Abstract
Cardiometabolic diseases are primarily linked to enlarged visceral adipose tissue (VAT). However, some data suggest heterogeneity within the subcutaneous adipose tissue (SAT) depot with potential metabolic differences between the superficial SAT (sSAT) and deep SAT (dSAT) compartments. We aimed to investigate the heterogeneity of these three depots with regard to fatty acid (FA) composition and gene expression. Adipose tissue biopsies were collected from 75 obese women undergoing laparoscopic gastric bypass surgery. FA composition and gene expression were determined with gas chromatography and quantitative real-time-PCR, respectively. Stearoyl CoA desaturase-1 (SCD-1) activity was estimated by product-to-precursor FA ratios. All polyunsaturated FAs (PUFA) with 20 carbons were consistently lower in VAT than either SAT depots, whereas essential PUFA (linoleic acid, 18:2n-6 and α-linolenic acid, 18:3n-3) were similar between all three depots. Lauric and palmitic acid were higher and lower in VAT, respectively. The SCD-1 product palmitoleic acid as well as estimated SCD-1 activity was higher in VAT than SAT. Overall, there was a distinct association pattern between lipid metabolizing genes and individual FAs in VAT. In conclusion, SAT and VAT are two distinct depots with regard to FA composition and expression of key lipogenic genes. However, the small differences between sSAT and dSAT suggest that FA metabolism of SAT is rather homogenous.
Introduction
Excess adipose tissue and obesity predispose to metabolic disease.1 Adipose tissue is however a heterogenic tissue that can be divided into several depots.2 Although abdominal subcutaneous adipose tissue (SAT) is quantitatively the most important, visceral adipose tissue (VAT) is more closely linked to metabolic risk.3 The reasons for this are not entirely clear but may involve regional differences in gene expression as well as fatty acid (FA) handling.
Abdominal SAT can be further subdivided into deep SAT (dSAT) and superficial SAT (sSAT) based on its anatomical relation to a connective tissue layer termed Scarpa’s fascia.2 Some data suggest that there may be differences in gene expression4, 5 and FA metabolism6 between the two SAT depots. Recently, it was also reported that dSAT harbors more saturated FAs than sSAT as assessed by magnetic resonance imaging spectroscopy7 or gas chromatography.6 However, these findings were obtained in rather small study samples that need confirmation in larger populations. In Spanish overweight and obese subjects, there were specific differences in FA composition between omental VAT, perivisceral VAT and SAT.8 Although some of the differences were related to dietary intake, they also appeared to reflect differences in de novo lipogenesis. However, the authors did not investigate dSAT and sSAT.
FAs constitute important receptor agonists that regulate gene expression and thereby tissue function via auto- and/or paracrine mechanisms.9 In fact, FAs can trigger transcriptional profiles linked to the pathophysiology of obesity, such as inflammation and adipogenesis.10 Variations in the expression and activity of elongase and desaturase activities may partly explain depot-specific differences in FA composition. FA composition and gene expression have previously been compared between different adipose depots6, whereas knowledge about the associations between FAs and gene expressions within each depot is lacking.
The aim of the present study was to determine if FA composition, desaturase activity, and the association between FAs and expression of key lipogenic genes differ between the three different abdominal adipose tissue depots in a large sample of obese subjects undergoing laparoscopic gastric bypass surgery.
Materials and methods
Subjects
Obese women (n=75) undergoing laparoscopic Roux-en-Y gastric bypass surgery at Uppsala University Hospital were recruited for the current study. All patients met the national inclusion criteria (body mass index >35, age 18–60 years and preoperative metabolic evaluation) for bariatric surgery. Exclusion criteria for bariatric surgery were several, and included severe psychiatric disease and uncontrolled binge eating. The mean age of the study population was 39±10 years and body mass index was 41.9±4.5 kg m−2. All subjects were assigned a low-calorie diet (LCD) (Modifast, Nutrition&Santé, Revel, France) consisting of 800–1100 kcal per day for 1 month prior to surgery according to national clinical guidelines. The subjects approved participation through written informed consent.
Adipose tissue biopsies
All biopsies were collected during the Roux-en-Y gastric bypass surgery. The biopsies from sSAT and dSAT were obtained from the upper left side of the abdomen, through the opening used for accessing the abdominal cavity with surgical instruments. The VAT biopsies were taken from the greater omentum in conjunction with its division. No complications occurred. All biopsies were immediately snap frozen on dry ice with ethanol and directly transferred to the laboratory for storage in −80 °C until the time of analysis.
FA composition
Aliquots of the biopsies were used to measure FA composition. Percentages of individual FAs were measured in total adipose tissue triglycerides using gas chromatography as previously described.11 Stearoyl CoA desaturase (SCD-1) activity was estimated by using product-to-precursor FA ratios, that is, the 16:1n-7/16:0 ratio as well as the 18:1n-9/18:0 ratio, which have been shown in adipose tissue and in very low-density lipoproteins to be reflected by the SCD-1 expression in adipose tissue and liver respectively.12, 13, 14
RNA extraction, cDNA synthesis and real-time reverse transcription-PCR
The real-time reverse transcription-PCR and pre-PCR procedures were performed on a representative subgroup consisting of the 47 patients (38±10 years and 40.4±3.4 kg m−2) initially included to the study, as previously described15 with one exception: the expression was normalized according to the ΔCt method (2(target gene−control gene)) instead of using a standard curve. TaqMan probes from Applied Biosystems, CA, USA were used. The probes targeted the genes SCD-1 (Hs01682761_m1), FASN (Hs01005622_m1), LPL (Hs00173425_m1), HSD11B1 (Hs01547870_m1), TLR4 (Hs00152939_m1), FFAR4 (Hs00699184_m1), PLIN1 (Hs00160173_m1), MLXIPL (Hs00975714_m1), CEBPA (Hs00269972.s1) and LRP10 (Hs00204094_m1). The expression was normalized against LRP10 expression.
Statistical analysis
Data are presented as mean±s.d. Before analyses, all variables were checked for normality using the Shapiro−Wilk test. Non-parametric tests were used since many of the variables were not normally distributed even after log transformation. Statistical differences between adipose tissue depots were analyzed with pairwise comparisons of Kruskal−Wallis one-way analysis of variance test. Correlations were analyzed using Spearman’s rho. A P-value of <0.05 was regarded statistically significant. All statistical tests were analyzed using IBM SPSS Statistics 22.0 (Armonk, NY, USA).
Results
FA composition in adipose tissue depots
Table 1 presents FA composition in the three examined depots. Notably, FA profiles differed mainly between VAT and SAT. The only FAs displaying differential levels between sSAT and dSAT were 18:3n-6 and 20:4n-6, which were slightly lower in dSAT. The most consistent variations between the depots were a significantly lower proportion of non-essential long-chain polyunsaturated FA (PUFA) with ≥20 carbons (Table 1). The proportions of the essential diet-derived PUFA 18:2n-6 and 18:3n-3, which are also precursors for longer PUFA, were similar between all adipose tissue depots. In addition to this, VAT showed lower 16:0, and higher 16:1n-7.
Table 1. The relative FA levels (%) within each adipose tissue depot.
| FA | sSAT | dSAT | oVAT | |
|---|---|---|---|---|
| Lauric acid | 12:0 | 0.43±0.15 | 0.48±0.16 | 0.57±0.23a,,b |
| Myristic acid | 14:0 | 2.79±0.42 | 2.84±0.46 | 2.89±0.50 |
| Pentadecanoic acid | 15:0 | 0.30±0.06 | 0.30±0.06 | 0.29±0.06 |
| Palmitic acid | 16:0 | 21.75±1.66 | 21.92±1.68 | 20.63±1.61a,,b |
| Palmitoleic acid | 16:1n-7 | 5.22±1.41 | 4.93±1.52 | 5.81±1.52a,,b |
| Margaric acid | 17:0 | 0.20±0.04 | 0.20±0.05 | 0.19±0.04 |
| Stearic acid | 18:0 | 2.94±0.66 | 2.94±0.81 | 2.97±0.75 |
| Oleic acid | 18:1n-9 | 53.28±1.93 | 53.49±2.02 | 54.25±2.14a |
| Linoleic acid | 18:2n-6 | 10.33±1.32 | 10.20±1.36 | 10.14±1.32 |
| γ-Linolenic acid | 18:3n-6 | 0.11±0.02 | 0.10±0.02a | 0.10±0.02 |
| α-Linolenic acid | 18:3n-3 | 1.09±0.23 | 1.09±0.24 | 1.09±0.25 |
| Di-homo-γ linolenic acid | 20:3n-6 | 0.31±0.10 | 0.31±0.10 | 0.18±0.08a,,b |
| Arachidonic acid | 20:4n-6 | 0.48±0.09 | 0.45±0.09a | 0.27±0.08a,,b |
| Eicosapentaenoic acid | 20:5n-3 | 0.13±0.04 | 0.12±0.04 | 0.10±0.03a,,b |
| Docosatetraenoic acid | 22:4n-6 | 0.17±0.05 | 0.19±0.06 | 0.13±0.05a,,b |
| Docosapentaenoic acid | 22:5n-3 | 0.29±0.08 | 0.30±0.09 | 0.23±0.09a,,b |
| Docosahexaenoic acid | 22:6n-3 | 0.17±0.07 | 0.17±0.08 | 0.14±0.08a,,b |
| SCD activity index | 16:1/16:0 | 0.24±0.07 | 0.23±0,08 | 0.28±0.08a,,b |
| Saturated fatty acids | SFA | 28.42±2.35 | 28.67±2.59 | 27.55±2.34b |
| Monounsaturated fatty acids | MUFA | 58.50±2.49 | 58.42±2.72 | 60.07±2.53a,,b |
| Polyunsaturated fatty acids | PUFA | 13.08±1.59 | 12.91±1.60 | 12.36±1.58a |
Abbreviations: dSAT, deep subcutaneous adipose tissue; FA, fatty acids; oVAT, omental visceral adipose tissue; SCD, stearoyl CoA desaturase; sSAT, superficial subcutaneous adipose tissue.
Significant differences differs from sSAT.
Differs from dSAT.
P<0.05 were considered as a significant.
Data presented as mean±s.d.
Gene expression in relation to FA composition
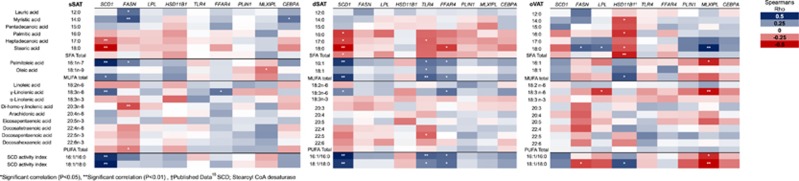
The gene expression differed between the depots but could not explain the regional differences in FA composition. For instance, although SCD-1 expression was lower in VAT, 16:1 levels were higher (data not shown). Therefore, the FA levels within each depot were compared with the expression of a few key lipogenic genes selected from hypothesis-based interactions with FA composition, that is, genes involved in FA uptake, signaling and metabolism (Figure 1). The correlation pattern differed between the depots with a distinct pattern for VAT. SCD-1 expression was consistently associated with estimated SCD activity (including 16:1n-7) in sSAT and dSAT, but not significantly in VAT. Only the correlations with 18:0 and 16:1 with SCD-1 expression in the SAT depots remained significant after a Bonferroni correction of P-values.
Figure 1.
Correlations between relative gene expression and relative FA level (%) within the adipose tissue from three depots. Each FA−gene pair is illustrated by a color which represents Spearman's rho, with dark blue indicating strong positive correlations and dark red indicating strong negative correlations.
The saturation of SAT was predominantly associated with SCD-1 expression whereas the saturation of VAT was associated with MLXIPL expression. Expression of TLR4 and FFAR4 were more pronouncedly correlated with FA levels in dSAT in comparison to sSAT and VAT.
Discussion
This is the first study that has compared FA composition and its relationship with the expression of genes involved in desaturation and lipogenesis in all three abdominal adipose tissue depots. We observed differences in FA composition between SAT and VAT but, in contrast to what has been previously suggested,6, 7 sSAT and dSAT displayed similar FA profiles. However, the relationships between FAs and gene expression differed among all depots suggesting some heterogeneity within SAT. The consistently lower proportions of non-essential PUFA in VAT versus the SAT depots have not been reported previously. The fact that essential FAs—18:2n-6 and 18:3n-3—were similar between the depots indicates that the variations in the non-essential PUFA are due to differences in endogenous FA turnover rather than dietary intake.
The FA composition of SAT and omental VAT has previously been investigated in a Mediterranean population.8 However they did not find clear differences in FA profiles between these depots. These discrepancies might be explained by important differences in the study design, for instance that the subjects in the current study underwent an LCD intervention prior to biopsy. Speculatively, a hypocaloric state may decrease the more easily oxidized PUFA in VAT, a depot which has an elevated lipolytic activity compared with SAT.16 However, a higher oxidation rate of PUFA does not explain why only the C20 PUFA, but not C18 PUFA were lower in VAT compared with SAT. In fact, 18:3n-3 has been shown to be the most readily oxidized PUFA and would therefore have been expected to be lower in VAT. Also, the sample size of the Mediterranean population (n=24) might have been insufficient to detect significant differences8.
The minor differences between sSAT and dSAT in FA composition and FA−gene expression associations suggest that SAT is rather homogenous. Noteworthy is that the present study compared the sub-depots of SAT in 75 participants compared with 30–43 subjects in previous reports. 6, 7 Thus the statistical power to detect possible differences in FA composition was clearly higher in the current study. Other differences between the studies include the methodology used for assessment of FA composition, and phenotype, that is, gas chromatography versus magnetic resonance imaging and obese versus non-obese, respectively.
We investigated if FA composition associates with gene expression of lipogenic genes, but we could not observe any consistent associations across the depots. Studies have previously shown that gene expression levels vary between the depots studied herein.4, 17, 18 Yet, to our knowledge, comparisons of correlations between FA species and gene expressions within the depots are novel. These findings may give insight into why FA composition differs between the depots and/or on the transcriptional regulation mediated by FAs per se. In fact, the transcriptional response to stimuli, such as glucocorticoids, differs between VAT and SAT.19 The SFA/MUFA ratio in SAT associated with SCD-1 expression is in agreement with previous findings12 but this relationship was less clear in VAT. This should be taken into consideration when comparing the regulation of FA composition in different depots since the responsible factors may differ. Furthermore, expression levels of TLR4, which SFA bind and induce inflammation through20, were negatively associated with SFA and positively associated with MUFA levels in dSAT but not in the other depots. Speculatively, this may be explained by a protective feature of dSAT against FA induced inflammation. An interesting observation was the correlation pattern across the depots. Although not entirely consistent, SFA exhibited a negative association with lipogenic genes in dSAT and a positive association in VAT with reversed observations for MUFAs. This observation was more apparent concerning the 16:1/16:0, 18:1/18:0 ratios. Similar patterns were observed for 18:3n-6 PUFA. Further, although inconclusive, VAT seemed to be more similar to sSAT than dSAT with regard to associations between FAs and gene expressions.
The link between FA composition, cellular/organ function and metabolism is complex.9 Firstly, FA composition is not only regulated by endogenous processes but also by diet. Secondly, FA species are ligands to nuclear receptors and modulate cellular processes directly through transcriptional regulation. Hence, the causality between FA levels and gene expression cannot be established in the present cross-sectional study. However, our data may serve as a hypothesis-generating resource for mechanistic studies in cell and/or animal models.
This study had some limitations. First, all participants were females, hence we cannot exclude possible gender differences. Second, all subjects underwent preoperative LCD according to standard clinical protocols. However, compliance to this regimen was not recorded. In addition, it is conceivable that LCD may alter FA composition and gene expression compared with habitual isocaloric diets. However, we find this less likely because previous studies have shown that LCD does not affect fasting NEFA uptake rates in VAT.21 Finally, the causal link between FAs and gene expression is lacking and warrants further investigation.
In conclusion, our results indicate that FA composition, as well as the link between FA species and expression of certain lipogenic genes differ between SAT and VAT, but with little variation between the SAT sub-depots. We further show that the association between FA composition and key lipogenic gene expression differ between SAT and VAT. Thus, at least in relation to FA composition, SAT can be regarded as a rather homogeneous tissue where the mechanisms regulating FA composition are most likely different from those in VAT.
Acknowledgments
This study was supported by the Swedish Research Council.
Footnotes
The authors declare no conflict of interest.
References
- Kopelman PG. Obesity as a medical problem. Nature 2000; 404: 635–643. [DOI] [PubMed] [Google Scholar]
- Lee M-J, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med 2013; 34: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Tseng Y-H, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell 2007; 131: 242–256. [DOI] [PubMed] [Google Scholar]
- Cancello R, Zulian A, Gentilini D, Maestrini S, Della Barba A, Invitti C et al. Molecular and morphologic characterization of superficial- and deep-subcutaneous adipose tissue subdivisions in human obesity. Obesity 2013; 21: 2562–2570. [DOI] [PubMed] [Google Scholar]
- Walker GE, Verti B, Marzullo P, Savia G, Mencarelli M, Zurleni F et al. Deep subcutaneous adipose tissue: a distinct abdominal adipose depot. Obesity 2007; 15: 1933–1943. [DOI] [PubMed] [Google Scholar]
- Marinou K, Hodson L, Vasan SK, Fielding Ba, Banerjee R, Brismar K et al. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care 2014; 37: 821–829. [DOI] [PubMed] [Google Scholar]
- Lundbom J, Hakkarainen A, Lundbom N, Taskinen M-R. Deep subcutaneous adipose tissue is more saturated than superficial subcutaneous adipose tissue. Int J Obes 2013; 37: 620–622. [DOI] [PubMed] [Google Scholar]
- Garaulet M, Pérez-Llamas F, Pérez-Ayala M, Martínez P, de Medina FS, Tebar FJ et al. Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am J Clin Nutr 2001; 74: 585–591. [DOI] [PubMed] [Google Scholar]
- Afman La, Müller M. Human nutrigenomics of gene regulation by dietary fatty acids. Prog Lipid Res 2012; 51: 63–70. [DOI] [PubMed] [Google Scholar]
- Shaw B, Lambert S, Wong MHT, Ralston JC, Stryjecki C, Mutch DM. Individual saturated and monounsaturated fatty acids trigger distinct transcriptional networks in differentiated 3T3-L1 preadipocytes. J Nutrigenet Nutrigenomics 2013; 6: 1–15. [DOI] [PubMed] [Google Scholar]
- Boberg M, Croon LB, Gustafsson IB, Vessby B. Platelet fatty acid composition in relation to fatty acid composition in plasma and to serum lipoprotein lipids in healthy subjects with special reference to the linoleic acid pathway. Clin Sci 1985; 68: 581–587. [DOI] [PubMed] [Google Scholar]
- Sjögren P, Sierra-Johnson J, Gertow K, Rosell M, Vessby B, de Faire U et al. Fatty acid desaturases in human adipose tissue: relationships between gene expression, desaturation indexes and insulin resistance. Diabetologia 2008; 51: 328–335. [DOI] [PubMed] [Google Scholar]
- Peter A, Cegan A, Wagner S, Lehmann R, Stefan N, Königsrainer A et al. Hepatic lipid composition and stearoyl-coenzyme A desaturase 1 mRNA expression can be estimated from plasma VLDL fatty acid ratios. Clin Chem 2009; 55: 2113–2120. [DOI] [PubMed] [Google Scholar]
- Bjermo H, Risérus U. Role of hepatic desaturases in obesity-related metabolic disorders. Curr Opin Clin Nutr Metab Care 2010; 13: 703–708. [DOI] [PubMed] [Google Scholar]
- Petrus P, Rosqvist F, Edholm D, Mejhert N, Arner P, Dahlman I et al. Saturated fatty acids in human visceral adipose tissue are associated with increased 11- β-hydroxysteroid-dehydrogenase type 1 expression. Lipids Health Dis 2015; 14: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S, Nakamura T, Shimomura I, Nishida M, Yoshida S, Kotani K et al. Insulin resistance and body fat distribution. Diabetes Care 1996; 19: 287–291. [DOI] [PubMed] [Google Scholar]
- Boulet N, Estève D, Bouloumié A, Galitzky J. Cellular heterogeneity in superficial and deep subcutaneous adipose tissues in overweight patients. J Physiol Biochem 2013; 69: 575–583. [DOI] [PubMed] [Google Scholar]
- Gerhard GS, Styer AM, Strodel WE, Roesch SL, Yavorek A, Carey DJ et al. Gene expression profiling in subcutaneous, visceral and epigastric adipose tissues of patients with extreme obesity. Int J Obes 2014; 38: 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindroos J, Husa J, Mitterer G, Haschemi A, Rauscher S, Haas R et al. Human but not mouse adipogenesis is critically dependent on LMO3. Cell Metab 2013; 18: 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol 2007; 27: 84–91. [DOI] [PubMed] [Google Scholar]
- Bucci M, Karmi AC, Iozzo P, Fielding BA, Viljanen A, Badeau RM et al. Enhanced fatty acid uptake in visceral adipose tissue is not reversed by weight loss in obese individuals with the metabolic syndrome. Diabetologia 2014; 58: 158–164. [DOI] [PubMed] [Google Scholar]