Abstract
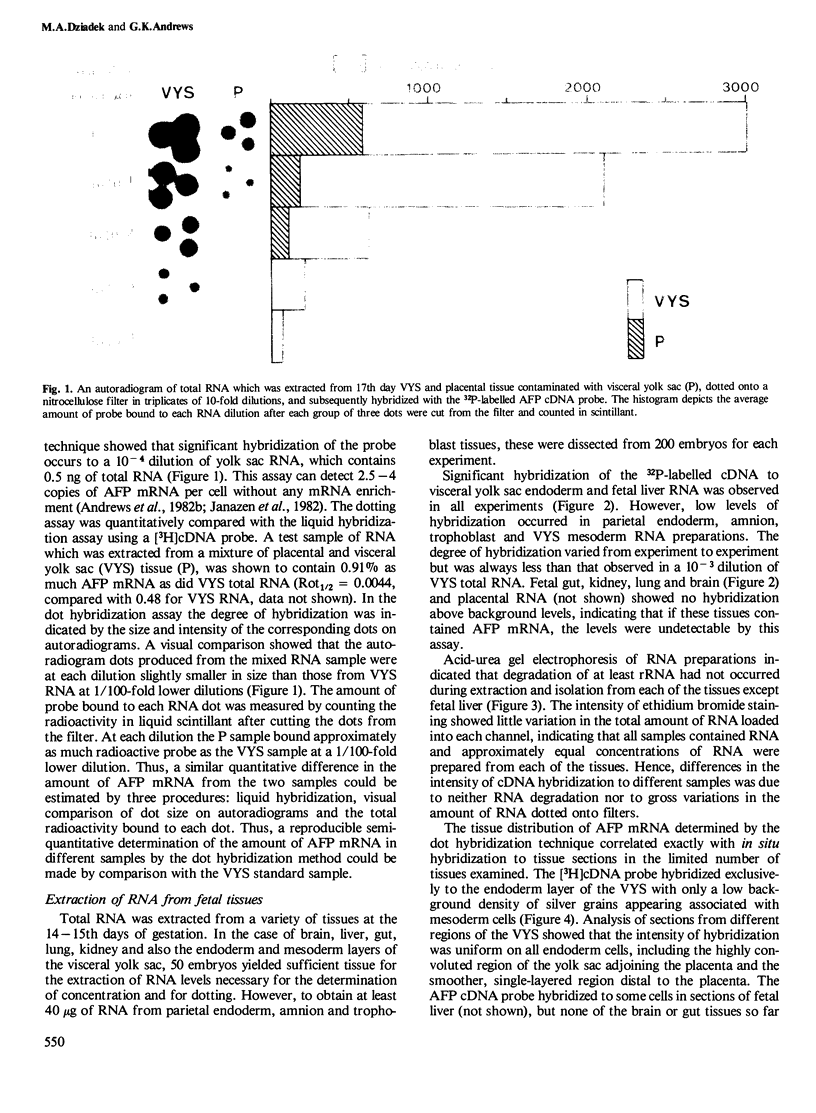
Alpha-fetoprotein (AFP) is a synthetic product of only the visceral yolk sac endoderm and fetal liver during mouse embryogenesis. To examine the involvement of transcriptional and post-transcriptional mechanisms in the regulation of tissue-specific expression of the AFP gene, the distribution of AFP mRNA in tissues at different stages of development was determined. Total RNA was isolated from mid-gestation tissues, and a microextraction procedure was developed for earlier stage embryos where the amount of tissue was limited. AFP mRNA was measured by a dot-hybridization assay using a 32P-labelled AFP cDNA clone as probe. Results show that only visceral yolk sac endoderm and fetal liver of midgestation embryos contained AFP mRNA. At earlier stages AFP mRNA was confined to regions within the visceral endoderm which have previously been shown to be AFP positive. These results indicate that expression of the AFP gene during post-implantation development is probably controlled at the level of AFP gene transcription. In addition, we show by in situ hybridization to tissue sections that all endoderm cells of the visceral yolk sac contain AFP mRNA, indicating that the visceral endoderm layer is a homogeneous population of cells with respect to transcription of the AFP gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelev G. I. Alpha-fetoprotein in ontogenesis and its association with malignant tumors. Adv Cancer Res. 1971;14:295–358. doi: 10.1016/s0065-230x(08)60523-0. [DOI] [PubMed] [Google Scholar]
- Adamson E. D., Ayers S. E. The localization and synthesis of some collagen types in developing mouse embryos. Cell. 1979 Apr;16(4):953–965. doi: 10.1016/0092-8674(79)90110-7. [DOI] [PubMed] [Google Scholar]
- Andrews G. K., Dziadek M., Tamaoki T. Expression and methylation of the mouse alpha-fetoprotein gene in embryonic, adult, and neoplastic tissues. J Biol Chem. 1982 May 10;257(9):5148–5153. [PubMed] [Google Scholar]
- Andrews G. K., Janzen R. G., Tamaoki T. Stability of alpha-fetoprotein messenger RNA in mouse yolk sac. Dev Biol. 1982 Jan;89(1):111–116. doi: 10.1016/0012-1606(82)90299-8. [DOI] [PubMed] [Google Scholar]
- Andrews G. K., Teng C. S. Studies on sex-organ development. Prenatal effect of oestrogenic hormone on tubular-gland cell morphogenesis and ovalbumin-gene expression in the chick Müllerian duct. Biochem J. 1979 Aug 15;182(2):271–286. doi: 10.1042/bj1820271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantine J. E., Woodland H. R., Sturgess E. A. Changes in protein synthesis during the development of Xenopus laevis. J Embryol Exp Morphol. 1979 Jun;51:137–153. [PubMed] [Google Scholar]
- Beebe D. C., Piatigorsky J. Translational regulation of delta-crystallin synthesis during lens development in the chicken embryo. Dev Biol. 1981 May;84(1):96–101. doi: 10.1016/0012-1606(81)90374-2. [DOI] [PubMed] [Google Scholar]
- Blumberg D. D., Margolskee J. P., Barklis E., Chung S. N., Cohen N. S., Lodish H. F. Specific cell-cell contacts are essential for induction of gene expression during differentiation of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1982 Jan;79(1):127–131. doi: 10.1073/pnas.79.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate accumulation of contractile protein mRNAs during myoblast differentiation. Dev Biol. 1979 Mar;69(1):202–216. doi: 10.1016/0012-1606(79)90286-0. [DOI] [PubMed] [Google Scholar]
- Dziadek M. A. Use of glycine as a non-enzymic procedure for separation of mouse embryonic tissues and dissociation of cells. Exp Cell Res. 1981 Jun;133(2):383–393. doi: 10.1016/0014-4827(81)90331-1. [DOI] [PubMed] [Google Scholar]
- Dziadek M., Adamson E. Localization and synthesis of alphafoetoprotein in post-implantation mouse embryos. J Embryol Exp Morphol. 1978 Feb;43:289–313. [PubMed] [Google Scholar]
- Dziadek M. Modulation of alphafetoprotein synthesis in the early postimplantation mouse embryo. J Embryol Exp Morphol. 1978 Aug;46:135–146. [PubMed] [Google Scholar]
- Enders A. C., Given R. L., Schlafke S. Differentiation and migration of endoderm in the rat and mouse at implantation. Anat Rec. 1978 Jan;190(1):65–77. doi: 10.1002/ar.1091900107. [DOI] [PubMed] [Google Scholar]
- Engelhardt N. V., Goussev A. I., Shipova L. J., Abelev G. I. Immunofluorescent study of alpha-foetoprotein (alpha-fp) in liver and liver liver tumours. I. Technique of alpha-fp localization in tissue sections. Int J Cancer. 1971 Mar 15;7(2):198–206. doi: 10.1002/ijc.2910070203. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. R., Conkie D., Paul J., Jones K. Localisation of cellular globin messenger RNA by in situ hybridisation to complementary DNA. FEBS Lett. 1973 May 15;32(1):109–112. doi: 10.1016/0014-5793(73)80749-5. [DOI] [PubMed] [Google Scholar]
- Hastings K. E., Emerson C. P., Jr cDNA clone analysis of six co-regulated mRNAs encoding skeletal muscle contractile proteins. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1553–1557. doi: 10.1073/pnas.79.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B. L. High molecular weight extracellular proteins synthesized by endoderm cells derived from mouse teratocarcinoma cells and normal extraembryonic membranes. Dev Biol. 1980 May;76(2):275–285. doi: 10.1016/0012-1606(80)90379-6. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Tilly R. Cell interactions and endoderm differentiation in cultured mouse embryos. J Embryol Exp Morphol. 1981 Apr;62:379–394. [PubMed] [Google Scholar]
- Howe C. C., Solter D. Identification of noncollagenous basement membrane glycopolypeptides synthesized by mouse parietal entoderm and an entodermal cell line. Dev Biol. 1980 Jun 15;77(2):480–487. doi: 10.1016/0012-1606(80)90489-3. [DOI] [PubMed] [Google Scholar]
- Innis M. A., Miller D. L. alpha-Fetoprotein gene expression. Control of alpha-fetoprotein mRNA levels in cultured rat hepatoma cells. J Biol Chem. 1979 Sep 25;254(18):9148–9154. [PubMed] [Google Scholar]
- Jaenisch R., Jähner D., Nobis P., Simon I., Löhler J., Harbers K., Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981 May;24(2):519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Janzen R. G., Andrews G. K., Tamaoki T. Synthesis of secretory proteins in developing mouse yolk sac. Dev Biol. 1982 Mar;90(1):18–23. doi: 10.1016/0012-1606(82)90207-x. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E., Sherman M. I. Analyses of cell surface and secreted proteins of primary cultures of mouse extraembryonic membranes. Dev Biol. 1979 May;70(1):89–104. doi: 10.1016/0012-1606(79)90009-5. [DOI] [PubMed] [Google Scholar]
- John H. A., Patrinou-Georgoulas M., Jones K. W. Detection of myosin heavy chain mRNA during myogenesis in tissue culture by in vitro and in situ hybridization. Cell. 1977 Oct;12(2):501–508. doi: 10.1016/0092-8674(77)90126-x. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Law S., Tamoaki T., Kreuzaler F., Dugaiczyk A. Molecular cloning of DNA complementary to a mouse alpha-fetoprotein mRNA sequence. Gene. 1980 Jun;10(1):53–61. doi: 10.1016/0378-1119(80)90143-2. [DOI] [PubMed] [Google Scholar]
- Liao W. S., Conn A. R., Taylor J. M. Changes in rat alpha 1-fetoprotein and albumin mRNA levels during fetal and neonatal development. J Biol Chem. 1980 Nov 10;255(21):10036–10039. [PubMed] [Google Scholar]
- Mackey J. K., Brackmann K. H., Green M. R., Green M. Preparation and characterization of highly radioactive in vitro labeled adenovirus DNA and DNA restriction fragments. Biochemistry. 1977 Oct 4;16(20):4478–4483. doi: 10.1021/bi00639a023. [DOI] [PubMed] [Google Scholar]
- Mackiewicz A., Breborowicz J. The in vitro production of alpha--fetoprotein variants by human fetal organs. Oncodev Biol Med. 1980;1(4-5):251–261. [PubMed] [Google Scholar]
- Mangiarotti G., Lefebvre P., Lodish H. F. Differences in the stability of developmentally regulated mRNAs in aggregated and disaggregated Dictyostelium discoideum cells. Dev Biol. 1982 Jan;89(1):82–91. doi: 10.1016/0012-1606(82)90296-2. [DOI] [PubMed] [Google Scholar]
- Marotti K. R., Belin D., Strickland S. The production of distinct forms of plasminogen activator by mouse embryonic cells. Dev Biol. 1982 Mar;90(1):154–159. doi: 10.1016/0012-1606(82)90220-2. [DOI] [PubMed] [Google Scholar]
- Miura K., Law S. W., Nishi S., Tamaoki T. Isolation of alpha-fetoprotein messenger RNA from mouse yolk sac. J Biol Chem. 1979 Jun 25;254(12):5515–5521. [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Nucleic acid hybridization to the DNA of cytological preparations. Methods Cell Biol. 1975;10:1–16. doi: 10.1016/s0091-679x(08)60727-x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Holder J. W., Means A. R., O'Malley B. W. Preparation and preliminary characterization of purified ovalbumin messenger RNA from the hen oviduct. Biochemistry. 1975 Jan 14;14(1):69–78. doi: 10.1021/bi00672a012. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. T., Hunt T., Ruderman J. V. Selective translation of mRNA controls the pattern of protein synthesis during early development of the surf clam, Spisula solidissima. Cell. 1980 Jun;20(2):487–494. doi: 10.1016/0092-8674(80)90635-2. [DOI] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Dever J., Sargent T. D., Thomas K., Sell S., Bonner J. Changes in expression of albumin and alpha-fetoprotein genes during rat liver development and neoplasia. Biochemistry. 1979 May 29;18(11):2167–2178. doi: 10.1021/bi00578a006. [DOI] [PubMed] [Google Scholar]
- Sell S., Sala-Trepat J. M., Sargent T. D., Thomas K., Nahon J. L., Goodman T. A., Bonner J. Molecular mechanisms of control of albumin and alphafetoprotein production: a system to study the early effects of chemical hepatocarcinogens. Cell Biol Int Rep. 1980 Mar;4(3):235–254. doi: 10.1016/0309-1651(80)90056-9. [DOI] [PubMed] [Google Scholar]
- Sherman M. I., Atienza-Samols S. B. Enzyme analysis of mouse extra-embryonic tissues. J Embryol Exp Morphol. 1979 Aug;52:127–139. [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Swaneck G. E., Nordstrom J. L., Kreuzaler F., Tsai M. J., O'Malley B. W. Effect of estrogen on gene expression in chicken oviduct: evidence for transcriptional control of ovalbumin gene. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1049–1053. doi: 10.1073/pnas.76.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Belayew A. Transcriptional control of the murine albumin/alpha-fetoprotein locus during development. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5254–5257. doi: 10.1073/pnas.79.17.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. R., Zimmerman E. F. Yolk sac: site of developmental microheterogeneity of mouse alpha-fetoprotein. Dev Biol. 1976 Dec;54(2):187–199. doi: 10.1016/0012-1606(76)90298-0. [DOI] [PubMed] [Google Scholar]