in 1C11 precursor cells, 1C115-HT neuronal cells and primary CGNs.
| LD50 TNFα (ng ml−1) | ||
|---|---|---|
| WT | PrPnull/PrP0/0 | |
| 1C11 | 70 ± 10 | 8.1 ± 1.5 |
| 1C115-HT | 8.1 ± 1.2 | 1.5 ± 0.3 |
| CGN | 100 ± 20 | 5.2 ± 3.1 |
LD50 TNFα values correspond to the concentration of sTNFα inducing a 50% cell death in 1C11 and 1C115-HT cells or inducing dendritic fragmentation for 50% of neuronal cells in CGNs. Data are the mean ± SEM of three independent experiments performed in triplicate.
Through immunofluorescence experiments, we recorded an increase of TNFR1 at the plasma membrane of PrPnull-1C11 and 1C115-HT cells as well as PrP0/0-CGNs compared to their wild-type counterparts (Fig. 2c). Of note, the level of sTNFR1 in the cell culture medium of PrPnull-1C11 and 1C115-HT cells was ∼10- to 25-fold lower than that measured with wild type cells (Fig. 2d), indicating reduced shedding of cell surface TNFR1 in the absence of PrPC.
Corroborating the augmentation of plasma membrane TNFR1 level in PrPnull-1C11/1C115-HT cells and PrP0/0-CGNs associated with the increased vulnerability of PrPC-depleted cells to sTNFα, we found that TNFR1 signaling is exacerbated in the absence of PrPC. In response to sTNFα exposure for 2 h, the activation of caspase-3, a downstream effector of TNFR1 signaling20, was ∼2 to 4-fold enhanced in PrPnull-1C11 (Supplementary Fig. 2) and PrPnull-1C115-HT cells (Fig. 2e) compared to wild type cells.
Loss of PrPC therefore triggers a deficit of TNFR1 shedding, leading to plasma membrane accumulation of TNFR1 and enhanced TNFR1 death signaling, that renders PrPC-depleted cells highly vulnerable to sTNFα toxicity. Our data thus argue for a protective function of PrPC against sTNFα-associated inflammation.
Cancellation of TNFR1 shedding in PrPnull-cells is associated with TACE internalization induced by ROCK-I and PDK1 kinases
Defect in TNFR1 shedding caused by the absence of PrPC prompted us to examine the status of the TACE α-secretase in PrPnull-cells. While no significant variation in TACE expression was measured at the mRNA and protein levels between PrPnull- and PrPC-expressing cells (Fig. 3a), immunolabeling experiments revealed that TACE was quite absent at the plasma membrane of PrPnull-cells but found intracellularly after cell permeabilization with saponin 0.05% (Fig. 3b). Transmission electron microscopy experiments further indicated that TACE was internalized in vesicles enriched with the caveolin-1 protein (Cav-1) in PrPnull-1C11 cells (Fig. 3c). These observations suggest that loss of PrPC promotes TACE internalization.
Figure 3.
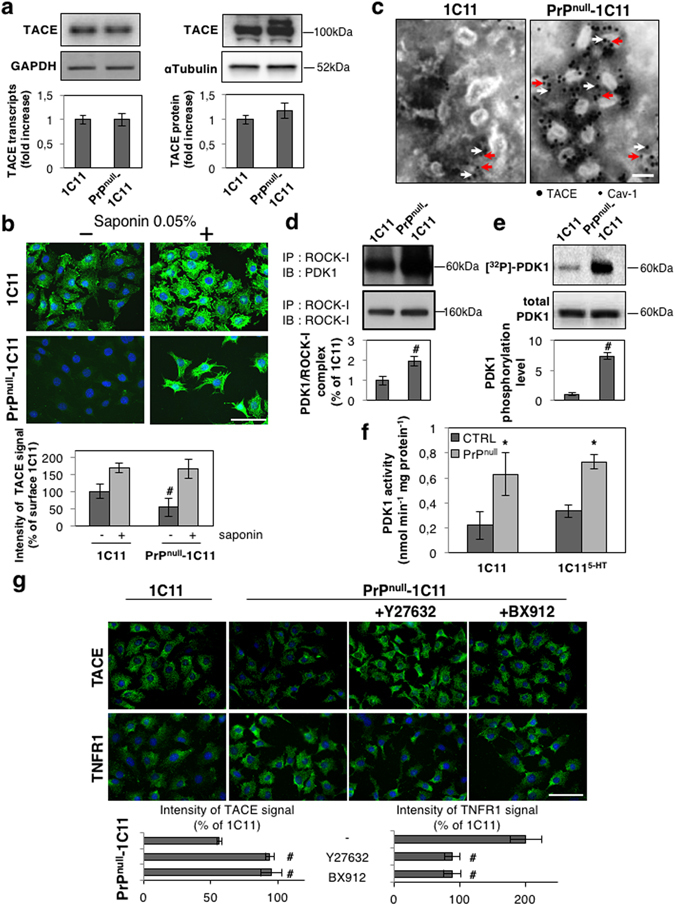
Overactivation of the ROCK-I-PDK1 signaling module in the absence of PrPC promotes TACE internalization. (a) RT-PCR (left) and Western-blot (right) experiments showing that siRNA-based PrPC silencing in 1C11 cells does not impact on TACE expression at the mRNA and protein levels. (b) Immunolabeling experiments indicating that TACE level is reduced at the cell surface of PrPnull-1C11 cells vs. 1C11 cells. Cell permeabilization with saponin reveals that TACE is internalized in the absence of PrPC. Scale bar = 50 µm. # p < 0.05 vs. PrPC expressing 1C11 cells. (c) Transmission electron micrographs showing TACE (7-nm gold particles, white arrows) accumulation in Caveolin-1-enriched vesicles (labeled by 5-nm gold particles, red arrows) in PrPnull-1C11 cells. Scale bar = 100 nm. (d) Immunoprecipitation of ROCK-I followed by immunoblotting of PDK1 reveals enhanced interaction between ROCK-I and PDK1 in PrPnull-1C11 cells compared to 1C11 cells. # p < 0.05. (e) Cell 32P metabolic labeling followed by PDK1 immunoprecipitation and western blotting indicates higher PDK1 phosphorylation level in PrPnull-1C11 cells than in 1C11 cells. # p < 0.05. (f) Augmented PDK1 activity in PrPnull- vs. 1C11/1C115-HT cells. *p < 0.01 vs. PrPC expressing cells. (g) Immunolabelings of cell surface TACE and TNFR1 showing that inhibition of either ROCK (Y-27632 100 µM for 2 h) or PDK1 (BX912 1 µM for 2 h) targets TACE back to the plasma membrane of PrPnull-1C11 cells and rescues TNFR1 shedding. Scale bar = 50 µm. # p < 0.05 vs. nontreated cells. Data shown are the mean ± SEM from three experiments performed in triplicate.
Such internalization of TACE in PrPC-depleted cells is reminiscent of what we observed in prion-infected neurons13, 21. We showed that pathogenic prions (PrPSc) overstimulate ROCK-I, which binds and phosphorylates PDK1, leading to PDK1 overactivity21. Overstimulated PDK1 promotes the phosphorylation and displacement of TACE from the plasma membrane to intracellular Cav-1-enriched vesicles in prion-infected neurons13. We thus probed whether the internalization of TACE and subsequent deficit in TNFR1 shedding in PrPnull-cells would also relate to overactivation of the ROCK-I/PDK1 duo.
Immunoprecipitation experiments revealed that the pool of PDK1 molecules interacting with ROCK-I subtype was ~2-fold more abundant in PrPnull-1C11 cells than in PrPC expressing cells (Fig. 3d). Such enhanced interaction between ROCK-I and PDK1 in PrPnull-1C11 cells was accompanied by an increased PDK1 phosphorylation level (Fig. 3e), leading to a ~2- to 3-fold increase in PDK1 activity in PrPnull-cells compared to their PrPC expressing counterparts (Fig. 3f).
In PrPnull-cells, overactivation of the ROCK-I/PDK1 module compromises TACE localization at the plasma membrane. The inhibition of either ROCK-I with Y-27632 (100 µM) or PDK1 with BX912 (1 µM) for 1 h indeed allowed to direct TACE back to the plasma membrane of PrPnull-cells (Fig. 3g). In addition, inhibition of ROCK-I or PDK1 in PrPnull-cells rescued TACE cleavage activity towards TNFR1 as assessed by reduced level of TNFR1 at the plasma membrane (Fig. 3g) and desensitization of PrPnull-1C11 and 1C115-HT cells from sTNFα-induced caspase-3 activation (Fig. 2e and Supplementary Fig. 2).
These results indicate that in the absence of PrPC overactivated ROCK-I and PDK1 kinases promote the internalization of TACE and neutralize TACE activity towards TNFR1. Beyond PrPC capacity to stimulate TACE-mediated TNFR1 shedding, the protective role of PrPC against sTNFα further depends on PrPC ability to maintain TACE α-secretase at the cell surface in an active state for TNFR1 cleavage.
The presence of active TACE α-secretase at the plasma membrane depends on PrPC-mediated regulation of β1 integrin signaling
In lipid rafts of the plasma membrane, PrPC is assumed to function as a dynamic platform for the assembly and modulation of the signaling activity of various modules4. Such PrPC role possibly relies on the interaction between PrPC and the membrane protein Cav-17, 22 that also mediates the recruitment of β1 integrins to rafts and activates β1 integrin signaling23, 24. By controlling Cav-1 availability for β1 integrins22, PrPC exerts a negative regulatory action on β1 integrin signaling6. Such interplay between PrPC and β1 integrins in 1C11 neuronal stem cells and PC12 cells fine-tunes the ROCK activity necessary for neurite sprouting6, 25. We next wondered whether loss of PrPC modulatory action on β1 integrin signaling would account for the ROCK-I/PDK1-dependent TACE internalization and subsequent defect in TNFR1 shedding in PrPnull-cells.
Exposure of PrPnull-1C11 cells to neutralizing antibodies towards β1 integrins (MAB1965) relocated TACE to the plasma membrane of PrPnull-1C11 cells (Fig. 4a), arguing that β1 integrin overactivity in the absence of PrPC triggers TACE internalization. Redirection of TACE to the cell surface started after 60 min exposure to ΜΑΒ1965. Immunofluorescence experiments indicated that TACE signal measured at the plasma membrane of PrPnull-1C11 cells after 120 and 240 min exposure to MAB1965 was comparable to that of PrPC expressing 1C11 cells. Correlating TACE relocation to the cell surface, neutralization of β1 integrins rescued TNFR1 shedding as assessed by the progressive disappearance of TNFR1 immunostaining at the plasma membrane of PrPnull-1C11 cells exposed to MAB 1965 (Fig. 4a). After β1 integrin neutralization for 120 to 240 min, cell surface TNFR1 level in PrPnull-cells was highly comparable to that measured with PrPC expressing cells. Manganese (Mn2+) is widely used to investigate conformational changes associated with the activation of integrins and the recruitment of signaling pathways26, 27, as Mn2+ binds integrins and strongly up-regulates integrin function by mimicking inside-out signaling events28, 29. In 1C11 and 1C115-HT cells expressing PrPC, forced stimulation of β1 integrin activity with 100 µM Mn2+ for 4 h promoted the internalization of TACE (Supplementary Fig. 3a,c) and abrogated TACE-mediated shedding of TNFR1 (Supplementary Fig. 3b,c) in a ROCK-I/PDK1-dependent manner. This set of experiments demonstrates that misregulation of β1 integrin signaling activity caused by loss of PrPC regulatory action over β1 integrins (in PrPnull-cells or in Mn2+-treated PrPC expressing cells) is at the root of TACE internalization.
Figure 4.
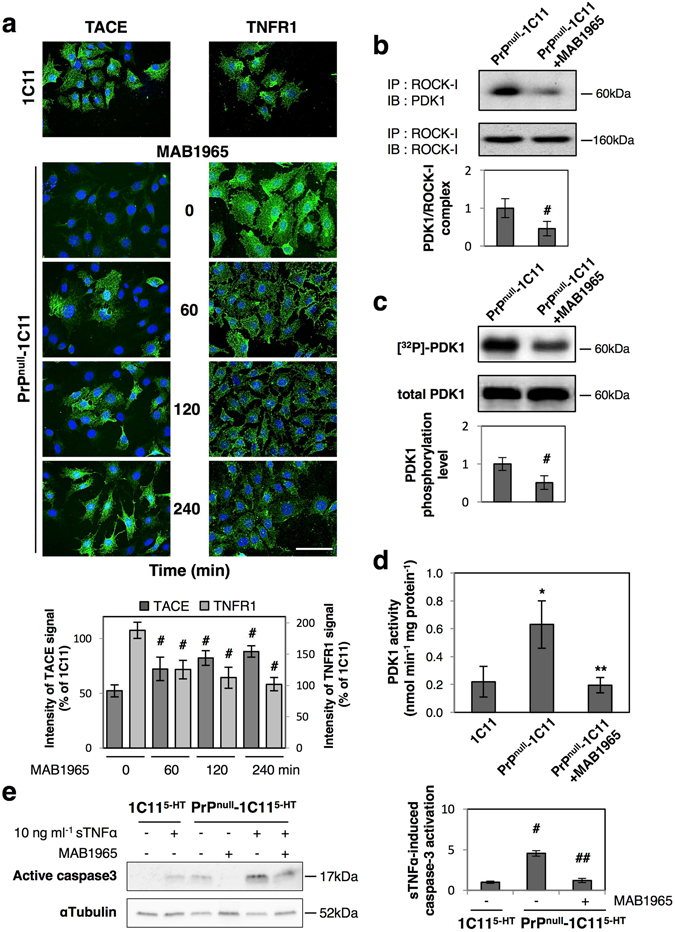
Misregulation of β1 integrin signaling in PrPnull-cells causes TACE internalization and defect of TNFR1 shedding. (a) Immunolabeling experiments showing time courses of TACE relocation to the plasma membrane and concomitant rescue of TNFR1 shedding upon β1 integrin neutralization with MAB 1965 antibodies (1 µg ml−1). # p < 0.05 vs. nontreated PrPnull-1C11 cells. (b) Immunoprecipitation of ROCK-I followed by PDK1 western-blotting indicating reduced ROCK-I and PDK1 interaction in PrPnull-1C11 cells treated with neutralizing β1 integrin antibodies. # p < 0.05. (c) Neutralization of β1 integrins in PrPnull-1C11 cells decreases phosphorylation of PDK1 as assessed by 32P metabolic labeling followed by PDK1 immunoprecipitation and western-blotting. # p < 0.05. (d) PDK1 activity returns to basal level in PrPnull-1C11 cells exposed to MAB 1965 antibodies. *p < 0.01 vs. 1C11 cells. **p < 0.01 vs. untreated PrPnull-1C11 cells. (e) Reduced sTNFα-induced caspase-3 activation in PrPnull-1C115-HT cells exposed to MAB 1965 antibodies for 4 h. # p < 0.05 vs. PrPC expressing 1C115-HT-cells exposed to sTNFα. ## p < 0.05 vs. PrPnull-1C115-HT cells treated with sTNFα. Data shown are the mean ± SEM from three experiments performed in triplicate.
Finally, we monitored that restoration of TACE α-secretase at the plasma membrane and subsequent recovery of TNFR1 shedding in PrPnull-cells exposed to the neutralizing β1 integrin antibody MAB1965 (240 min) were associated with disruption of the ROCK-I/PDK1 complex (Fig. 4b), reduced phosphorylation of PDK1 (Fig. 4c), decrease in PDK1 activity in PrPnull-1C11 cells (Fig. 4d), and lower cell sensitivity to sTNFα, as inferred by the decreased sTNFα-induced activation of caspase-3 (Fig. 4e).
These overall data provide the prime evidence that loss of PrPC regulatory function towards β1 integrin signaling and downstream overactivation of ROCK-I trigger (i) PDK1 overactivation, (ii) PDK1-dependent TACE internalization and (iii) abrogation of TACE shedding activity. By modulating β1 integrin signaling and activation of the ROCK-I/PDK1 module, PrPC physiologically ensures bioavailability of active TACE α-secretase at the plasma membrane for TNFR1 shedding that thereby protects neuronal stem cells and neurons from sTNFα toxicity.
Enhanced sensitivity of PrP0/0 mice to sTNFα inflammatory challenge can be counteracted upon PDK1 inhibition
To corroborate our in vitro data with the in vivo situation, we next probed in the brain of PrP0/0 mice the status of PDK1, the TACE shedding activity towards TNFR1, and the sensitivity to sTNFα-mediated inflammation.
First, we measured a ∼3-fold increase in PDK1 activity in brain extracts from 20 weeks-old FVB PrP0/0-mice (Fig. 5a) and a ∼2.5-fold decrease in soluble TNFR1 (sTNFR1) level in the cerebrospinal fluid (CSF) of FVB PrP0/0-mice (Fig. 5b) compared to their wild type counterparts. Intracerebroventricular (icv) injection of the PDK1 inhibitor BX912 (1 µM) in FVB PrP0/0-mice provoked a ∼2-fold increase in CSF sTNFR1 level (Fig. 5b), indicating that deficit of TNFR1 shedding in the brain of PrP0/0-mice originates from PDK1 overactivity.
Figure 5.
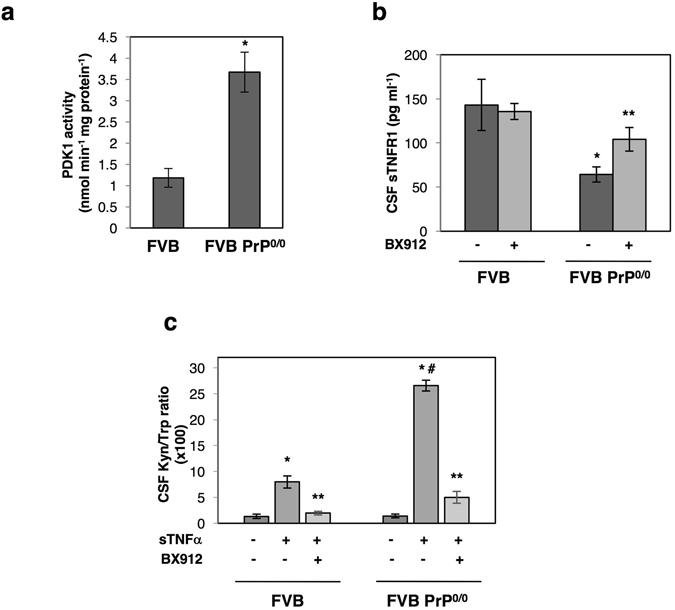
PDK1 inhibition protects FVB PrP0/0 mice from sTNFα-induced inflammation. (a) Measure of PDK1 activity indicating a rise of PDK1 activity in the brain of 20-weeks old FVB PrP0/0 mice compared to FVB wild type mice (n = 6 for each group). Values are means ± SEM. *p < 0.01 vs. FVB mice. (b) Concentration of sTNFR1 in the CSF of 20 weeks-old FVB PrP0/0 and wild type mice intracerebroventricular (icv) injected or not with the PDK1 inhibitor BX912 (n = 6 for each group). Values are means ± SEM. *p < 0.01 vs. nontreated FVB mice. **p < 0.01 vs. nontreated FVB PrP0/0 mice. (c) [kynurenine]/[tryptophan] ratio in the CSF of 20 weeks-old FVB PrP0/0 and wild type mice icv injected or not with BX912 following a sTNFα challenge (200 ng in 10 µl saline buffer) for 24 h (n = 6 for each group). Values are means ± SEM. *p < 0.01 vs. mice nontreated with sTNFα. # p < 0.01 vs. FVB mice treated with sTNFα. **p < 0.01 vs. sTNFα-treated mice.
We next challenged FVB PrP0/0-mice and their PrPC expressing FVB counterparts (n = 6) with an icv dose of sTNFα (200 ng in 10 µl saline buffer) for 24 h. The neuroinflammation effect of sTNFα was evaluated through measure of the concentrations of kynurenine and tryptophan in the CSF as the kynurenine pathway of tryptophan metabolism was shown to mediate the action of pro-inflammatory cytokines, including sTNFα, in the brain30–32. Following the challenge with sTNFα, FVB animals expressing PrPC showed a ∼7-fold increase in the CSF [kynurenine]/[tryptophan] ratio, while PrP0/0-animals showed an exaggerated response with a ∼17-fold elevation of the [kynurenine]/[tryptophan] ratio (Fig. 5c). This suggests that in the absence of PrPC excessive TNFR1 signaling combined with deficit of the anti-inflammatory sTNFR1 factor caused by PDK1 overactivation would exacerbate the pro-inflammatory action of sTNFα in the brain. Accordingly, we showed that PDK1 inhibition and subsequent restoration of TNFR1 shedding (Fig. 5b) reversed the exaggerated kynurenine response induced by sTNFα icv injection in FVB PrP0/0 mice (Fig. 5c).
As a whole, these in vivo data indicate that loss of PrPC is associated with a defect of TNFR1 shedding, which in turn exacerbates cell sensitivity to sTNFα-mediated inflammation.
Discussion
Although corruption of normal function(s) of cellular prion protein (PrPC) plays a central role in TSEs, PrPC role(s) remain(s) elusive. This study discloses that PrPC limits the sensitivity of cells to the pro-inflammatory cytokine sTNFα by restricting the level of TNFR1 present at the plasma membrane. Such protective action of PrPC towards TNFα toxicity depends on the signaling activity of PrPC (i) to stimulate the cleavage of TNFR1 and the release of soluble TNFR1 (sTNFR1) through PrPC coupling to the NADPH oxidase–TACE α-secretase pathway and (ii) to ensure active TACE bioavailability at the plasma membrane through negative control of β1 integrin coupling to ROCK-I and PDK1 kinases.
PrPC acts as a signaling molecule at the cell surface and activates diverse effectors involved in neuronal homeostasis, including NADPH oxidase and TACE α-secretase5, 8, 9. Through coupling to the NADPH oxidase-TACE pathway, PrPC promotes the release of sTNFα into the cell microenvironment9, 33. With 1C11-derived neuronal cells, sTNFα behaves as an autocrine modulator of neurotransmitter-associated functions devoid of any toxicity9. Here, we describe that PrPC also takes part to the regulated cleavage of transmembrane TNFR1 and the subsequent release of sTNFR1 into the cell microenvironment through TACE activation. The identification of TNFR1 as a novel target of the PrPC/NADPH oxidase/TACE coupling sheds light on how PrPC fine-tunes the cell response to sTNFα. By controlling the levels of shed TNFα and plasma membrane TNFR1, PrPC thus confines the role of sTNFα to modulation of neuronal functions. Accordingly, due to the peculiar binding stoichiometry between sTNFR1 and sTNFα (2:3), sTNFR1 molecules released by PrPC signaling (3000 molecules per 1C115-HT cell) neutralize PrPC-induced shed TNFα (4000 molecules per 1C115-HT cell) present in the cell microenvironment and, thereby, help to limit neuronal sTNFα signaling34. Dual control of TNFα release and TNFR1 shedding by TACE was also reported to protect liver from lipopolysaccharide (LPS)-induced inflammatory injury35. The present study also shows that the PrPC/TACE-mediated TNFR1 shedding ensures cell protection against an exogenous sTNFα insult with increased sensitivity of PrPnull-cells and PrP0/0 mice towards sTNFα. This is in line with increased vulnerability of PrP0/0 mice to LPS-induced septic shock compared to PrPC expressing mice2 associated with hyperactive inflammatory responses36. Our data add to the global idea that PrPC exerts stress protection in a physiological context37–40 by adjusting the cell response to sTNFα of endogenous or exogenous origin.
We further evidence that the protective role of PrPC towards sTNFα also depends on its capacity to maintain TACE α-secretase at the plasma membrane. The rise of sensitivity of PrPnull-cells to exogenous sTNFα is associated with an increased level of TNFR1 molecules present at the plasma membrane caused by deficit of TACE activity. From a mechanistic point of view, defect of TACE shedding activity in PrPnull-cells originates from the displacement of TACE from the plasma membrane to intracellular compartments. The internalization of TACE in the absence of PrPC depends on a gain of plasma membrane β1 integrin signaling. Lowering β1 integrin activity in PrPnull-cells directs TACE back to the plasma membrane and rescues TACE-mediated TNFR1 shedding. Acting as a scaffolding protein, PrPC limits β1 integrin microclustering at the plasma membrane and negatively regulates β1 integrin signaling6. We further show that in the absence of PrPC excessive β1 integrin signaling and downstream ROCK-I overactivity promote overactivation of PDK1, which in turn triggers TACE internalization. Our work thus supports the view that PrPC cytoprotective effect against sTNFα toxicity is intimately linked to functional interactions between PrPC and β1 integrin.
In prion diseases, it is now widely acknowledged that the subversion of PrPC normal functions by PrPSc takes a critical part in neuronal cell demise41–45. Whether loss of PrPC function upon its conversion into PrPSc or gain of PrPC function by PrPSc lies at the root of neurodegeneration is still debated. The phenotypic proximity of PrPnull-cells with prion-infected cells lends support for loss-of-PrPC cytoprotective role towards TACE-mediated TNFR1 shedding along TSEs. Of note, is the increased sensitivity to sTNFα toxicity related to plasma membrane TNFR1 overexpression13 highly comparable between PrPnull- and prion-infected cells. Correlatively, the overactivation of ROCK-I and PDK1, as well as subsequent internalization of TACE13, 21 occur with comparable intensities between PrPC-depleted and prion-infected cells. Such a loss-of-PrPC cytoprotective function towards inflammation in TSEs is further supported in vivo by increase in PDK1 activity and deficit of TNFR1 shedding in the brain of PrP0/0 mice (Fig. 5a,b), as for prion-infected mice13. The exaggerated sensitivity to sTNFα-induced inflammation can be reversed upon PDK1 inhibition similarly between PrP0/0 mice (Fig. 5c) and prion-infected mice13.
Contrasting with other amyloid-based neurodegenerative diseases, inflammation in TSEs is atypical-qualified46 with low levels of inflammatory cytokines (sTNFα, IL1, IL6) released by activated microglia47, 48 in response to diverse signals emitted by prion-infected neurons49. Our data support the view that abrogation of PrPC cytoprotective function against sTNFα by PrPSc is a priming event that renders prion-infected neurons sensitive to low doses of sTNFα. In line with this, the quickened death of prion-infected mice challenged with LPS50, 51 could be due to an accelerated degeneration of TNFR1 overexposing infected neurons provoked by the LPS-induced release of sTNFα by reactive microglial cells or peripheral production of sTNFα.
Methods
Antibodies
The mouse monoclonal SAF61 PrP antibody was from SPI-Bio (Montigny le Bretonneux, France). The rabbit polyclonal antibody to TNFR1 was from MBL International (Woburn, MA, USA). Rabbit polyclonal antibodies to TACE and active caspase-3 were purchased from QED Bioscience Inc. (San Diego, CA, USA) and Biovision (Mountain View, CA, USA), respectively. The rabbit polyclonal antibody to caveolin-1 (Cav-1) (610059) was obtained from Transduction Laboratories (Lexington, KY, USA). The rabbit polyclonal anti-MAP2 antibody and the mouse monoclonal neutralizing antibody towards β1 integrins (MAB1965) were from EMD Millipore (Darmstadt, Germany). The mouse monoclonal anti-actin antibody was from Novus Biologicals (Littleton, CO, USA). The rabbit monoclonal ROCK-I and polyclonal PDK1 antibodies were from Cell Signaling (Beverly, MA, USA). When non-specified, primary antibodies were used at 0.5 µg ml−1 for Western blot experiments and at 5 µg ml−1 for immunofluorescence experiments.
Mice
Adult wild type FVB and PrP0/0 FVB mice were bred and underwent experiments, respecting European guidelines for the care and ethical use of laboratory animals (Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes). All animal procedures were approved by the Animal Care and Use Committee at Basel University (Switzerland).
Treatment of mice and sample collection
Recombinant murine sTNFα (Biosource International, Camarillo, CA, USA) was intracerebroventricular (icv) injected at a dose of 200 ng in 10 µl saline buffer in combination or not with the PDK1 inhibitor (1 µM). At either 24 h following saline or sTNFα injection combined or not with BX912, mouse CSF was collected from the cisterna magna under anesthesia with 3% isoflurane.
Measure of CSF tryptophan and kynurenine concentrations
CSF was analyzed by HPLC to quantify tryptophan (Trp) and kynurenine (Kyn). Briefly CSF samples were deproteinized by treatment with 86% methanol (1:6, vol/vol) to avoid Kyn diazotization induced by the usual acidic treatment of samples52. The resulting supernatant was filtered through 0.2 µm nylon membranes. Chomatography was performed with a ThermoFinnigan solvent delivery Spectra Series P100 pump. Sample injection was controlled by a Spark Holland Triathlon autosampler. A C18 reverse-phase HPLC column (Supelcosil LC-18-DB, 15 cm × 4.6 mm, 3 µm bead size; Supelco, Buchs, Switzerland) with a guard column (Supelguard LC-18-DB, 2 cm; Supelco, Buchs, Switzerland). Trp catabolites were eluted isocratically at a flow rate of 0.8 ml min−1 with a mobile phase consisting of a 94:6 mixture (by volume) of 16.2 mmol l−1 KH2PO4 and acetonitrile. The coulometric detection system consisted of a thin-layer flow-through electrochemical ESA Coulochem II detector connected to an ESA Model 5011 analytical cell containing two working electrodes made of porous graphite. The analytical cell voltage was set at +0.45 V for the first detector and +0.60 V for the second detector. Kyn and Trp were detected at +0.60 V. Chromatograms were generated and analyzed using D-7000 HPLC System Manager software.
Soluble TNFα receptor type 1 (sTNFR1) quantification
The amount of soluble TNFR1 was measured in cell culture media or CSF by ELISA using the Mouse/Rat TNFR1/TNFRSF1A Quantikine ELISA kit (MRT10) according to the manufacturer’s instructions (R&D System, Minneapolis, MN, USA).
Cell culture
1C11 cells were grown and induced to differentiate along the serotonergic (1C115-HT) pathway as described in ref. 15. Primary CGNs were isolated from dissociated cerebella of 4–5 days-old FVB and PrP0/0 FVB mice as in ref. 53.
Cell viability assays
The viability of ~1.105 1C11 or 1C115-HT cells expressing or not PrPC exposed to recombinant murine sTNFα (Biosource International, Camarillo, CA, USA) was evaluated by the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Invitrogen, Carlsbad, CA, USA)13.
Neuronal dysfunction in CGNs and PrP0/0-CGNs was evaluated by sTNFα-induced dendritic fragmentation. CGNs and PrP0/0-CGNs seeded (5.105 cells per well) in 12-well plates coated with polyD-lysine (Sigma-Aldrich, St. Louis, MO, USA) were exposed to sTNFα. Cells were then fixed and stained with an anti-MAP2 antibody. After imaging with a fluorescence microscope (Zeiss Leica), cells exhibiting fragmented vs. non-fragmented dendrites were counted using ImageJ software (http://rsb.info.nih.gov/ij).
Immunofluorescent experiments
Immunofluorescent labelings of PrPC, TNFR1, TACE and MAP2 were performed using standard protocols. Briefly, for cell surface detection of PrPC, TNFR1 and TACE, cells grown on glass coverslides were washed with cold PBS and fixed with 3.6% formaldehyde. Cells were incubated for 1 h at room temperature with the primary antibody in blocking buffer (PBS enriched with 2% FCS) and then with AlexaFluor 488-conjugated secondary immunoglobulins (1 µg ml−1; Molecular Probes, Eugene, OR, USA). For the intracellular detection of TACE or MAP2, cells fixed with 3.6% formaldehyde were permeabilized with 0.05% saponin or 0.1% Triton X-100, respectively, in PBS for 15 min at room temperature prior TACE or MAP2 immunostaining. Cell preparations were mounted under coverslips with Fluoromount G (Fisher Scientific, Pittsburgh, PA, USA) and analyzed by wide-field indirect immunofluorescence using a Leica DMI6000 B microscope (Wetzlar, Germany). For all images, out-of-focus haze was reduced by digital deconvolution of sets of 16 serial optical sections recorded at 0.3 µm intervals using the Adaptative Blind Deconvolution in the program Autoquant X (Meyer Instruments, Houston, TX, USA). All pixel values in each focal plane were then summed along z-axis to obtain the final image. Deconvoluted images were subjected to image analysis with the AQUA software54.
Cell extract preparation and western blot analyses
Cells were washed in PBS/Ca/Mg and incubated for 30 min at 4 °C in lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1 mM Na3VO4 and protease inhibitors [Roche]). After centrifugation of the lysate (14,000 × g, 15 min), the concentration of the proteins in the supernatant was measured with the bicinchoninic acid method (Pierce, Rockford, IL, USA).
PrPC silencing and enzyme inhibition
We exploited 1C11 precursor cells stably expressing shRNA towards PrPC in which PrPC expression is repressed by more than 90% (referred to as PrPnull-1C11 cells)6. Because PrPnull-1C11 cells fail to implement a neuronal phenotype upon exposure to serotonergic inducers6, 1C11 cells were converted into serotonergic 1C115-HT neuronal cells and then transfected with a siRNA against PrPC 41 using lipofectamine 2000 reagent following manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). These cells refer to as PrPnull-1C115-HT cells.
NADPH oxidase activity was switched off using Diphenyleneiodonium (DPI). TACE activity was inhibited with TNFα processing inhibitor-2 (TAPI-2; Peptides International, Louisville, KY, USA). ADAM10 activity was blocked with GI254023X (Tocris Bioscience, Ellisville, MO, USA). γ-secretase activity was inhibited using DAPT (Tocris Bioscience, Ellisville, MO, USA). Guanylate cyclase activity was antagonized using NS-2028 (Tocris Bioscience, Ellisville, MO, USA). ROCK activity was inhibited with Y-27632 (Tocris Bioscience, Ellisville, MO, USA). PDK1 activity was switched-off with BX912 (Axon Medchem BV, Groningen, The Netherlands).
Immunoelectron microscopy
Cells, grown to ~80% confluency, were rinsed twice with PBS, collected in PBS and 10 mM EDTA, and rinsed twice with PBS. The cell pellet was fixed with 0.2% phosphate-buffered glutaraldehyde for 20–120 s and blocked with bovine albumin. Processing of cells for ultrathin cryosectioning and immunolabeling was performed indirectly55, with 5- or 7-nm gold particles conjugated with affinity-purified goat anti-mouse or anti-rabbit IgG (Invitrogen, Carlsbad, CA, USA)56. The labeled specimens were negatively stained with sodium silicotungstate, and images were captured with a JEOL CX100 transmission electron microscope.
RT-PCR analyses
RNA was isolated by using the RNase Easy Kit (Qiagen), including a RNase-free DNase I digestion step, as recommended by the manufacturer. For RT-PCR analysis, first-strand cDNA was synthesized from 5 µg of RNA with oligo(dT)17 primer, using 400 U of Superscript II reverse transcriptase (Invitrogen). PCR amplifications were then carried out in a 25 µl volume containing 1 µl of the reverse transcription products, using TaqDNA polymerase (Invitrogen). PCR products were analyzed on 1% agarose gels. Primers used for the PCR reactions included GAPDH, forward 5′-TGAAGGTCGGTGTGAACGGAT-3′ and reverse 5′-CATGTAGGCCATGAGGTCCAC-3′; TACE, forward 5′-CCAGCATCTGCTAAGTTGCTTCC-3′ and reverse 5′-CAGCACAGCTGCCAAGTCCTT-3′.
ROCK-I immunoprecipitation
ROCK-I immunoprecipitation was performed according to standard protocols by using protein A-Sepharose beads (Amersham Pharmacia Biotech, Picataway, NJ, USA) coupled to anti-ROCK-I antibody and 100 μg of cell lysates or brain extracts. Immunoprecipitates were analyzed by western blotting using anti-ROCK-I and anti-PDK1 antibodies.
Cell metabolic labeling with [32P]-orthophosphate
[32P]-orthophosphate labeling was performed as in ref. 57. Briefly, the cell culture medium was removed and cells were thoroughly washed with phosphate-free DMEM to eliminate any residual phosphate containing medium. [32P]-orthophosphate (40.7 Gbq mmol−1, GE Healthcare, Little Chalfont, UK) was added to the cell culture at a final concentration of 18.5 Mbq ml−1. After 2 h, the labeling medium was removed and the cells were lyzed after extensive washing.
Measurement of PDK1 activity
PDK1 activity was measured in cell lysates or brain extracts using a fluorescent-labeled PDK1 substrate (5FAM-ARKRERTYSFGHHA-COOH, Caliper Life Sciences, Hanover, MD, USA)58. The relative amounts of substrate peptide and product phospho-peptide were determined using a Caliper EZ-reader (Caliper Life Sciences, Hanover, MD, USA).
Data analysis
An analysis of variance of the cell/animal response group was performed using the Kaleidagraph software (Synergy Software, Reading, PA, USA). Values are given as means ± SEM. Significant responses (P < 0.05) are marked by symbols (#, *) and their corresponding p-values are provided in figure legends. When non-specified experiments were performed in three to five times in triplicates.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
We thank M. Bühler, N. Pierron, B. Gieux, F. d’Agostini and R. Kettler for skillfull methodological assistance and stimulating discussions. This work was supported by Inserm, the French Agence Nationale de la Recherche - ANR (Prions&SensiTNF, no. 131201), the European Joint Program on Neurodegenerative Diseases (PrPC & PDK1, no. ANR-14-JPCD-0003-01) and the patient association LECMA-Vaincre Alzheimer (no. FR-15033). JE is funded by Domaine d’Intérêt Majeur - Cerveau et Pensée - Région Ile de France. AAB is a post-doctoral fellow of the ANR (mirDEP, no. ANR-13-SAMA-0001-01).
Author Contributions
J.E., F.B.D., M.P., A.B., V.B., A.A.B., N.D., J.M.L. and B.S. did the experiments. J.E., F.B.D., M.P., A.B., V.B., A.A.B., J.M.L. and B.S. analyzed the data. J.M.L. and B.S. designed the experiments. M.P., A.B., O.K. and B.S. wrote the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08110-x
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen WW, Zhang X, Huang WJ. Role of neuroinflammation in neurodegenerative diseases (Review) Mol Med Rep. 2016;13:3391–3396. doi: 10.3892/mmr.2016.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J, et al. Prion protein participates in the protection of mice from lipopolysaccharide infection by regulating the inflammatory process. J Mol Neurosci. 2015;55:279–287. doi: 10.1007/s12031-014-0319-2. [DOI] [PubMed] [Google Scholar]
- 3.Shi F, et al. Prion protein participates in the regulation of classical and alternative activation of BV2 microglia. J Neurochem. 2013;124:168–174. doi: 10.1111/jnc.12053. [DOI] [PubMed] [Google Scholar]
- 4.Linden R, et al. Physiology of the prion protein. Physiol Rev. 2008;88:673–728. doi: 10.1152/physrev.00007.2007. [DOI] [PubMed] [Google Scholar]
- 5.Schneider, B. et al. Understanding the neurospecificity of Prion protein signaling. Front Biosci16, 169–186, doi:3682 (2011). [DOI] [PubMed]
- 6.Loubet, D. et al. Neuritogenesis: the prion protein controls beta1 integrin signaling activity. Faseb J26, 678–690, doi:fj.11-185579 (2012). [DOI] [PubMed]
- 7.Mouillet-Richard S, et al. Signal transduction through prion protein. Science. 2000;289:1925–1928. doi: 10.1126/science.289.5486.1925. [DOI] [PubMed] [Google Scholar]
- 8.Schneider B, et al. NADPH oxidase and extracellular regulated kinases 1/2 are targets of prion protein signaling in neuronal and nonneuronal cells. Proc Natl Acad Sci USA. 2003;100:13326–13331. doi: 10.1073/pnas.2235648100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pradines E, et al. Cellular prion protein coupling to TACE-dependent TNF-alpha shedding controls neurotransmitter catabolism in neuronal cells. J Neurochem. 2009;110:912–923. doi: 10.1111/j.1471-4159.2009.06176.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang, Y., Herrera, A. H., Li, Y., Belani, K. K. & Walcheck, B. Regulation of mature ADAM17 by redox agents for L-selectin shedding. J Immunol182, 2449–2457, doi:182/4/2449 (2009). [DOI] [PMC free article] [PubMed]
- 11.Reddy, P. et al. Functional analysis of the domain structure of tumor necrosis factor-alpha converting enzyme. J Biol Chem275, 14608–14614, doi:275/19/14608 [pii] (2000). [DOI] [PubMed]
- 12.Scheller J, Chalaris A, Garbers C, Rose-John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32:380–387. doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Pietri, M. et al. PDK1 decreases TACE-mediated alpha-secretase activity and promotes disease progression in prion and Alzheimer’s diseases. Nat Med19, 1124–1131, doi:nm.3302 (2013). [DOI] [PubMed]
- 14.MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal. 2002;14:477–492. doi: 10.1016/S0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- 15.Mouillet-Richard S, et al. Regulation by neurotransmitter receptors of serotonergic or catecholaminergic neuronal cell differentiation. J Biol Chem. 2000;275:9186–9192. doi: 10.1074/jbc.275.13.9186. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, et al. Reactive oxygen species mediate tumor necrosis factor alpha-converting, enzyme-dependent ectodomain shedding induced by phorbol myristate acetate. Faseb J. 2001;15:303–305. doi: 10.1096/fj.01-0260com. [DOI] [PubMed] [Google Scholar]
- 17.Deng M, Loughran PA, Zhang L, Scott MJ, Billiar TR. Shedding of the tumor necrosis factor (TNF) receptor from the surface of hepatocytes during sepsis limits inflammation through cGMP signaling. Sci Signal. 2015;8:ra11. doi: 10.1126/scisignal.2005548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang WS, et al. 1,25-dihydroxyvitamin D3 causes ADAM10-dependent ectodomain shedding of tumor necrosis factor receptor 1 in vascular smooth muscle cells. Mol Pharmacol. 2015;87:533–542. doi: 10.1124/mol.114.097147. [DOI] [PubMed] [Google Scholar]
- 19.Chhibber-Goel J, et al. gamma-Secretase Activity Is Required for Regulated Intramembrane Proteolysis of Tumor Necrosis Factor (TNF) Receptor 1 and TNF-mediated Pro-apoptotic Signaling. J Biol Chem. 2016;291:5971–5985. doi: 10.1074/jbc.M115.679076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorburn, A. Death receptor-induced cell killing. Cell Signal16, 139–144, doi:S0898656803001475 (2004). [DOI] [PubMed]
- 21.Alleaume-Butaux A, et al. Double-Edge Sword of Sustained ROCK Activation in Prion Diseases through Neuritogenesis Defects and Prion Accumulation. PLoS Pathog. 2015;11:e1005073. doi: 10.1371/journal.ppat.1005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pantera B, et al. PrPc activation induces neurite outgrowth and differentiation in PC12 cells: role for caveolin-1 in the signal transduction pathway. J Neurochem. 2009;110:194–207. doi: 10.1111/j.1471-4159.2009.06123.x. [DOI] [PubMed] [Google Scholar]
- 23.Salani B, et al. IGF-I induced rapid recruitment of integrin beta1 to lipid rafts is Caveolin-1 dependent. Biochem Biophys Res Commun. 2009;380:489–492. doi: 10.1016/j.bbrc.2009.01.102. [DOI] [PubMed] [Google Scholar]
- 24.Park JH, Ryu JM, Han HJ. Involvement of caveolin-1 in fibronectin-induced mouse embryonic stem cell proliferation: role of FAK, RhoA, PI3K/Akt, and ERK 1/2 pathways. J Cell Physiol. 2011;226:267–275. doi: 10.1002/jcp.22338. [DOI] [PubMed] [Google Scholar]
- 25.Kim HJ, et al. Regulation of RhoA activity by the cellular prion protein. Cell Death Dis. 2017;8:e2668. doi: 10.1038/cddis.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mould AP, et al. Integrin activation involves a conformational change in the alpha 1 helix of the beta subunit A-domain. J Biol Chem. 2002;277:19800–19805. doi: 10.1074/jbc.M201571200. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez AM, Claiborne J, Jones JC. Integrin cross-talk in endothelial cells is regulated by protein kinase A and protein phosphatase 1. J Biol Chem. 2008;283:31849–31860. doi: 10.1074/jbc.M801345200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dransfield I, Cabanas C, Craig A, Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol. 1992;116:219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu C, Shimaoka M, Zang Q, Takagi J, Springer TA. Locking in alternate conformations of the integrin alphaLbeta2 I domain with disulfide bonds reveals functional relationships among integrin domains. Proc Natl Acad Sci USA. 2001;98:2393–2398. doi: 10.1073/pnas.041618598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connor TJ, Starr N, O’Sullivan JB, Harkin A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: a role for IFN-gamma? Neurosci Lett. 2008;441:29–34. doi: 10.1016/j.neulet.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Vecsei L, Szalardy L, Fulop F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov. 2013;12:64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- 32.Parrott JM, et al. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry. 2016;6:e918. doi: 10.1038/tp.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stella, R., Massimino, M. L., Sandri, M., Sorgato, M. C. & Bertoli, A. Cellular prion protein promotes regeneration of adult muscle tissue. Mol Cell Biol30, 4864–4876, doi:MCB.01040-09 (2010). [DOI] [PMC free article] [PubMed]
- 34.Van Zee KJ, et al. Effects of intravenous IL-8 administration in nonhuman primates. J Immunol. 1992;148:1746–1752. [PubMed] [Google Scholar]
- 35.McMahan RS, Riehle KJ, Fausto N, Campbell JS. A disintegrin and metalloproteinase 17 regulates TNF and TNFR1 levels in inflammation and liver regeneration in mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G25–34. doi: 10.1152/ajpgi.00326.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rittirsch D, et al. Acute lung injury induced by lipopolysaccharide is independent of complement activation. J Immunol. 2008;180:7664–7672. doi: 10.4049/jimmunol.180.11.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuwahara C, et al. Prions prevent neuronal cell-line death. Nature. 1999;400:225–226. doi: 10.1038/22241. [DOI] [PubMed] [Google Scholar]
- 38.McLennan, N. F. et al. Prion protein accumulation and neuroprotection in hypoxic brain damage. Am J Pathol165, 227–235, doi:S0002-9440(10)63291-9 (2004). [DOI] [PMC free article] [PubMed]
- 39.Roucou X, et al. Neuroprotective functions of prion protein. J Neurosci Res. 2004;75:153–161. doi: 10.1002/jnr.10864. [DOI] [PubMed] [Google Scholar]
- 40.Lopes, M. H. et al. Interaction of cellular prion and stress-inducible protein 1 promotes neuritogenesis and neuroprotection by distinct signaling pathways. J Neurosci25, 11330–11339, doi:25/49/11330 (2005). [DOI] [PMC free article] [PubMed]
- 41.Pietri M, et al. Overstimulation of PrPC signaling pathways by prion peptide 106-126 causes oxidative injury of bioaminergic neuronal cells. J Biol Chem. 2006;281:28470–28479. doi: 10.1074/jbc.M602774200. [DOI] [PubMed] [Google Scholar]
- 42.Mallucci G, et al. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science. 2003;302:871–874. doi: 10.1126/science.1090187. [DOI] [PubMed] [Google Scholar]
- 43.Chesebro B, et al. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science. 2005;308:1435–1439. doi: 10.1126/science.1110837. [DOI] [PubMed] [Google Scholar]
- 44.Rambold AS, et al. Stress-protective signalling of prion protein is corrupted by scrapie prions. EMBO J. 2008;27:1974–1984. doi: 10.1038/emboj.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pradines E, et al. Pathogenic prions deviate PrP(C) signaling in neuronal cells and impair A-beta clearance. Cell Death Dis. 2013;4:e456. doi: 10.1038/cddis.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perry VH, Cunningham C, Boche D. Atypical inflammation in the central nervous system in prion disease. Curr Opin Neurol. 2002;15:349–354. doi: 10.1097/00019052-200206000-00020. [DOI] [PubMed] [Google Scholar]
- 47.Mabbott NA, McGovern G, Jeffrey M, Bruce ME. Temporary blockade of the tumor necrosis factor receptor signaling pathway impedes the spread of scrapie to the brain. J Virol. 2002;76:5131–5139. doi: 10.1128/JVI.76.10.5131-5139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tribouillard-Tanvier D, et al. Early cytokine elevation, PrPres deposition, and gliosis in mouse scrapie: no effect on disease by deletion of cytokine genes IL-12p40 and IL-12p35. J Virol. 2012;86:10377–10383. doi: 10.1128/JVI.01340-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marella M, et al. Pathological prion protein exposure switches on neuronal mitogen-activated protein kinase pathway resulting in microglia recruitment. J Biol Chem. 2005;280:1529–1534. doi: 10.1074/jbc.M410966200. [DOI] [PubMed] [Google Scholar]
- 50.Cunningham, C., Wilcockson, D. C., Campion, S., Lunnon, K. & Perry, V. H. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci25, 9275–9284, doi:25/40/9275 (2005). [DOI] [PMC free article] [PubMed]
- 51.Murray, C. L., Skelly, D. T. & Cunningham, C. Exacerbation of CNS inflammation and neurodegeneration by systemic LPS treatment is independent of circulating IL-1beta and IL-6. J Neuroinflammation8, 50, doi:1742-2094-8-50 (2011). [DOI] [PMC free article] [PubMed]
- 52.Hara T, et al. Diazotization of kynurenine by acidified nitrite secreted from indoleamine 2,3-dioxygenase-expressing myeloid dendritic cells. J Immunol Methods. 2008;332:162–169. doi: 10.1016/j.jim.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 53.Cronier S, Beringue V, Bellon A, Peyrin JM, Laude H. Prion strain- and species-dependent effects of antiprion molecules in primary neuronal cultures. J Virol. 2007;81:13794–13800. doi: 10.1128/JVI.01502-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCabe, A., Dolled-Filhart, M., Camp, R. L. & Rimm, D. L. Automated quantitative analysis (AQUA) of in situ protein expression, antibody concentration, and prognosis. J Natl Cancer Inst97, 1808–1815, doi:97/24/1808 (2005). [DOI] [PubMed]
- 55.Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slot JW, Geuze HJ. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985;38:87–93. [PubMed] [Google Scholar]
- 57.Moore, D. D. & BM, S. Analysis of protein phosphorylation. Vol. 78:18.0:18.0.1–18.0.2 (J Wiley & Sons, Inc, 1997).
- 58.Hofler, A. et al. Study of the PDK1/AKT signaling pathway using selective PDK1 inhibitors, HCS, and enhanced biochemical assays. Anal Biochem414, 179–186, doi:S0003-2697(11)00170-9 (2011). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


