Abstract
Objectives:
White adipose tissue (WAT) expands through hypertrophy (increased adipocyte size) and/or hyperplasia (increased adipocyte number). Hypertrophy has been associated with insulin resistance and dyslipidemia independently of body composition and fat distribution. In contrast, hyperplasia protects against metabolic alterations. Proanthocyanidins, which are the most abundant flavonoids in the human diet, improve metabolic disturbances associated with diet-induced obesity without reducing body weight or adiposity. The aim of this study was to determine whether grape seed proanthocyanidin extract (GSPE) can modulate WAT expandability. Because GSPE also contains gallic acid, we also studied the capacity of gallic acid to remodel WAT.
Design:
Male Wistar rats were fed a standard chow diet (n=6) or a cafeteria diet (CAF) for 11 weeks. After 8 weeks, the CAF-fed animals were supplemented with 25 mg GSPE/kg body weight (n=6), 7 mg gallic acid/kg body weight (n=6) or the vehicle (n=6) for 3 weeks. Histological analyses were performed in the retroperitoneal (rWAT) and inguinal (iWAT) WAT to determine adipocyte size and number. Specific markers for adipogenesis and WAT functionality were analysed in rWAT using quantitative RT-PCR.
Results:
GSPE or gallic acid supplementation did not reduce weight gain or reverse and adiposity. However, GSPE reduced adipocyte size significantly in rWAT and moderately in iWAT and tripled the adipocyte number in rWAT. Gallic acid slightly reduced adipocyte size in rWAT and iWAT and doubled the adipocyte number in both WATs. In accordance with this adipogenic activity, Pref-1 and PPARγ tended to be overexpressed in rWAT of rats supplemented with GSPE. Moreover, GSPE supplementation increased Plin1 and Fabp4 expression and restored adiponectin expression completely, indicating a better functionality of visceral WAT.
Conclusions:
GSPE supplementation has anti-hypertrophic and hyperplasic activities in rats with established obesity, mainly in visceral WAT inducing a healthier expansion of WAT to match the surplus energy provided by the cafeteria diet.
Introduction
Obesity is a risk factor for many metabolic diseases, such as dyslipidemia, hypertension, type 2 diabetes and cardiovascular disease.1 It has been assumed that the prevalence of metabolic diseases increases with white adipose tissue (WAT) accretion. However, some obese individuals do not display metabolic alterations and are referred to as metabolically healthy obese.2 Therefore, new theories linking obesity and metabolic diseases have been proposed. One theory suggests that metabolic disorders appear when the storage capacity of WAT is exceeded, thereby generating dysfunctional adipose tissue.3 This phenomenon is even called WAT failure by some authors.4 Thus, the expansion of WAT to match the surplus energy may confer metabolic benefits despite making individuals more obese.
WAT expansion occurs by hypertrophy (increase in adipocyte size by lipid accumulation in adipocytes) and/or hyperplasia (increase in adipocyte number by pre-adipocyte differentiation, that is, adipogenesis). Several studies have shown that adipocyte size is an independent predictor of metabolic diseases, and adipocyte hypertrophy has been associated with insulin resistance5 and dyslipidemia6 independently of body composition and fat distribution. In fact, hypertrophic adipocytes have greater capacity to attract inflammatory cells7 and are more lipolytic and resistant to insulin action than are small adipocytes.8 Moreover, the capacity of adipocytes to store and mobilize lipids is disturbed in dysfunctional WAT. In this regard, a reduced expression of peroxisome proliferator-activated receptor γ (PPARγ) and fatty acid synthase (FASN) has been observed in obese humans with insulin resistance.4 Thus, it is assumed that hyperplasia protects against metabolic alterations, whereas adipocyte hypertrophy results in metabolic dysfunctions. Consequently, adipogenesis is a key factor in preserving suitable WAT expandability, and several studies have shown that obese subjects exhibit lower adipogenic potential.4
Hypoxia is also associated with impaired WAT functionality; thus, WAT expansion requires angiogenesis. Vascular endothelial growth factors (VEGFs) promote angiogenesis, and the expression of VEGF-A in WAT is higher in obese subjects with low insulin resistance than in those with insulin resistance.4 In addition, mice overexpressing VEGF-A in WAT have an increased number and size of blood vessels and are consequently protected against hypoxia and insulin resistance induced by a high-fat diet.9
Proanthocyanidins, which are flavonoids that are very abundant in the human diet, have been defined as healthy bioactive compounds.10 Several studies have reported many beneficial effects of grape seed proanthocyanidin extract (GSPE) on various obesity-associated diseases, such as insulin resistance,11 dyslipidaemia,12 hypertension,13 inflammation14 and leptin resistance.15 Interestingly, these beneficial effects of GSPE have been observed without significant reductions in body weight and adiposity. Thus, the objective of this study was to determine whether GSPE can improve expandability in retroperitoneal (rWAT) and inguinal (iWAT) adipose tissues in the case of obesity and thereby avoid metabolic diseases associated with high-fat diet-induced obesity. In addition to proanthocyanidins, GSPE also contains gallic acid. Thus, the objective of this work was further extended to investigate the contribution of gallic acid to the capacity of GSPE to remodel WAT.
Materials and methods
Grape seed proanthocyanidin extract and gallic acid
GSPE was kindly provided by Les Dérives Résiniques et Terpéniques (Dax, France). The composition of the GSPE was previously characterized by Margalef et al16 and is described in Supplementary Table 1. Gallic acid (98.5% purity) was purchased from Sigma-Aldrich (G7384, Madrid, Spain).
Animal experimental procedure
The investigation was carried out in accordance with the ethical standards and the Declaration of Helsinki and was approved by the Ethics Review Committee for Animal Experimentation of the Universitat Rovira i Virgili (reference number 7959 by Generalitat de Catalunya).
Five-week-old male Wistar rats were purchased from Charles River Laboratories (Barcelona, Spain) and housed in animal quarters at 22 °C with a light/dark period of 12 h (light from 0800 to 2000 hours). Animals were housed for 1 week and fed chow diet for adaptation. The animals were then were divided into four groups randomly: the standard group (STD, n=6) was fed a standard chow diet (STD Panlab A04, Panlab, Barcelona, Spain) and tap water ad libitum, and all other groups were fed a cafeteria diet (CAF) consisting of sausage, bacon, biscuits with paté, cheese, ensaïmada (sweetened pastry), carrots and sweetened milk (20% sucrose w/v) in addition to the standard chow diet. The composition of the cafeteria diet was 14% protein, 35% fat and 51% carbohydrates. This highly palatable diet is able to induce voluntary hyperphagia. CAF was freshly provided to the animals daily, and animals could choose and eat ad libitum. After 8 weeks, CAF-fed animals were divided into three new groups: GSPE group (GSPE, n=6), which received a supplementation of CAF diet with an oral dose of 25 mg GSPE/kg body weight in sweetened milk; gallic acid group (gallic acid, n=6), which received an oral dose of 7 mg gallic acid/kg body weight in sweetened milk; and CAF group, which received sweetened milk alone. All groups received the compounds or the vehicle throughout the remainder of the experiment. After 3 weeks, the rats were fasted for 3 h after the oral dose and then killed by live decapitation. Total blood was collected using heparin (DeltaLab, Barcelona, Spain) as an anticoagulant. Plasma was obtained by centrifugation (1500 g, 15 min, 4 °C), and all adipose tissue depots were excised and immediately frozen in liquid nitrogen. Both plasma and tissues were stored at −80 °C until further use.
The adiposity index was computed for each animal as the sum of the mesenteric, retroperitoneal and epididymal WAT depot weights expressed as a percentage of the total body weight.
Quantification of plasma parameters
The plasma levels of insulin, adiponectin and leptin were measured using an immunometric sandwich enzyme-linked immunosorbent assay (ELISA) using a rat/mouse insulin ELISA kit (EZRMI-13K), rat leptin ELISA kit (EZRL-83K) and rat adiponectin ELISA kit (EZRADP-62K) purchased from Millipore Ibérica (Madrid, Spain). Plasma samples were diluted, and immunoassays were performed in duplicate according to the manufacturer’s protocols. Plasma triglycerides (TGs), total cholesterol (TC), HDL-cholesterol (HDL-C) and glucose were measured with enzymatic colorimetric kits following the manufacturer’s protocols (QCA, Barcelona, Spain). Plasma NEFAs were analysed with the enzymatic colorimetric HR NEFA series kit (Wako, CA, USA).
Adipose tissue morphology
Small pieces of frozen rWAT and iWAT (−80 °C) were sent to ELDINE Patologia (Tarragona, Spain), where they were thawed and fixed in 4% diluted formaldehyde. After 24 h of fixation, tissues underwent successive dehydration (Alcohol/Ethanol 70, 96 and 100% plus xylol/dimethyl benzene) and paraffin infiltration and immersion at 52 °C (Citadel 2000. HistoStar, Thermo Scientific, Madrid, Spain). The paraffin blocks were subsequently cut into successive 2-μ thick sections (Microm HM 355S. Thermo Scientific). The sections were deposited on slides (JP Selecta Paraffin Bath) and subjected to automated hematoxylin-eosin staining (Varistain Gemini. Shandom. Thermo).17 Images of the adipose sections were acquired using AxioVision Zeiss Imaging software (Carl Zeiss Iberia, S.L, Madrid, Spain). Finally, the images captured at × 20 magnification were stored and analysed with the Adiposoft software (CIMA, University of Navarra, Spain) to quantify adipocyte number and areas. Five fields per sample and three samples from each group (STD, CAF, GSPE and gallic acid) were measured. The area was calculated from the average value of the cell area in all measured fields. The total cell number in the rWAT and iWAT fat depots was calculated from the fat cell volume.
Fat cell volume was obtained for each captured field using the formula 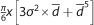 18 (where d is the mean diameter of 100 measured cells in the field, and σ is the standard deviation of the diameter). Then, fat cell density was applied (0.92 g/mL) to determinate fat cell weight. Finally, the total fat cell number in the whole rWAT and iWAT depot of each animal was determined by dividing the total fat depot weight by the mean cell weight of all captured fields.
18 (where d is the mean diameter of 100 measured cells in the field, and σ is the standard deviation of the diameter). Then, fat cell density was applied (0.92 g/mL) to determinate fat cell weight. Finally, the total fat cell number in the whole rWAT and iWAT depot of each animal was determined by dividing the total fat depot weight by the mean cell weight of all captured fields.
RNA extraction and mRNA quantification by real-time qRT-PCR
Total RNA was isolated from the frozen rWAT and iWAT using trizol reagent (Ambion, MA, USA) according to the manufacturer’s protocol. The quality of total RNA was checked using a NanoDrop 1000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
Relative mRNA levels of Perilipin 1 (Plin1), Fatty acid binding protein 4 (Fabp4), Peroxisome proliferator-activated receptor gamma (Pparγ), Fatty acid synthase (Fasn), Wnt family member 10 Beta (Wnt10β), Collagen type VI alpha 2 chain (Col6A2), Vascular endothelial growth factor A (Vegfa), Leptin, Uncoupling protein 1 (Ucp1), Interleukin 6 (IL-6), Tumour necrosis factor alpha (TNFα), Adiponectin (Adipoq), Pre-adipocyte factor-1 (Pref-1) and Adhesion G protein-coupled receptor E1 (Adgre1) were analysed by real-time PCR in rWAT using cyclophilin (Ppia) as the endogenous control.
Total RNA was reverse-transcribed using the TaqMan Reverse Transcription Reagents kit (Applied Biosystems, MA, USA) according to the manufacturer’s protocol. Gene expression was evaluated with the Bio-Rad CFX96 Real-time PCR System (Bio-Rad Laboratories, Barcelona, Spain) using the SsoFast EvaGreen Supermix (Bio-Rad Laboratories, Barcelona, Spain) and gene-specific SYBR primers designed for each gene using the FastPCR software (Supplementary Table 2). The results were normalized to PPIA. Amplification was performed following the temperature steps of 95 °C for 30 s followed by 40 cycles at 95 °C for 5 s and 60 °C for 5 s. The fold-change in the mRNA level was calculated in the log 2 scale using the equation 2−ΔΔCt (where ΔCt=Ct mRNA − Ct Ppia and ΔΔCt=ΔCt treated samples − ΔCt untreated controls).
Statistical analysis
The results are reported as the mean±s.e.m. of six animals per group for mRNA levels and as the mean±s.e.m. of three animals per group for histology Adiposoft analysis. Group means were compared using one-way analysis of variance (ANOVA) with IBM SPSS statistics 20.0 software (SPSS, Inc, Chicago, IL, USA). The comparisons were considered significant at P⩽0.05.
Results
GSPE or gallic acid supplementation did not reduce body weight and adiposity but improved hyperglycemia and dyslipidemia in cafeteria diet-fed rats
GSPE or gallic acid supplementation for 3 weeks did not reduce body weight or reverse the subcutaneous and visceral fat-pad accretion induced by the cafeteria diet (Table 1). However, GSPE and gallic acid supplementations were effective in modulating the plasma level of hormones and metabolites related to glucose and lipid homeostasis (Table 2). Specifically, GSPE and gallic acid reduced plasma TGs, TC and non-HDL-C levels by more than 20%. This resulted in a more than 20% reduction of the atherogenic index TC/HDL-C in the animals supplemented with GSPE. Moreover, GSPE and gallic acid reduced the plasma glucose levels by 11 and 21% and insulin levels by 27 and 23%, respectively. Thus, GSPE or gallic acid supplementation improved the insulin resistance indexes HOMA-IR, QUICKI and R-QUICKI. However, the adiponectin and leptin levels were not affected by GSPE or gallic acid administration.
Table 1. Effects of grape seed proanthocyanidins (GSPE) or gallic acid supplementation on body weight and fat accretion in obese rats.
| STD | CAF | CAF+GSPE | CAF+gallic acid | |
|---|---|---|---|---|
| Body weight (g) | 438.5±17.02a | 518.4±14.66b | 538.7±45.48b | 521.6±19.21b |
| iWAT weight (g) | 1.00±0.12a | 5.49±0.71b | 6.63±2.18b | 5.13±0.71b |
| rWAT weight (g) | 3.54±0.08a | 9.69±1.51b | 9.22±1.69b | 12,53±2.10b |
| eWAT weight (g) | 8.99±0.95a | 18.64±1.42b | 18.60±3.27b | 24.11±2.43b |
| Adiposity index | 2.95±0.10a | 6.35±0.61b | 6.20±0.76b | 7.72±0.68b |
Abbreviations: eWAT, epididymal fat depot. iWAT, inguinal fat depot; rWAT, retroperitoneal fat depot.
Rats were fed a cafeteria diet for 11 weeks and supplemented with GSPE (25 mg GSPE/kg body weight) or gallic acid (7 mg gallic acid/kg body weight) for the last 3 weeks. Each value is the mean of six animals±s.e.m. a,bDifferent letters denote significant differences between groups by one-way ANOVA followed by Tukey or Dunnett’s T3 post hoc analysis according to Levene’s test.
Table 2. Effects of grape seed proanthocyanidins (GSPE) or gallic acid supplementation on plasma metabolites, hormones, and atherogenic and insulin resistance indexes in obese rats.
| STD | CAF | CAF+GSPE | CAF+gallic acid | |
|---|---|---|---|---|
| Glucose (mg/dl) | 126.12±4.05a | 186.52±14.81b | 165.51±12.83a,b | 147.12±5.85a,b |
| Insulin (ng/ml) | 2.68±0.22a | 7.48±1.14b | 5.43±1.00a,b | 5.74±0.69b |
| HOMA-IR | 21.35±2.73a | 66.93±5.79b | 58.14±14.16a,b | 42.18±5.65a,b |
| QUICKI | 0.255±0.004a | 0.222±0.005b | 0.234±0.007a,b | 0.237±0.004a,b |
| R-QUICKI | 0.274±0.008a | 0.229±0.009b | 0.242±0.009a,b | 0.239±0.004a,b |
| Adiponectin (μg/ml) | 28.15±2.17a | 57.73±5.46b | 53.40±4.28b | 47.71±4.25b |
| Leptin (ng/ml) | 10.88±0.40a | 40.84±4.40b | 34.90±3.76b | 50.10±5.18b |
| Triglyceride (mg/dL) | 69.27±12.70a | 151.99±7.23b | 128.95±18.09a,b | 98.97±9.04a |
| NEFAs (mmol/l) | 0.55±0.05 | 0.78±0.13 | 0.96±0.1 | 0.81±0.05 |
| Total cholesterol (TC) (mg/dl) | 79.63±4.52a,b | 99.74±7.17a | 76.57±5.60b | 79.25±2.19a,b |
| HDL-c | 40.96±4.06a | 24.81±1.42b | 26.47±2.39b | 18.97±2.89b |
| Non-HDL-C | 34.91±1.73a | 70.97±8.82b | 51.69±4.38a,b | 56.92±7.17a,b |
| HDL-C/non-HDL-C | 1.24±0.11a | 0.42±0.01b | 0.49±0.04b | 0.35±0.07b |
| TC/HDL-C | 1.20±0.18a | 4.08±0.70b | 3.11±0.20a,b | 3.67±0.44b |
Rats were fed a cafeteria diet for 11 weeks and supplemented with GSPE (25 mg GSPE/kg body weight) or gallic acid (7 mg gallic acid/kg body weight) for the last 3 weeks. Each value is the mean of six animals±s.e.m. a,bDifferent letters denote significant differences between groups by one-way ANOVA followed by Tukey or Dunnett’s T3 post hoc analysis according to Levene’s test.
Altogether, these results indicate that GSPE and gallic acid supplementation improved hyperglycemia and dyslipidemia induced by the cafeteria diet without affecting body weight and fat mass accretion. Because dysfunctional adipose tissues can be determinants of metabolic impairments associated with obesity, we next explored whether GSPE or gallic acid supplementation could modulate adipocyte morphology and WAT expansion in obesity.
GSPE or gallic acid supplementation reduced adipocyte size and increased adipocyte number
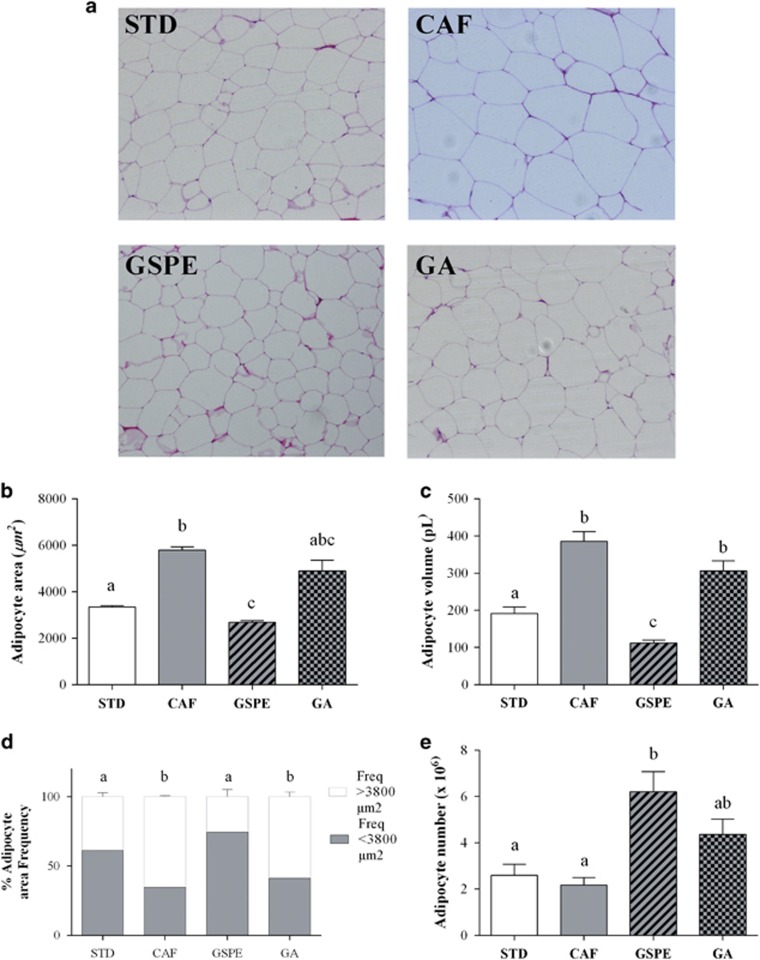
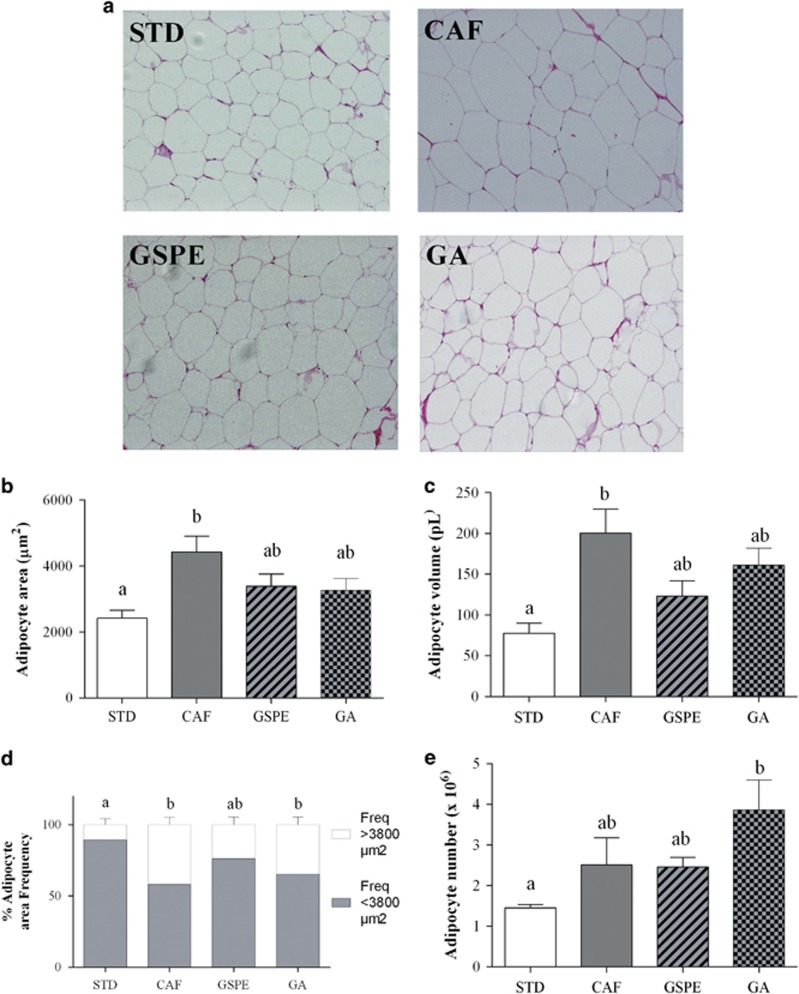
There are metabolic and functional differences between WAT depots. Thus, adipocyte morphology was studied in rWAT and iWAT fat depots representing visceral and subcutaneous WAT, respectively (Figures 1 and 2). We evaluated hyperplasia in visceral and subcutaneous WAT by extrapolating the total number of adipocytes from the size of adipocytes (via histology) and the weight of the fat pad in rWAT and iWAT. Figures 1a and 2a show a representative histological image of rWAT and iWAT for each group of rats.
Figure 1.
Effect of GSPE or gallic acid supplementation on adipocyte size and number in rWAT. Rats were fed a standard chow diet (STD group) or cafeteria diet (CAF) for 11 weeks. After 8 weeks, CAF-fed animals were supplemented with 25 mg GSPE/kg body weight (GSPE group), 7 mg gallic acid/kg body weight (GA group) or the vehicle (CAF group) for 3 weeks. Samples of rWAT were stained with hematoxylin and eosin. Representative light microscopy images (a) from each group were used to measure adipocyte area (b). Adipocyte volume (c) and the frequency of adipocyte size (d) were calculated from adipocyte area. Total adipocyte number (e) was extrapolated from the size of adipocytes and the weight of rWAT. The values are the mean±s.e.m. of five fields per animal from three animals of each group. Different letters indicate significant differences between groups at P⩽0.05 using one-way ANOVA.
Figure 2.
Effect of GSPE or gallic acid supplementation on adipocyte size and number in iWAT. Rats were fed a standard chow diet (STD group) or cafeteria diet (CAF) for 11 weeks. After 8 weeks, CAF-fed animals were supplemented with 25 mg GSPE/kg body weight (GSPE group), 7 mg gallic acid/kg body weight (GA group) or the vehicle (CAF group) for 3 weeks. Samples of iWAT were stained with hematoxylin and eosin. Representative light microscopy images (a) from each group were used to measure adipocyte area (b). Adipocyte volume (c) and the frequency of adipocyte size (d) were calculated from adipocyte area. Total adipocyte number (e) was extrapolated from the size of adipocytes and the weight of iWAT. The values are the mean±s.e.m. of five fields per animal from three animals of each group. Different letters indicate significant differences between groups at P⩽0.05 using one-way ANOVA.
Feeding rats the cafeteria diet for 11 weeks significantly increased adipocyte area and volume in both rWAT (Figures 1b and c) and iWAT (Figures 2b and c) relative to the control lean animals, resulting in an elevated frequency of adipocytes of higher than 3800 μm2, mainly in rWAT (Figures 1d and 2d). In contrast, the cafeteria diet almost doubled adipocyte number in iWAT alone, although this increase was not statistically significant (Figures 1e and 2e). These values indicate that the cafeteria diet induced visceral and subcutaneous fat accretion mainly by hypertrophy.
GSPE supplementation for the last 3 weeks, when obesity was already established, totally reversed the adipocyte hypertrophy of rWAT induced by the cafeteria diet alone (Figures 1b and c). Thus, the adipocyte area was normalized due to an increased frequency of adipocytes to lower than 3800 μm2 (Figure 1d). Moreover, GSPE supplementation tripled the adipocyte numbers in the rWAT depot (Figure 1e). In contrast, GSPE supplementation tended to reduce adipocyte area and volume in iWAT without any significant effect on adipocyte number (Figures 2b–e).
Effects of gallic acid supplementation on adipocyte morphology were not as evident as those of GSPE supplementation. Rats fed cafeteria diet supplemented with gallic acid for the last 3 weeks displayed a slight reduction in adipocyte area and volume (approximately 15–20%) compared to those of rats fed with cafeteria diet alone in both rWAT (Figures 1b–d) and iWAT (Figures 2b–d). Moreover, gallic acid supplementation doubled the adipocyte number in both rWAT and iWAT. However, this increase was only significant in iWAT when compared to lean rats (Figures 1e and 2e).
Altogether, these results indicate that the supplementation of GSPE or gallic acid was able to modulate visceral and subcutaneous WAT, reducing hypertrophy and increasing hyperplasia. However, rWAT was more sensitive than iWAT to GSPE, whereas iWAT was more sensitive than rWAT to gallic acid.
To determine whether these morphological changes in WAT expansion induced by GSPE or gallic acid supplementation could be related to the improvement of dyslipidemia and hyperglycemia, we calculated the correlation between the volume and number of adipocytes with plasma metabolic parameters (Supplementary Table 3). Interestingly, the iWAT adipocyte volume correlated negatively with the QUICKI index and positively with TG and the TC/HDL-C ratio. In addition, the adipocyte number in iWAT correlated negatively with HDL-C and the HDL-C/non-HDL-C ratio. In contrast, no significant correlation was found between adipocyte number and size in rWAT.
Mechanisms by which GSPE or gallic acid supplementation could remodel visceral adipose tissue
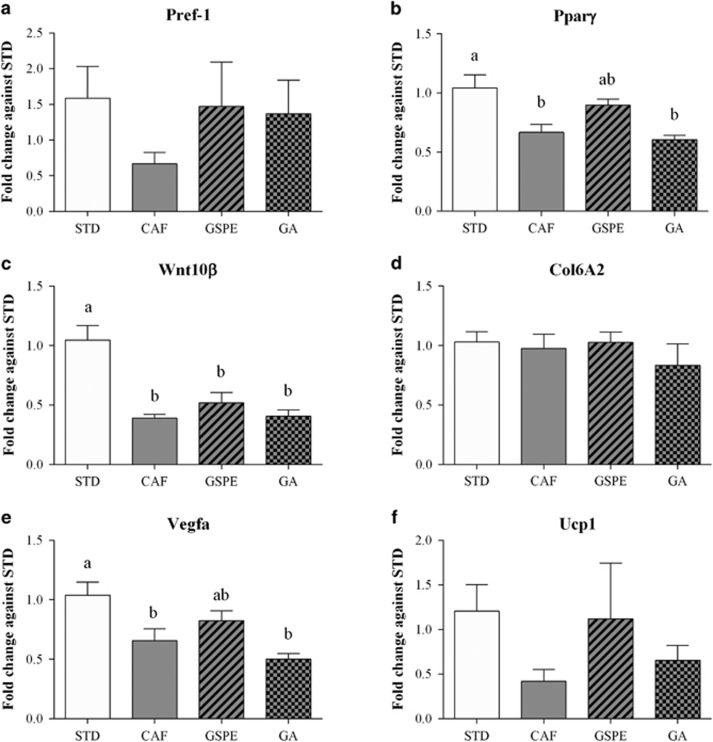
The large modifications of adipocyte size and number in rWAT induced by GSPE supplementation, and to a lesser extent by gallic acid supplementation, prompted us to investigate some of the processes involved in adipose remodelling by these compounds. These processes include adipogenesis (pre-adipocyte differentiation), extracellular matrix and vascularization. Moreover, visceral WAT, in contrast to subcutaneous WAT, is more closely associated with obesity-related metabolic disorders.19
The expression of pre-adipocyte factor-1 (Pref-1), a pre-adipocyte marker, was increased in the rWAT of rats supplemented with GSPE or gallic acid, although the differences were not significant because of the high variability in expression between rats of the same group (Figure 3a). Moreover, the expression of PPARγ, which is considered the master regulator of adipogenesis, tended to be upregulated by GSPE supplementation but not by that of gallic acid (Figure 3b). In contrast, the Wnt signalling pathway, which inhibits adipogenesis, was not affected by GSPE or gallic acid supplementation (Figure 3c). These observations indicate that GSPE increased the number of pre-adipocytes and favoured their differentiation into mature adipocytes.
Figure 3.
Effect of GSPE or gallic acid supplementation on the expression of genes related to adipose remodelling in rWAT. Rats were fed a standard chow diet (STD group) or cafeteria diet (CAF) for 11 weeks. After 8 weeks, CAF-fed animals were supplemented with 25 mg GSPE/kg body weight (GSPE group), 7 mg gallic acid/kg body weight (GA group) or the vehicle (CAF group) for 3 weeks. (a) Pre-adipocyte factor-1 (Pref-1) mRNA levels. (b) Peroxisome proliferator-activated receptor gamma (Pparγ) mRNA levels. (c) Wnt family member 10 Beta (Wnt10β) mRNA levels. (d) Collagen type VI alpha 2 chain (Col6A2) mRNA levels. (e) Vascular endothelial growth factor A (Vegfa) mRNA levels. (f) Uncoupling protein 1 (Ucp1) mRNA levels. The values are the mean±s.e.m. of six animals per group. Statistical analyses were performed using one-way ANOVA. Different letters (a–c) indicate significant differences between groups considering P⩽0.05.
GSPE or gallic acid supplementation did not affect the extracellular matrix, as measured by the expression of Collagen 6A2 gene (Figure 3d). In contrast, GSPE, but not gallic acid, increased the expression of the vascular endothelial growth factor A (VEGF-A) (Figure 3e), suggesting increased angiogenesis in the rWAT of rats supplemented with GSPE.
Furthermore, we also investigated whether GSPE or gallic acid supplementation could modulate the fate of pre-adipocytes to the beige phenotype by analysing UCP1 expression (Figure 3f). However, because of the high variability in UCP1-expression between rats of the same group, no significant differences were observed.
Although the study was focused in rWAT, the PPARγ and UCP1 expression was also analysed in iWAT (Supplementary Figure 1). According to the less sensitivity of iWAT to these compounds, no statistical differences were observed in these gene expression in iWAT.
GSPE supplementation improved visceral adipose functionality
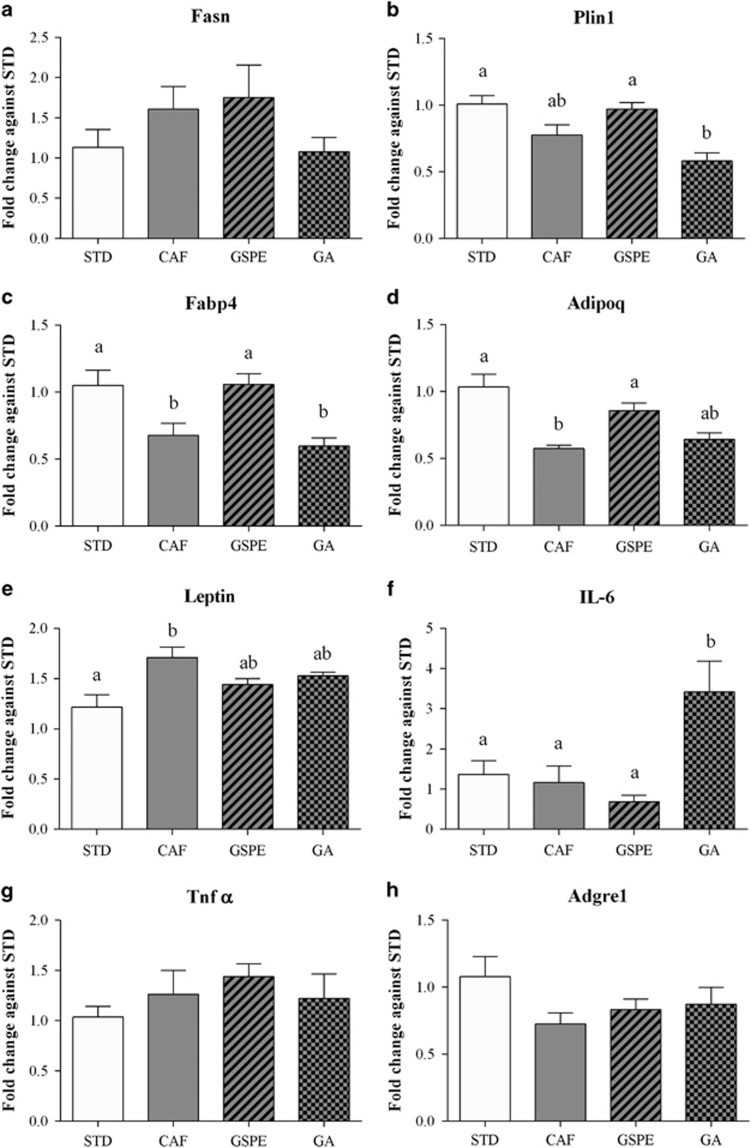
Adipose hypertrophy is associated with adipose tissue dysfunction. Thus, we investigated the capacity of GSPE or gallic acid supplementation to improve several markers of adipose tissue functionality, focusing on rWAT.
A reduced capacity to store and mobilize lipids is associated with WAT dysfunction. Thus, we analysed the expression of Fasn, Plin1 and Fabp4 in rWAT (Figures 4a–c). Interestingly, GSPE supplementation significantly increased the expression of Plin1 and Fabp4, thereby indicating an increased capacity of visceral fat to store and mobilize triglycerides. In contrast, gallic acid supplementation did not modify these processes.
Figure 4.
Effect of GSPE or gallic acid supplementation on the expression of genes related to WAT functionality in the retroperitoneal fat depot. Rats were fed a standard chow diet (STD group) or cafeteria diet (CAF) for 11 weeks. After 8 weeks, CAF-fed animals were supplemented with 25 mg GSPE/kg body weight (GSPE group), 7 mg gallic acid/kg body weight (GA group) or the vehicle (CAF group) for 3 weeks. (a) Fatty acid synthase (Fasn) mRNA levels. (b) Perilipin 1 (Plin1) mRNA levels. (c) Fatty acid binding protein 4 (Fabp4) mRNA levels. (d) Adiponectin (Adipoq) mRNA levels. (e) Leptin mRNA levels. (f) Interleukin 6 (IL-6) mRNA levels. (g) Tumour necrosis factor alpha (TNF-α) mRNA levels. (h) the macrophage surface marker gene Adhesion G protein-coupled receptor E1 (Adgre1) mRNA levels. The values are the mean±s.e.m. of six animals per group. Statistical analyses were performed using one-way ANOVA. Different letters indicate significant differences between groups considering P⩽0.05.
Moreover, GSPE supplementation also completely restored adiponectin expression and partially restored leptin expression (Figures 4d and e). Thus, GSPE normalized visceral functionality relative to adipokine secretion. However, gallic acid supplementation only partially restored both leptin and adiponectin expressions.
Inflammation and macrophage infiltration are characteristics of dysfunctional adipose tissue. However, feeding rats a cafeteria diet did not increase IL-6 or TNFα expression in these animals (Figures 4f and g), and GSPE supplementation did not alter the expression of these markers any further. Nevertheless, it is important to note that gallic acid worsened inflammation, as its supplementation significantly increased IL-6 expression in rWAT. The expression of Adgre1 (Figure 4h), a macrophage marker, indicated that dietary treatments did not induce immune cell infiltration.
Taken together, these results clearly indicate that GSPE supplementation improved visceral adipocyte functionality. In contrast, gallic acid supplementation was not as effective as GSPE and even worsened inflammation markers in rWAT.
Discussion
Previous studies have demonstrated that GSPE improves hyperglycemia, dyslipidemia and even the disruption of central leptin signalling induced by a cafeteria diet without affecting body weight or adipose index.11, 12, 15 Impaired WAT expandability is considered causative of metabolic disorders associated with obesity. Thus, in this study, we investigated whether the beneficial effects of GSPE could be associated with improved WAT expandability.
Hyperplasia and hypertrophy can contribute to WAT expansion in obesity. In our model of obesity using cafeteria diet-fed rats, rWAT expanded by hypertrophy, whereas iWAT expanded by a combination of hypertrophy and hyperplasia. Studies in rodents fed a high-fat diet indicate that hyperplasia is relevant mainly in subcutaneous WAT expansion,20 whereas others demonstrate that hyperplasia is significant only in visceral WAT expansion.21 This discrepancy has been ascribed to the methodology used to measure WAT hyperplasia.22
Importantly, the supplementation of cafeteria diet with GSPE for 3 weeks, when obesity was noticeable, deeply altered the pattern of the CAF-induced rWAT expansion by normalizing adipocyte size and increasing the number of adipocytes. Thus, to match surplus energy, GSPE induced a healthier expansion of rWAT than the cafeteria diet alone. This could be concluded because GSPE supplementation clearly increased the capacity to store and mobilize TGs and restored adiponectin and leptin expression in rWAT. In contrast, in iWAT, GSPE supplementation modulated adipocyte number and size to a much lower extent than in rWAT. These results highlight that visceral fat was more sensitive than subcutaneous fat to GSPE.
Gallic acid, a non-proanthocyanidin component of GSPE, is known to have beneficial effects on metabolic syndrome23 and to modulate 3T3-L1 adipocyte differentiation.24 Thus, we reasoned that gallic acid could also participate in the modulation of WAT morphology induced by GSPE. To test this, a group of rats was supplemented with 10 times the quantity of gallic acid present in 25 mg of GSPE. Gallic acid supplementation affected the adipocyte number more than it affected the adipocyte size, suggesting that the anti-hypertrophic activity of GSPE could be ascribed mainly to proanthocyanidins, whereas gallic acid and proanthocyanidins could contribute to the hyperplasic activity of GSPE.
Other authors have focused on the capacity of pure polyphenols and polyphenol-rich extracts to modulate adipocyte hypertrophy, but not hyperplasia, in rodent models of diet-induced obesity. For example, polyphenol-rich grape pomace,25 resveratrol26 or piceatannol27 supplementation reduces epididymal adipocyte size. Moreover, consistent with our results, long-term resveratrol supplementation reduces adipocyte size in visceral, but not subcutaneous, WAT in rhesus monkeys fed a high-fat, high-sugar diet.28 The capacity of resveratrol to reduce adipocyte size has also been confirmed in abdominal subcutaneous WAT of healthy humans.29 However, in all these animal studies, polyphenols were administered from the beginning of the diet, indicating that these polyphenols prevented the development of hypertrophy associated with obesity. In contrast, in our study, GSPE was only administered when obesity was noticeable, demonstrating that GSPE had clear anti-hypertrophic and hyperplasic activities.
Visceral WAT is more closely related than subcutaneous WAT to metabolic disorders associated with obesity.19 Thus, by mainly targeting rWAT expandability, GSPE could have a high potential to avoid metabolic disturbances. The anti-hypertrophic action of GSPE on visceral fat could restore glucose and lipid homeostasis because hypertrophic adipocytes are associated with insulin resistance5 and with an increased risk of having metabolic syndrome.30 Moreover, the hyperplasic action of GSPE could also increase insulin sensitivity because thiazolidinedione drugs lead to insulin sensitivity by increasing adipogeneis.31 This suggests that having more adipocytes can be beneficial. Notably, this modification of adipocyte number and size induced by GSPE in rWAT was concomitant with the overexpression of VEGF, a factor that induces angiogenesis and increases WAT adipose vasculature,32 indicating that GSPE supplementation increased the oxygen supply to adipocytes and thus improved rWAT functionality. In accordance with these results, measurements of plasma parameters also indicate an improvement of dyslipidemia and hyperglycemia by GSPE, even though the correlation found between adipocyte number and size in rWAT and these plasma parameters was not significant. In contrast, iWAT adipocyte volume correlated positively with TG and the TC/HDL-C ratio, and iWAT adipocyte number correlated negatively with HDL-C and the HDL-C/non-HDL-C ratio. Therefore, moderate changes in iWAT expandability induced by GSPE and gallic acid could be relevant to the improved lipid and glucose homeostasis by supplementation with these dietary compounds.
Interestingly, the effect of GSPE on increasing the adipocyte number and reducing adipocyte size was very fast, that is, within 3 weeks of GSPE supplementation. Although the life span of adipocytes in rodents is not well defined, this speed of action of GSPE is in agreement with the results of some studies investigating epididymal fat that indicate a very rapid adipogenesis after cold exposure.21
Until now, the mechanisms that control adipogenesis have been studied mainly in vitro, and little information is known under in vivo conditions. Some studies have identified adipocyte precursors in the WAT stromal-vascular fraction that are able to differentiate into mature adipocytes.22 In the present study, the rWAT of rats supplemented with GSPE showed a tendency to increase the expression of Pref-1, which is a pre-adipocyte marker,33 suggesting that GSPE supplementation increased the number of adipocyte precursors in visceral WAT. Although Pref-1 has been considered an adipogenic inhibitor,34 the concomitant overexpression of PPARγ, the master regulator of adipogenesis, indicated that adipogenesis was active in the rWAT of rats supplemented with GSPE.
The adipogenic activity of GSPE observed in this study contradicts previous in vitro studies using 3T3-L1 cells, which postulated proanthocyanidins and GSPE as anti-adipogenic agents.35 Although in vitro and in vivo conditions are not equivalent, several factors can account for these contradictory results. One important factor could be the specific molecules that reach adipocytes under each condition, that is, parental proanthocyanidins or their metabolites. Under in vivo conditions, proanthocyanidins are actively metabolized by the intestine, the liver and the microbiota, generating a large set of metabolites, such as phenyl-valerolactones.36, 37, 38 Thus, the adipocytes in vivo are in contact with proanthocyanidin metabolites and not with parental proanthocyanidins. In contrast, in the in vitro studies, the pre-adipocytes were cultured directly with GSPE, that is, with parental proanthocyanidin. An additional factor that could explain this disparity is the presence of mature adipocytes under in vivo conditions. These mature adipocytes secrete adipogenic signals that can affect adipocyte formation either positively or negatively39, 40, 41 and even control adipocyte size.42 Thus, GSPE could increase adipogenesis in vivo by modulating some of these factors in mature adipocytes without directly affecting the differentiation of pre-adipocytes, which is the process observed under in vitro conditions.
In this experiment, GSPE was administered at 25 mg of GSPE/kg of body weight. This dose, using a translation of animal to human doses43 and estimating the daily intake for a 70-kg human, corresponds to an intake of 284 mg of GSPE/day. This GSPE intake can be achieved in humans with a polyphenol-rich diet. For example, in Spanish adults, the mean dietary flavonoid intake was 313.26 mg/day, with proanthocyanidins comprising 60.1%.44 Although experimental data obtained in rats cannot be directly translatable to humans, the fact that proanthocyanidins reverse adipocyte hypertrophy suggests that the inclusion of proanthocyanidin-rich foods in the diets of obese humans could be a good strategy for improving their metabolic alterations.
To summarize, GSPE supplementation has anti-hypertrophic and adipogenic activities in rats with established obesity, mainly in visceral WAT. Because hypertrophy is associated with insulin resistance and metabolic syndrome, GSPE supplementation induced a healthier expansion of WAT to match the surplus energy provided by the cafeteria diet. Moreover, GSPE supplementation improved visceral WAT functionality, increasing the capacity of visceral WAT to store and to mobilize TGs and restoring the expression of adiponectin.
Acknowledgments
The research was financially supported by grant AGL2013-40707-R (AEI/FEDER, UE) from the Spanish Ministry of Economy and Competitiveness. AP-S and SS-G are recipients of a fellowship from the Catalan Government.
Footnotes
Supplementary Information accompanies this paper on International Journal of Obesity website (http://www.nature.com/ijo)
The authors declare no conflict of interest.
Supplementary Material
References
- King RJ, Ajjan RA. Vascular risk in obesity: facts, misconceptions and the unknown. Diabetes Vasc Dis Res 2017; 14: 2–13. [DOI] [PubMed] [Google Scholar]
- Phillips CM. Metabolically healthy obesity across the life course: epidemiology, determinants, and implications. Ann NY Acad Sci 2017; 1391: 85–100. [DOI] [PubMed] [Google Scholar]
- Blüher M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract Res Clin Endocrinol Metab 2013; 27: 163–177. [DOI] [PubMed] [Google Scholar]
- Moreno-Indias I, Tinahones FJ. Impaired adipose tissue expandability and lipogenic capacities as ones of the main causes of metabolic disorders. J Diabetes Res 2015; 2015: 970375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren M, Svensson M, Lindmark S, Renström F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia 2007; 50: 625–633. [DOI] [PubMed] [Google Scholar]
- Veilleux A, Caron-Jobin M, Noël S, Laberge PY, Tchernof A. Visceral adipocyte hypertrophy is associated with dyslipidemia independent of body composition and fat distribution in women. Diabetes 2011; 60: 1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Yang X, Lin Y, Li S, Jiang J, Qian S et al. Large adipocytes function as antigen-presenting cells to activate CD4+ T cells via upregulating MHCII in obesity. Int J Obes 2016; 40: 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JR, Douagi I, Andersson DP, Bäckdahl J, Rydén M, Arner P et al. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia 2016; 59: 560–570. [DOI] [PubMed] [Google Scholar]
- Elias I, Franckhauser S, Ferre T, Vila L, Tafuro S, Munoz S et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes 2012; 61: 1801–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladé C, Aragonès G, Arola-Arnal A, Muguerza B, Bravo FI, Salvadó MJ et al. Proanthocyanidins in health and disease. Biofactors 2016; 42: 5–12. [DOI] [PubMed] [Google Scholar]
- Montagut G, Bladé C, Blay M, Fernández-Larrea J, Pujadas G, Salvadó MJ et al. Effects of a grapeseed procyanidin extract (GSPE) on insulin resistance. J Nutr Biochem 2010; 21: 961–967. [DOI] [PubMed] [Google Scholar]
- Quesada H, del Bas JM, Pajuelo D, Díaz S, Fernandez-Larrea J, Pinent M et al. Grape seed proanthocyanidins correct dyslipidemia associated with a high-fat diet in rats and repress genes controlling lipogenesis and VLDL assembling in liver. Int J Obes (Lond) 2009; 33: 1007–1012. [DOI] [PubMed] [Google Scholar]
- Pons Z, Guerrero L, Margalef M, Arola L, Arola-Arnal A, Muguerza B. Effect of low molecular grape seed proanthocyanidins on blood pressure and lipid homeostasis in cafeteria diet-fed rats. J Physiol Biochem 2014; 70: 629–637. [DOI] [PubMed] [Google Scholar]
- Martinez-Micaelo N, González-Abuín N, Ardèvol A, Pinent M, Blay MT. Procyanidins and inflammation: molecular targets and health implications. Biofactors 38: 257–265. [DOI] [PubMed] [Google Scholar]
- Ibars M, Ardid-Ruiz A, Suárez M, Muguerza B, Bladé C, Aragonès G. Proanthocyanidins potentiate hypothalamic leptin/STAT3 signalling and Pomc gene expression in rats with diet-induced obesity signalling. Int J Obes (Lond) 2017; 41: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalef M, Pons Z, Iglesias-Carres L, Bravo FI, Muguerza B, Arola-Arnal A. Lack of tissue accumulation of grape seed flavanols after daily long-term administration in healthy and cafeteria-diet obese rats. J Agric Food Chem 2015; 63: 9996–10003. [DOI] [PubMed] [Google Scholar]
- Fortuño-Mar A, P. Pasquali P. Cryobiopsy, cryoanesthesia and cryoanalgesia. In: Cryosurgery. A Practical Manual 2015. pp 85–91.
- Eriksson-Hogling D, Andersson DP, Bäckdahl J, Hoffstedt J, Rössner S, Thorell A et al. Adipose tissue morphology predicts improved insulin sensitivity following moderate or pronounced weight loss. Int J Obes (Lond) 2015; 39: 893–898. [DOI] [PubMed] [Google Scholar]
- Amato MC, Guarnotta V, Giordano C. Body composition assessment for the definition of cardiometabolic risk. J Endocrinol Invest 36: 537–543. [DOI] [PubMed] [Google Scholar]
- Joe AWB, Yi L, Even Y, Vogl AW, Rossi FMV Depot-Specific. Differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells 2009; 27: 2563–2570. [DOI] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013; 19: 1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R, Jeffery E, Rodeheffer MS. Weighing in on adipocyte precursors. Cell Metab 2014; 19: 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan KV, Ko CM, Kinyua AW, Yang DJ, Choi Y-H, Oh IY et al. Gallic acid regulates body weight and glucose homeostasis through AMPK activation. Endocrinology 2015; 156: 157–168. [DOI] [PubMed] [Google Scholar]
- Makihara H, Koike Y, Ohta M, Horiguchi-Babamoto E, Tsubata M, Kinoshita K et al. Gallic acid, the active ingredient of Terminalia bellirica, enhances adipocyte differentiation and adiponect in secretion. Biol Pharm Bull 2016; 39: 1137–1143. [DOI] [PubMed] [Google Scholar]
- Rodriguez Lanzi C, Perdicaro DJ, Antoniolli A, Fontana AR, Miatello RM, Bottini R et al. Grape pomace and grape pomace extract improve insulin signaling in high-fat-fructose fed rat-induced metabolic syndrome. Food Funct 2016; 7: 1544–1553. [DOI] [PubMed] [Google Scholar]
- Lv Z, Wang Q, Chen Y, Wang S, Huang D. Resveratrol attenuates inflammation and oxidative stress in epididymal white adipose tissue: implications for its involvement in improving steroidogenesis in diet-induced obese mice. Mol Reprod Dev 2015; 82: 321–328. [DOI] [PubMed] [Google Scholar]
- Tung Y-C, Lin Y-H, Chen H-J, Chou S-C, Cheng A-C, Kalyanam N et al. Piceatannol exerts anti-obesity effects in C57BL/6 mice through modulating adipogenic proteins and gut microbiota. Molecules 2016; 21: 1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Gomez Y, Mattison JA, Pearson KJ, Martin-Montalvo A, Palacios HH, Sossong AM et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab 2013; 18: 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings E, Timmers S, Boekschoten MV, Goossens GH, Jocken JW, Afman LA et al. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int J Obes (Lond) 2014; 38: 470–473. [DOI] [PubMed] [Google Scholar]
- O’Connell J, Lynch L, Cawood TJ, Kwasnik A, Nolan N, Geoghegan J et al. The relationship of omental and subcutaneous adipocyte size to metabolic disease in severe obesity. PLoS ONE 2010; 5: e9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin TM, Liu T, Yee G, Abbasi F, Lamendola C, Reaven GM et al. Pioglitazone increases the proportion of small cells in human abdominal subcutaneous adipose tissue. Obesity (Silver Spring) 2010; 18: 926–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H-K, Doh K-O, Son JE, Park JG, Bae Y, Choi S et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab 2013; 17: 61–72. [DOI] [PubMed] [Google Scholar]
- Sul HS, Minireview, Pref-1. Role in adipogenesis and mesenchymal cell fate. Mol Endocrinol 2009; 23: 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell J, Lynch L, Hogan A, Cawood TJ, O’Shea D. Preadipocyte factor-1 is associated with metabolic profile in severe obesity. J Clin Endocrinol Metab 2011; 96: E680–E684. [DOI] [PubMed] [Google Scholar]
- Pinent M, Bladé MC, Salvadó MJ, Arola L, Hackl H, Quackenbush J et al. Grape-seed derived procyanidins interfere with adipogenesis of 3T3-L1 cells at the onset of differentiation. Int J Obes (Lond) 2005; 29: 934–941. [DOI] [PubMed] [Google Scholar]
- Margalef M, Pons Z, Bravo FI, Muguerza B, Arola-Arnal A. Tissue distribution of rat flavanol metabolites at different doses. J Nutr Biochem 2015; 26: 987–995. [DOI] [PubMed] [Google Scholar]
- Margalef M, Pons Z, Bravo FI, Muguerza AA-A. B. Plasma kinetics and microbial biotransformation of grape seed flavanols in rats. J Funct Foods 2015; 12: 478–488. [Google Scholar]
- Del Rio D, Rodriguez-Mateos A, Spencer JPE, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 2013; 18: 1818–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challa TD, Straub LG, Balaz M, Kiehlmann E, Donze O, Rudofsky G et al. Regulation of de novo adipocyte differentiation through cross talk between adipocytes and preadipocytes. Diabetes 2015; 64: 4075–4087. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Mark AL, Haynes WG, Sigmund CD. Adipose depot-specific modulation of angiotensinogen gene expression in diet-induced obesity. Am J Physiol - Endocrinol Metab 2004; 286: E891–E895. [DOI] [PubMed] [Google Scholar]
- Janke J, Engeli S, Gorzelniak K, Luft FC, Sharma AM. Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes 2002; 51: 1699–1707. [DOI] [PubMed] [Google Scholar]
- Mori Y, Itoh Y, Tajima N. Angiotensin II receptor blockers downsize adipocytes in spontaneously type 2 diabetic rats with visceral fat obesity. Am J Hypertens 2007; 20: 431–436. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008; 22: 659–661. [DOI] [PubMed] [Google Scholar]
- Zamora-Ros R, Forouhi NG, Sharp SJ, González CA, Buijsse B, Guevara M et al. Dietary intakes of individual flavanols and flavonols are inversely associated with incident type 2 diabetes in European populations. J Nutr 2014; 144: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.