Abstract
It is unclear how treatment sequencing for metastatic castration-resistant prostate cancer (mCRPC) affects real-world patient outcomes. We assessed treatment sequences, patient characteristics and overall survival (OS) in post-docetaxel mCRPC patients. mCRPC patients receiving second-line cabazitaxel or androgen receptor-targeted therapy (ART; abiraterone/enzalutamide) post-docetaxel were identified using electronic medical records. OS was assessed from second-line therapy initiation using Cox regressions adjusting for: metastases; prostate-specific antigen (PSA); hemoglobin; alkaline phosphatase (ALP); albumin; second-line therapy initiation year. Following docetaxel (n = 629), 123 (19.6%) and 506 (80.4%) patients received cabazitaxel and ART, respectively. One hundred and ninety-five patients received additional treatments thereafter (54 following cabazitaxel; 141 following ART). Although patients receiving second-line cabazitaxel versus ART had similar disease characteristics at first-line therapy initiation, at second-line therapy initiation they had higher mean PSA (386.6 vs. 233.9 ng/mL) and ALP (182.0 vs. 167.3 u/L), lower mean hemoglobin (10.8 vs. 11.5 g/dL), and more frequently had intermediate/high-risk Halabi scores (61.8 vs. 48.4%); all p < 0.05. Overall, crude survival was not significantly different. Among Halabi high-risk patients, adjusted median OS was significantly longer in patients receiving cabazitaxel versus ART (HR 0.48; 95% CI 0.24–0.93; p = 0.030). Low albumin and hemoglobin led to similar findings (HR 0.43; 95% CI 0.23–0.80; p = 0.0077; HR 0.60; 95% CI 0.40–0.90; p = 0.014). Most post-docetaxel patients received second-line ART. Patients receiving second-line cabazitaxel had more high-risk features; however, second-line cabazitaxel administered after docetaxel may improve OS in patients with Halabi high-risk scores or low albumin/hemoglobin.
Keywords: mCRPC, Chemotherapy, AR-targeted therapy, Real-world, Cabazitaxel
Introduction
It has been estimated that 180,890 new cases of prostate cancer will be diagnosed in the USA in 2016, and that these will represent 21% of all new cancer diagnoses in American men [1]. Despite significant progress in the discovery of novel therapeutic agents, patients with metastatic castration-resistant prostate cancer (mCRPC) continue to have a poor prognosis, and their treatment remains a significant clinical challenge [2]. Until recently, treatment options that improve survival were limited to docetaxel-based regimens. However, recent advances have led to the approval of several new agents shown to improve survival, including the taxane chemotherapy, cabazitaxel, and the androgen receptor (AR)-targeted agents, abiraterone and enzalutamide [3]. As these three agents were developed simultaneously and largely in isolation, limited data are available on their optimal sequencing in the treatment of mCRPC.
A number of retrospective studies have sought to define the association between different treatment sequences of abiraterone, enzalutamide, docetaxel and cabazitaxel and patient outcomes such as overall survival (OS), progression-free survival (PFS) and prostate-specific antigen (PSA) response [4–8]. However, in the absence of prospective sequencing studies, the available literature assessing the optimal sequencing of treatments to achieve the best possible patient outcomes in mCRPC remains limited and inconclusive (as extensively reviewed by Lorente et al. [2]). For example, some studies have suggested that, in patients with mCRPC who have progressed on docetaxel, the sequence of subsequent therapies can affect patient outcomes. It has been shown that, after docetaxel, administration of cabazitaxel before, rather than after, AR-targeted therapy is associated with prolonged OS [9–11]. However, other studies have shown that cabazitaxel retains activity in patients who have received prior docetaxel and AR-targeted therapy suggesting that prior AR-targeted therapy does not influence the efficacy of cabazitaxel [12, 13].
Understanding the potential link between treatment sequences and patient outcomes is important to help achieve the most optimal patient benefit, particularly in the post-docetaxel setting; most patients with mCRPC have received docetaxel as part of their long-term treatment regimen as it is a standard treatment for patients with symptomatic metastases [14, 15]. In addition, recent studies have shown that a combination of androgen-deprivation therapy and docetaxel as first-line therapy for hormone-sensitive metastatic prostate cancer can improve OS, suggesting that in the future more patients may have received docetaxel by the time they have developed castration-resistant disease [16, 17]. In patients with mCRPC who have progressed after first-line docetaxel, it is unclear whether they would benefit from receiving a further line of chemotherapy (cabazitaxel) or second-line AR-targeted therapy. This study aimed to evaluate the real-world effectiveness of mCRPC treatment sequences. Specifically, we assessed whether the choice of second-line treatment post-docetaxel is associated with OS in patients with mCRPC in order to provide information on the optimal placement of cabazitaxel in the treatment sequence for patients with mCRPC.
Methods
Data source
This study used de-identified electronic medical records (EMR) data obtained from Altos Solutions, Inc (Silicon Valley, CA, USA). This database includes information from patients treated in community oncology practices that use OncoEMR™, an oncology-specific EMR system developed by Altos. The network of Altos includes 150 medical oncology practices across the USA and represents approximately 20% of all oncologists practicing in the community setting. Because our study involved retrospective analysis of existing data with no patient intervention or interaction, and patient data were de-identified, the New England Independent Review Board (NEIRB) determined that this study was exempt from NEIRB review (per Title 45 of CFR, Part 46).
Study design
This study used a retrospective, longitudinal, open cohort design. The study index date was defined as the initiation of first-line docetaxel and was restricted to be no later than 6 months prior to the data cut-off date (October 17, 2014). To avoid selection bias based on treatment availability, the initiation of docetaxel was required to have occurred on or after May 2011 (when abiraterone was approved for use post-docetaxel in the USA). The analytic index date was defined as the initiation of second-line therapy, and the observation period began with the analytic index date and extended to the end of data availability (loss to follow-up), the data cut-off date or death, whichever came first (Fig. 1).
Fig. 1.
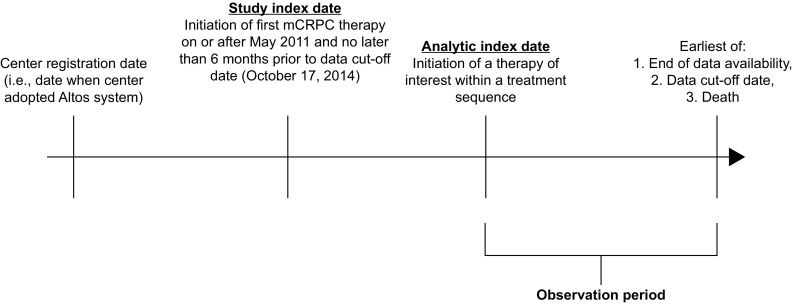
Study design. mCRPC metastatic castration-resistant prostate cancer
Study sample
The study sample cohorts were driven by the observed treatment patterns among patients who received first-line docetaxel. Firstly, the types of therapies and treatment sequences received were characterized. Secondly, based on the observed sequence distribution, patients were stratified into two cohorts: (1) cabazitaxel cohort: patients who received first-line docetaxel, followed by second-line cabazitaxel and any or no subsequent treatment (2) AR-targeted therapy cohort: patients who received first-line docetaxel, followed by second-line AR-targeted therapy (abiraterone or enzalutamide) and any or no subsequent treatment. Patients who received a combination of AR-targeted therapy and chemotherapy in either the first- or second-line setting were excluded from the analysis.
Eligibility criteria
Patients included in the study had at least one medical record with a diagnosis of prostate cancer and had received at least two mCRPC treatments of interest (docetaxel, cabazitaxel, abiraterone or enzalutamide) including docetaxel as first-line therapy, followed by any other treatment of interest in the second-line setting. Patients had to be at least 18 years old at the time of prostate cancer diagnosis.
Study endpoints
Treatment effectiveness was assessed in terms of OS, which was defined as the time from initiation of second-line therapy to death from any cause.
Statistical methods
Descriptive analyses were conducted to characterize the landscape of therapies that mCRPC patients received following first-line docetaxel. Descriptive statistics were generated to summarize patient demographic and clinical characteristics at the initiation of first- and second-line therapy. Comparisons between cohorts were conducted using Chi-squared tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. OS was assessed using the Kaplan–Meier method and compared using log-rank tests. Multivariate Cox proportional hazard models were also conducted to compare OS between cohorts, adjusting for the year of the initiation of second-line therapy, site of metastasis, and PSA, hemoglobin, alkaline phosphatase (ALP) and albumin levels. OS was also assessed for specific patient subgroups based on Halabi risk score [18] at the initiation of second-line therapy, as well as its underlying prognostic factors including PSA (>50 ng/mL), hemoglobin (<11 g/dL), ALP (>1 × upper limit of normal [ULN]), lactate dehydrogenase (LDH; >1 × ULN), and albumin levels (<1 × lower limit of normal), use of opioids, and the presence of bone or visceral metastases. Statistical analyses were performed using SAS software version 9.3 or more recent.
Results
Study population and cohorts
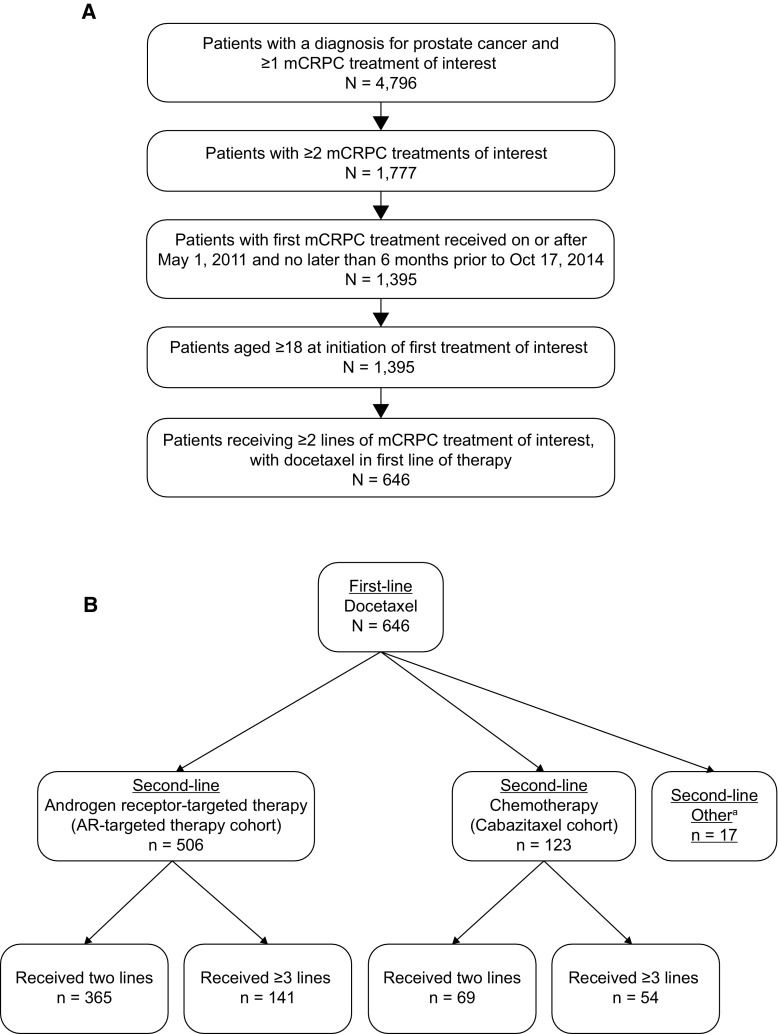
A total of 646 patients received first-line docetaxel of which 629 received either cabazitaxel or AR-targeted therapy in the second-line setting (Fig. 2a, b). The remaining 17 patients received a combination of cabazitaxel and AR-targeted therapy as second-line treatment and were excluded from further analyses. Of the 629 eligible patients, 19.6% (n = 123) received cabazitaxel followed by any or no further treatment and 80.4% (n = 506) received AR-targeted therapy followed by any or no further treatment. Of the patients in the cabazitaxel cohort, 56.1% (n/N = 69/123) received no further therapy of interest following second-line cabazitaxel, whereas 72.1% of patients (n/N = 365/506) in the AR-targeted therapy cohort received no further treatment of interest following second-line AR-targeted therapy (Table 1). Of the patients in the AR-targeted therapy cohort receiving only two lines of therapy of interest (n = 365), 233 patients (63.8%) received second-line abiraterone, 129 (35.3%) received second-line enzalutamide and 3 (0.8%) received both AR-targeted agents in the second-line setting (Table 1). Among the 629 eligible patients who received second-line cabazitaxel or AR-targeted therapy, 434 patients (69.0%) received a total of two treatment lines of interest only, and 195 (31.0%) received a total of three or more treatment lines of interest (54 patients in the cabazitaxel cohort and 141 patients in the AR-targeted therapy cohort) (Fig. 2b).
Fig. 2.
Study sample selection (a) and cohort allocation (b). AR andrgoen receptor, mCRPC metastatic castration-resistant prostate cancer. aExcluded from the further analyses
Table 1.
Treatment sequences received by patients with docetaxel as first observed mCRPC therapy
| n (%) | |
|---|---|
| Sequence of two therapies | |
| Docetaxel → AR-targeted therapy | 365 (81.8) |
| Abiraterone | 233 (52.2) |
| Enzalutamide | 129 (28.9) |
| Abiraterone/enzalutamide | 3 (0.7) |
| Docetaxel → cabazitaxel | 69 (15.5) |
| Other (e.g., cabazitaxel/AR-targeted therapy) | 12 (2.7) |
| Total patients with a sequence of two therapies | 446 (69.0) |
| Sequence of three therapies | |
| Docetaxel → AR-targeted therapy → alternative AR-targeted therapy | 57 (37.5) |
| Abiraterone → enzalutamide | 40 (26.3) |
| Enzalutamide → abiraterone | 15 (9.9) |
| Abiraterone → abiraterone/enzalutamide | 2 (1.3) |
| Docetaxel → AR-targeted therapy → chemotherapy | 44 (28.9) |
| Abiraterone → cabazitaxel | 20 (13.2) |
| Enzalutamide → cabazitaxel | 13 (8.6) |
| Abiraterone → docetaxel | 6 (3.9) |
| Enzalutamide → docetaxel | 5 (3.3) |
| Docetaxel → AR-targeted therapy → other (e.g., chemotherapy/alternative AR-targeted therapy) | 3 (2.0) |
| Docetaxel → cabazitaxel → AR-targeted therapy | 42 (27.6) |
| Cabazitaxel → abiraterone | 28 (18.4) |
| Cabazitaxel → enzalutamide | 14 (9.2) |
| Docetaxel → cabazitaxel → other (e.g., chemotherapy/AR-targeted therapy) | 1 (0.7) |
| Other (e.g., chemotherapy/AR-targeted therapy) | 5 (3.3) |
| Total patients with a sequence of three therapies | 152 (23.5) |
| Sequence of four or more therapies | |
| Total patients with a sequence of four therapies | 37 (5.7) |
| Total patients with a sequence of five therapies | 8 (1.2) |
| Total patients with a sequence of six therapies | 3 (0.5) |
| Total | 646 (100) |
| Patients with second-line cabazitaxel or AR-targeted therapy | 629 (97.4) |
AR androgen receptor, mCRPC metastatic castration-resistant prostate cancer
The median duration of first-line docetaxel therapy was similar between the two patient cohorts (4.2 months for both the cabazitaxel and AR-targeted therapy cohorts; p = 0.548) (Table 2). The median duration of second-line therapy was significantly shorter for those receiving second-line cabazitaxel versus second-line AR-targeted therapy (2.8 vs. 4.7 months; p = 0.001) (Table 3).
Table 2.
Characteristics of patients receiving second-line cabazitaxel versus second-line androgen receptor-targeted therapy at initiation of first-line docetaxel therapy
| Characteristics | Second-line therapy received | p value | |
|---|---|---|---|
| Cabazitaxel N = 123 |
AR-targeted therapy N = 506 |
||
| Age, years, mean ± SD (median) | 70.8 ± 9.3 (72.0) | 71.6 ± 9.0 (72.0) | 0.503 |
| Age, n (%) | |||
| <65 years | 29 (23.6) | 109 (21.5) | 0.625 |
| 65–74 years | 46 (37.4) | 190 (37.5) | 0.975 |
| ≥75 years | 48 (39.0) | 207 (40.9) | 0.703 |
| ECOG performance status, n (%) | |||
| 0 | 24 (19.5) | 74 (14.6) | 0.180 |
| 1 | 11 (8.9) | 62 (12.3) | 0.304 |
| 2 | 2 (1.6) | 17 (3.4) | 0.314 |
| 3 | 1 (0.8) | 2 (0.4) | 0.546 |
| Unknown | 85 (69.1) | 351 (69.4) | 0.955 |
| Number of metastases, mean ± SD (median) | 1.1 ± 0.3 (1.0) | 1.1 ± 0.4 (1.0) | 0.505 |
| Bone metastasis, n (%) | 82 (66.7) | 296 (58.5) | 0.097 |
| Visceral metastasis, n (%) | 7 (5.7) | 20 (4.0) | 0.394 |
| Lymph node metastasis, n (%) | 3 (2.4) | 29 (5.7) | 0.136 |
| Unknown, n (%) | 38 (30.9) | 193 (38.1) | 0.135 |
| PSA, ng/mL, mean ± SD (median) | 359.7 ± 1211.2 (85.3) | 262.3 ± 962.8 (63.4) | 0.193 |
| ALP, u/L, mean ± SD (median) | 231.1 ± 233.9 (139.0) | 206.4 ± 269.6 (111.0) | 0.039* |
| LDH, u/L, mean ± SD (median) | 373.8 ± 248.0 (256.0) | 333.2 ± 448.1 (222.0) | 0.048* |
| Albumin, g/dL, mean ± SD (median) | 3.8 ± 0.4 (3.9) | 3.9 ± 0.5 (3.9) | 0.219 |
| Hemoglobin, g/dL, mean ± SD (median) | 11.7 ± 1.5 (11.9) | 11.7 ± 1.6 (11.7) | 0.999 |
| Halabi risk score, mean ± SD (median)a | 127.6 ± 51.8 (126.7) | 126.1 ± 50.4 (121.6) | 0.243 |
| Low risk, n (%) | 70 (56.9) | 325 (64.2) | 0.132 |
| Intermediate risk, n (%) | 43 (35.0) | 139 (27.5) | 0.100 |
| High risk, n (%) | 10 (8.1) | 42 (8.3) | 0.951 |
| Opioid use in prior 365 days, n (%) | 39 (31.7) | 136 (26.9) | 0.284 |
| Duration of first-line therapy, months, mean ± SD (median) | 5.4 ± 4.4 (4.2) | 5.2 ± 4.4 (4.2) | 0.548 |
AR androgen receptor, ALP alkaline phosphatase, ECOG Eastern Cooperative Oncology Group, LDH lactate dehydrogenase, PSA prostate-specific antigen, SD standard deviation
aBased on Halabi et al. [18]
* Denotes significant p value at the 5% level
Table 3.
Characteristics of patients receiving second-line cabazitaxel versus second-line androgen receptor-targeted therapy at initiation of second-line therapy
| Characteristics | Second-line therapy received | p value | |
|---|---|---|---|
| Cabazitaxel N = 123 |
AR-targeted therapy N = 506 |
||
| Age, years, mean ± SD (median) | 71.5 ± 9.2 (72.0) | 72.3 ± 9.0 (73.0) | 0.478 |
| Age, n (%) | |||
| <65 years | 27 (22.0) | 98 (19.4) | 0.520 |
| 65–74 years | 42 (34.1) | 188 (37.2) | 0.534 |
| ≥75 years | 54 (43.9) | 220 (43.5) | 0.932 |
| ECOG performance status, n (%) | |||
| 0 | 15 (12.2) | 60 (11.9) | 0.918 |
| 1 | 19 (15.4) | 83 (16.4) | 0.796 |
| 2 | 7 (5.7) | 24 (4.7) | 0.663 |
| 3 | 0 (0.0) | 15 (3.0) | – |
| Unknown | 81 (65.9) | 323 (63.8) | 0.675 |
| Number of metastases, mean ± SD (median) | 1.1 ± 0.4 (1.0) | 1.1 ± 0.4 (1.0) | 0.860 |
| Bone metastasis, n (%) | 90 (73.2) | 325 (64.2) | 0.061 |
| Visceral metastasis, n (%) | 10 (8.1) | 24 (4.7) | 0.136 |
| Lymph node metastasis, n (%) | 3 (2.4) | 35 (6.9) | 0.062 |
| Unknown, n (%) | 30 (24.4) | 160 (31.6) | 0.117 |
| PSA, ng/mL, mean ± SD (median) | 386.6 ± 760.5 (126.6) | 233.9 ± 659.4 (47.0) | 0.001* |
| ALP, u/L, mean ± SD (median) | 182.0 ± 189.7 (131.0) | 167.3 ± 240.2 (91.0) | 0.016* |
| LDH, u/L, mean ± SD (median) | 347.7 ± 291.4 (241.0) | 589.8 ± 1904.2 (221.0) | 0.122 |
| Albumin, g/dL, mean ± SD (median) | 3.9 ± 3.4 (3.7) | 3.9 ± 3.5 (3.8) | 0.522 |
| Hemoglobin, g/dL, mean ± SD (median) | 10.8 ± 1.5 (10.9) | 11.5 ± 2.0 (11.4) | <0.001* |
| Halabi risk score, mean ± SD (median)a | 153.6 ± 49.2 (154.5) | 143.8 ± 53.8 (136.4) | 0.013* |
| Low risk, n (%) | 47 (38.2) | 261 (51.6) | 0.008* |
| Intermediate risk, n (%) | 55 (44.7) | 169 (33.4) | 0.019* |
| High risk, n (%) | 21 (17.1) | 76 (15.0) | 0.572 |
| Opioid use in prior 365 days, n (%) | 53 (43.1) | 231 (45.7) | 0.609 |
| Duration of second-line therapy, months, mean ± SD (median) | 4.2 ± 1.9 (2.8) | 6.1 ± 1.9 (4.7) | 0.001* |
AR androgen receptor, ALP alkaline phosphatase, ECOG Eastern Cooperative Oncology Group, LDH lactate dehydrogenase, PSA prostate-specific antigen, SD standard deviation
aBased on Halabi et al. [18]
* Denotes significant p value at the 5% level
Patient baseline characteristics
At the initiation of first-line docetaxel therapy, patient characteristics were generally similar across cohorts. Only ALP and LDH were significantly different, with patients receiving second-line cabazitaxel presenting with higher mean levels versus patients receiving second-line AR-targeted therapy (ALP: 231.1 vs. 206.4 u/L, p = 0.039; LDH: 373.8 vs. 333.2 u/L, p = 0.048). This, however, did not translate into a significant difference in the mean Halabi risk score at baseline (127.6 vs. 126.1 points, p = 0.243, for the cabazitaxel and AR-targeted therapy cohorts, respectively) (Table 2).
At the initiation of second-line therapy, patients in the cabazitaxel cohort presented with poorer prognostic features compared with patients in the AR-targeted therapy cohort (Table 3). Of note, patients in the cabazitaxel cohort had significantly higher mean PSA and ALP levels (PSA: 386.6 vs. 233.9 ng/mL, p = 0.001; ALP: 182.0 vs. 167.3 u/L, p = 0.016), and significantly lower mean hemoglobin levels (10.8 vs. 11.5 g/dL, p < 0.001). The mean Halabi risk score was 153.6 versus 143.8 for the cabazitaxel and AR-targeted therapy cohorts, respectively (p = 0.013), and a greater proportion of patients in the cabazitaxel cohort was categorized into the intermediate- or high-risk Halabi groups (61.8 vs. 48.4%, p = 0.008).
Overall survival
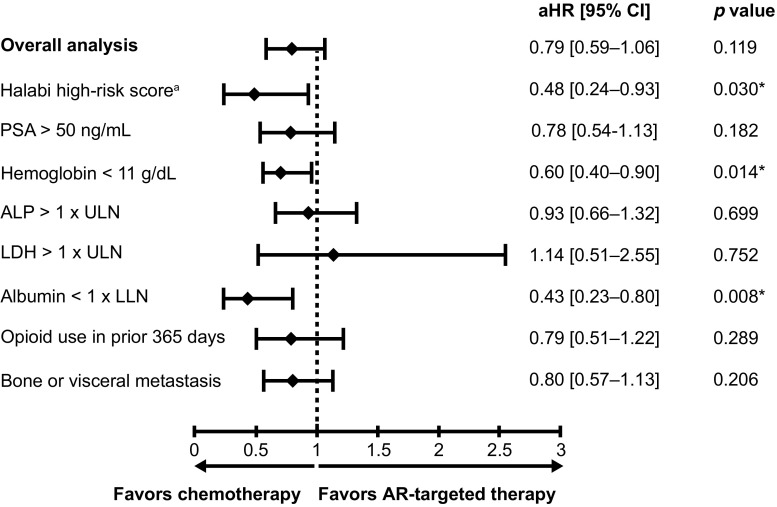
The difference in overall median OS for patients receiving second-line cabazitaxel versus patients receiving second-line AR-targeted therapy did not reach statistical significance (10.2 vs. 15.1 months; p = 0.155). Results from the multivariate Cox regression showed a trend for OS in favor of cabazitaxel, although the results did not reach statistical significance (adjusted hazard ratio for cabazitaxel vs. AR-targeted therapy: 0.79; 95% Confidence Interval [CI] 0.59–1.06, p = 0.119) (Fig. 3).
Fig. 3.
Adjusted hazard ratios for overall survival by subgroup for patients receiving second-line chemotherapy versus second-line androgen receptor-targeted therapy. aHR adjusted hazard ratio, ALP alkaline phosphatase, CI confidence interval, LDH lactate dehydrogenase, LLN lower limit of normal, PSA prostate-specific antigen, ULN upper limit of normal. a Based on Halabi et al. [18]. * Denotes significant p value at the 5% level
In the subgroup of patients defined as Halabi high risk, median OS was 6.9 months for patients receiving second-line cabazitaxel and 4.7 months for patients receiving second-line AR-targeted therapy (p = 0.320). After adjustment for observed differences in risk factors, median OS was significantly longer for Halabi high-risk patients receiving second-line cabazitaxel compared with Halabi high-risk patients receiving second-line AR-targeted therapy (Hazard Ratio [HR]: 0.48; 95% CI 0.24–0.93; p = 0.030). These findings, indicating a benefit with second-line cabazitaxel, were similar in the subgroups of patients with albumin levels below the lower limit of normal (HR: 0.43; 95% CI 0.23–0.80; p = 0.008) and hemoglobin levels <11 g/dL (HR: 0.60; 95% CI 0.40–0.90; p = 0.014) (Fig. 3).
Discussion
This retrospective study described the real-world treatment sequences received by patients with mCRPC following first-line docetaxel and outlined the characteristics of patients who received either cabazitaxel or AR-targeted therapy as second-line treatment. This study also assessed the real-world effectiveness in terms of OS of cabazitaxel versus AR-targeted therapy in the post-docetaxel setting. Data showed that after first-line docetaxel, more patients received AR-targeted therapy as a second-line treatment versus cabazitaxel in the community oncology setting. The patients who received second-line cabazitaxel, post-docetaxel, had more high-risk features and a worse prognosis compared with the patients who received second-line AR-targeted therapy. This suggested that more patients with aggressive cancer progression were included in the cabazitaxel cohort, and that oncologists favored second-line chemotherapy as a treatment for these patients. In addition, our findings suggest that, for patients presenting with worse prognostic features, including a Halabi high-risk score, and low albumin and/or low hemoglobin levels, there may be an OS benefit associated with second-line cabazitaxel compared with second-line AR-targeted therapy, following first-line docetaxel. Trends favoring patients receiving second-line cabazitaxel were also observed in patients with PSA > 50 mg/mL and bone or visceral metastases at the initiation of second-line therapy, and in patients who had used opioid pain medication in the 365 days prior to second-line therapy; however, these trends were not significant.
Consistent with our findings, a number of recent studies have suggested that, in the post-docetaxel setting, there may be a survival benefit when receiving cabazitaxel earlier in the treatment regimen. One retrospective study, for example, found that among patients receiving three lines of therapy including first-line docetaxel, followed by second-line and third-line cabazitaxel or abiraterone, those receiving second-line cabazitaxel followed by third-line abiraterone experienced significantly prolonged OS compared with patients receiving the opposite sequence of second-line abiraterone followed by third-line cabazitaxel (median OS: 18.2 vs. 11.8 months; p = 0.021) [10]. Another study showed a survival benefit associated with receiving cabazitaxel prior to either abiraterone or enzalutamide in the post-docetaxel setting with a median OS of 49.0 months reported in patients receiving cabazitaxel prior to abiraterone or enzalutamide versus 20.8 months in patients receiving abiraterone or enzalutamide prior to cabazitaxel [9]. When other patient outcome parameters were assessed, such as PFS, among patients who received three lines of therapy, including first-line docetaxel, there was no difference in PFS for patients receiving second-line cabazitaxel followed by third-line abiraterone (4.6 months) versus second-line abiraterone followed by third-line cabazitaxel (4.9 months; p = 0.488), though the sample size was small [19]. Our results are not directly comparable with the above studies as we did not distinguish between the specific AR-targeted therapies received and did not condition on the number of lines of therapy received when the cohorts were established, as did the cited studies. Keeping these differences in study design in mind, while we found no statistically significant differences in terms of OS for patients in the cabazitaxel versus AR-targeted therapy cohorts in the full patient population, we did find that the subgroup of patients with worse disease prognosis at initiation of second-line therapy benefitted from receiving second-line cabazitaxel versus second-line AR-targeted therapy, post-docetaxel. These findings are consistent with a separate analysis of this study, which assessed the effectiveness of second-line taxane chemotherapy versus an alternative AR-targeted therapy in chemotherapy-naïve patients who received first-line AR-targeted therapy. In this patient population, second-line chemotherapy (docetaxel or cabazitaxel), compared with second-line AR-targeted therapy, was also associated with improved OS in patients with worse prognostic features, namely low albumin or hemoglobin levels [20]. Findings from our study, in the post-docetaxel and chemotherapy-naïve settings, are in line with the results of a separate study that found an association between second-line cabazitaxel and prolonged PFS in patients who had a worse prognosis, i.e., a higher Gleason score (≥8), compared with patients who had a lower Gleason score (<7; HR: 0.36; 95% CI 0.18–0.72; p = 0.004) [21].
A key strength of this study is that it is one of the largest of its kind. Moreover, as it is based on the EMRs of patients treated in a USA community oncology setting, it provides a unique insight into the link between real-world treatment patterns and patient outcomes. Use of EMR data also allowed for inclusion of clinical parameters typically unavailable in other information resources such as administrative healthcare claims databases. Despite these strengths, there were a number of limitations to our methodology that should be considered. Firstly, therapy information in the EMR database was obtained from physician prescription data rather than from filled prescription files taken from claims databases. This means that prescriptions for oral medications were not necessarily filled and taken as directed. Secondly, some of the fields in the EMR database were not well populated; dummy variables indicating missing values were therefore included in the multivariate analyses (residual confounding may, however, still be present as only recorded variables could be adjusted for as a means to control for confounding). For comparison of treatment effectiveness, confounding by indication may be present and may affect the validity of the results. For instance, cabazitaxel administration may have been influenced by disease severity. All analyses were therefore adjusted for indicators of disease severity. Finally, our study focused on community oncology settings, and results may therefore not be generalizable to care received in other settings.
In conclusion, although no significant difference in OS was observed between the two treatment groups overall, our findings suggest that following treatment with docetaxel, second-line cabazitaxel versus second-line AR-targeted therapy may be associated with an improved survival benefit in patients with poorer prognostic features (Halabi high-risk score or low albumin/hemoglobin levels). Prospective randomized trials are warranted to validate these results. Future research should also consider other approved therapies, such as sipuleucel-T and radium-223, as well as features such as adverse events and health-related quality of life.
Acknowledgments
This study was supported by Sanofi. Editorial support in the preparation of this manuscript was provided by Anna Longjaloux and Danielle Lindley of MediTech Media funded by Sanofi. The authors were responsible for all content and editorial decisions and received no honoraria related to the development of this manuscript.
Author contributions
WKO, RM, JS and RD have provided substantial contributions to the conception and design of the study and have been involved in the analysis and interpretation of the data. FV, WYC, MGL and MSD have been involved in the development and implementation of the analytical models and acquisition, analysis and interpretation of the data. All authors have critically reviewed the manuscript and provided final approval of the version to be published. All authors are accountable for all aspects of the work, including the accuracy and integrity of the manuscript.
Compliance with ethical standards
Conflict of interest
WKO has consulted for Astellas, Bayer, Janssen and Sanofi. RM is an employee of Sanofi, owns Sanofi stock and is a researcher involved in Sanofi-sponsored research. JS was an employee of Sanofi at the time this research and study was undertaken, and owns Sanofi and Novartis stock. FV, WYC, MGL and MSD are employees of Analysis Group Inc., which has received research funding from Sanofi. RD was an employee of Sanofi at the time this research and study was undertaken.
Ethical approval
No prospective studies with human participants or animals were performed by any of the authors for this article. The New England Independent Review Board (NEIRB) determined that this study was exempt from NEIRB review.
Informed consent
Informed consent was waived for individual participants included in the study given the retrospective nature of this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Lorente D, Mateo J, Perez-Lopez R, et al. Sequencing of agents in castration-resistant prostate cancer. Lancet Oncol. 2015;16:279–292. doi: 10.1016/S1470-2045(15)70033-1. [DOI] [PubMed] [Google Scholar]
- 3.Sternberg CN, Petrylak DP, Madan RA, et al. Progress in the treatment of advanced prostate cancer. Am Soc Clin Oncol Educ Book. 2014;117–31. doi:10.14694/EdBook_AM.2014.34.117. [DOI] [PubMed]
- 4.Loriot Y, Bianchini D, Ileana E, et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100) Ann Oncol. 2013;24:1807–1812. doi: 10.1093/annonc/mdt136. [DOI] [PubMed] [Google Scholar]
- 5.Petrelli F, Coinu A, Borgonovo K, et al. Enzalutamide after docetaxel and abiraterone acetate treatment in prostate cancer: a pooled analysis of 10 case series. Clin Genitourin Cancer. 2015;13:193–198. doi: 10.1016/j.clgc.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 6.de Bono JS, Smith MR, Saad F, et al. Response to taxane chemotherapy as first subsequent therapy after abiraterone acetate (AA) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): post-hoc analysis of COU-AA-302. J Clin Oncol. 2015; 33:abstract 184.
- 7.Gizzi M, Baciarello G, Flechon A, et al. Previous enzalutamide therapy and response to subsequent taxane therapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2015;33:abstract 227.
- 8.Pezaro CJ, Le Moulec S, Albiges L, et al. Response to cabazitaxel in CRPC patients previously treated with docetaxel and abiraterone acetate. J Clin Oncol. 2013;31:abstract 155.
- 9.Angelergues A, Maillet D, Flechon A, et al. Prognostic factors of survival in patients with metastatic castration resistant prostate cancer (mCRPC) treated with cabazitaxel: sequencing might matter. J Clin Oncol. 2013;31:abstract 5063.
- 10.Sonpavde G, Bhor M, Hennessy D, et al. Sequencing of cabazitaxel and abiraterone acetate after docetaxel in metastatic castration-resistant prostate cancer: treatment patterns and clinical outcomes in multicenter community-based US oncology practices. Clin Genitourin Cancer. 2015;13:209–318. doi: 10.1016/j.clgc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Wissing MD, Coenen JL, van den Berg P, et al. CAST: a retrospective analysis of cabazitaxel and abiraterone acetate sequential treatment in patients with metastatic castrate-resistant prostate cancer previously treated with docetaxel. Int J Cancer. 2015;136:E760–E772. doi: 10.1002/ijc.29231. [DOI] [PubMed] [Google Scholar]
- 12.van Soest RJ, Nieuweboer AJ, de Morree ES, et al. The influence of prior novel androgen receptor targeted therapy on the efficacy of cabazitaxel in men with metastatic castration-resistant prostate cancer. Eur J Cancer. 2015;17:2562–2569. doi: 10.1016/j.ejca.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 13.Pezaro CJ, Omlin AG, Altavilla A, et al. Activity of cabazitaxel in castration-resistant prostate cancer progressing after docetaxel and next-generation endocrine agents. Eur Urol. 2014;66:459–465. doi: 10.1016/j.eururo.2013.11.044. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines®) Prostate Cancer (Version 2.2016). 2016. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed May 2016.
- 15.Taxotere® Prescribing Information. 2015. http://products.sanofi.us/Taxotere/taxotere.html. Accessed May 2016.
- 16.James ND, Sydes MR, Mason MD, et al. Docetaxel and/or zoledronic acid for hormone-naïve prostate cancer: first overall survival results from STAMPEDE. J Clin Oncol. 2014;33:abstract 5001.
- 17.Sweeney C, Chen Y-H, Carducci M, et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): an ECOG-led Phase III randomized trial. J Clin Oncol. 2014;32:abstract LBA2.
- 18.Halabi S, Lin C-Y, Kelly K, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32:671–677. doi: 10.1200/JCO.2013.52.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houts A, Hennessy D, Walker MS, et al. Treatment patterns and clinical effectiveness in metastatic castrate resistant prostate cancer after first-line docetaxel. J Community Support Oncol. 2014;12:321–328. doi: 10.12788/jcso.0072. [DOI] [PubMed] [Google Scholar]
- 20.Oh W, Miao R, Vekeman F, et al. Is there a difference in outcomes in metastatic castration-resistant prostate cancer (mCRPC) patients (pts) who receive chemotherapy (CT) vs androgen receptor-targeted therapy (ART) after 1st-line ART in the community setting? Eur J Cancer. 2015;51:S478. doi: 10.1016/S0959-8049(16)31332-6. [DOI] [Google Scholar]
- 21.Buonerba C, Pond G, Sonpavde G, et al. Potential value of Gleason score in predicting the benefit of cabazitaxel in metastatic castration-resistant prostate cancer. Future Oncol. 2013;9:889–897. doi: 10.2217/fon.13.39. [DOI] [PubMed] [Google Scholar]