Abstract
Background/objectives:
Despite the clear health benefits of exercise, exercised-induced weight loss is often less than expected. The term ‘exercise energy compensation’ is used to define the amount of weight loss below what is expected for the amount of exercise energy expenditure. We examined the dose–response effects of exercise volume on energy compensation in postmenopausal women.
Participants/methods:
Data from Alberta Physical Activity and Breast Cancer Prevention (ALPHA) and Breast Cancer and Exercise Trial in Alberta (BETA) were combined for the present analysis. The ALPHA and BETA trials were two-centred, two-armed, 12-month randomized controlled trials. The ALPHA trial included 160 participants randomized to 225 min per week of aerobic exercise, and the BETA trial randomized 200 participants to each 150 and 300 min per week of aerobic exercise. All participants were aged 50–74 years, moderately inactive (<90 min per week of exercise), had no previous cancer diagnosis and a body mass index between 22 and 40 kg m−2. Energy compensation was based on changes in body composition (dual-energy X-ray absorptiometry scan) and estimated exercise energy expenditure from completed exercise volume. Associations between Δenergy intake, ΔVO2peak and Δphysical activity time with energy compensation were assessed.
Results:
No differences in energy compensation were noted between interventions. However, there were large inter-individual differences in energy compensation between participants; 9.4% experienced body composition changes that were greater than expected based on exercise energy expenditure, 64% experienced some degree of energy compensation and 26.6% experienced weight gain based on exercise energy expenditure. Increases in VO2peak were associated with reductions in energy compensation (β=−3.44 ml kg−1 min−1, 95% confidence interval for β=−4.71 to −2.17 ml kg−1 min−1; P=0.0001).
Conclusions:
Large inter-individual differences in energy compensation were noted, despite no differences between activity doses. In addition, increases in VO2peak were associated with lower energy compensation. Future studies are needed to identify behavioral and metabolic factors that may contribute to this large inter-individual variability in energy compensation.
Introduction
Public health agencies recommend at least 150 min per week of moderate or 75 min per week of vigorous intensity physical activity for overall health benefits.1, 2, 3 Some organizations also recommend >300 min per week of physical activity to promote weight loss and/or avoid weight gain.4, 5, 6, 7 The American Institute for Cancer Research8 has identified regular physical activity participation as a ‘probable’ factor for decreasing the risk of postmenopausal breast cancer. A recent pooled analysis of prospective cohort studies conducted by the National Cancer Institute cohort consortium also found a decreased risk of many types of cancers, including breast cancer, with higher leisure-time physical activity levels.9 More specifically, physical activity participation may lead to reductions in a number of biomarkers associated with increased breast cancer risk (for example, obesity and/or weight gain, sex-steroid and metabolic hormone levels, and inflammation).10, 11, 12
Despite the benefits of physical activity participation for overall health, exercise-induced weight loss may often be less than expected for the amount of energy expended from increased physical activity participation.13, 14, 15 The term ‘exercise energy compensation’ is used to define the amount of weight loss that is less than expected for the amount of energy expended from increased exercise participation.13, 16 Systematic reviews have reported that ~50–80% less weight loss than that predicted for the amount of energy expended from exercise occurs as a result of long-term exercise interventions (⩾6 months).13, 17, 18 Varying degrees of inter-individual weight loss in response to the same prescription of exercise volume were also noted within a number of exercise intervention trials.16, 19, 20, 21 A few studies have previously assessed the degree of energy compensation following different prescribed doses of exercise and have reported no additional weight loss with greater exercise volumes.19, 20, 22 However, these studies employed short-term exercise interventions (≈12 weeks)22 or reported the degree of energy compensation as a result of changes in overall body weight, not body composition.19, 20 In addition, no study to date has assessed exercise energy compensation as a result of individualized energy expenditure determined from completed exercise sessions rather than prescribed exercise volume, which provides a more accurate assessment of energy compensation that takes into account individual adherence rates to the prescribed exercise interventions.13
The Alberta Physical Activity and Breast Cancer Prevention (ALPHA) trial and the Breast Cancer and Exercise Trial in Alberta (BETA) were primary prevention trials designed to determine the effects of 12-month exercise interventions on biomarkers hypothesized to mediate the inverse association between physical activity and breast cancer risk.23, 24, 25 The ALPHA trial randomized 320 postmenopausal women to either a 225 min per week aerobic exercise intervention or usual care, and the BETA trial randomized 400 postmenopausal women to either 150 or 300 min per week of aerobic exercise. Previous reports from the ALPHA and BETA trials indicated that 225 min per week of aerobic exercise versus usual care, as well as 300 versus 150 min per week of aerobic exercise, led to greater reductions in total fat mass and other adiposity measures.23, 24 The exercise arms of the ALPHA and BETA trials were combined in the present paper, with the aim of comparing the effects of incremental increases in prescribed and completed exercise volume on exercise energy compensation. We hypothesized that there would be no differences in exercise energy compensation between the three exercise arms, despite incremental increases in exercise energy expenditure. We also hypothesized that the degree of exercise energy compensation will vary between participants, and that changes in total energy intake, VO2peak and/or total physical activity time would be associated with this large degree of variance in exercise energy compensation between individuals.
Materials and methods
Setting and participants
The design and methods for the ALPHA and BETA trials are described elsewhere.23, 25, 26 Briefly, these trials involved 12-month randomized controlled exercise interventions conducted in healthy, postmenopausal women living in Calgary and Edmonton, Alberta, Canada. The study protocols for both trials were approved by the Alberta Cancer Research Ethics Committee, the Conjoint Health Research Ethics Board of the University of Calgary and the Health Research Ethics Board of the University of Alberta. Written informed consent was obtained from all participants. Eligibility criteria for both trials included: age 50–74 years, postmenopausal, no previous cancer diagnosis, moderately inactive (<90 min per week of exercise or if between 90 and 120 min per week, having a VO2peak<34 ml kg−1 min−1 as measured by a submaximal fitness test), a body mass index between 22 and 40 kg m−2, non-smoker, able to do unrestricted physical activity as assessed by physician screening, and not planning to undertake a weight loss or dietary program. Participants in the ALPHA trial were randomized to either a 12-month exercise intervention (225 min per week of moderate-to-vigorous intensity aerobic exercise) or a usual care (control) group, whereas participants in the BETA trial were randomized to one of two 12-month exercise interventions (150 vs 300 min per week of moderate-to-vigorous intensity aerobic exercise). The exercise arms in the ALPHA and BETA trials were combined in the present analyses, with the aim of comparing the effects of incremental increases in exercise prescription and participation on exercise energy compensation.
Exercise interventions
Participants randomized to the exercise intervention in the ALPHA trial were asked to take part in at least 45 min of moderate-to-vigorous intensity aerobic exercise (70–80% of heart rate reserve) on 5 days per week for a total of 225 min/week. Participants in the BETA trial were randomized to either a moderate (150 min per week) or high (300 min per week) volume of aerobic exercise intervention. These participants exercised on 5 days per week at 65–75% of heart rate reserve for either 30 min (moderate volume group) or 60 min (high volume group). In both trials, the exercise intervention was supervised by certified exercise trainers at fitness facilities in Calgary and Edmonton on 3 days per week, and 2 days per week of exercise were unsupervised and completed at a location chosen by the participants. Exercise volume in both trials was increased gradually over a 12-week ramp-up period.23, 25, 26 Heart rate monitors were worn to provide continuous measurements of heart rate during the exercise trial and to ensure that the exercise was completed within the prescribed target heart rate zones. These monitors also provided objective measurements of exercise time and the type of aerobic exercise that was completed by the participants (for example, running and rowing). Exercise adherence was monitored with weekly exercise logs completed by the trainers. The trainers also recorded all information collected by the heart rate monitors (exercise time, continuous heart rate, time spent in the pre-determined heart rate zone and the aerobic activities that were completed) during supervised and unsupervised exercise sessions into a database that was maintained at the recreational facilities in Edmonton and Calgary. The mean heart rate values for each exercise session were used to estimate mean VO2 during exercise.27 These values, in addition to body weight assessed at baseline, were used to estimate exercise energy expenditure of each participant over the 12-month exercise intervention with the following equation:
N.B.: ExEE, exercise energy expenditure; VO2, volume of oxygen consumption. The variables in this equation are defined as: VO2 is the volume of oxygen consumption associated with mean exercise intensity, or heart rate, measured over the course of the 12-month intervention, body weight at baseline, as well as total exercise time over the course of the 12-month intervention. Two different estimations of energy expenditure were computed based on total exercise time: (1) total exercise time based on data collected by the heart rate monitors and recorded by the exercise trainers (used to determine exercise energy compensation based on completed exercise volume) and (2) total prescribed exercise time (used to determine exercise energy compensation based on prescribed exercise volume).
Outcome measures
All outcomes were assessed at baseline and post-intervention in both trials. Body fat mass and body fat-free mass were assessed with full-body dual-energy X-ray absorptiometry scans (Hologic Discovery A dual-energy X-ray absorptiometry system and Hologic QDR software or a GE Healthcare Lunar Prodigy dual-energy X-ray absorptiometry and GE Healthcare encore software, Marlborough, MA, USA). Changes in body fat mass and body fat-free mass following the exercise interventions, combined with exercise energy expenditure based on prescribed or completed exercise volume and estimated with the abovementioned equation, were used to determine exercise energy compensation, the primary outcome of interest in the present paper. The degree of exercise energy compensation (%) for each participant was assessed with the following equation13 and used energy equivalents for body fat mass and body fat-free mass that were previously described by Hall28 and Thomas et al.:29
N.B.: ExEC, exercise energy compensation; ExEE, exercise energy expenditure; FM, fat mass; FFM, fat-free mass. An exercise energy compensation of 0% indicates that the changes in body weight varied perfectly according to the amount of energy expended from exercise (100% of expected weight loss). Conversely, an exercise energy compensation of 100% indicates that there were no changes in body weight, despite increased exercise energy expenditure. Finally, a negative exercise energy compensation is indicative of changes in body weight that are greater than that expected for the amount of energy expended from exercise, whereas an energy compensation above 100% indicates increases in body weight despite increases in exercise energy expenditure.30
Changes in certain secondary outcomes that may be associated with the degree of exercise energy compensation were also included in the present analyses. These include changes in: estimated VO2peak, as assessed with a submaximal cardiorespiratory test using a multistage modified Balke treadmill protocol;31 total energy intake (kcal) assessed with the Canadian Diet History Questionnaire-II32 and the Diet*Calc Analysis Program (Version 1.4.3; National Cancer Institute Applied Research Program, Bethesda, MD, USA); total physical activity time (MET-hours per week) assessed with the Past Year Total Physical Activity Questionnaire.33 Accelerometers (Actigraph GT3X+, Pensacola, FL, USA) were also used to objectively assess total physical activity time in BETA (62% of all participants included in the energy compensation analysis and 2/3 randomization groups). Actigraph Vector Magnitude calculations34 were used to derive total physical activity time (MET-hours per day) from the accelerometry-measured activity counts. Changes in these secondary outcomes were determined by subtracting baseline to post-intervention values.
Covariates and proposed moderators
Standard demographic items included in the present analyses as covariates were obtained from a self-administered questionnaire at baseline and have been reported elsewhere.23, 25 These include age, marital status (married or common law vs unmarried), ethnicity (Caucasian vs other), education (>high school vs ⩽high school) and study site (Calgary vs Edmonton). Covariates also included baseline fat mass (kg) and baseline VO2peak (ml kg−1 min−1).
Statistical analyses
Sample sizes for the ALPHA and BETA trials were based on the primary endpoint of adiposity.23, 24 For the present analyses, it is estimated that the sample size of 530 participants with a pre-determined power of 0.80 and a two-tailed alpha of 0.05 provides an effect size of Cohen’s f=0.14 (medium effect) to detect differences in energy compensation between three groups. An analysis of covariance was used to assess the effects of prescribed exercise volumes (150 vs 225 vs 300 min per week) on completed (calculated based on reported exercise time by participants in the weekly exercise logs over 12 months) and prescribed (calculated based on prescribed exercise time over 12 months) exercise energy compensation. Differences in baseline covariates between exercise interventions were compared using an analysis of variance test for continuous variables and binary logistic regression for comparisons of frequencies for categorical variables. An intention-to-treat analysis that included all participants randomized to an exercise intervention in the ALPHA and BETA trials that had baseline and post-intervention body composition data regardless of their protocol adherence was used.
As a result of very high inter-individual variances in exercise energy compensation (prescribed exercise: −213 to 297% completed exercise: −1206 to 1176%), completed and prescribed exercise energy compensation data were truncated for participants with values greater or smaller than 3 s.d. from the mean, and replaced with values at ±3 s.d.’s from the mean (prescribed exercise: ±145%, n=19; completed exercise: 411%, n=11). A sensitivity analysis revealed no differences in results when using truncated values for the identified outliers for our main adjusted analysis (results with non-truncated values: 76±116 vs 69±172 vs 73±125% P=0.97, results with truncated values: 73±91 vs 65±88 vs 70±83% P=0.90). In addition, no significant differences in completed and prescribed estimates of energy compensation were noted between interventions (Figure 1), therefore only completed exercise energy expenditure was used as the primary outcome in subsequent analyses. Effect modification was assessed with an analysis of variance test, by estimating the P-values for the multiplicative interaction term between the abovementioned covariates and exercise intervention group on the primary outcome (completed exercise energy compensation). A median split for all continuous covariates (age, baseline fat mass and baseline VO2peak) was computed for this analysis. Multivariable linear regression analyses were used to examine the strength of the associations between changes in self-reported total energy intake, VO2peak and self-reported total physical activity time with completed exercise energy compensation. In a subsequent multivariable linear regression analysis, total physical activity time assessed with accelerometry was added to the model instead of self-reported total physical activity time. A multivariable analysis of covariance was also used to determine potential differences in Δenergy intake, ΔVO2peak and Δ self-reported total physical activity time between completed exercise energy compensation groups (1—exercise energy compensation<0%, 2—0%<exercise energy compensation⩽50%, 3—50%<exercise energy compensation⩽100%, 4—exercise energy compensation>100%). In an exploratory dose–response analysis that combined the three exercise intervention groups, we conducted a multivariable linear regression analysis to assess the strength of the association between mean completed exercise time (min per week) over the 12-month intervention with completed exercise energy compensation. A subsequent sensitivity analysis removing participants with <50 min per week of completed exercise time was also computed. Statistical analyses were performed using SPSS (version 19.0; SPSS, Chicago, IL, USA). Statistical significance was set at P<0.05.
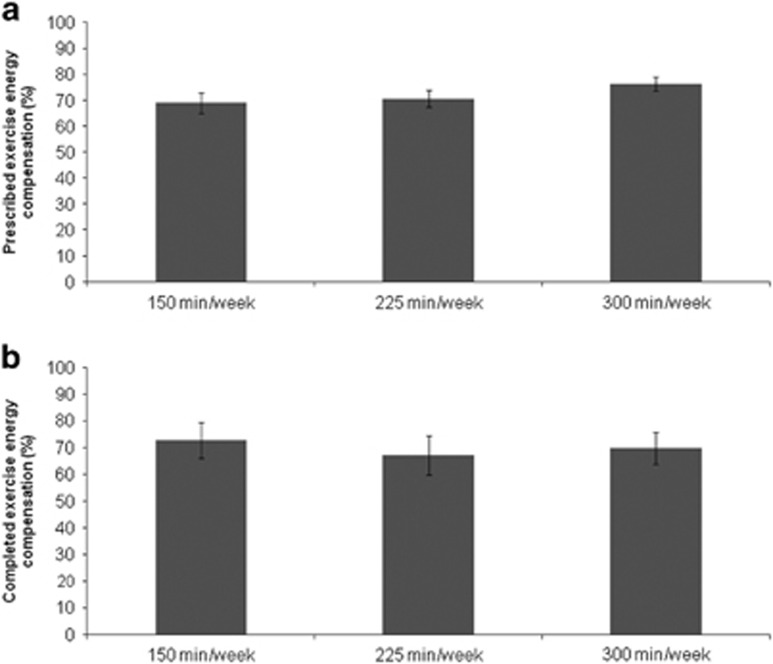
Figure 1.
Differences in (a) prescribed and (b) completed exercise energy compensation between exercise interventions. Prescribed exercise energy compensation: F (2, 519)=1.79; P=0.17; Completed exercise energy compensation: F (2, 519)=0.11; P=0.90. The values are presented as means±s.e.m.
Results
A flow chart of participant inclusion for the ALPHA and BETA trials is presented elsewhere.23, 24, 25 For the present analyses, participants who were randomized to exercise interventions and had baseline and post-intervention body composition assessments were included (total n=530; n for 150 min per week=187, n for 225 min per week=151, n for 300 min per week=192). There were no differences in baseline participant characteristics between groups, except for age and ethnicity (Table 1). No differences in prescribed and completed exercise energy compensation were noted between exercise interventions after adjusting for covariates (Figure 1). In addition, there was no evidence of effect modification in the association between completed exercise energy compensation and exercise group assignment by any of the covariates presented in Table 1 (results not shown).
Table 1. Baseline covariates for the participants randomized to exercise interventions in the ALPHA and BETA trials (n=530).
| Baseline characteristic | 150 min per week (n=187) | 225 min per week (n=151) | 300 min per week (n=192) | Prescribed exercise group comparison |
|---|---|---|---|---|
| Study site | ||||
| Calgary; n (%) | 52 (28)a | 76 (50)a | 51 (27)a | χ2 (1)=0.09; P=0.77 |
| Edmonton; n (%) | 135 (72)a | 75 (50)a | 141 (73)a | |
| Education category | ||||
| >High school; n (%) | 145 (77)a | 107 (71)a | 151 (79)a | χ2 (1)=0.09; P=0.77 |
| ⩽High school; n (%) | 42 (23)a | 43 (29)a | 41 (21)a | |
| Ethnic category | ||||
| Caucasian; n (%) | 173 (92)a | 135 (89)a | 164 (85)b | χ2 (1)=5.03; P=0.03 |
| Other; n (%) | 14 (8)a | 15 (11)a | 28 (15)a | |
| Marital status | ||||
| Married, common law; n (%) | 129 (69)a | 109 (72)a | 130 (68)a | χ2 (1)=0.08; P=0.78 |
| Other; n (%) | 58 (31)a | 41 (28)a | 62 (32)a | |
| Age (years); mean±s.d. | 60±5a | 61±5b | 59±5a | F (2, 527)=6.4; P=0.002 |
| Baseline fat mass (kg); mean±s.d. | 31±9a | 31±8a | 31±8a | F (2, 527)=0.1; P=0.91 |
| Baseline maximal oxygen uptake (ml kg-1 min-1); mean±s.d. | 27±5a | 27±6a | 27±5a | F (2, 527)=0.1; P=0.89 |
Note: means±standard deviations not sharing the same letter are significantly different from each other (P<0.05).
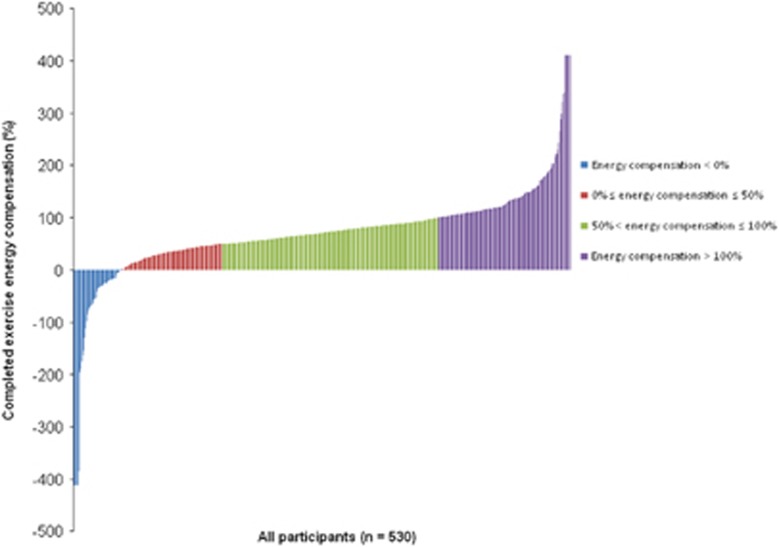
Figure 2 presents individual data for completed exercise energy compensation. In all participants, 9.4% experienced body composition changes that were greater than expected based on completed exercise energy expenditure, 64% experienced some degree of energy compensation in response to the exercise intervention (20.4% and 43.6% had 0–50% and 50.1–100% exercise energy compensation, respectively, in response to the amount of energy expended from exercise), and 26.6% of participants had an exercise energy compensation >100%, indicating weight gain based on completed exercise energy expenditure from the 12-month intervention.
Figure 2.
Distribution of completed exercise energy compensation between participants.
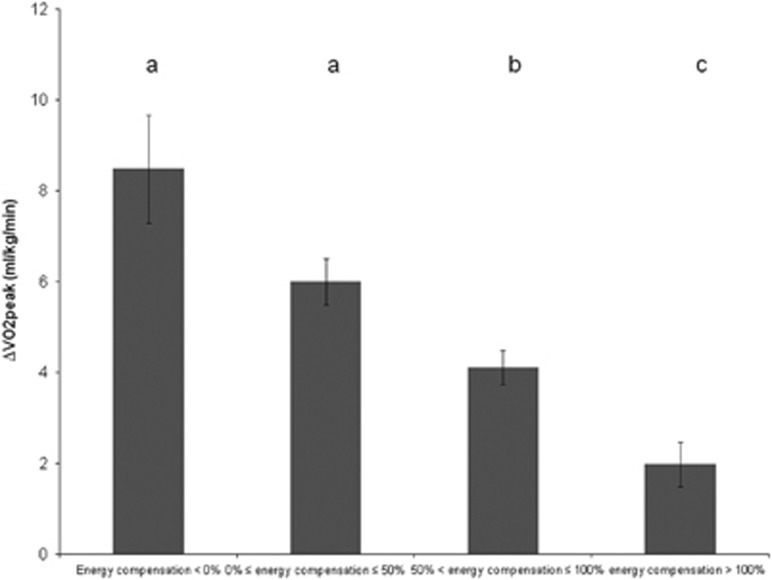
Increases in VO2peak were associated with lower degrees of completed exercise energy compensation in the adjusted linear regression model (β=−3.45 ml kg−1 min−1, 95% confidence interval (CI) for β=−4.67 to −2.22 ml kg−1 min−1; P=0.0001). No significant associations were noted between changes in self-reported total energy intake (β=0.01 kcal, 95% CI for β=−0.01 to 0.02 kcal; P=0.41) and self-reported total physical activity time (β=−0.05 MET-hours per week, 95% CI for β=−0.18 to 0.07 MET-hours per week; P=0.42) with completed exercise energy compensation. When substituting self-reported for objectively assessed total physical activity time in the adjusted multivariable linear regression model, a trend towards an inverse association between total physical activity time and exercise energy compensation was observed (β=−2.38 MET-hours per day, 95% CI for β=−5.08 to 0.31 MET-hours per day; P=0.08). In the between-group analysis adjusted for covariates, a significant main effect for changes in these outcomes between completed exercise energy compensation groups was noted (F (10, 476)=6.1; P=0.0001; Wilk’s Λ=0.89, partial η2=0.04). More specifically, changes in VO2peak were smallest in participants with>100% completed exercise energy compensation, and highest in participants with <0% completed exercise energy compensation (F (3, 476)=17.9; P=0.0001; Figure 3). No significant differences in Δenergy intake and Δself-reported total physical activity time were noted between completed exercise energy compensation groups (results not shown).
Figure 3.
Changes in VO2peak between completed exercise energy compensation groups. The values are presented as means±s.e.m. Note: Means not sharing the same letter are significantly different from each other (P<0.05).
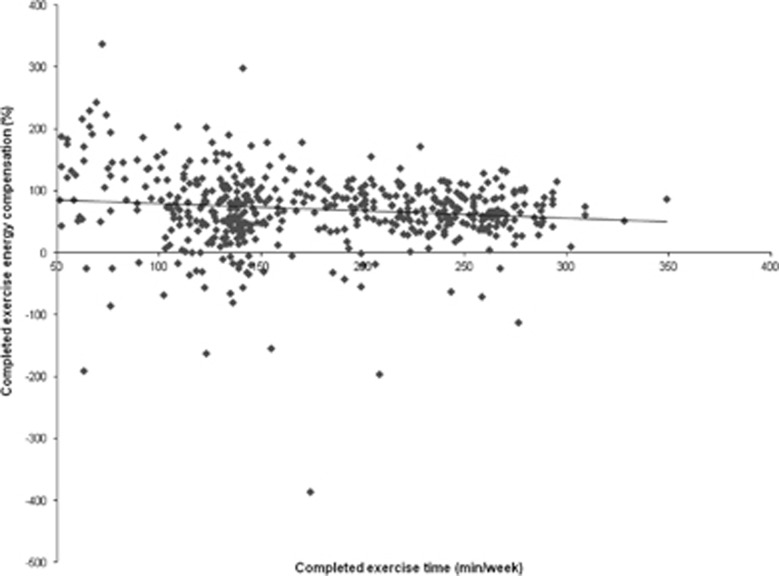
Our exploratory analysis revealed no significant association between completed exercise time with completed exercise energy compensation in the adjusted linear regression model (β=−0.09%, 95% CI for β=−0.19 to 0.02% P=0.12). However, the exclusion of participants with <50 min per week of completed exercise time (n=505 participants remaining) did reveal a significant inverse association between completed exercise time with completed exercise energy compensation in the adjusted linear regression model (β=−0.12%, 95% CI for β=−0.20 to −0.04% P=0.004; Figure 4).
Figure 4.
Inverse association between completed exercise time (min per week) and completed exercise energy compensation (%). Note: participants with a mean completed exercise time <50 min per week were excluded from this Figure.
Discussion
The primary finding from this paper indicates that there is no statistically significant difference in prescribed and completed energy compensation between randomization groups. However, our exploratory analysis revealed a statistically significant inverse association between completed exercise time (mean min per week) and energy compensation when removing participants with very low mean exercise adherence (<50 min per week) from the model. These results suggest that exercise energy compensation is lower when completed exercise volumes are higher, implying that greater amounts of weight loss may be achieved with greater exercise volumes, as previously reported in both the ALPHA24 and BETA23 trials.
These findings do not corroborate results from previous exercise trials that have reported no additional weight loss with greater exercise volumes.19, 20, 22 Certain participant (for example, age, sex and initial fat mass) and exercise intervention (for example, intervention duration and weekly exercise volume) characteristics have been previously associated with the degree of exercise energy compensation13, 35 and may, in part, explain the discrepancy between our studies and the literature. Participants of the ALPHA and BETA trials most closely resemble those recruited for the DREW trial (that is, overweight, previously inactive postmenopausal women).20 Differences in the prescribed exercise volumes (that is, 72–194 min per week over 6 months in the DREW trial vs 150–300 min per week over 12 months in the ALPHA and BETA trials) between these trials influenced the degree of exercise energy compensation calculated based on prescribed exercise energy expenditure. Greater amounts of weight loss are expected to occur with greater volumes of prescribed exercise, which results in higher degrees of exercise energy compensation if not achieved. In addition, a meta-analysis by Riou et al.13 reported increased exercise energy compensation with interventions of longer duration (that is, the span of the entire intervention), which may also partially explain the greater proportion of participants with some degree of exercise energy compensation noted in our research compared to the DREW trial.20 Last, the use of different equations to estimate exercise energy compensation (for example, exercise energy expenditure/7700 kcal kg−1 of body weight change in the DREW trial20) may also contribute to discrepancies in findings between studies.
To our knowledge, this paper is the first to compare prescribed to completed exercise energy compensation. Even though the results noted between these two methods were identical, the range of completed exercise time was variable within each prescribed exercise intervention group (range: ≈10–120% mean adherence over 12 months), thus implying that completed, rather than prescribed, exercise volume provides more precise estimations of exercise energy compensation. In addition, our exploratory analysis revealed that lower degrees of exercise energy compensation may be achieved with increased amounts of completed exercise. However, this was only observed when excluding participants with <50 min per week of completed exercise, thus suggesting that non-adherence may have contributed to the null findings initially reported.
Only one other randomized controlled exercise intervention (13-week exercise intervention in overweight men)22 and a meta-analysis13 used changes in body composition rather than body weight to determine inter-individual exercise energy compensation. The use of body composition to determine energy compensation is especially beneficial for exercise trials, as many studies have reported reductions in fat mass coupled with no changes or increases in fat-free mass following an exercise intervention compared to control and/or diet interventions.19, 35, 36, 37, 38, 39, 40, 41 Indeed, King et al.16 reported an increase in fat-free mass in compensators, whereas non-compensators experienced a decrease in fat-free mass following a 12-week exercise intervention. The degree of expected weight loss and the determination of exercise energy compensation may therefore be undermined in exercise trials as a result of the potential increases or maintenance of fat-free mass following the intervention.
Despite no differences in completed exercise energy compensation between exercise groups, we did note a large degree of variance in exercise energy compensation between participants, which corroborates our second hypothesis and previous findings.16, 19, 20, 21, 22, 40 These results indicate that the amount of weight loss expected to result from an identical prescription or completion of exercise volume is highly variable between participants. There are a multitude of factors that can contribute to this inter-individual variation in exercise energy compensation, including increased energy intake, decreased energy expenditure from activities outside of the prescribed exercise volume and/or metabolic adaptations to weight loss (for example, reductions in resting metabolic rate, upregulation of orexigenic and/or downregulation of anorexigenic peptides).13, 14, 15, 42, 43 However, recent systematic reviews suggest that changes in both energy intake and energy expenditure in response to exercise interventions are conflicting and inconclusive.44, 45 More specifically, studies have reported no significant differences in energy intake,20, 22, 35, 46 despite differences in energy compensation,20, 22 between exercise groups. Conversely, others reported an increase in energy intake in compensators vs non-compensators.16, 47 Similarly, no changes in energy expenditure across time were noted in some studies,20, 22, 35, 48, 49 whereas others reported lower energy expenditure in compensators vs non-compensators.47, 50 Given the large degree of variability in these behavioral components across time, discrepancies in these results may be in part explained by the type of measurement tools used (for example, self-reported questionnaires, in-laboratory test meals, accelerometry, doubly labeled water), in addition to the frequency of measurement administration.42
In the present study, we explored the strength of associations between changes in total energy intake, VO2peak and total physical activity time with completed exercise energy compensation. A trend towards an inverse association between objectively assessed total physical activity time and exercise energy compensation was noted, suggesting that reductions in total physical activity participation may be associated with greater degrees of exercise energy compensation. However, these result should be interpreted with caution, as objective measurements of total physical activity were only collected in BETA, which represents 2/3 of the randomized groups or 62% of the total sample included in the present paper. This analysis may therefore be statistically underpowered to detect any associations between these variables using an adjusted multivariable linear regression model. Changes in VO2peak were inversely associated with exercise energy compensation in the adjusted linear regression model. These results were confirmed by greater increases in VO2peak in participants with the lowest vs highest degree of exercise energy compensation, as well as the inverse association observed between completed exercise time (mean min per week) and energy compensation. These novel results suggest that greater increases in VO2peak, or fitness levels, in response to an exercise intervention are associated with lower degrees of energy compensation, independently of baseline covariates including baseline VO2peak. These results may have important implications for exercise interventions used as weight loss trials as improvements in fitness levels may lead to smaller degrees of exercise energy compensation. Therefore, more emphasis should be placed on the development of interventions to promote improvements in health benefits besides weight loss, including improvements in fitness levels. These results need to be interpreted with caution, as VO2peak (ml kg−1 min−1) is directly related to body weight or, more specifically, fat-free mass.51 Although we were unable to add weight loss as a covariate in the statistical models due to its high collinearity with exercise energy compensation, it is possible that the degree of change in VO2peak may be a direct result of the degree of weight lost.
Strengths of the ALPHA and BETA trials include the implementation of a mostly supervised 12-month exercise intervention, minimal loss to follow-up, a large sample size, and the inclusion of objective measurements of VO2peak and body composition. In addition, estimations of exercise energy compensation included three exercise arms from two large trials to illustrate the dose–response effects of exercise volume on exercise energy compensation. Estimations of exercise energy compensation were also based on both prescribed and completed exercise volume, in addition to changes in body composition rather than body weight. Limitations of our two trials include the use of a select population (previously inactive, healthy, postmenopausal women) that limits generalizability of the results to other populations and the large number of analyses that increases the chances of false-positive findings. The prescribed heart rate zones reflect moderate–vigorous intensity physical activity (65–80% of heart rate reserve); however, the prescribed exercise dose was not adjusted according to the intensity of the exercise performed to align with international guidelines (for example, 150 min per week of moderate intensity vs 75 min per week of vigorous intensity physical activity). Exercise energy expenditure was not directly measured, but estimated based on baseline VO2peak, in addition to mean heart rate and exercise time over the 12-month period. In addition, the use of self-reported measurements of total energy intake and physical activity time does not account for day-to-day variability in these outcomes, and may not properly capture small changes in these outcomes as a result of the intervention that would contribute to varying degrees of energy compensation. There are also a number of behavioral (for example, appetite and food reward) and metabolic (for example, reductions in resting metabolic rate and changes in (an)orexigenic peptides) variables42 that were not assessed in these trials and may directly impact acute and/or long-term energy compensation responses to exercise. Last, the ALPHA and BETA trials were not designed to be weight loss interventions or to examine the effectiveness of exercise interventions in promoting weight loss.
In conclusion, there was no dose–response effect of prescribed and completed exercise volume on exercise energy compensation. However, large inter-individual differences in exercise energy compensation were noted, thus suggesting that weight loss responses to the same volume of prescribed or completed exercise is highly variable among individuals. Last, greater changes in VO2peak were associated with lower degrees of exercise energy compensation. Future studies are needed to identify the behavioral (for example, energy intake and physical activity energy expenditure) and metabolic (for example, appetite peptides and resting metabolic rate) factors that may contribute to this large inter-individual variability in energy compensation in response to exercise interventions. The E-mechanic trial,52 a randomized controlled trial designed to evaluate the degree of energy compensation and its contributing factors (that is, changes in energy intake, energy expenditure and resting metabolic rate) following a 6-month exercise intervention in overweight men and women, is currently underway. Findings from the E-mechanic and other trials designed to evaluate the causes of energy compensation will provide the evidence needed to develop and personalize maximally effective exercise interventions.
Acknowledgments
The ALPHA trial was funded by a research grant (no. 017468) from the Canadian Breast Cancer Research Alliance. The BETA trial was funded by a research grant from the Alberta Cancer Foundation (#24404). CMF holds a Health Senior Scholar Award from Alberta Innovates-Health Solutions and the Alberta Cancer Foundation Weekend to End Women’s Cancers Breast Cancer Chair. KSC holds a Tier I Canada Research Chair. DRB is supported by a Career Development Award in Prevention (#703917) from the Canadian Cancer Society Research Institute. JM is a recipient of Postdoctoral Fellowship Awards from the Canadian Institutes of Health Research and Alberta Innovates-Health Solutions. Clinical trial registration number: clinicaltrials.gov identifier: NCT 00522262 (ALPHA trial); NCT1435005 (BETA trial).
Footnotes
The authors declare no conflict of interest.
References
- Canadian Society for Exercise Physiology. Canadian physical activity guidelines for adults 18–64 years. 2011.
- World Health OrganizationGlobal Recommendations on Physical Activity for Health. WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43: 1334–1359. [DOI] [PubMed] [Google Scholar]
- Ross R, Freeman JA, Janssen I. Exercise alone is an effective strategy for reducing obesity and related comorbidities. Exerc Sport Sci Rev 2000; 28: 165–170. [PubMed] [Google Scholar]
- World Health OrganizationObesity: Preventing and Managing the Global Epidemic. World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Saris WH, Blair SN, van Baak MA, Eaton SB, Davies PS, Di Pietro L et al. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the IASO 1st Stock Conference and consensus statement. Obes Rev 2003; 4: 101–114. [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2009; 41: 459–471. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research (AICR)Continuous Update Project Report: Food, Nutrition, Physical Activity, and the Prevalence of Breast Cancer. AICR: Washington, DC, USA, 2010. [Google Scholar]
- Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern Med 2016; 176: 816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol 2015; 16: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K, Bassett JK, MacInnis RJ, English DR, Hopper JL, McLean C et al. Associations between weight in early adulthood, change in weight, and breast cancer risk in postmenopausal women. Cancer Epidemiol Biomarkers Prev 2013; 22: 1409–1416. [DOI] [PubMed] [Google Scholar]
- McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer 2008; 8: 205–211. [DOI] [PubMed] [Google Scholar]
- Riou ME, Jomphe-Tremblay S, Lamothe G, Stacey D, Szczotka A, Doucet E. Predictors of energy compensation during exercise interventions: A systematic review. Nutrients 2015; 7: 3677–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutcher SH, Dunn SL. Factors that may impede the weight loss response to exercise-based interventions. Obes Rev 2009; 10: 671–680. [DOI] [PubMed] [Google Scholar]
- King NA, Caudwell P, Hopkins M, Byrne NM, Colley R, Hills AP et al. Metabolic and behavioral compensatory responses to exercise interventions: barriers to weight loss. Obesity 2007; 15: 1373–1383. [DOI] [PubMed] [Google Scholar]
- King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int J Obes 2008; 32: 177–184. [DOI] [PubMed] [Google Scholar]
- Dhurandhar EJ, Kaiser KA, Dawson JA, Alcorn AS, Keating KD, Allison DB. Predicting adult weight change in the real world: a systematic review and meta-analysis accounting for compensatory changes in energy intake or expenditure. Int J Obes (Lond) 2015; 39: 1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Janssen I. Physical activity, total and regional obesity: dose-response considerations. Med Sci Sports Exerc 2001; 33 (6 Suppl): S521–S527. [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Honas JJ, Smith BK, Mayo MS, Gibson CA, Sullivan DK et al. Aerobic exercise alone results in clinically significant weight loss for men and women: midwest exercise trial 2. Obesity 2013; 21: E219–E228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS One 2009; 4: e4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira PJ, Palmeira AL, Branco TL, Martins SS, Minderico CS, Barata JT et al. Who will lose weight? A reexamination of predictors of weight loss in women. Int J Behav Nutr Phys Act 2004; 1: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkilde M, Auerbach P, Reichkendler MH, Ploug T, Stallknecht BM, Sjodin A. Body fat loss and compensatory mechanisms in response to different doses of aerobic exercise—a randomized controlled trial in overweight sedentary males. Am J Physiol Regul Integr Comp Physiol 2012; 303: R571–R579. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM, Neilson HK, O'Reilly R, Duha A, Yasui Y, Morielli AR et al. Effects of a high vs moderate volume of aerobic exercise on adiposity outcomes in postmenopausal women: A Randomized Clinical Trial. JAMA Oncol 2015; 1: 766–776. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM, Woolcott CG, McTiernan A, Terry T, Brant R, Ballard-Barbash R et al. Adiposity changes after a 1-year aerobic exercise intervention among postmenopausal women: a randomized controlled trial. Int J Obes 2011; 35: 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenreich CM, Woolcott CG, McTiernan A, Ballard-Barbash R, Brant RF, Stanczyk FZ et al. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol 2010; 28: 1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenreich CM, MacLaughlin S, Neilson HK, Stanczyk FZ, Yasui Y, Duha A et al. Study design and methods for the Breast Cancer and Exercise Trial in Alberta (BETA). BMC Cancer 2014; 14: 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain DP, Leutholtz BC, King ME, Haas LA, Branch JD. Relationship between % heart rate reserve and % VO2 reserve in treadmill exercise. Med Sci Sports Exerc 1998; 30: 318–321. [DOI] [PubMed] [Google Scholar]
- Hall KD. Diet versus exercise in ‘the biggest loser’ weight loss competition. Obesity 2013; 21: 957–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Bouchard C, Church T, Slentz C, Kraus WE, Redman LM et al. Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obes Rev 2012; 13: 835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu K, Hopkins M, Blundell J, Finlayson G. Does habitual physical activity increase the sensitivity of the appetite control system? A systematic review. Sports Med 2016; 46: 1897–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock ML, Foster C, Schmidt D, Hellman C, Linnerud AC, Ward A. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. Am Heart J 1982; 103: 363–373. [DOI] [PubMed] [Google Scholar]
- Csizmadi I, Kahle L, Ullman R, Dawe U, Zimmerman TP, Friedenreich CM et al. Adaptation and evaluation of the National Cancer Institute's Diet History Questionnaire and nutrient database for Canadian populations. Public Health Nutr 2007; 10: 88–96. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM, Courneya KS, Neilson HK, Matthews CE, Willis G, Irwin M et al. Reliability and validity of the past year total physical activity questionnaire. Am J Epidemiol 2006; 163: 959–970. [DOI] [PubMed] [Google Scholar]
- Sasaki JE, John D, Freedson PS. Validation and comparison of ActiGraph activity monitors. J Sci Med Sport 2011; 14: 411–416. [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Hill JO, Jacobsen DJ, Potteiger J, Sullivan DK, Johnson SL et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med 2003; 163: 1343–1350. [DOI] [PubMed] [Google Scholar]
- Velthuis MJ, Schuit AJ, Peeters PH, Monninkhof EM. Exercise program affects body composition but not weight in postmenopausal women. Menopause 2009; 16: 777–784. [DOI] [PubMed] [Google Scholar]
- Slentz CA, Duscha BD, Johnson JL, Ketchum K, Aiken LB, Samsa GP et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE—a randomized controlled study. Arch Intern Med 2004; 164: 31–39. [DOI] [PubMed] [Google Scholar]
- Wilmore JH, Stanforth PR, Hudspeth LA, Gagnon J, Daw EW, Leon AS et al. Alterations in resting metabolic rate as a consequence of 20 wk of endurance training: the HERITAGE Family Study. Am J Clin Nutr 1998; 68: 66–71. [DOI] [PubMed] [Google Scholar]
- Potteiger JA, Kirk EP, Jacobsen DJ, Donnelly JE. Changes in resting metabolic rate and substrate oxidation after 16 months of exercise training in overweight adults. Int J Sport Nutr Exerc Metab 2008; 18: 79–95. [DOI] [PubMed] [Google Scholar]
- Martins C, Kulseng B, King NA, Holst JJ, Blundell JE. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. J Clin Endocrinol Metab 2010; 95: 1609–1616. [DOI] [PubMed] [Google Scholar]
- Foster-Schubert KE, Alfano CM, Duggan CR, Xiao L, Campbell KL, Kong A et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity 2012; 20: 1628–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melanson EL, Keadle SK, Donnelly JE, Braun B, King NA. Resistance to exercise-induced weight loss: compensatory behavioral adaptations. Med Sci Sports Exerc 2013; 45: 1600–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet E, Cameron J. Appetite control after weight loss: what is the role of bloodborne peptides? Appl Physiol Nutr Metab 2007; 32: 523–532. [DOI] [PubMed] [Google Scholar]
- Washburn RA, Lambourne K, Szabo AN, Herrmann SD, Honas JJ, Donnelly JE. Does increased prescribed exercise alter non-exercise physical activity/energy expenditure in healthy adults? A systematic review. Clin Obes 2014; 4: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly JE, Herrmann SD, Lambourne K, Szabo AN, Honas JJ, Washburn RA. Does increased exercise or physical activity alter ad-libitum daily energy intake or macronutrient composition in healthy adults? A systematic review. PLoS One 2014; 9: e83498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn RA, Honas JJ, Ptomey LT, Mayo MS, Lee J, Sullivan DK et al. Energy and Macronutrient Intake in the Midwest Exercise Trial 2 (MET-2). Med Sci Sports Exerc 2015; 47: 1941–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann SD, Willis EA, Honas JJ, Lee J, Washburn RA, Donnelly JE. Energy intake, nonexercise physical activity, and weight loss in responders and nonresponders: The Midwest Exercise Trial 2. Obesity 2015; 23: 1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis EA, Herrmann SD, Honas JJ, Lee J, Donnelly JE, Washburn RA. Nonexercise energy expenditure and physical activity in the Midwest Exercise Trial 2. Med Sci Sports Exerc 2014; 46: 2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollowell RP, Willis LH, Slentz CA, Topping JD, Bhakpar M, Kraus WE. Effects of exercise training amount on physical activity energy expenditure. Med Sci Sports Exerc 2009; 41: 1640–1644. [DOI] [PubMed] [Google Scholar]
- Jakicic JM, Otto AD, Lang W, Semler L, Winters C, Polzien K et al. The effect of physical activity on 18-month weight change in overweight adults. Obesity 2011; 19: 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goran M, Fields DA, Hunter GR, Herd SL, Weinsier RL. Total body fat does not influence maximal aerobic capacity. Int J Obes Relat Metab Disord 2000; 24: 841–848. [DOI] [PubMed] [Google Scholar]
- Myers CA, Johnson WD, Earnest CP, Rood JC, Tudor-Locke C, Johannsen NM et al. Examination of mechanisms (E-MECHANIC) of exercise-induced weight compensation: study protocol for a randomized controlled trial. Trials 2014; 15: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]