Abstract
The yeast exocyst complex (also called Sec6/8 complex in higher eukaryotes) is a multiprotein complex essential for targeting exocytic vesicles to specific docking sites on the plasma membrane. It is composed of eight proteins (Sec3, -5, -6, -8, -10, and -15, and Exo70 and -84), with molecular weights ranging from 70 to 144 kDa. Mammalian orthologues for seven of these proteins have been described and here we report the cloning and initial characterization of the remaining subunit, Sec3. Human Sec3 (hSec3) shares 17% sequence identity with yeast Sec3p, interacts in the two-hybrid system with other subunits of the complex (Sec5 and Sec8), and is expressed in almost all tissues tested. In yeast, Sec3p has been proposed to be a spatial landmark for polarized secretion (1), and its localization depends on its interaction with Rho1p (2). We demonstrate here that hSec3 lacks the potential Rho1-binding site and GFP-fusions of hSec3 are cytosolic. Green fluorescent protein (GFP)-fusions of nearly every subunit of the mammalian Sec6/8 complex were expressed in Madin–Darby canine kidney (MDCK) cells, but they failed to assemble into a complex with endogenous proteins and localized in the cytosol. Of the subunits tested, only GFP-Exo70 localized to lateral membrane sites of cell–cell contact when expressed in MDCK cells. Cells overexpressing GFP-Exo70 fail to form a tight monolayer, suggesting the Exo70 targeting interaction is critical for normal development of polarized epithelial cells.
Vesicles mediate protein transport along the secretory pathway in eukaryotic cells. Transport vesicles bud from a donor organelle and are translocated to an acceptor organelle where they dock, fuse, and thereby deliver their cargo (3). Proteins that mediate different steps in vesicle trafficking are highly conserved from yeast to man. For example, proteins that are crucial for neurosecretion in mammals (nSec1, Vamp1, Vamp2, SNAP-25, NSF, and α-SNAP) are homologous to proteins required for vesicle trafficking to the yeast plasma membrane (Sec1p, Snc1p, Snc2p, Sec9p, Sec18p, and Sec17p, respectively). Another group of proteins involved in this transport step in yeast includes Sec3p, Sec5p, Sec6p, Sec8p, Sec10p, Sec15p, Exo70p, and Exo84p, which form a stable complex called the exocyst (4). A mammalian homolog of this protein complex (Sec6/8 complex) has been described (5, 6), and in both yeast and mammals each subunit is represented once, resulting in protein complexes of 845 kDa (yeast) and 736 kDa (rat).
Accumulating evidence indicates that the Sec6/8 complex is required for post-Golgi vesicle trafficking (7, 8). Subcellular localization of the complex correlates with sites of polarized membrane growth. In yeast, Sec3p is present at plasma membrane sites of active vesicle fusion, and the location of these sites changes during the cell cycle. At the beginning of a new cell cycle, the exocyst localizes in a patch at the prebud site, and as the bud emerges the exocyst is localized to its tip. When the growth pattern switches from apical to isotropic the patch disperses around the membrane of the bud. During cytokinesis, the exocyst subunits reconcentrate in a ring-like structure at the neck separating the mother cell and the bud. Bud tip, isotropic bud, and mother–daughter neck represent sites of directed membrane growth that is coordinated with the cell cycle (1). In mammalian cells the sec6/8 complex is also present on plasma membranes at sites of membrane growth. In cultured hippocampal neurons, the Sec6/8 complex was shown to be present in regions of membrane addition—i.e., at neurite outgrowth and potential active zones during synaptogenesis (9). In differentiated PC12 cells the complex is found in the cell body, in the extending neurite, and at the growth cone, whereas it shows a perinuclear localization in undifferentiated PC12 cells (10). Best characterized however is the localization of the Sec6/8 complex in Madin–Darby canine kidney (MDCK) epithelial cells (8). Here the complex is rapidly recruited from the cytosol to cell–cell contacts on initiation of calcium-dependent cell–cell adhesion. As cell polarity develops, the localization of the complex becomes restricted to the apical junctional complex, which includes adherens junctions and tight junctions. It has been proposed that localization of Sec6/8 complex to cell–cell junctions serves to direct trafficking of transport vesicles containing basal-lateral proteins to the developing lateral membrane domain (11).
Functionally, the Sec6/8 complex probably acts as a tethering complex at the plasma membrane. In line with the localization studies, it has been shown that the Sec6/8 complex is involved in specifying docking and/or tethering of postGolgi transport vesicles to the plasma membrane. In yeast exocyst mutants, there is an accumulation of transport vesicles in the cytoplasm, when the cells are shifted to the restrictive temperature (12). And in streptolysin-O permeabilized MDCK cells, antibodies to Sec8 inhibit delivery of vesicles to the basal-lateral membrane, but not the apical membrane (8).
In addition to a primary localization on the plasma membrane, components of the exocyst complex may be present on other membranes. Overexpressed Sec15p cofractionates in sucrose gradients with Sec4p and Sncp, the rab protein, and v-SNARE associated with secretory vesicles. Because Sec15p also binds to activated Sec4p, the exocyst might be an effector for this Rab-like GTPase that is necessary for the targeting or tethering of secretory vesicles to sites of secretion. Sec10p also exists in a free pool, as has been shown by subcellular fractionations in yeast. Sec15p and Sec10p interact with each other in the two-hybrid system and in vitro synthesized Sec15p coimmunoprecipitates with epitope-tagged Sec10p (13). These findings suggest that Sec10p and Sec15p exist in a subcomplex that might act as a bridge between Sec4p on the vesicle and other subunits bound to the plasma membrane.
The localization of Sec3p in yeast to sites at the plasma membrane is reported to be independent of a functional secretory pathway, the actin cytoskeleton, and the other exocyst subunits (1). This led to the model that Sec3p is a spatial landmark for exocytosis and that it may be the component of the complex most proximal to the target membrane. Purification of mammalian Sec6/8 complex hinted at the existence of a Sec3 protein, but the corresponding gene was not previously cloned (14). Coomassie blue-stained SDS/PAGE of purified Sec6/8 complex reveals eight individual bands, seven of which comprise the components of the exocyst. But peptide sequencing of the remaining protein, p106, did not easily lead to its identification in protein databases (14). Now, as whole genomes from higher organisms are sequenced, a blast2 search with the yeast Sec3p sequence lead to the identification of the SEC3 genes from fly, worm, and man. Here we report the cloning of the human SEC3 gene, its expression pattern in different tissues, and a network of two-hybrid interactions that link Sec3 to other subunits of the Sec6/8 complex. Our localization studies employing green fluorescent protein (GFP) fusions of several Sec6/8 complex subunits revealed that only GFP-Exo70 becomes localized to the plasma membrane in polarized epithelial MDCK cells, whereas all other GFP-tagged subunits are cytosolic. These studies suggest that regulation of Sec6/8 complex assembly and localization at the plasma membrane depends on Exo70 targeting interactions, not Sec3 as in yeast.
Materials and Methods
Cloning of Human SEC3.
A blast2 search was done at the European Molecular Biology Laboratory (EMBL) web site, using the entire ySec3p sequence and default parameters to identify the human Sec3. The corresponding gene was then amplified by PCR out of human cDNA (CLONTECH), using the following oligonucleotide pairs and the proofreading Herculase polymerase blend (Stratagene). HM156 (ATGACAGCAATCAAGC ATGCATTACAAAGAG)/HM158 (CCTTTGGCCAAGTCCATGTGGTTCACAGG), HM159 (GGATCAGATCTCTGAAAGCAACCACCTAATTC)/HM160 (CTCCTAATGCAATTAGGTTGTTCAGCTC), and HM161 (GTGGCACACCACTGCCT GTTTCATCTGAG)/HM165 (GGGCAAATAAAACTGCTATATAGGTTGG). The obtained PCR products were then cloned, sequenced, and put together by using appropriate restriction enzymes and conventional cloning.
Northern Blot Analysis.
The mRNA blot of different human tissues was purchased from CLONTECH and used according to the manufacturer's instructions. The blot was probed with a 940-nucleotide hSEC3 cDNA fragment (from bp 920 to 1860) labeled with [32P]dCTP using random primers (Megaprime RPN, Amersham Pharmacia). To show an equal loading of RNA, the blot was stripped and tested again with human β-actin cDNA as control.
Two-Hybrid Interactions.
To clone the Sec6/8 genes into two-hybrid vectors, suitable restriction sites were created by PCR, the modified regions sequenced, and the complete ORFs subsequently cloned into pACTII (GAL4 activation domain vector) and pGBKT7 (GAL4 DNA-binding domain vector). The two-hybrid yeast strains Y187 and AM109 were then transformed with these plasmids, respectively. The expression of the fusion protein was confirmed by Western blot analysis using antibodies directed against the HA epitope of GAL4 activation domain fusion proteins and anti c-Myc antibody to detect GAL4 DNA-binding domain fusions. To check interactions between any two Sec6/8 subunits, all possible combinations were obtained by mating Y187 (a) with AM109 (α) and independently by cotransformation of AM109 with any two plasmids. X-Gal (5-bromo-4-chloro-3-indolyl β-D-galactoside) filter assays and quantification of interactions with o-nitrophenyl-β-D-galactopyranoside (ONPG) were performed as described (15). As negative control for self-activation, we used a combination of the Sec6/8 subunits with CLONTECH vectors pGAD-T-antigen and pGBKT7-p53, while these two plasmids together served as a positive control.
Expression of GFP Fusion Proteins and Immunofluorescent Staining.
N- and C-terminal-tagged enhanced green fluorescent fusion proteins were made by using pEGFP-N3 and pEGFP-C1 vectors from CLONTECH. Specifically, we created N- and C-terminal GFP fusion proteins of Sec3, -5, -8, and -10, and Exo70, as well as a C-terminal Sec15-GFP fusion protein. Transfection of MDCK IIG epithelial cells was either performed by using lipofectAMINE PLUS reagent (GIBCO/BRL Life Technologies) or Ca2+-phospate. Stably transfected MDCK IIG cells expressing GFP-tagged Exo70 were selected in 500 μg/ml G418 sulfate. Stably transfected T23 MDCK cells expressing GFP-tagged Sec3 or Sec10 under control of the tetracycline-repressible transactivator were selected in hygromycin and maintained in DMEM containing 10% FBS and 20 ng/ml doxycycline. Cells were induced to express GFP fusion proteins by removing doxycycline from culture medium of low-density cultures for 16–18 h before replating cells on either coverslips or 12-mm-diameter Transwell filters (Costar). Immunofluorescent staining of Sec6, E-cadherin, and ZO-1 was performed as described (8).
Results and Discussion
Cloning of Human SEC3.
In a blast2 search at the European Molecular Biology Laboratory (EMBL) web site, using the entire ySec3p sequence, we identified CG3885 [DNA Data Base in Japan (DDBJ)/EMBL/GenBank accession no. AAF49347], F52E4.7 (accession no. T16430), and FLJ10893 (accession no. NP 060731/BAA91886) as the Drosophila, Caenorhabditis elegans, and human Sec3 proteins, respectively. We then cloned the gene (AK001755) that encodes BAA91886 by PCR using human brain cDNA as template. Stop codons in all three reading frames upstream of the ATG start codon indicate that we indeed predict the full-length protein. BAA91886 is an 894-aa protein with a predicted molecular weight of 101.97 kDa (GCG: mol wt). The now complete calculated weight of the mammalian Sec6/8 complex is 736 kDa and therefore roughly 110 kDa smaller then its yeast counterpart (845 kDa). The Sec3 proteins from multicellular eukaryotes share 17–22% sequence identity with the yeast protein. These sequence identities are low, but additional evidence that BAA91886 represents a bona fide ortholog of ySec3p came from re-examining the peptide sequences we originally reported for p106, the unidentified subunit of the Sec6/8 complex isolated from rat brain (14). Three of these peptide sequences (ELPEFNLHFF, XLQDVDLASXR, and XNRXNEPAVNVL) match (around 70% identity) within the identified human Sec3 sequence and are preceded by lysine residues that are recognized by trypsin to generate peptides. The deviations between the peptides and the predicted human sequence might be due to a combination of protein sequencing errors and species variations, as the peptides are derived from rat. A sequence comparison of the yeast, fly, and human Sec3 proteins is given in Fig. 1; the position of the three peptides is marked by a line above the corresponding region. The newly identified proteins are of similar length (841–894 aa) and each lacks ≈480 aa found at the N terminus of the yeast protein. Interestingly, this N-terminal domain of yeast Sec3p has recently been shown to interact with Rho1p, whereas its deletion leads to a mislocalization of the protein (2).
Figure 1.
Sequence alignment of Sec3 proteins. A blast2 search with the ySec3p sequence revealed (in order): C. elegans F52E4.7, accession T16430, high score 105, e value 4.0e-08 with 19% identities; Homo sapiens BAA91886/FLJ10893, accession NP 060731, high score 83, e value 9.0e-08 with 17% identities; and Drosophila melanogaster CG3885 protein, accession AAF49347, high score 112, e value 1.4e-06 with 22% identities. The predicted amino acid sequence of human Sec3 was compared with the respective fly and yeast homologues by using the GCG programs PILEUP and PRETTYBOX. Identical residues are in a black box with white letters and similar residues are shaded. Dotted regions represent gaps. Lines above the amino acid sequences indicate the peptides determined by amino-acid sequencing of the p106 subunit purified from rat brain (14). The Sec3 proteins from higher organisms lack an equivalent of the 480-aa N-terminal domain of yeast Sec3p. Therefore, this part of ySec3p is not shown here.
Tissue Distribution of hSEC3 Transcripts.
The distribution of mRNA transcripts encoding human Sec3 was investigated by RNA blotting (Fig. 2). The SEC3 gene is expressed as one transcript of ≈4 kb. It is detectable in almost all tissues, but is most abundant in brain, heart, placenta, skeletal muscle, and kidney. This expression pattern is similar to that of other Sec6/8 subunits examined (6). A reblotting of the filter with a labeled probe against β-actin served as a control to show equal mRNA levels in all lines.
Figure 2.
Multiple-tissue Northern blot analysis. Size markers are on the left in Kb. (Upper) hSEC3, one transcript of about 4 Kb is observed in almost all tissues. (Lower) To show an equal loading of mRNA in all lanes, the filter was stripped and reblotted with human β-actin.
Molecular Interactions Between Sec6/8 Subunits.
To identify the binding partners of Sec3 and those of all other subunits within the complex, all eight genes were subcloned into both two-hybrid vectors (pGBKT7 as bait and pACTII as prey). These constructs were then used to test all possible pairwise interactions between individual subunits. An X-Gal (5-bromo-4-chloro-3-indolyl β-D-galactoside) filter assay that shows these interactions is given in Fig. 3A. All interactions were quantified by using a liquid β-galactosidase assay; but only those where the calculated interaction was at least 10× stronger than background (i.e., ≥1.0) are given here. As shown in Fig. 3A, hSec3 interacts with Sec5 and Sec8 from rat, providing further evidence that the identified protein is part of the Sec6/8 complex. Other two-hybrid interactions were found between Sec15 and Sec10, between Sec8 and Sec10, between Sec5 and Sec6, and between Sec6 and Exo70. In addition to these strong interactions, numerous weak or transient interactions are detectable. The most plausible explanation for these weak interactions is that the stability of the intact complex is achieved through a series of higher order interactions not achieved in the pairwise two-hybrid system. Intriguingly, there is only one very strong interaction, between Sec10 and Sec15, two proteins that in yeast have been suggested to form a subcomplex outside the whole complex. In contrast to the other exocyst subunits, Sec10p and Sec15p exist in a cytosolic pool, interact with each other in the two-hybrid system, and in vitro synthesized Sec15p coimmunoprecipitates with epitope-tagged Sec10p (13).
Figure 3.
Map of two-hybrid interactions. (A) X-Gal filter assays of all pairwise two-hybrid interactions between individual Sec6/8 subunits. CLONTECH vectors pBAD-T-antigene and pGBKT7-p53 combined with Sec6/8 subunits served as negative control, whereas the two vectors together served as positive control. A quantification of the two-hybrid interaction was done by a liquid o-nitrophenyl-β-D-galactopyranoside (ONPG) assay. The calculated units are given below the individual dots. Although all interactions were measured, only those 10× higher than background (≥1) are given here. (B) Schematic representation of these two-hybrid interactions. Arrows indicate the direction from bait to prey. (Known two-hybrid interactions in yeast are: Sec3p–Sec5p, Sec5p–Sec10p, Sec10p–Sec15p, Exo84p–Sec5p, and Exo84–Sec10p.)
A schematic representation of the stronger and therefore more reliable two-hybrid-interactions between the mammalian Sec6/8 subunits is given in Fig. 3B. Given the fact that both the yeast exocyst and the mammalian Sec6/8 complexes are composed of the same number of subunits in equal stoichiometry, we expected to find identical patterns of interactions between those subunits. This is however only partially true. The strongest interaction in the mammalian complex (Sec10–Sec15) was also shown in yeast. The interactions between Sec6–Sec5 and Sec8–Sec6 from rat were also seen by immunoprecipitation of in vivo-synthesized yeast exocyst proteins (13). Exo84p, however, was shown to interact with Sec5p and Sec10p in yeast (16). In the mammalian complex, Exo84 binds Exo70 and Sec15. Additional interactions observed between mammalian subunits that are not seen in yeast include Sec6 and Sec15 with Exo70, and Sec8 with Sec10 and vice versa. The interactions of Sec6 and Sec10 with Sec8 were confirmed recently by in vitro binding studies (10). In Saccharomyces cerevisiae, Sec3p interacts with Sec5p and it may bind Sec6p and/or Sec8p because, in a sec5 mutant, only Sec3p, Sec6p, and Sec8p are in the immunoisolated complex (13). In the mammalian complex, Sec3 interacts with Sec5 and Sec8, which parallels the yeast data.
Further studies are needed to better understand the organization of this large complex; in particular, two-hybrid studies need to be confirmed by other methods. As the data stand today some, but not all of the interactions between components of the two complexes are conserved between yeast and mammals. This could be due to technical limitations, but may also indicate that the structure (and function) of the complex in yeast and mammals has likely evolved to fulfill different demands of spatial regulation of exocytosis in these eukaryotes.
Localization of GFP-Fusion Proteins in MDCK Cells.
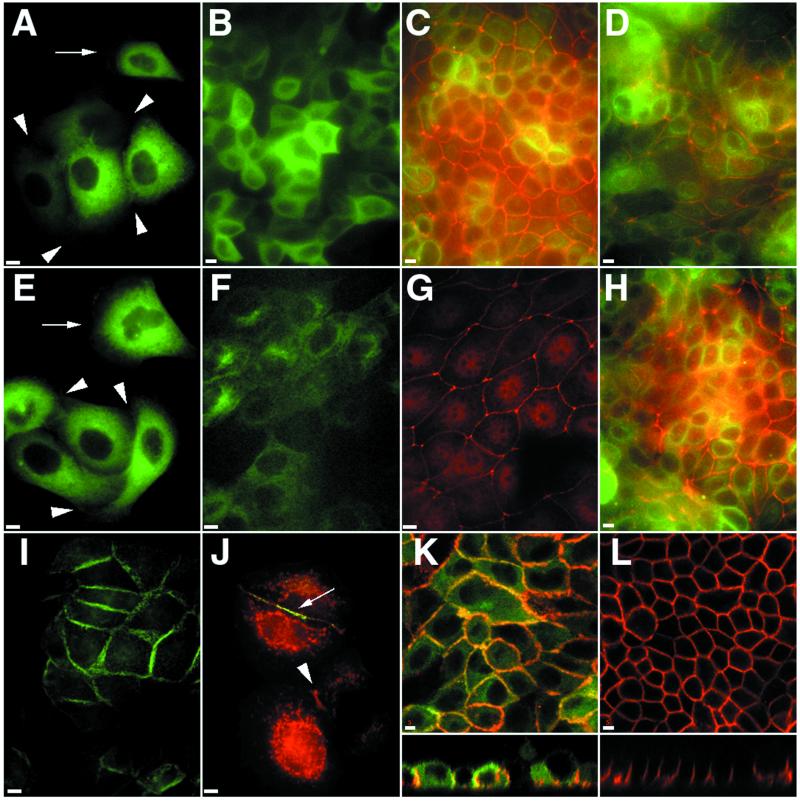
Because Sec3p is proposed to serve as a spatial landmark for polarized membrane growth in yeast (1), we were interested in determining whether the mammalian homolog has this function as well. Therefore, we expressed Sec3 tagged at either the N or C terminus with GFP in MDCK cells and examined its subcellular distribution during development of cell polarity. We reported previously that in contact-naive MDCK cells endogenous Sec6/8 complex is cytosolic and that upon induction of E-cadherin-mediated cell–cell adhesion the complex is recruited to lateral membrane cell–cell contacts where it becomes assembled into a detergent-insoluble structure (8). In both contact-naive (Fig. 4A, arrows) and early-contacting (arrowheads) MDCK cells, GFP-tagged Sec3 is found exclusively in the cytosol. No accumulation on plasma membranes or other organelles was observed for Sec3, regardless of whether the GFP tag was present at the C terminus (Fig. 4) or the N terminus (data not shown). Sec3-GFP was cytosolic at all time points examined over the course of 72 h following induction of cell–cell adhesion, and was cytosolic in fully polarized MDCK cells (Fig. 4B). Fractionation of MDCK cell homogenates in self-forming iodixanol density gradients confirmed that Sec3-GFP was present almost exclusively in cytosolic fractions (data not shown). Overexpression of Sec3-GFP had no affect on recruitment of Sec6 (Fig. 4C) or the tight junction associated protein ZO-1 (Fig. 4D) to lateral membranes. We considered the possibility that overexpressed Sec3 fusion proteins were cytosolic because limiting plasma membrane binding sites were saturated. However, when different levels of GFP-tagged Sec3 fusion proteins were expressed by varying the concentration of doxycycline in the medium, Sec3 was found to be cytosolic at even the lowest detectable expression levels. Examination of GFP-tagged Sec3 in live MDCK cells revealed no membrane-associated Sec3 during a 1-h imaging period (data not shown). One plausible explanation for the failure to detect exogenously expressed Sec3 fusion proteins on the plasma membrane is that placement of a GFP tag at either terminus interferes with the ability of Sec3 to associate with binding partners on the membrane or within the Sec6/8 complex. We disfavor this explanation, however, because a similarly modified Sec3p is functional and appropriately localized in yeast (1). A more interesting explanation is that the function of Sec3 in mammalian cells does not involve plasma membrane recruitment, or that its recruitment to the membrane is regulated by mechanisms different from those that recruit other subunits of the Sec6/8 complex. One remarkable difference between Sec3 proteins from different species is that an N-terminal region of 480 aa found in the yeast protein is lacking from higher eukaryotic organisms. Recent work has shown that a region spanning the first 320 aa of yeast Sec3p is essential for its interaction with Rho1p (2). When this domain is deleted, Sec3p no longer associates with Rho1p and is no longer restricted to sites of polarized membrane growth. Indeed, it is then diffusely distributed throughout the yeast cytosol, similar to hSec3-GFP expressed in MDCK cells. These results suggest that Sec3 from higher eukaryotic cells does not recruit the other subunits to the plasma membrane and does not function as a spatial landmark for secretion.
Figure 4.
Expression of GFP-tagged Sec6/8 complex subunits in MDCK cells. Stably transfected MDCK II cells expressing Sec3-GFP (A–D), GFP-Sec10 (E–H), or Exo70-GFP (I–L); all GFP stainings are shown in green. Cells were fixed with 4% paraformaldehyde before extraction with 1% Triton X-100. Cells in C, G, and J were stained with monoclonal antibody 9H5 against endogenous Sec6, and bound antibodies were detected with Texas red donkey anti-mouse IgG. Cells in D and H were stained with polyclonal anti-ZO-1 antibodies, which were detected with Texas red donkey anti-rabbit IgG. Cells in K and L were stained with monoclonal antibody 3G8 against E-cadherin followed by Texas red donkey anti-mouse IgG. (Scale bars, 5 μm.)
We also examined the distribution of Sec10 tagged at its N terminus with GFP (Fig. 4 E and F). In two-hybrid screens, this subunit interacted strongly with Sec8. However, Sec10-GFP did not colocalize with endogenous Sec6 (Fig. 4G) or Sec8 when expressed in MDCK cells. Instead, most of the protein was cytosolic in both contact-naive (arrow) and early-contacting (arrowheads) cells (Fig. 4E). In polarized cells, a concentration of Sec10-GFP was occasionally observed in a perinuclear localization (Fig. 4F). Perinuclear distributions of Sec6/8 complex subunits have been reported, although the precise identity of this compartment remains to be demonstrated (10, 17). As with Sec3-GFP fusion proteins, overexpression of GFP-Sec10 had no affect on the recruitment and organization of endogenous Sec6 (Fig. 4G) or ZO-1 (Fig. 4H) at the lateral membrane.
Of all of the Sec6/8 complex subunits that we expressed as GFP fusion proteins (see Materials and Methods) in MDCK cells, only Exo70-GFP was recruited to plasma membrane sites of cell–cell contact (Fig. 4I). The behavior of Exo70-GFP during development of cell polarity is superficially similar to what we have previously reported for endogenous Sec6 and Sec8 (8). However, the association of Exo70-GFP with the lateral membranes is in many ways different from the endogenous proteins. First, during early stages of cell–cell contact establishment, endogenous Sec6 is found throughout the cytosol in association with particulate structures and also along the entire length of cell–cell contacts in a very fine distribution pattern (Fig. 4J). In contrast, Exo70-GFP is not observed in the cytosol, and at cell–cell contacts it is observed in a much thicker distribution pattern that overlaps with that of Sec6 only in the oldest (middle) part of the contact (Fig. 4J, arrow). Often, very short cell–cell contacts were observed that were positive for Sec6, but lacked Exo70-GFP (Fig. 4J, arrowhead). A biochemical difference was also observed in the behaviors of Sec6 and Exo70-GFP. Whereas extraction with Triton X-100 before fixation fails to remove Sec6 from cell–cell contact sites (8), Exo70-GFP is completely solubilized by this treatment (data not shown). Therefore, although Exo70 is recruited into developing cell–cell contact sites, this subunit appears to arrive slightly later than Sec6 and Sec8, and does not seem to be bound to the membrane by the same type of interactions mediating Sec6 and Sec8 membrane association. This result suggests that individual subunits of the complex are recruited to and maintained at the membrane by different mechanisms, rather then arriving there as a fully assembled complex.
In polarized MDCK cells, Exo70-GFP remains enriched along lateral plasma membranes and was not observed at either apical or basal membranes (Fig. 4K, green). However, in contrast to the distributions observed for endogenous Sec6 and Sec8 (8) and Sec10 (18), Exo70-GFP distribution was not confined to the apex of the lateral membrane. Instead, Exo70-GFP appeared to be uniformly distributed along the length of the lateral plasma membrane in a distribution similar to that of E-cadherin (Fig. 4K, red).
Overexpression of Exo70-GFP had profound effects on the morphology of MDCK cells. In contrast to the crisp staining observed for E-cadherin in parental MDCK cells (Fig. 4L), E-cadherin staining in Exo70-GFP expressing cells was more diffuse, suggesting that the membranes of adjacent cells were not in close proximity. Measurement of transepithelial resistances in parental vs. Exo70-GFP-expressing clones revealed that the junctions between transfectants were much less tight than parental MDCK cells. Whereas parental MDCK cells developed a transepithelial resistance of 252 Ω/cm2 24 h after plating, Exo70-GFP-expressing cells had only developed a resistance of 100 Ω/cm2. This failure of Exo70-GFP-expressing cells to develop a snug monolayer is further evidenced by the lower cell density achieved by these cells at confluence. Although parental (Fig. 4L) and transfected (Fig. 4K) cells were seeded at identical densities, the packing density of parental cells was significantly higher than Exo70 overexpressers. Although the ultimate height of the cells was similar, the diameter of Exo70-GFP-transfected MDCK cells was typically 1.4× that of parental cells. These results show that overexpression of Exo70-GFP in MDCK cells has dramatic effects on organization of lateral membranes and function of junctional complexes and demonstrate a crucial function of Exo70 in establishing and maintaining cell–cell contacts.
In Summary, the development of cells and tissues with spatially organized membranes requires the polarized delivery of cargo-laden vesicles to the cell surface. A common feature of the mechanism of polarized vesicle targeting in eukaryotic organisms is the exocyst or Sec6/8 complex, which acts as a tethering factor at the plasma membrane for vesicles to be secreted. With this work, all eight subunits of this complex are known from yeast to man. The function of the Sec6/8 complex in polarized vesicle targeting is clear, yet the mechanism whereby this set of proteins acts is still largely unknown. Although the general mechanism of action of the Sec6/8 complex in yeast and mammals is likely to be similar, differences revealed here may include Sec3 interactions with Rho1p, details of the molecular organization of the complex, and Exo70 binding to target molecules. These and perhaps other mechanistic differences between yeast and mammalian vesicle targeting likely evolved to facilitate the more highly regulated development and physiology of multicellular organisms. Understanding the function of the exocyst or Sec6/8 complex in a variety of species will therefore not only provide insight into the cell biology of membrane trafficking, but will also lead to an appreciation of the ways in which fundamental cellular mechanisms are modified during the evolution of complex organisms.
Abbreviations
- MDCK
Madin–Darby canine kidney
- GFP
green fluorescent protein
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on May 2, 2000.
References
- 1.Finger F P, Hughes T E, Novick P. Cell. 1998;92:559–571. doi: 10.1016/s0092-8674(00)80948-4. [DOI] [PubMed] [Google Scholar]
- 2.Guo W, Tamanoi F, Novick P. Nat Cell Biol. 2001;3:353–360. doi: 10.1038/35070029. [DOI] [PubMed] [Google Scholar]
- 3.Bock J B, Matern H T, Peden A A, Scheller R H. Nature (London) 2001;409:839–841. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- 4.TerBush D R, Novick P. J Cell Biol. 1995;130:299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu S C, Hazuka C D, Roth R, Foletti D L, Heuser J, Scheller R H. Neuron. 1998;20:1111–1122. doi: 10.1016/s0896-6273(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 6.Kee Y, Yoo J S, Hazuka C D, Peterson K E, Hsu S C, Scheller R H. Proc Natl Acad Sci USA. 1997;94:14438–14443. doi: 10.1073/pnas.94.26.14438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.TerBush D R, Maurice T, Roth D, Novick P. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- 8.Grindstaff K K, Yeaman C, Anandasabapathy N, Hsu S C, Rodriguez-Boulan E, Scheller R H, Nelson W J. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- 9.Hazuka C D, Foletti D L, Hsu S C, Kee Y, Hopf F W, Scheller R H. J Neurosci. 1999;19:1324–1334. doi: 10.1523/JNEUROSCI.19-04-01324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vega I E, Hsu S C. J Neurosci. 2001;21:3839–3848. doi: 10.1523/JNEUROSCI.21-11-03839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeaman C, Grindstaff K K, Nelson W J. Physiol Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- 12.Finger F P, Novick P. Mol Biol Cell. 1997;8:647–662. doi: 10.1091/mbc.8.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo W, Roth D, Walch-Solimena C, Novick P. EMBO J. 1999b;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu S C, Ting A E, Hazuka C D, Davanger S, Kenny J W, Kee Y, Scheller R H. Neuron. 1996;17:1209–1219. doi: 10.1016/s0896-6273(00)80251-2. [DOI] [PubMed] [Google Scholar]
- 15.Fields S, Sternglanz R. Trends Genet. 1994;10:286–292. doi: 10.1016/0168-9525(90)90012-u. [DOI] [PubMed] [Google Scholar]
- 16.Guo W, Grant A, Novick P. J Biol Chem. 1999;274:23558–23564. doi: 10.1074/jbc.274.33.23558. [DOI] [PubMed] [Google Scholar]
- 17.Shin D M, Zhao X S, Zeng W, Mozhayeva M, Muallem S. J Cell Biol. 2000;150:1101–1112. doi: 10.1083/jcb.150.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipschutz J H, Guo W, O'Brien L E, Nguyen Y H, Novick P, Mostov K E. Mol Biol Cell. 2000;11:4259–4275. doi: 10.1091/mbc.11.12.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]