Abstract
Sedentary behaviour (SB) is increasing in Western societies and some studies suggest a deleterious effect of SB on bone. The aim of this systematic review was to examine the association between SB and bone health in children, adolescents and young adults. Electronic databases (PubMed, MEDLINE, PsycINFO and Science Citation Index) were searched for relevant articles up to January 9, 2017. Studies were included when results on bone health (e.g. strength, mass and structure) and either subjectively (questionnaires) or objectively (accelerometry) measured SB were reported in healthy participants ≤24 years. Two reviewers independently screened titles and abstracts for eligibility, rated methodological quality and extracted data. Seventeen observational studies were included. Several studies that used DXA or quantitative ultrasound suggested that objectively measured SB was negatively associated with lower extremity bone outcomes, such as femoral neck bone mineral density. The magnitude of this negative association was small and independent of moderate-to-vigorous physical activity. In contrast to the lower extremities, there was insufficient evidence for an association of lumbar spine bone outcomes with objectively measured SB. In high-quality studies that used DXA, no association was observed between objectively measured SB and total body bone outcomes. In studies using questionnaires, none of these relationships were observed. Well-designed longitudinal studies, objectively measuring SB, are needed to further unravel the effect of SB, physical activity and their interaction on bone health.
Electronic supplementary material
The online version of this article (doi:10.1007/s00198-017-4076-2) contains supplementary material, which is available to authorized users.
Keywords: Adolescent, Bone, Child, Osteoporosis, Sedentary behaviour, Unloading
Introduction
Lifestyle factors are estimated to influence 20–40% of adult peak bone mass and suboptimal bone deposition due to unfavourable lifestyle factors can increase the risk of osteoporosis and associated fractures [1]. To optimize bone accrual, physical activity (PA) is important. According to the mechanostat theory, bone is continuously adapting its content, mass and structure to the loads to which it is exposed [2–4]. Unfortunately, lack of engagement in PA is becoming increasingly common in childhood: Canadian and Finnish data suggest that only 7–23% of children and adolescents meet the current PA guidelines of at least 60 min of moderate-to-vigorous physical activity (MVPA) per day [5–7]. Furthermore, current guidelines provide no guidance on how to spend the remaining 23 h/day, while the opportunity to spend time sedentary, rather than active, has enormously increased [6, 8, 9]. The Canadian data also demonstrate that children and adolescents spend on average 8.6 h, or 62% of their waking time, in sedentary pursuits. Moreover, the amount of sedentary time increases while the time spent in MVPA becomes less during childhood [5]. This rise in sedentary behaviour (SB) is a case of concern, as accumulating evidence suggests that SB is associated with deleterious health effects (e.g. diabetes mellitus, cardiovascular disease and increased mortality), independent of PA levels [10, 11]. Moreover, studies on extremes of SB such as bed rest observed that in young and older adults, bone responds to unloading by a rapid and sustained increase in bone resorption and a subtle decrease in bone formation [12–14]. However, bed rest represents complete removal of loading and is likely to have different effects than habitual lack of PA or increase in SB.
The effect of SB on several metabolic diseases has received much attention, while little is known about the effect of SB on bone health [15]. Recently, several studies have been performed on the relation between SB and bone health in the young with diverse results. To the best of our knowledge, a systematic review specifically aimed at this topic has not been performed. Therefore, the aim of this systematic review was to examine the association between SB and bone health in children, adolescents and young adults, as these individuals are in a crucial phase of life for bone accrual.
Methods
Search strategy and inclusion criteria
We performed a comprehensive search to identify all studies published in peer-reviewed journals that examined associations between total sedentary time or patterns of SB and bone outcomes in children, adolescents and young adults. SB was defined as any waking behaviour characterized by an energy expenditure ≤1.5 METs (metabolic equivalents) while in a sitting or reclining posture [16]. This review was reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [17]. Four electronic databases were searched for relevant studies up to January 9, 2017: PubMed (from 1966), MEDLINE (from 1946), PsycINFO (from 1967) and Science Citation Index (from 1988). The following combinations of MeSH and free terms were used: bone, bone area, bone (mineral) density, bone strength, bone mass, peak bone mass, bone architecture, sedentary lifestyle, motor activity, exercise, physical activity, child, adolescent and young adult. The search was restricted to human studies written in English. We included studies that contained healthy participants ≤24 years, had results on bone health (e.g. strength, mass and structure) and measured SB either subjectively or objectively. We only considered studies that used a specific measure of SB and not just a lack of PA or the lowest PA level. There were no restrictions on study design. Reference lists of included studies were hand-searched to identify additional articles. Two reviewers (J.K. and J.R.) independently assessed each article and discussed any discrepancies to reach consensus on inclusion.
Quality assessment of studies and data extraction
After a preliminary screening and exclusion of irrelevant studies, the quality of relevant studies (high, moderate or low) was assessed using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies of the National Institutes of Health [18]. Two reviewers (J.K. and J.R.) independently evaluated the quality and discussed until consensus was reached in case of any discrepancies. An initial high rating was given to longitudinal studies, while a moderate rating was given to case-control and cross-sectional studies. The initial rating was either up- or downgraded based on the risk of bias, which was based on the following 14 components: (1) clearly stated research question; (2) specified and defined study population; (3) participation rate of eligible persons; (4) selection of subjects; (5) sample size justification; (6) exposure measured prior to outcome(s); (7) sufficient timeframe between exposure and outcome; (8) levels of exposure; (9) exposure measures defined, valid, reliable and consistently implemented; (10) exposure(s) assessed more than once over time; (11) outcome measures defined, valid, reliable and consistently implemented; (12) blinding of outcome assessors; (13) loss to follow-up and (14) adjustment for key confounders.
We extracted descriptive information regarding the study reference (authors and year of publication), study design, participants (sex, sample size, maturity and age), method of measurements and outcomes of SB, bone-imaging tools, anatomical sites assessed and bone variables reported (such as strength, mass and structure). In case of unclear results, the primary author was contacted by e-mail. Results were summarized and results that were and those that were not adjusted for MVPA were reported separately.
Best evidence synthesis and clustering
We noted several differences regarding the types of outcomes, the bone-imaging tools and the anatomical sites assessed in the included studies. Therefore, we combined several screen-related outcomes (TV or video time, screen time no game, screen time game and total screen time) as screen time. We clustered the assessed anatomical sites into three different categories: the lower extremities (include the femoral neck, proximal femur, total hip, distal tibia and calcaneus), the lumbar spine and the total body. As there was only one study examining bone outcomes at the arm, we did not cluster these results in any of the categories but described these in the section ‘Other bone-related outcomes’. The same applies to the two studies that reported on the relation between interruptions of SB and bone outcomes; these results are presented in a separate section called ‘Interruptions of SB and bone outcomes’. Furthermore, 13 studies used dual-X-energy absorptiometry (DXA) including one study that also used high-resolution peripheral quantitative computed tomography (HR-pQCT) and one study that also used quantitative ultrasound (QUS). There was one other study that used HR-pQCT and three other studies that used QUS. We did not differentiate between bone-imaging tools in the results. The statistical methods used in the included studies varied, resulting in different types of effect sizes (regression coefficients and odds ratios), making statistical pooling impossible.
To be able to draw conclusions regarding the association between SB and bone health, we applied the approach of a best evidence synthesis, as used by Singh et al. [19]. There are three levels of evidence in this rating system taking the number, the quality and the consistency of outcomes of the studies into account. Results were considered consistent when at least 75% of significant outcomes (p < 0.05) had the same direction:
Strong evidence: consistent findings in ≥2 high-quality studies
Moderate evidence: consistent findings in one high-quality study and at least one moderate-quality study, or consistent findings in ≥3 moderate-quality studies
Insufficient evidence: only one study available or inconsistent findings in ≥2 studies
Some studies presented their results separately for boys and girls, while others only presented results for both genders combined. For the best evidence synthesis, we did not differentiate between genders as this would result in more subcategories with too few studies. Furthermore, when studies presented multiple outcomes, we clustered comparable results to prevent a disproportional influence of one study reporting multiple comparable outcomes at the same anatomical location. For instance, one study reported nine outcomes at the distal tibia of which six outcomes represented bone architecture, two outcomes bone mineral density (BMD) and one outcome strength [20]. In this case, bone architecture, BMD and strength were all counted as one outcome, resulting in three outcomes at the distal tibia. There was an association if at least 75% of significant outcomes (p < 0.05) in a subcategory (e.g. bone architecture) had the same direction.
Results
Search results and quality assessment
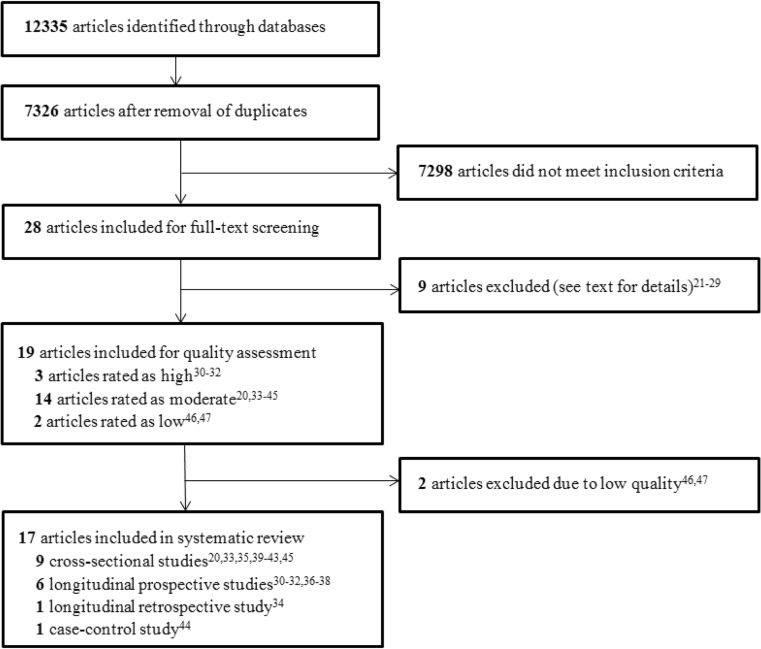
We identified 12,335 potentially relevant articles. After the duplicates were removed, 7326 articles remained of which the titles and abstracts were reviewed. Of those, 7298 were found irrelevant based on title or abstract, leaving 28 articles for full-text review. Of these, 19 studies were included initially (Fig. 1). The excluded articles (n = 9) did not report on bone outcomes in the results, did not investigate children or investigated children with low birth weight [21–29]. The hand-search of reference lists of included studies did not yield additional relevant articles.
Fig. 1.
Flow chart of study selection
Only high- and moderate-rated studies were included for this review. Three longitudinal studies received a high rating [30–32], and 14 studies were given a moderate rating [20, 33–45]. Two cross-sectional studies received a low rating and were therefore excluded from this review (for the ratings, see Online Resource 1) [46, 47]. Thus, 17 articles were included in this review. All 17 included articles had an observational design: nine cross-sectional studies [20, 33, 35, 39–43, 45], six longitudinal prospective cohort studies [30–32, 36–38], one longitudinal retrospective cohort study [34] and one case-control study [44]. During the review process, we noticed that two high-quality studies possibly had an overlap between the cohorts [31, 32]. We contacted the authors, who confirmed the largely overlapping cohorts. Therefore, we have considered these two high-quality studies as one high-quality study in the best evidence synthesis.
Characteristics of included studies
Of the 17 included studies, participants of 14 studies were recruited from birth announcements, paediatric practices and elementary or high schools [20, 30–32, 35–37, 39–45]. One study included participants that were part of the National Health and Nutrition Examination Survey [33], while another included participants of the Berkeley Bone Health Study [34]. The Raine Study included offspring of pregnant women which were recruited in antenatal clinics [38].
The age of the participants varied between 2 and 24 years. The mean sample size was 760, with a range of 52 and 4465 participants. As shown in Table 1, sedentary time was objectively measured by wearable monitors/accelerometers in nine studies. The requirements for accelerometry data to be valid for each study were heterogeneous: minimal number of wear days varied between 3 and 5 days and minimal wear time varied between 6 and 10 h/day. The accelerometers were worn long enough in all accelerometry-based studies to generate reliable average PA and SB [48]. Moreover, these studies used the same classification for SB (<100 counts per minute). In two of the accelerometry-based studies, SB was further characterized using questionnaires [20, 33]. Eight studies used only questionnaires to assess the type of SB [34–38, 40, 41, 43]. None of the studies used the same questionnaire. Questionnaires contained questions to inquire information about the average daily engagement in sedentary patterns such as the time spent watching TV or using computers. Only one study used a questionnaire that was validated with accelerometry [49]. Sixteen studies developed regression models to assess the contribution of SB to bone outcomes, while one study used ANOVA and ANCOVA to investigate differences in bone mineral content (BMC) between TV-watching trajectories [38]. The other characteristics and the main results (described below) are presented in Table 1.
Table 1.
Descriptive characteristics and main results of the reviewed studies
| First author (year of publication) | Study design (follow-upa) | Sample size (♂/♀); maturity status (age range) | Outcomes of SB; results presented separately or combined for ♂/♀ | Assessment of SB (in case of accelerometry: type, epochs in s) | Bone assessment techniques | Anatomical sites assessed: bone outcomes | Main results |
|---|---|---|---|---|---|---|---|
| Gabel (2015) [20] | Cross-sectional | 328 (154/174); pre-, peri-, postpubertal (9–20 years) | Total sedentary time, screen time; ♂ and ♀ together | Accelerometry (actigraph, 15), questionnaire | HR-pQCT | Distal tibia: BMD, bone strength and architecture | Total sedentary time and screen time: no association with any bone outcome. Adjusted for MCSA, tibia length, maturity, ethnicity, dietary calcium and MVPA. |
| Heidemann (2013)b [30] | Longitudinal prospective (2 years) | 742 (not given); pre-, peripubertal (9.7–13.9 years) | Total sedentary time; ♂ and ♀ together | Accelerometry (actigraph, 2) | DXA | Total body: BA, BMC, BMD | Total sedentary time and: TB BMC b = 27.77, SE 8.17 p < 0.01, TB BMD and TB BA NS. Adjusted for height, weight, age, puberty at follow-up, bone outcome at baseline, activity levels, interaction between gender and activity levels and interaction between gender and puberty. |
| Ivuskans (2015)b [31] | Longitudinal prospective (1 year) | 169 (169/0); peripubertal (11–13 years) | Total sedentary time; ♂ | Accelerometry (actigraph, 60) | DXA | Total body, lumbar spine, femoral neck: BA, BMC, BMD | The change in total sedentary time over a 1-year period and: ΔTB BMD, BMC, BA NS ΔLS BMD, BMC, BA NS ΔFN BMD b = −0.185 p < 0.03, BMC b = −0.167 p < 0.03, BA NS All adjusted for Δchronical age, Δpubertal status, Δbody mass, baseline PA levels, body mass and pubertal stage. |
| Vaitkeviciute (2014)b [32] | Longitudinal prospective (2 years) | 206 (206/0); peripubertal (11–14 years) | Total sedentary time; ♂ | Accelerometry (actigraph, 60) | DXA | Total body, lumbar spine, femoral neck: BMD | Total sedentary time and: TB BMD NS, and LS BMD NS, and FN BMD b = −0.0001 p < 0.03. Adjusted for age, LBM, weight at baseline and MVPA. |
| Binkley (2016) [41] | Cross-sectional | 155 (79/76); pre-, peripubertal (6–20 years) | Total sedentary time; ♂ and ♀ together | Questionnaire | HR-pQCT | Distal tibia: bone strength, Endo C, Peri C | Total sedentary time and: Endo C b = −0.20 (−0.37;−0.03) p < 0.05 Peri C b = −0.17 (−0.32;−0.02) p < 0.05 pSSI b = −7.1 (−13.4;−0.8) p < 0.05 The results above are MVPA adjusted. All other bone variables NS. All adjusted for age, sex and fat mass. |
| DXA | Lumbar spine, femoral neck, total hip: BA, BMC | ||||||
| Chastin (2014) [33] | Cross-sectional | 1348 (671/677); pre-, peri-, postpubertal (8–22 years) | Total sedentary time, TV time, screen time no game, total screen time, total non-screen time; ♂ and ♀ separately | Accelerometry (actigraph, 60), questionnaire | DXA | Lumbar spine, proximal femur: BMC | Total sedentary time and: ♂: FE BMC b = 15.93 (18.64;23.11) p < 0.001, and LS BMC b = 24.10 (13.22;34.99) p < 0.001 ♀: FE BMC b = 8.07 (3.18;12.97) p < 0.001, and LS BMC b = 23.63 (12.91;34.35) p < 0.001 TV time and: ♂: FE BMC b = −0.45 (−0.83;−0.06) p < 0.05, and LS BMC NS ♀: FE BMC b = −0.26 (−0.48;−0.05) p < 0.05, and LS BMC b = −0.58 (−1.13;−0.03) p < 0.05 Total non-screen time and: ♂: FE BMC b = 0.327 (0.141;0.513) p < 0.01, and LS BMC NS ♀: FE BMC b = 0.327 (0.141;0.513) p < 0.01, and LS BMC b = 0.58 (0.35;0.805) p < 0.001 Models were adjusted for age, BMI, ethnicity, MVPA and age of first menstrual cycle (♀) or vitamin D (♂). Only MVPA adjusted results are shown in this table. |
| Gracia-Marco (2012) [40] | Cross-sectional | 359 (178/181); peripubertal (12.5–17.5 years) | Total sedentary time, TV time, screen game time, screen time no game, time spent studying (non-school); ♂ and ♀ separately | Questionnaire | DXA | Total body, lumbar spine, femoral neck: BMC | Sedentary activity variables and: FE BMC ♂: all NS ♀: study time: b = −0.119 p < 0.05 LS BMC ♂/♀: all NS TB BMC: ♂: internet for non-study: b = −0.075 p < 0.05; others NS ♀: all NS Results were adjusted for height, sexual maturation, total lean mass and MVPA. Only MVPA-adjusted results are shown in this table. |
| Herrmann A (2015) [45] | Cross-sectional | 4465 (2213/2252); prepubertal (2–10 years) | Total sedentary time; ♂ and ♀ together | Accelerometry (actigraph, 60), questionnaire | QUS | Calcaneus: SI | Total sedentary time and: SI preschool b = −0.84 p < 0.02 and SI school b = −0.60 p < 0.01. Screen time and: SI preschool and SI school NS. All adjusted for age, sex and country. After MVPA adjustment: Total sedentary time and: SI preschool NS, SI school b = −0.42 p < 0.02. Screen time: SI preschool and school NS |
| Herrmann B (2015) [44] | Case-control | 1819 (960/859); prepubertal (2–9 years) | Total sedentary time; ♂ and ♀ together | Accelerometry (actigraph, 60) | QUS | Calcaneus: SI | Odds ratio for poor SI for children that sit >42.6% of total wearing time (cases vs. controls): OR = 0.86 (0.57;1.30). Adjusted for age, sex, country, fat-free mass, daylight duration, consumption of dairy products and MVPA. |
| Sioen (2015) [42] | Cross-sectional | 210 (105/105); pre-, peripubertal (6–12 years) | Total sedentary time; ♂ and ♀ together | Accelerometry (actigraph, 15) | DXA | Total body: BMC, BMD | Total sedentary time and: TB BMC and BMD NS Adjusted for age, gender, tanner stage, height, fat mass index, fat-free mass index and MVPA. |
| Vicente-Rodriguez (2015) [43] | Cross-sectional | 277 (168/109); peri-, postpubertal (13–18.5 years) | TV time, screen game time, time spent studying (non-school); ♂ and ♀ separately | Questionnaire | DXA | Total body: BMC | TV time and low bone mass, before PA adjustment: ♂: OR 7.01 (1.73–28.40), and ♀: NS After PA adjustment: ♂/♀: NS All other activity variables were not associated with low bone mass before and after adjusting for PA. Adjusted for body mass, height, lean mass, fat mass, sex, tanner stage and PA participation. |
| Wang (2003) [34] | Longitudinal retrospective (10 years) | 341 (0/341); pre-, peri-, postpubertal (11–24 years) | TV/video time; ♀ | Questionnaire | DXA | Total body, lumbar spine, proximal femur: BMC, BMD | TV/video time and: FE BMD b = −0.001, SE 0.0004 p < 0.05 LS BMD NS, and TB BMD NS CA BUA, SOS and SI NS Adjusted for race, weight, height and dietary calcium in midpuberty. |
| QUS | Calcaneus: BUA, SOS, SI | ||||||
| Winther (2016) [35] | Cross-sectional | 747 (359/388); peri-, postpubertal ♂, postpubertal ♀ (15–18 years) | Total screen time; ♂ and ♀ separately | Questionnaire | DXA | Total body, femoral neck, total hip: BMD | >6 h/day screen time and: Cohort 1: ♂ TH b = −0.062 (−0.120;−0.004) p < 0.05 ♂ FN b = −0.064 (−0.121;−0.007) p < 0.05 ♂ TB NS ♀ FN b = 0.058 (0.010;0.105) p < 0.05, TH and TB NS Cohort 2: All bone variables for ♂/♀ NSAdjusted for age, BMI, height, sexual maturation, leisure time PA, smoking habits, alcohol consumption, vitamin D levels, calcium intake and carbonated drink consumption. Only PA-adjusted results are shown in this table. |
| Wosje (2009) [36] | Longitudinal prospective (3.5 years) | 214 (109/105); prepubertal (3–7 years) | TV/video time; ♂ and ♀ together | Questionnaire | DXA | Total body: BA, BMC | High TV watching (>2 h/day) was associated with lower BMC: b = −10.74, SE 5.09 p < 0.05, and lower BA: b = −14.1, SE 5.07 p < 0.01. Adjusted for fat mass, BA, race, sex, age, height and calcium intake. |
| De Smet (2014) [39] | Cross-sectional | 306 (153/153); prepubertal (6–12 years) | Total sedentary time; ♂ and ♀ together | Accelerometry (actigraph, 15) | QUS | Calcaneus: BUA, SI, SOS | Total screen time and: CA BUA b = −0.20 p < 0.05, CA SI b = −0.26 p < 0.02, CA SOS NS Adjusted for age, sex, fat mass and education. |
| McVeigh (2016) [38] | Longitudinal prospective (15 years) | 1181 (607/574); pre-, peri-, postpubertal (5–20 years) | TV time; ♂ and ♀ separately | Questionnaire | DXA | Total body, total leg, total arm: BA, BMC, BMD | High TV watchers (>14 h/week) were compared with low TV watchers (<14 h/week): Leg BMC (g): ♂: 569, SE 6 vs. 612, SE 12 p < 0.01 ♀: 447, SE 6 vs. 471, SE 6 p < 0.01 Arm BMC (g): ♂: 214, SE 3 vs. 234, SE 5 p < 0.01 ♀: 153, SE 2 vs. 160, SE 2 p < 0.03 TB BMC (g): ♂: 3111, SE 31 vs. 3338, SE 59 p < 0.01 ♀: 2610, SE 26 vs. 2711, SE 38 p < 0.01 Adjusted for height, body mass, PA, calcium intake, vitamin D levels, alcohol and smoking. Only PA-adjusted results are shown in this table. |
| Bounds (2005) [37] | Longitudinal prospective (2 years) | 52 (25/27); prepubertal (6–8 years) | Total sedentary time; ♂ and ♀ together | Questionnaire | DXA | Total body: BMC, BMD | Total sedentary time and: TB BMC and BMD NSAdjusted for children’s longitudinal dietary intakes, sex, height, weight, BMI, age and mother’s TB BMC or BMD. |
Results between brackets indicate the 95% confidence interval
b beta coefficient, BA bone area, BMC bone mineral content, BMD bone mineral density, BMI body mass index, BUA broadband ultrasound attenuation, CA calcaneus, DXA dual-X-energy absorptiometry, Endo C endosteal circumference, FE femoral, FN femoral neck, HR-pQCT high-resolution peripheral quantitative computed tomography, LBM lean body mass, LS lumbar spine, MCSA muscle cross-sectional area, MVPA moderate-to-vigorous physical activity, NS non-significant, OR odds ratio, PA physical activity, Peri C periosteal circumference, pSSI polar strength strain index, QUS quantitative ultrasound, SB sedentary behaviour, SE standard error, SI stiffness index, SOS speed of sound, TB total body, TH total hip, TV television
aOnly applicable to longitudinal studies
bIndicates a high-quality study; all other studies are moderate quality
Association between SB and lower extremity bone outcomes
Twelve studies examined the relationship between total sedentary time and/or (non-)screen time and lower extremity bone outcomes [20, 31–35, 38–41, 44, 45]. Two high-quality articles indicated that objectively measured total sedentary time was negatively associated with bone outcomes at the lower extremities and that these associations were independent of MVPA [31, 32]. The first high-quality article investigated whether the change in SB over a 1-year period was associated with the change in lower extremity bone outcomes and reported that an additional hour of sedentary time was associated with 0.06 g lower femoral neck BMC in 11–13-year-old boys [31]. In the second high-quality article, the association between absolute values of SB and bone outcomes was investigated over a 2-year period [32]. An additional hour of sedentary time was associated with 0.006 g/cm2 lower femoral neck BMD in 11–14-year-old boys; every additional hour of MVPA was associated with 0.02 g/cm2 increase in femoral neck BMD. Compared to reducing sedentary time, increasing MVPA was associated with a 3.3 times greater increase in femoral neck BMD. These results suggest that 1 h less sedentary time per day has the same effect on femoral neck BMD as 18 min of MVPA in boys. Another moderate-quality study that was included in the best evidence synthesis reported that an additional hour of sedentary time was associated with a decrease in the bone stiffness index (SI) at the calcaneus of 0.42 in 6–10-year-old boys and girls [45]. This association was independent of MVPA. There was no such independent association in 2–5-year-old boys and girls in the same study.
An overview of all lower extremity bone outcomes and whether they were associated with total sedentary time or screen time is presented in Online Resource 1. The above-described two high-quality articles were considered as one high-quality study in the best evidence synthesis, because of the overlapping cohorts. According to the best evidence synthesis, we found moderate evidence for a negative association between objectively measured total sedentary time and lower extremity bone outcomes in schoolchildren that was independent of MVPA. There was insufficient evidence for this association in studies that did not adjust for MVPA, which is likely to be caused by the small amount of studies (n = 3). Insufficient evidence was also found for an association between subjectively measured total sedentary time or (non-)screen and lower extremity bone outcomes (Online Resource 1).
Association between SB and lumbar spine bone outcomes
Six studies examined the relationship between total sedentary time and/or (non-)screen time and lumbar spine bone outcomes [31–34, 40, 41]. In the above-described two high-quality articles, no association was observed between objectively measured total sedentary time and lumbar spine bone outcomes [31, 32]. An overview of all lumbar spine bone outcomes and whether they were associated with total sedentary time or patterns of SB is presented in Online Resource 1. We found insufficient evidence for an association between objectively and subjectively measured total sedentary time or (non-)screen time and lumbar spine bone outcomes.
Association between SB and total body bone outcomes
Eleven studies examined the relationship between total sedentary time and/or (non-)screen time and total body bone outcomes [30–32, 34–38, 40, 42, 43]. The above-described two high-quality articles observed no association between total sedentary time and total body BMC, bone area (BA) and BMD [31, 32]. In the third high-quality study, a significant positive association between total sedentary time and total body BMC was observed in 9.7–13.9-year-old boys and girls, while there was no significant association with total body BMD or BA [30].
An overview of all total body bone outcomes and whether they were associated with total sedentary time or patterns of SB is presented in Online Resource 1. According to the best evidence synthesis, we found strong evidence to suggest no association between objectively measured total sedentary time and total body bone outcomes in schoolchildren. There was insufficient evidence for an association between subjectively measured total sedentary time or (non-)screen time and total body bone outcomes.
Other bone-related outcomes
One moderate-quality study in boys and girls observed that ‘high’ TV watchers (>14 h/week) had lower arm BMC vs. ‘low’ TV watchers (<14 h/week) after adjustment for MVPA [38]. This result was not observed for arm BMD; for this variable, only data unadjusted for MVPA were provided.
Interruptions of SB and bone outcomes
Two moderate-quality studies looked at the effect of the frequency of breaks in sedentary time on bone outcomes [20, 33]. In one study, there was a negative association (MVPA adjusted) between both femoral neck and lumbar spine BMC in boys and girls with longer periods of SB [33]. The other study observed that breaks in sedentary time were not associated with distal tibia strength, BMD and architecture in boys and girls when controlling for MVPA [20].
Discussion
The aim of this systematic review was to examine the association between SB and bone health in children, adolescents and young adults. For lumbar spine bone outcomes, we did not find evidence for an association with total sedentary time, neither subjectively nor objectively assessed. Furthermore, no evidence was found for an association between subjectively measured total sedentary time and lower extremity or total body bone outcomes. However, we did find moderate evidence to suggest a negative association between objectively measured total sedentary time and lower extremity bone outcomes in schoolchildren that was independent of MVPA. Also, there was strong evidence to suggest no association between objectively measured total sedentary time and total body bone outcomes in the studies that used DXA in schoolchildren.
To the best of our knowledge, our study is the first to review the relation between sedentary time and bone outcomes per anatomical region. Therefore, there is little material for comparison. Prolonged bed rest causes bone loss in most skeletal regions including the lumbar spine, but in our systematic review, we could not find a clear association between SB and lumbar spine bone outcomes [50]. It is conceivable that this difference between SB and bed rest could be explained by increased muscle tension on the spinal skeletal structures during SB, while the lower limb muscles are unloaded in both positions [51].
Unfortunately, when a statistically significant result was observed, the clinical relevance was not discussed in all 17 included articles. Of the seven studies that measured both sedentary time objectively and bone outcomes at the lower extremities, only three studies presented results in such a way that we were able to make an estimation of the effect size [31, 32]. In the first study, an additional hour of sedentary time resulted in −0.06 g femoral neck BMC in boys, which is a −1.3% change of the femoral neck BMC per hour SB [31]. The second study observed that every additional hour of sedentary time resulted in −0.006 g/cm2 femoral neck BMD in boys [32]. This is a −0.7% change of the femoral neck BMD per hour SB. The third study reported that an additional hour of sedentary time was associated with a decrease in the bone SI at the calcaneus of 0.42 in boys and girls, which is a −0.5% change per hour SB [45]. These effect sizes seem small but may theoretically be relevant for bone accrual in the young, in particular when the SB is maintained over several years. If confirmed in other studies, reducing sedentary time could be helpful to optimize bone accrual in children who are unable or less interested to engage in PA. The potential effect of MVPA seemed to be larger than that of sedentary time; we calculated, based on data of one high-quality study, that 1 h less sedentary time per day had the same effect on femoral neck BMD as 18 min of MVPA [32].
It should be noted that the negative association between objectively measured total sedentary time and lower extremity bone outcomes was independent of MVPA. This association suggests that engaging in MVPA while remaining seated the rest of the day cannot prevent the negative effects of SB [38]. However, as argued by Chastin et al., it is also possible that the accelerometry underestimated the amount of PA. All studies in our review reporting an independent effect of SB used data obtained per minute, which could have resulted in misclassifying short periods of PA and underestimating the total time spent in MVPA [33]. Also, it should be noted that in growing children, the observed negative association with SB suggests that more time spent sedentary is associated with less bone accrual and not necessarily with bone loss.
Screen time was one of the most surveyed measures of SB. We found insufficient evidence for an association between screen time and bone outcomes, but screen time only represents a limited portion of all SB as children engage in many other sedentary activities such as doing homework, sitting in the classroom, eating and reading. Furthermore, screen time was a self-reported measure and most studies used questionnaires that were not validated, which may have biased the relationship to zero [52, 53].
Research into the effect of the frequency of breaks in sedentary time is novel in the field of bone health, but already common in studies investigating cardio-metabolic health [54–56]. Recently, a study in postmenopausal women reported that more breaks in sedentary time were associated with a lower risk of osteopenia and osteoporosis at the femoral neck, but not at the lumbar spine [57]. In our review, too few studies reported on this outcome to draw a conclusion, but since relations between interruptions of SB and cardio-metabolic and bone variables have been observed, further investigation is warranted.
There are only two other systematic reviews that examined the association between SB and bone health in youth, which both concluded that there was insufficient evidence for an association [58, 59]. However, these reviews focused on multiple health outcomes in which bone health received relatively little attention, included only one respectively eight studies and did not differentiate per anatomical region. Our review is more comprehensive, we included more studies (n = 17) and could therefore investigate the association more specifically. Furthermore, we differentiated between results that were and those that were not adjusted for MVPA.
Our review is limited by the quality of the included studies. Only three out of 17 studies were rated as high quality (18%). Studies in this review measured SB objectively and subjectively, resulting in different categories in the best evidence synthesis with fewer studies per category. Furthermore, some studies only presented results that were adjusted for MVPA, which again led to fewer results for each category. We are aware that there are differences in the metabolic state of bone at different pubertal stages with periods of rapid bone mineral accretion and periods with relatively less accretion [1]. Unfortunately, most included studies had participants with different combinations of pubertal statuses, making it impossible to do a sub-analysis based on pubertal status. The evidence in this review is to a large extent based on high-quality studies which used DXA and this technique can lead to misinterpretation of BMD values in growing children. Two-dimensional projection techniques like DXA determine density in a pre-specified area to calculate areal BMD (the most widely used marker for bone mineralization). Consequently, DXA evaluation of younger children with smaller bones will result in lower areal BMD values compared to older children with larger bones, while volumetric BMD can be equal [60]. Although two out of three high-quality studies controlled for age, weight and pubertal stage, only one controlled for height. Therefore, this omission is a limitation of the strength of the evidence. At last, the included studies used different bone-imaging tools to assess bone outcomes, but due to the small number of studies, it was not feasible to differentiate between bone-imaging tools.
Although the evidence base on the potential detrimental effects of excessive sedentary time on bone health in youth is growing, there is still a lack of quality evidence. This review highlights the heterogeneity of the available evidence; moreover, most studies had a cross-sectional design and the follow-up of longitudinal studies was limited. In order to make progress in this field, we need well-designed longitudinal studies in different age groups of children and adults, with objective measures of SB and PA, including usage of short measurement epochs, and HR-pQCT and DXA to assess bone outcomes.
Conclusion
This systematic review suggests that objectively measured total sedentary time is negatively associated with bone outcomes of the lower extremities in schoolchildren. This association seems rather small and independent of MVPA but clearly needs further study. Based on the available literature, we calculated that 1 h less sedentary time per day is associated with the same effect on femoral neck BMD as 18 min of MVPA, but it should be noted that this conclusion is based on one high-quality longitudinal study. In high-quality studies that used DXA, no association was observed between objectively measured SB and total body bone outcomes. For lumbar spine bone outcomes, there was insufficient evidence for an association with objectively measured SB.
Electronic supplementary material
(DOCX 41 kb)
Acknowledgements
The authors gratefully thank A. Koster for her advice on conducting the review and comments on the manuscript.
Abbreviations
- BA
Bone area
- BMC
Bone mineral content
- BMD
Bone mineral density
- DXA
Dual-X-energy absorptiometry
- HR-pQCT
High-resolution peripheral quantitative computed tomography
- MET
Metabolic equivalent
- MVPA
Moderate-to-vigorous physical activity
- PA
Physical activity
- QUS
Quantitative ultrasound
- SB
Sedentary behaviour
- SI
Stiffness index
Compliance with ethical standards
Conflicts of interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s00198-017-4076-2) contains supplementary material, which is available to authorized users.
Contributor Information
J. B. Koedijk, Phone: +316 34385324, Email: j.koedijk@student.maastrichtuniversity.nl
J. van Rijswijk, Email: j.vanrijswijk@student.maastrichtuniversity.nl
W. A. Oranje, Email: w.oranje@etz.nl
J. P. van den Bergh, Email: j@vdbergh.org
S. P. Bours, Email: s.bours@mumc.nl
H. H. Savelberg, Email: hans.savelberg@maastrichtuniversity.nl
N. C. Schaper, Email: n.schaper@mumc.nl
References
- 1.Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O'Karma M, Wallace TC, Zemel BS. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int: J Established Result Cooperation Eur Found Osteoporos Natl Osteoporos Found USA. 2016;27:1281–1386. doi: 10.1007/s00198-015-3440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rauch F, Bailey DA, Baxter-Jones A, Mirwald R, Faulkner R. The ‘muscle-bone unit’ during the pubertal growth spurt. Bone. 2004;34:771–775. doi: 10.1016/j.bone.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Schoenau E, Frost HM. The “muscle-bone unit” in children and adolescents. Calcif Tissue Int. 2002;70:405–407. doi: 10.1007/s00223-001-0048-8. [DOI] [PubMed] [Google Scholar]
- 4.Frost HM. Wolff’s law and bone’s structural adaptations to mechanical usage: an overview for clinicians. Angle Orthod. 1994;64:175–188. doi: 10.1043/0003-3219(1994)064<0175:WLABSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Colley RC, Garriguet D, Janssen I, Craig CL, Clarke J, Tremblay MS. Physical activity of Canadian children and youth: accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2011;22:15–23. [PubMed] [Google Scholar]
- 6.(2010) Global recommendations on physical activity for health. World Health Organization, Geneva [PubMed]
- 7.Tammelin T, Ekelund U, Remes J, Nayha S. Physical activity and sedentary behaviors among Finnish youth. Med Sci Sports Exerc. 2007;39:1067–1074. doi: 10.1249/mss.0b13e318058a603. [DOI] [PubMed] [Google Scholar]
- 8.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitt-Glover MC, Taylor WC, Floyd MF, Yore MM, Yancey AK, Matthews CE. Disparities in physical activity and sedentary behaviors among US children and adolescents: prevalence, correlates, and intervention implications. J Public Health Policy. 2009;30(Suppl 1):S309–S334. doi: 10.1057/jphp.2008.46. [DOI] [PubMed] [Google Scholar]
- 10.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc. 2009;41:998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 11.Owen N, Bauman A, Brown W. Too much sitting: a novel and important predictor of chronic disease risk? Br J Sports Med. 2009;43:81–83. doi: 10.1136/bjsm.2008.055269. [DOI] [PubMed] [Google Scholar]
- 12.Zerwekh JE, Ruml LA, Gottschalk F, Pak CY. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J Bone Miner Res: Off J Am Soc Bone Miner Res. 1998;13:1594–1601. doi: 10.1359/jbmr.1998.13.10.1594. [DOI] [PubMed] [Google Scholar]
- 13.Zwart SR, Hargens AR, Lee SMC, Macias BR, Watenpaugh DE, Tse K, Smith SM. Lower body negative pressure treadmill exercise as a countermeasure for bed rest-induced bone loss in female identical twins. Bone. 2007;40:529–537. doi: 10.1016/j.bone.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rittweger J, Winwood K, Seynnes O, de Boer M, Wilks D, Lea R, Rennie M, Narici M. Bone loss from the human distal tibia epiphysis during 24 days of unilateral lower limb suspension. J Physiol. 2006;577:331–337. doi: 10.1113/jphysiol.2006.115782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tremblay MS, Colley RC, Saunders TJ, Healy GN, Owen N. Physiological and health implications of a sedentary lifestyle. Appl Physiol, Nutr Metab = Physiol Appl, Nutr Metab. 2010;35:725–740. doi: 10.1139/H10-079. [DOI] [PubMed] [Google Scholar]
- 16.Sedentary Behaviour Research N Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab. 2012;37:540–542. doi: 10.1139/h2012-024. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339 [DOI] [PMC free article] [PubMed]
- 18.(2014) Quality assessment tool for observational cohort and cross-sectional studies. U.S. Department of Health & Human Services. Accessed 18-09 2016
- 19.Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev: Off J Int Assoc Study Obes. 2008;9:474–488. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 20.Gabel L, McKay HA, Nettlefold L, Race D, Macdonald HM. Bone architecture and strength in the growing skeleton: the role of sedentary time. Med Sci Sports Exerc. 2015;47:363–372. doi: 10.1249/MSS.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy K, Shepherd S, Williams JE, Ahmed SF, Wells JC, Fewtrell M. Activity, body composition and bone health in children. Arch Dis Child. 2013;98:204–207. doi: 10.1136/archdischild-2012-302823. [DOI] [PubMed] [Google Scholar]
- 22.Chastin SF, Mandrichenko O, Helbostadt JL, Skelton DA. Associations between objectively-measured sedentary behaviour and physical activity with bone mineral density in adults and older adults, the NHANES study. Bone. 2014;64:254–262. doi: 10.1016/j.bone.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Zanker CL, Osborne C, Cooke CB, Oldroyd B, Truscott JG. Bone density, body composition and menstrual history of sedentary female former gymnasts, aged 20-32 years. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2004;15:145–154. doi: 10.1007/s00198-003-1524-y. [DOI] [PubMed] [Google Scholar]
- 24.Ma D, Jones G. Television, computer, and video viewing; physical activity; and upper limb fracture risk in children: a population-based case control study. J Bone Miner Res Off J Am Soc Bone Miner Res. 2003;18:1970–1977. doi: 10.1359/jbmr.2003.18.11.1970. [DOI] [PubMed] [Google Scholar]
- 25.Ye S, Song A, Yang M, Ma X, Fu X, Zhu S. Duration of television viewing and bone mineral density in Chinese women. J Bone Miner Metab. 2014;32:324–330. doi: 10.1007/s00774-013-0504-3. [DOI] [PubMed] [Google Scholar]
- 26.Chin KY, Soelaiman IN, Mohamed IN, Ibrahim S, Wan Ngah WZ. The effects of age, physical activity level, and body anthropometry on calcaneal speed of sound value in men. Arch Osteoporos. 2012;7:135–145. doi: 10.1007/s11657-012-0091-2. [DOI] [PubMed] [Google Scholar]
- 27.Farr JN, Laddu DR, Blew RM, Lee VR, Going SB. Effects of physical activity and muscle quality on bone development in girls. Med Sci Sports Exerc. 2013;45:2332–2340. doi: 10.1249/MSS.0b013e31829c32fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonseca H, Goncalves D, Figueiredo P, Mota MP, Duarte JA. Lifelong sedentary behaviour and femur structure. Int J Sports Med. 2011;32:344–352. doi: 10.1055/s-0031-1271679. [DOI] [PubMed] [Google Scholar]
- 29.Babaroutsi E, Magkos F, Manios Y, Sidossis LS. Lifestyle factors affecting heel ultrasound in Greek females across different life stages. Osteoporos Int: J Established Result Cooperation Eur Found Osteoporos Natl Osteoporos Found USA. 2005;16:552–561. doi: 10.1007/s00198-004-1720-4. [DOI] [PubMed] [Google Scholar]
- 30.Heidemann M, Molgaard C, Husby S, Schou AJ, Klakk H, Moller NC, Holst R, Wedderkopp N. The intensity of physical activity influences bone mineral accrual in childhood: the childhood health, activity and motor performance school (the CHAMPS) study, Denmark. BMC Pediatr. 2013;13:32. doi: 10.1186/1471-2431-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivuskans A, Maestu J, Jurimae T, Latt E, Purge P, Saar M, Maasalu K, Jurimae J. Sedentary time has a negative influence on bone mineral parameters in peripubertal boys: a 1-year prospective study. J Bone Miner Metab. 2015;33:85–92. doi: 10.1007/s00774-013-0556-4. [DOI] [PubMed] [Google Scholar]
- 32.Vaitkeviciute D, Latt E, Maestu J, Jurimae T, Saar M, Purge P, Maasalu K, Jurimae J. Physical activity and bone mineral accrual in boys with different body mass parameters during puberty: a longitudinal study. PLoS One. 2014;9:e107759. doi: 10.1371/journal.pone.0107759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chastin SF, Mandrichenko O, Skelton DA. The frequency of osteogenic activities and the pattern of intermittence between periods of physical activity and sedentary behaviour affects bone mineral content: the cross-sectional NHANES study. BMC Public Health. 2014;14:4. doi: 10.1186/1471-2458-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang MC, Crawford PB, Hudes M, Van Loan M, Siemering K, Bachrach LK. Diet in midpuberty and sedentary activity in prepuberty predict peak bone mass. Am J Clin Nutr. 2003;77:495–503. doi: 10.1093/ajcn/77.2.495. [DOI] [PubMed] [Google Scholar]
- 35.Winther A, Ahmed LA, Furberg AS, Grimnes G, Jorde R, Nilsen OA, Dennison E, Emaus N. Leisure time computer use and adolescent bone health—findings from the Tromso Study, Fit Futures: a cross-sectional study. BMJ Open. 2015;5:e006665. doi: 10.1136/bmjopen-2014-006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wosje KS, Khoury PR, Claytor RP, Copeland KA, Kalkwarf HJ, Daniels SR. Adiposity and TV viewing are related to less bone accrual in young children. The Journal of Pediatrics. 2009;154:79–85.e72. doi: 10.1016/j.jpeds.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bounds W, Skinner J, Carruth BR, Ziegler P. The relationship of dietary and lifestyle factors to bone mineral indexes in children. J Am Diet Assoc. 2005;105:735–741. doi: 10.1016/j.jada.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 38.McVeigh JA, Zhu K, Mountain J, Pennell CE, Lye SJ, Walsh JP, Straker LM. Longitudinal trajectories of television watching across childhood and adolescence predict bone mass at age 20 years in the Raine Study. J Bone Miner Res: Off J Am Soc Bone Miner Res. 2016;31:2032–2040. doi: 10.1002/jbmr.2890. [DOI] [PubMed] [Google Scholar]
- 39.De Smet S, Michels N, Polfliet C, D’Haese S, Roggen I, De Henauw S, Sioen I. The influence of dairy consumption and physical activity on ultrasound bone measurements in Flemish children. J Bone Miner Metab. 2015;33:192–200. doi: 10.1007/s00774-014-0577-7. [DOI] [PubMed] [Google Scholar]
- 40.Gracia-Marco L, Rey-López JP, Santaliestra-Pasías AM, Jiménez-Pavón D, Díaz LE, Moreno LA, Vicente-Rodríguez G. Sedentary behaviours and its association with bone mass in adolescents: the HELENA cross-sectional study. BMC Public Health. 2012;12:971. doi: 10.1186/1471-2458-12-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Binkley TL, Specker BL. The negative effect of sitting time on bone is mediated by lean mass in pubertal children. J Musculoskelet Neuronal Interact. 2016;16:18–23. [PMC free article] [PubMed] [Google Scholar]
- 42.Sioen I, Michels N, Polfliet C, De Smet S, D’Haese S, Roggen I, Deschepper J, Goemaere S, Valtueña J, De Henauw S. The influence of dairy consumption, sedentary behaviour and physical activity on bone mass in Flemish children: a cross-sectional study. BMC Public Health. 2015;15:717. doi: 10.1186/s12889-015-2077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vicente-Rodriguez G, Ortega FB, Rey-Lopez JP, Espana-Romero V, Blay VA, Blay G, Martin-Matillas M, Moreno LA. Extracurricular physical activity participation modifies the association between high TV watching and low bone mass. Bone. 2009;45:925–930. doi: 10.1016/j.bone.2009.07.084. [DOI] [PubMed] [Google Scholar]
- 44.Herrmann D, Pohlabeln H, Gianfagna F, et al. Association between bone stiffness and nutritional biomarkers combined with weight-bearing exercise, physical activity, and sedentary time in preadolescent children. A Case-Control study. Bone. 2015;78:142–149. doi: 10.1016/j.bone.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 45.Herrmann D, Buck C, Sioen I, et al. Impact of physical activity, sedentary behaviour and muscle strength on bone stiffness in 2-10-year-old children-cross-sectional results from the IDEFICS study. Int J Behav Nutr Phys Act. 2015;12:112. doi: 10.1186/s12966-015-0273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braun SI, Kim Y, Jetton AE, Kang M, Morgan DW. Prediction of bone mineral density and content from measures of physical activity and sedentary behavior in younger and older females. Prev Med Reports. 2015;2:300–305. doi: 10.1016/j.pmedr.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sardinha LB, Baptista F, Ekelund U. Objectively measured physical activity and bone strength in 9-year-old boys and girls. Pediatrics. 2008;122:e728–e736. doi: 10.1542/peds.2007-2573. [DOI] [PubMed] [Google Scholar]
- 48.Rich C, Geraci M, Griffiths L, Sera F, Dezateux C, Cortina-Borja M. Quality control methods in accelerometer data processing: defining minimum wear time. PLoS One. 2013;8:e67206. doi: 10.1371/journal.pone.0067206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rey-Lopez JP, Ruiz JR, Ortega FB, et al. Reliability and validity of a screen time-based sedentary behaviour questionnaire for adolescents: the HELENA study. Eur J Pub Health. 2012;22:373–377. doi: 10.1093/eurpub/ckr040. [DOI] [PubMed] [Google Scholar]
- 50.LeBlanc AD, Spector ER, Evans HJ, Sibonga JD. Skeletal responses to space flight and the bed rest analog: a review. J Musculoskelet Neuronal Interact. 2007;7:33–47. [PubMed] [Google Scholar]
- 51.Callaghan JP, McGill SM. Low back joint loading and kinematics during standing and unsupported sitting. Ergonomics. 2001;44:280–294. doi: 10.1080/00140130118276. [DOI] [PubMed] [Google Scholar]
- 52.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clark BK, Sugiyama T, Healy GN, Salmon J, Dunstan DW, Owen N. Validity and reliability of measures of television viewing time and other non-occupational sedentary behaviour of adults: a review. Obes Rev: Off J Int Assoc Stud Obes. 2009;10:7–16. doi: 10.1111/j.1467-789X.2008.00508.x. [DOI] [PubMed] [Google Scholar]
- 54.Bankoski A, Harris TB, McClain JJ, Brychta RJ, Caserotti P, Chen KY, Berrigan D, Troiano RP, Koster A. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care. 2011;34:497–503. doi: 10.2337/dc10-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, Owen N. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31:661–666. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 56.Healy GN, Matthews CE, Dunstan DW, Winkler EA, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J. 2011;32:590–597. doi: 10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Braun SI, Kimb Y, Jetton AE, Kang M, Morgan DW (2016) Sedentary behavior, physical activity, and bone health in post-menopausal women. J Aging Phys Act 1–30 [DOI] [PubMed]
- 58.Chinapaw MJ, Proper KI, Brug J, van Mechelen W, Singh AS. Relationship between young peoples’ sedentary behaviour and biomedical health indicators: a systematic review of prospective studies. Obes Rev: Off J Int Assoc Stud Obes. 2011;12:e621–e632. doi: 10.1111/j.1467-789X.2011.00865.x. [DOI] [PubMed] [Google Scholar]
- 59.Cliff DP, Hesketh KD, Vella SA, et al. Objectively measured sedentary behaviour and health and development in children and adolescents: systematic review and meta-analysis. Obes Rev: Off J Int Assoc Stud Obes. 2016;17:330–344. doi: 10.1111/obr.12371. [DOI] [PubMed] [Google Scholar]
- 60.Faulkner RA, Davison KS, Bailey DA, Mirwald RL, Baxter-Jones AD. Size-corrected BMD decreases during peak linear growth: implications for fracture incidence during adolescence. J Bone Miner Res: Off J Am Soc Bone Miner Res. 2006;21:1864–1870. doi: 10.1359/jbmr.060907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 41 kb)