Abstract
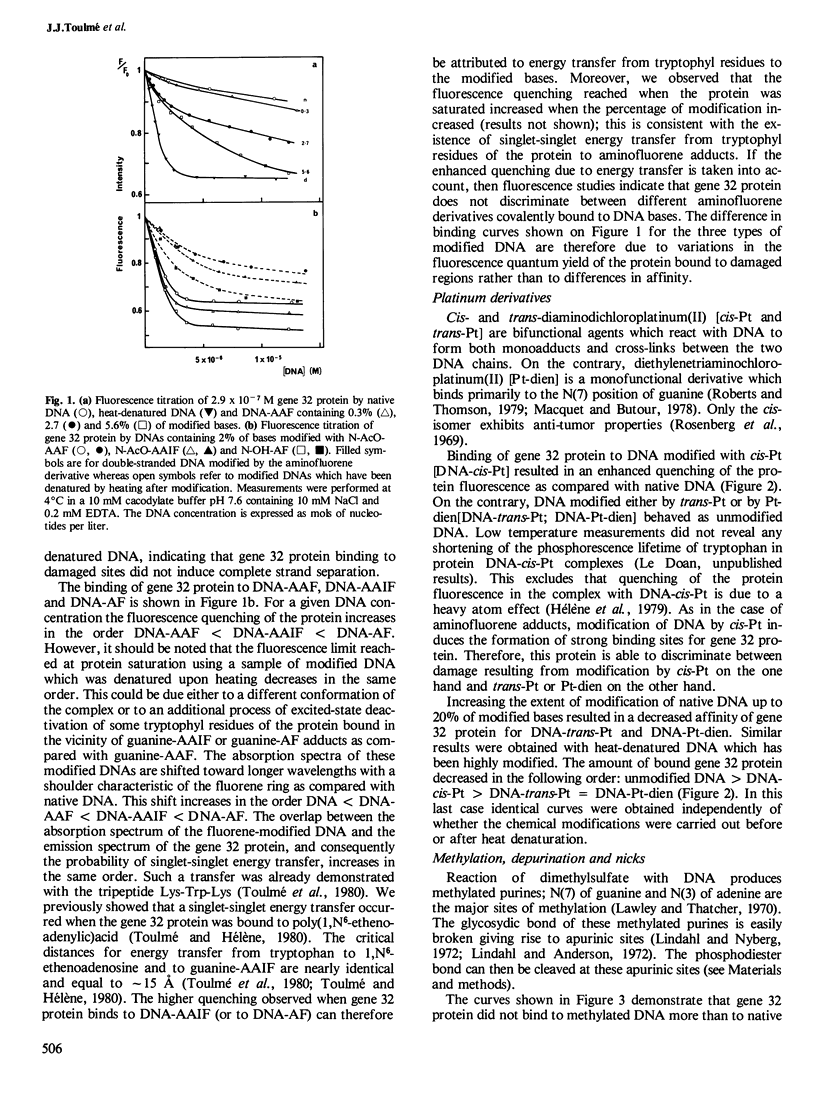
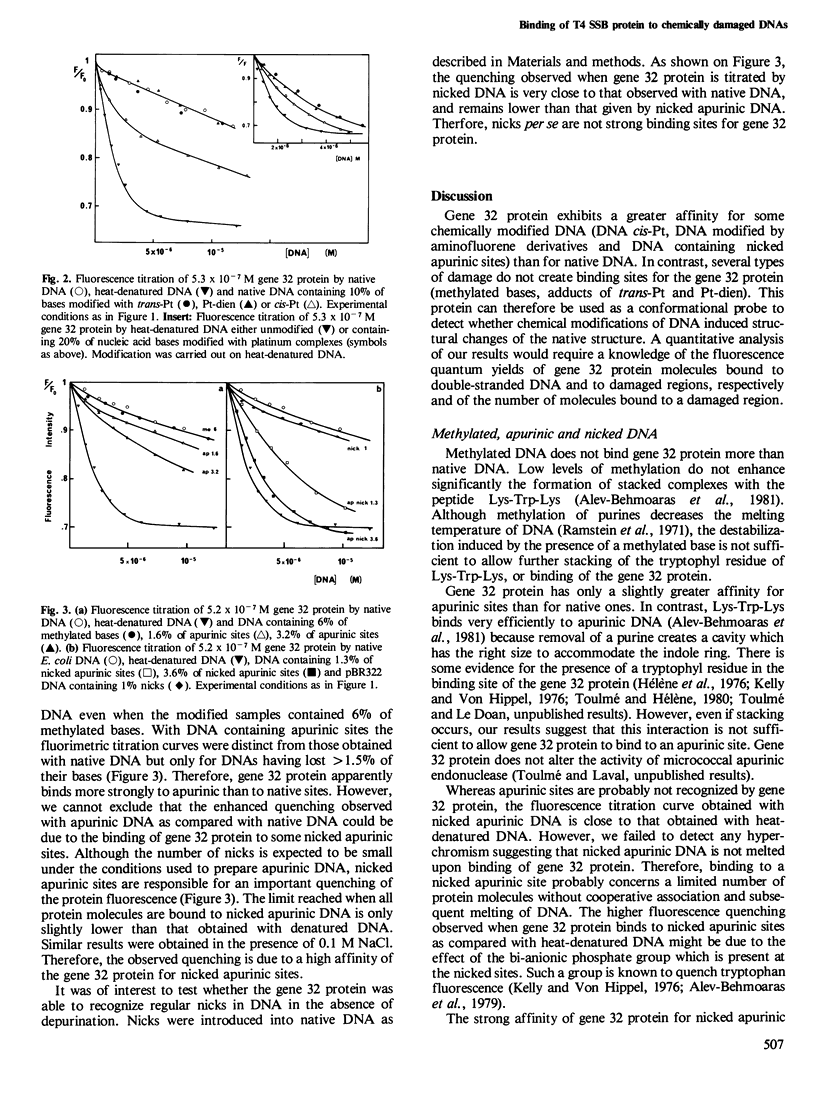
We have used fluorescence spectroscopy to investigate the binding of gene 32 protein from bacteriophage T4 to DNA which has been chemically modified with carcinogens or antitumor drugs. This protein exhibits a high specificity for single-stranded nucleic acids and binds more efficiently to DNA modified either with cis-diaminodichloroplatinum(II) or with aminofluorene derivatives than to native DNA. This increased affinity is related to the formation of locally unpaired regions which are strong binding sites for the single-strand binding protein. In contrast, gene 32 protein has the same affinity for native DNA, DNA containing methylated purines and DNA that has reacted with trans-diaminodichloroplatinum(II) or with chlorodiethylenetriaminoplatinum(II) chloride. These types of damage do not induce a sufficient structural change to allow gene 32 protein binding. Depurination of DNA does not create binding sites for the T4 gene 32 protein but nicked apurinic sites are strong ligands for the protein. This T4 single-strand binding protein does not exhibit a significantly increased affinity for nicked DNA as compared with native DNA. These results are discussed with respect to the recognition of DNA damage by proteins involved in DNA repair and to the possible role of single-strand binding proteins in DNA repair mechanisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Alev-Behmoaras T., Toulmé J. J., Hélène C. Effect of phosphate ions on the fluorescence of tryptophan derivatives. Implications in fluorescence investigation of protein-nucleic acid complexes. Biochimie. 1979;61(8):957–960. doi: 10.1016/s0300-9084(79)80246-1. [DOI] [PubMed] [Google Scholar]
- Baldy M. W. The UV sensitivity of some early-function temperature-sensitive mutants of phage T4. Virology. 1970 Feb;40(2):272–287. doi: 10.1016/0042-6822(70)90403-4. [DOI] [PubMed] [Google Scholar]
- Behmoaras T., Toulme J. J., Helene C. Specific recognition of apurinic sites in DNA by a tryptophan-containing peptide. Proc Natl Acad Sci U S A. 1981 Feb;78(2):926–930. doi: 10.1073/pnas.78.2.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun F., Toulmé J. J., Hélène C. Interactions of aromatic residues of proteins with nucleic acids. Fluorescence studies of the binding of oligopeptides containing tryptophan and tyrosine residues to polynucleotides. Biochemistry. 1975 Feb 11;14(3):558–563. doi: 10.1021/bi00674a015. [DOI] [PubMed] [Google Scholar]
- Delius H., Mantell N. J., Alberts B. Characterization by electron microscopy of the complex formed between T4 bacteriophage gene 32-protein and DNA. J Mol Biol. 1972 Jun 28;67(3):341–350. doi: 10.1016/0022-2836(72)90454-8. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P., Daune M. P. Dynamic structure of DNA modified with the carcinogen N-acetoxy-n-2-acetylaminofluorene. Biochemistry. 1974 Oct 8;13(21):4435–4440. doi: 10.1021/bi00718a028. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P. In vitro recognition of carcinogen-induced local denaturation sites native DNA by S1 endonuclease from Aspergillus oryzae. Nature. 1975 Sep 11;257(5522):151–152. doi: 10.1038/257151a0. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P., Lefevre J. F., Pouyet J., Daune M. P. Comparative orientation of the fluorene residue in native DNA modified by N-acetoxy-N-2-acetylaminofluorene and two 7-halogeno derivatives. Biochemistry. 1976 Jul 27;15(15):3347–3351. doi: 10.1021/bi00660a027. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Daune M. Physical studies on deoxyribonucleic acid after covalent binding of a carcinogen. Biochemistry. 1972 Jul 4;11(14):2659–2666. doi: 10.1021/bi00764a017. [DOI] [PubMed] [Google Scholar]
- Helene C., Toulme F., Charlier M., Yaniv M. Photosensitized splitting of thymine dimers in DNA by gene 32 protein from phage T 4. Biochem Biophys Res Commun. 1976 Jul 12;71(1):91–98. doi: 10.1016/0006-291x(76)90253-9. [DOI] [PubMed] [Google Scholar]
- Helene C., Toulme J. J., Le Doan T. A spectroscopic probe of stacking interactions between nucleic acid bases and tryptophan residues of proteins. Nucleic Acids Res. 1979 Dec 11;7(7):1945–1954. doi: 10.1093/nar/7.7.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda J., Moise H. Purification and physicochemical properties of limited proteolysis products of T4 helix destabilizing protein (gene 32 protein). J Biol Chem. 1978 Oct 25;253(20):7547–7558. [PubMed] [Google Scholar]
- Huberman J. A., Kornberg A., Alberts B. M. Stimulation of T4 bacteriophage DNA polymerase by the protein product of T4 gene 32. J Mol Biol. 1971 Nov 28;62(1):39–52. doi: 10.1016/0022-2836(71)90129-x. [DOI] [PubMed] [Google Scholar]
- Jensen D. E., Kelly R. C., von Hippel P. H. DNA "melting" proteins. II. Effects of bacteriophage T4 gene 32-protein binding on the conformation and stability of nucleic acid structures. J Biol Chem. 1976 Nov 25;251(22):7215–7228. [PubMed] [Google Scholar]
- Kelly R. C., von Hippel P. H. DNA "melting" proteins. III. Fluorescence "mapping" of the nucleic acid binding site of bacteriophage T4 gene 32-protein. J Biol Chem. 1976 Nov 25;251(22):7229–7239. [PubMed] [Google Scholar]
- Kowalczykowski S. C., Lonberg N., Newport J. W., von Hippel P. H. Interactions of bacteriophage T4-coded gene 32 protein with nucleic acids. I. Characterization of the binding interactions. J Mol Biol. 1981 Jan 5;145(1):75–104. doi: 10.1016/0022-2836(81)90335-1. [DOI] [PubMed] [Google Scholar]
- Kozinski A. W., Felgenhauer Z. Z. Molecular recombination in T4 bacteriophage deoxyribonucleic acid. II. Single-strand breaks and exposure of uncomplemented areas as a prerequisite for recombination. J Virol. 1967 Dec;1(6):1193–1202. doi: 10.1128/jvi.1.6.1193-1202.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriek E., Miller J. A., Juhl U., Miller E. C. 8-(N-2-fluorenylacetamido)guanosine, an arylamidation reaction product of guanosine and the carcinogen N-acetoxy-N-2-fluorenylacetamide in neutral solution. Biochemistry. 1967 Jan;6(1):177–182. doi: 10.1021/bi00853a029. [DOI] [PubMed] [Google Scholar]
- Krisch H. M., Van Houwe G. Stimulation of the synthesis of bacteriophage T4 gene 32 protein by ultraviolet light irradiation. J Mol Biol. 1976 Nov;108(1):67–81. doi: 10.1016/s0022-2836(76)80095-2. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Thatcher C. J. Methylation of deoxyribonucleic acid in cultured mammalian cells by N-methyl-N'-nitro-N-nitrosoguanidine. The influence of cellular thiol concentrations on the extent of methylation and the 6-oxygen atom of guanine as a site of methylation. Biochem J. 1970 Feb;116(4):693–707. doi: 10.1042/bj1160693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre J. F., Fuchs R. P., Daune M. P. Comparative studies on the 7-iodo and 7-fluoro derivatives of N-acetoxy-N-2-acetylaminofluorene: binding sites on DNA and conformational change of modified deoxytrinucleotides. Biochemistry. 1978 Jun 27;17(13):2561–2567. doi: 10.1021/bi00606a016. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Andersson A. Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3618–3623. doi: 10.1021/bi00769a019. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Malfoy B., Hartmann B., Leng M. The B goes to Z transition of poly(dG-dC) . poly(dG-dC) modified by some platinum derivatives. Nucleic Acids Res. 1981 Nov 11;9(21):5659–5669. doi: 10.1093/nar/9.21.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Nossal N. G. DNA synthesis on a double-stranded DNA template by the T4 bacteriophage DNA polymerase and the T4 gene 32 DNA unwinding protein. J Biol Chem. 1974 Sep 10;249(17):5668–5676. [PubMed] [Google Scholar]
- Paoletti C., LePecq J. B., Lehman I. R. The use of ethidium bromide-circular DNA complexes for the fluorometric analysis of breakage and joining of DNA. J Mol Biol. 1971 Jan 14;55(1):75–100. doi: 10.1016/0022-2836(71)90282-8. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramstein J., Héléne C., Leng M. A study of chemically methylated deoxyribonucleic acid. Eur J Biochem. 1971 Jul 15;21(1):125–136. doi: 10.1111/j.1432-1033.1971.tb01448.x. [DOI] [PubMed] [Google Scholar]
- Roberts J. J., Thomson A. J. The mechanism of action of antitumor platinum compounds. Prog Nucleic Acid Res Mol Biol. 1979;22:71–133. doi: 10.1016/s0079-6603(08)60799-0. [DOI] [PubMed] [Google Scholar]
- Sage E., Leng M. Conformation of poly(dG-dC) . poly(dG-dC) modified by the carcinogens N-acetoxy-N-acetyl-2-aminofluorene and N-hydroxy-N-2-aminofluorene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4597–4601. doi: 10.1073/pnas.77.8.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage E., Spodheim-Maurizot M., Rio P., Leng M., Fuchs R. P. Discrimination by antibodies between local defects in DNA induced by 2-aminofluorene derivatives. FEBS Lett. 1979 Dec 1;108(1):66–68. doi: 10.1016/0014-5793(79)81180-1. [DOI] [PubMed] [Google Scholar]
- Santella R. M., Kriek E., Grunberger D. Circular dichroism and proton magnetic resonance studies of dApdG modified with 2-aminofluorene and 2-acetylaminofluorene. Carcinogenesis. 1980;1(11):897–902. doi: 10.1093/carcin/1.11.897. [DOI] [PubMed] [Google Scholar]
- Spodheim-Maurizot M., Saint-Ruf G., Leng M. Conformational changes induced in DNA by in vitro reaction with N-hydroxy-N-2-aminofluorene. Nucleic Acids Res. 1979 Apr;6(4):1683–1694. doi: 10.1093/nar/6.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone P. J., Kelman A. D., Sinex F. M. Specific binding of antitumour drug cis-Pt(NH3)2C12 to DNA rich in guanine and cytosine. Nature. 1974 Oct 25;251(5477):736–737. doi: 10.1038/251736a0. [DOI] [PubMed] [Google Scholar]
- Tomizawa J. I., Anraku N., Iwama Y. Molecular mechanisms of genetic recombination in bacteriophage. VI. A mutant defective in the joining of DNA molecules. J Mol Biol. 1966 Nov 14;21(2):247–253. doi: 10.1016/0022-2836(66)90095-7. [DOI] [PubMed] [Google Scholar]
- Toulmé F., Hélène C., Fuchs R. P., Daune M. Binding of a tryptophan-containing peptide (lysyltryptophyllysine) to deoxyribonucleic acid modified by 2-(N-acetoxyacetylamino)fluorene. Biochemistry. 1980 Mar 4;19(5):870–875. doi: 10.1021/bi00546a007. [DOI] [PubMed] [Google Scholar]
- Toulmé J. J., Hélène C. Fluorescence study of the association between gene 32 protein of bacteriophage T4 and poly(1-N6-ethenoadenylic acid). Evidence for energy transfer. Biochim Biophys Acta. 1980;606(1):95–104. doi: 10.1016/0005-2787(80)90101-x. [DOI] [PubMed] [Google Scholar]
- Toulmé J. J., Hélène C. Specific recognition of single-stranded nucleic acids. Interaction of tryptophan-containing peptides with native, denatured, and ultraviolet-irradiated DNA. J Biol Chem. 1977 Jan 10;252(1):244–249. [PubMed] [Google Scholar]
- Wallace S. S., Melamede R. J. Host- and phage-mediated repair of radiation damage in bacteriophage T4. J Virol. 1972 Dec;10(6):1159–1169. doi: 10.1128/jvi.10.6.1159-1169.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



