Abstract
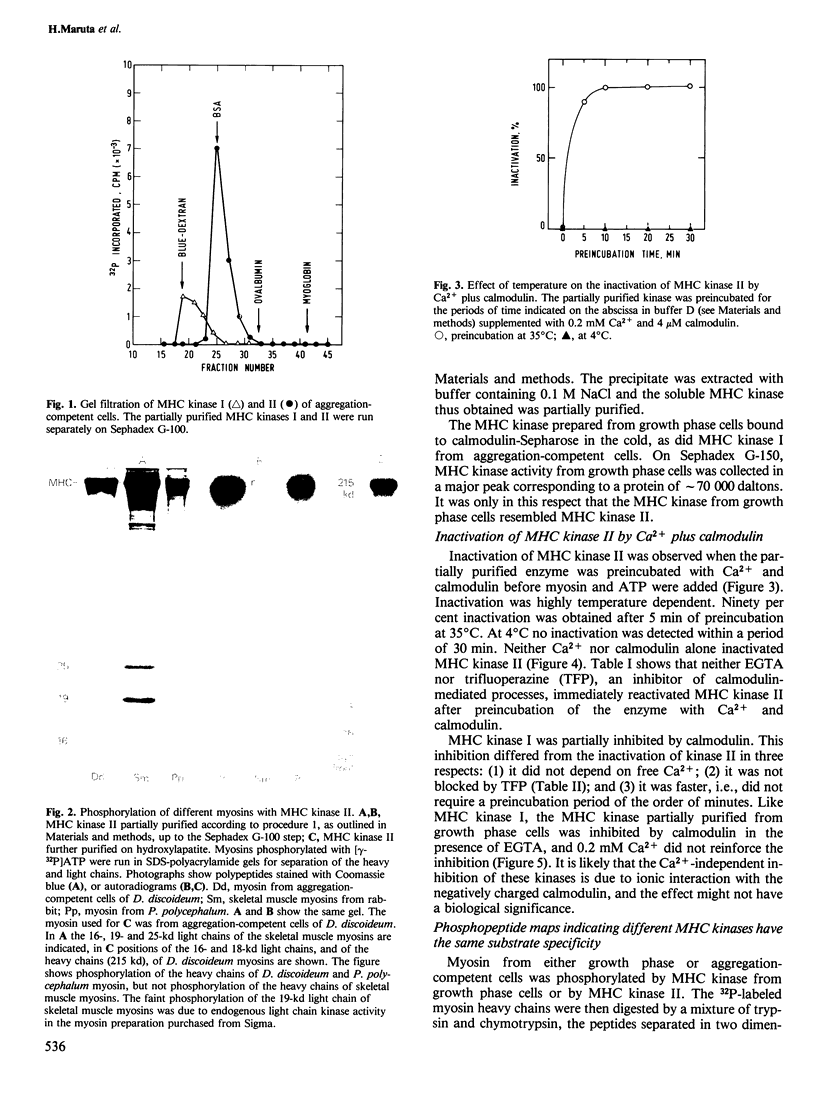
Soluble myosin heavy chain kinases (MHC kinases) were partially purified from growth phase and aggregation-competent cells of Dictyostelium discoideum. In the aggregation-competent cells, two MHC kinases were distinguishable. One of these enzymes, called MHC kinase II, was inactivated by Ca2+ and calmodulin in a highly temperature-dependent reaction. A MHC kinase found in growth phase cells did not have these regulatory properties. Substrate specificities were analysed for MHC kinase II and for the MHC kinase from growth phase cells. Both enzymes phosphorylated threonine residues of the myosin heavy chains of D. discoideum and Physarum polycephalum. Phosphopeptide mapping of D. discoideum myosin and determination of the stoichiometry of its phosphorylation suggested the presence of two phosphorylation sites per heavy chain. Both sites were contained within a 38-kd chymotryptic fragment. The inactivation of MHC kinase II by Ca2+ plus calmodulin suggests this enzyme has a role in the regulation of myosin functions during the chemotactic response of a cell. The phosphorylated myosin had about one third the actin-activated Mg2+-ATPase activity of the non-phosphorylated myosin. Previous findings indicated that stimulation of D. discoideum cells with the chemo-attractant cAMP increases the cytoplasmic Ca2+ concentration. Under these conditions MHC kinase II might be inhibited and the dephosphorylated, more active form of myosin would accumulate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Conti M. A. Phosphorylation of platelet myosin increases actin-activated myosin ATPase activity. Nature. 1975 Aug 14;256(5518):597–598. doi: 10.1038/256597a0. [DOI] [PubMed] [Google Scholar]
- Bazari W. L., Clarke M. Characterization of a novel calmodulin from Dictyostelium discoideum. J Biol Chem. 1981 Apr 10;256(7):3598–3603. [PubMed] [Google Scholar]
- Bonner J. T., Barkley D. S., Hall E. M., Konijn T. M., Mason J. W., O'Keefe G., 3rd, Wolfe P. B. Acrasin, Acrasinase, and the sensitivity to acrasin in Dictyostelium discoideum. Dev Biol. 1969 Jul;20(1):72–87. doi: 10.1016/0012-1606(69)90005-0. [DOI] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Biochemical and structural studies of actomyosin-like proteins from non-muscle cells. Isolation and characterization of myosin from amoebae of Dictyostelium discoideum. J Mol Biol. 1974 Jun 25;86(2):209–222. doi: 10.1016/0022-2836(74)90013-8. [DOI] [PubMed] [Google Scholar]
- Claviez M., Pagh K., Maruta H., Baltes W., Fisher P., Gerisch G. Electron microscopic mapping of monoclonal antibodies on the tail region of Dictyostelium myosin. EMBO J. 1982;1(8):1017–1022. doi: 10.1002/j.1460-2075.1982.tb01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. H., Côté G. P., Korn E. D. Localization of the three phosphorylation sites on each heavy chain of Acanthamoeba myosin II to a segment at the end of the tail. J Biol Chem. 1982 Apr 25;257(8):4529–4534. [PubMed] [Google Scholar]
- Collins J. H., Korn E. D. Actin activation of Ca2+-sensitive Mg2+-ATPase activity of Acanthamoeba myosin II is enhanced by dephosphorylation of its heavy chains. J Biol Chem. 1980 Sep 10;255(17):8011–8014. [PubMed] [Google Scholar]
- Darmon M., Brachet P., Da Silva L. H. Chemotactic signals induce cell differentiation in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3163–3166. doi: 10.1073/pnas.72.8.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G., Fromm H., Huesgen A., Wick U. Control of cell-contact sites by cyclic AMP pulses in differentiating Dictyostelium cells. Nature. 1975 Jun 12;255(5509):547–549. doi: 10.1038/255547a0. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Eisenberg E., Korn E. D. Characterization of cytoplasmic actin isolated from Acanthamoeba castellanii by a new method. J Biol Chem. 1976 Aug 10;251(15):4778–4786. [PubMed] [Google Scholar]
- Gracy R. W. Two-dimensional thin-layer methods. Methods Enzymol. 1977;47:195–204. doi: 10.1016/0076-6879(77)47024-1. [DOI] [PubMed] [Google Scholar]
- Hathaway D. R., Adelstein R. S. Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1653–1657. doi: 10.1073/pnas.76.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B., Greengard P. Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5402–5406. doi: 10.1073/pnas.76.10.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson G. A., Jr, Vanaman T. C. Calcium-dependent affinity chromatography of calmodulin on an immobilized phenothiazine. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1048–1056. doi: 10.1016/0006-291x(79)91932-6. [DOI] [PubMed] [Google Scholar]
- Khandelwal R. L., Vandenheede J. R., Krebs E. G. Purification, properties, and substrate specificities of phosphoprotein phosphatase(s) from rabbit liver. J Biol Chem. 1976 Aug 25;251(16):4850–4858. [PubMed] [Google Scholar]
- Kuczmarski E. R., Spudich J. A. Regulation of myosin self-assembly: phosphorylation of Dictyostelium heavy chain inhibits formation of thick filaments. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7292–7296. doi: 10.1073/pnas.77.12.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malchow D., Böhme R., Rahmsdorf H. J. Regulation of phosphorylation of myosin heavy chain during the chemotactic response of Dictyostelium cells. Eur J Biochem. 1981 Jun;117(1):213–218. doi: 10.1111/j.1432-1033.1981.tb06324.x. [DOI] [PubMed] [Google Scholar]
- Maruta H., Gadasi H., Collins J. H., Korn E. D. Multiple forms of Acanthamoeba myosin I. J Biol Chem. 1979 May 10;254(9):3624–3630. [PubMed] [Google Scholar]
- Maruta H., Korn E. D. Acanthamoeba myosin II. J Biol Chem. 1977 Sep 25;252(18):6501–6509. [PubMed] [Google Scholar]
- Nevaldine B., Kassell B. Bovine pepsinogen and pepsin. IV. A new method of purification of the pepsin. Biochim Biophys Acta. 1971 Oct;250(1):207–209. doi: 10.1016/0005-2744(71)90135-5. [DOI] [PubMed] [Google Scholar]
- Peltz G., Kuczmarski E. R., Spudich J. A. Dictyostelium myosin: characterization of chymotryptic fragments and localization of the heavy-chain phosphorylation site. J Cell Biol. 1981 Apr;89(1):104–108. doi: 10.1083/jcb.89.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit F. H., Hamilton L., Munk P., Namihira G., Eley M. H., Willms C. R., Reed L. J. Alpha-keto acid dehydrogenase complexes. XIX. Subunit structure of the Escherichia coli alpha-ketoglutarate dehydrogenase complex. J Biol Chem. 1973 Aug 10;248(15):5282–5290. [PubMed] [Google Scholar]
- Pollard T. D., Korn E. D. Acanthamoeba myosin. I. Isolation from Acanthamoeba castellanii of an enzyme similar to muscle myosin. J Biol Chem. 1973 Jul 10;248(13):4682–4690. [PubMed] [Google Scholar]
- Rahmsdorf H. J., Malchow D., Gerisch G. Cyclic AMP-induced phosphorylation in Dictyostelium of a polypeptide comigrating with myosin heavy chains. FEBS Lett. 1978 Apr 15;88(2):322–326. doi: 10.1016/0014-5793(78)80203-8. [DOI] [PubMed] [Google Scholar]
- Scholey J. M., Taylor K. A., Kendrick-Jones J. Regulation of non-muscle myosin assembly by calmodulin-dependent light chain kinase. Nature. 1980 Sep 18;287(5779):233–235. doi: 10.1038/287233a0. [DOI] [PubMed] [Google Scholar]
- Sherry J. M., Górecka A., Aksoy M. O., Dabrowska R., Hartshorne D. J. Roles of calcium and phosphorylation in the regulation of the activity of gizzard myosin. Biochemistry. 1978 Oct 17;17(21):4411–4418. doi: 10.1021/bi00614a009. [DOI] [PubMed] [Google Scholar]
- Stull J. T., Buss J. E. Phosphorylation of cardiac troponin by cyclic adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Feb 10;252(3):851–857. [PubMed] [Google Scholar]
- Suzuki H., Onishi H., Takahashi K., Watanabe S. Structure and function of chicken gizzard myosin. J Biochem. 1978 Dec;84(6):1529–1542. doi: 10.1093/oxfordjournals.jbchem.a132278. [DOI] [PubMed] [Google Scholar]
- Tsugita A., Scheffler J. J. A rapid method for acid hydrolysis of protein with a mixture of trifluoroacetic acid and hydrochloric acid. Eur J Biochem. 1982 Jun;124(3):585–588. doi: 10.1111/j.1432-1033.1982.tb06634.x. [DOI] [PubMed] [Google Scholar]
- Wick U., Malchow D., Gerisch G. Cyclic-AMP stimulated calcium influx into aggregating cells of Dictyostelium discoideum. Cell Biol Int Rep. 1978 Jan;2(1):71–79. doi: 10.1016/0309-1651(78)90086-3. [DOI] [PubMed] [Google Scholar]