Abstract
Dementia worry, an anxiety-related response to the possibility of developing dementia, represents an important yet underexplored health concern for an aging population. Such a construct is likely impacted by stereotypes concerning aging, including biased associations of aging with inevitable cognitive decline. The present article explores the impact of mixed positive and negative aging stereotype messages on levels of dementia worry. The Fear of Alzheimer’s Disease Scale (FADS) was used to measure impact of priming with different proportions of positive and negative aging stereotype words. The priming intervention was modeled after Levy (J Pers Soc Psychol 71:1092–1107, 1996, doi:10.1037/0022-3514.71.6.1092). Eighty older adult participants (M age = 71.65, SD = 6.57) were exposed to mostly positive aging stereotype words, half positive/half negative words, mostly negative words, all negative words, or non-stereotype words. Mean FADS item response was significantly impacted by priming such that those in the all negative condition had highest levels of dementia worry, F(4, 75) = 2.48, p = .05, . This effect was strengthened when relevance of aging stereotypes was controlled for, p < .01. Results suggested that brief exposure to negative aging stereotype content increased levels of dementia worry, particularly when stereotypes were self-relevant. These findings indicate addressing aging stereotypes may be one way of impacting dementia worry.
Keywords: Dementia worry, Stereotypes, Older adults, Ageism
Introduction
Although the aging process is an inevitable part of life, ageist attitudes may make the transition to older adulthood a process to be dreaded and avoided (North and Fiske 2012). Of particular concern is aging’s association with memory loss: Alzheimer’s disease (AD) ranks as one of the most feared diseases among Americans, with 31 % naming it as their most feared disease in a recent survey (MetLife 2011). This represents an increase over the 20 % who responded similarly just 5 years previously and may relate to a similar increase in knowledge of AD reported over the same time span (MetLife 2011). Furthermore, common stereotypes regarding older adults include the perception that they are cognitively impaired, among other negative traits (Whitbourne and Sneed 2004).
Dementia worry
Anxiety is a logical consequence of concern and negative stereotypes surrounding memory loss as part of the aging process, but this anxiety can result in maladaptive pre-occupation with cognitive functioning. Different facets of this anxiety have been explored in the literature as anticipatory dementia (Cutler and Hodgson 1996), perceived Alzheimer’s disease threat (Roberts 2000; Suhr and Kinkela 2007), and more recently, dementia worry (DW; Kessler et al. 2012). DW represents the broadest conceptualization of a set of related concepts, defined as:
… an emotional response to the perceived threat of developing dementia, independent of chronological age and cognitive status. Importantly, we suggest that DW constitutes an overlap of affective components (e.g., fear) as well as more cognitive components (e.g., associations, thoughts, images) related to the perceived threat of developing dementia. (Kessler et al. 2012, p. 277)
The authors specify that DW represents a spectrum of response intensity ranging from passing concern to outright phobia. This model of understanding has its roots in literature considering other health worries and differs from aging anxiety via its inextricable connection with concerns regarding memory/cognitive functioning as well as what such losses mean for identity (Kessler et al. 2012).
Correlates of dementia worry
Kessler and colleagues (2012) propose that DW could potentially be associated with both positive and negative outcomes. They suggest a moderate level of DW might motivate health-promoting behaviors and future planning, but high levels of dementia worry may negatively impact aging attitudes, life satisfaction, and interactions with the healthcare system (either through over- or underutilization). Furthermore, they theorize that high levels of DW may contribute to biased misattributions of common memory lapses and inflate subjective memory complaints (Kessler et al. 2012). Indeed, one of the strongest, most consistent links demonstrated in DW’s short history in the literature is its connection with subjective memory concerns: multiple studies have identified a link between lower subjective memory appraisals and greater concern about developing dementia (Cutler 2015; Cutler and Brăgaru 2015; Cutler and Hodgson 1996, 2001; Werner 2002). In addition to intraindividual factors, DW may also relate to the environmental context (e.g., frequent exposure to dementia; Kessler et al. 2014). Although DW could potentially result in positive outcomes (e.g., future planning) as conceptualized by Kessler et al., extant research on related constructs thus far is suggestive of associations with negative consequences including lower life satisfaction, self-reports of poorer health, depression, and caregiver burden (Cutler and Brăgaru 2015; Cutler and Hodgson 1996; Hodgson and Cutler 1997; Suhr and Kinkela 2007). However, it should be noted that existing DW-related research has been correlational in nature, and therefore, it is not possible to determine whether DW leads to outcomes like poorer well-being or whether it is a consequence of other factors—for example, greater DW may lead to depressive symptoms, or it may be one manifestation of depressive symptoms.
Aging stereotypes
The cognitive and affective concern for development of dementia (despite a lack of objective cognitive impairment) in DW may reflect common content of aging stereotypes. Aging stereotypes often focus on incompetence or memory loss regardless of the objective reality of cognitive decline (or lack thereof) among older adults (Cuddy et al. 2005; Whitbourne and Sneed 2004). Evidence suggests that aging stereotypes may be incorporated into one’s perceived future self as an older adult and that with increased age, current self-concept begins to reflect those stereotypes (Kornadt and Rothermund 2012). Indeed, aging stereotypes have the potential to become self-fulfilling prophecies for aging adults (Levy and Leifheit-Limson 2009).
Stereotype priming
A wealth of literature supports the notion that older adults’ attitudes, behaviors, and even physical health may be influenced by aging stereotype priming—with multiple studies also demonstrating impact on cognitive functioning (see Levy 2009 for a review). Studies typically compare the impact of exclusively positive versus exclusively negative aging stereotype content, with negative outcomes associated with negative stereotypes and neutral or positive outcomes associated with positive stereotypes. The magnitude of these effects is not equal, however; effects of negative stereotype content are stronger and more robust than those of positive (Meisner 2012). Additionally, while several influential studies have suggested more reliable effects for implicit rather than explicit priming (e.g., Levy 1996), a recent meta-analysis of these and other relevant literature reported comparable effects for both implicit and explicit priming (Meisner 2012).
Self-relevance of aging stereotypes
Worth noting is that at least within the domain of cognitive functioning, the effects of both positive and negative age stereotype priming hold for older, but not young adults—leading some researchers to propose that stereotypes must be self-relevant in order to be impactful (Hess et al. 2004; Levy 1996). Yet relevance is relative: the transition of aging stereotypes into self-stereotypes involves both inclusion in groups categorized as “old” (e.g., Social Security recipients), and the individual’s subjective perception of being old (Levy 2003). While negative age stereotypes may predict poorer outcomes for older adults in general, this effect may be strengthened when self-relevance of aging stereotypes is factored in (Levy et al. 2012).
The present study
Given the effects of aging stereotypes on not only cognitive functioning, but also perceptions of one’s own aging and future self, it stands to reason that aging stereotypes may also influence DW. The present research aims to address several gaps in the literature. First, no known studies exist using experimental methods to assess DW, and research is needed to better understand what factors may lead to its development. Second, research related to aging stereotypes has so far considered exclusively positive and/or exclusively negative stereotype content. What previous experimental literature has not considered is that older adults are operating under continually mixed stereotypic messages (Cuddy et al. 2005). Given that older adults are not surrounded by purely positive or negative stereotypes, the question becomes: how do concerns related to development of dementia change based on the mixture of both positive and negative stereotypes? Is any amount of negativity sufficient to undo any effects of positive stereotypes (and vice versa)?
The purpose of the present research was to explore the effect of mixed stereotype messages on DW. Given the theoretical significance of self-relevance of aging stereotypes, this study focused exclusively on DW among older adults, for whom stereotypes are presumably most relevant. We used four levels of stereotype exposure: (1) exposure to more positive stereotype words than negative (mostly positive condition); (2) exposure to half positive and half negative stereotype words (half positive/half negative condition); (3) exposure to more negative stereotype words than positive (mostly negative condition); and (4) exposure to all negative stereotype words (all negative condition). A fifth control condition entailed exposure to neutral words. Based on literature suggesting that positive priming is less impactful and that negative information is typically more influential (Baumeister et al. 2001; Meisner 2012), we elected not to include an all-positive stereotype group. We hypothesized that those exposed to more negative stereotypes would demonstrate greater DW such that there would be greatest DW among those in the all negative condition and lowest levels in the control condition, with levels of those in the mostly positive, half positive/half negative, and mostly negative conditions falling in between the two.
Methods
Participants
Eligible participants were those who agreed to participate in exchange for an hourly rate of pay and were age 60 or older. Data collection took place at a medium-sized public university in the western United States as part of a larger study exploring effects of aging stereotypes. A total of 88 community-dwelling older adults were recruited from a participant registry. Four were excluded from analyses due to experimenter administration error and two were excluded for failing to score above a pre-specified threshold score of 20 % on a single story administered from the Logical Memory immediate recall subtest of the Wechsler Memory Scale-4th Edition (Wechsler 2009), suggesting possible cognitive impairment. Another participant was excluded for potentially invalid responding on the DW measure, and a final participant was excluded as an outlier whose mean response to the DW measure was more than 2.5 standard deviations higher than other participants’. The final N was 80 participants ranging in age from 61 to 90 (M = 71.65, SD = 6.57); total sample size and cell size (detailed below) was comparable to previous research exploring the effects of aging stereotype priming (e.g., Hess et al. 2004; Stein et al. 2002). Participant characteristics are presented in Table 1. Overall, participants considered themselves to be in relatively good health; on a scale from 1 (very poor health) to 7 (excellent health), only four participants (5.0 %) rated themselves as less than somewhat healthy (M = 5.42, SD = 1.15).
Table 1.
Participant characteristics
| n | % | |
|---|---|---|
| Gender | ||
| Female | 61 | 76.3 |
| Male | 19 | 23.8 |
| Race/ethnicity | ||
| White/non-Hispanic | 70 | 87.5 |
| African-American | 2 | 2.5 |
| Latino/Hispanic | 2 | 2.5 |
| Other | 4 | 5.0 |
| Decline to answer | 2 | 2.5 |
| Religion | ||
| Christian | 65 | 81.3 |
| Hindu | 1 | 1.3 |
| Other | 11 | 13.8 |
| Decline to answer | 3 | 3.8 |
| Partner status | ||
| Married/partnered | 38 | 47.5 |
| Widowed | 18 | 22.5 |
| Divorced | 19 | 23.8 |
| Single | 4 | 5.0 |
| Decline to answer | 1 | 1.3 |
| Family history of dementia | 48 | 60.0 |
Measures
Dementia worry
The Fear of Alzheimer’s Disease Scale (FADS; French et al. 2012) is a 30-item scale assessing DW within the specific context of Alzheimer’s disease. Participants respond to items such as “I am afraid of losing my memories” and “I would rather die than develop Alzheimer’s disease” using a 5-point rating scale (0 = never, 4 = always). Overall reliability of scores on the FADS has been good with a Cronbach’s α of 0.94 (French et al. 2012). Initial validation of the FADS suggested convergent validity; scores correlated significantly but not tremendously high with scores on the State-Trait Anxiety Inventory-Form Y (r = 0.22, p = .03), suggesting a similar underlying anxiety element but still unique construct (French et al. 2012).
Affect
A measure of affect was included to better assess how aging stereotypes might impact emotional state. The Positive and Negative Affect Scale-Expanded Form (PANAS-X; Watson and Clark 1999) is a 60-item measure in which participants indicate to what extent they have felt certain feelings in the previous few weeks. Participants use a rating scale ranging from 1 (very slightly or not at all) to 5 (extremely) to rate feeling words such as “cheerful,” “disgusted,” and “sluggish.” Scores on the PANAS-X among non-clinical samples have demonstrated good reliability both for the past few weeks timeframe (Cronbach’s α ranging from 0.86 to 0.87 for the positive affect subscale and 0.87 for the negative affect subscale) and for present moment timeframes (Cronbach’s α of 0.88 for positive affect and 0.85 for negative affect; Watson and Clark 1999).
Research design, procedure, and experimental manipulations
Pre-priming procedure
All procedures were approved by the Institutional Research Board. Participants were told they were completing a study of cognitive functioning and attention. After providing informed consent, participants were administered a brief memory battery including both immediate and delayed recall tasks as part of a larger study; the PANAS-X was administered during the delay period.
Priming intervention
The priming manipulation was modeled after the procedure detailed in Levy (1996). Target words flashed either above or below a bull's-eye on the white background of a computer screen. Participants were instructed to press an up or down arrow on the keyboard to indicate whether they saw a flash above or below the bull's-eye. Upon pressing the button, a patterned mask filled the computer screen. Participants had to press another button to clear the pattern, and the process repeated until that condition’s list of words was exhausted. Response times and location accuracy were calculated for each word. While each participant in essence ran his or her own manipulation, a researcher was present to launch the software as well as to offer instructions and assistance as needed. Each priming task took approximately 5–10 min.
Participants were assigned to one of five conditions, four experimental and one control. Although the experimenter launched the priming software, the conditions were coded in such a way (e.g., A, B, C, D, E) that he or she did not know which condition a given participant was in. A predetermined sequence of randomly ordered conditions (e.g., 1-D; 2-C; 3-A; 4-E; 5-B; etc.) was generated and participants were assigned to the condition associated with their participant number. In this way, participants were essentially randomly assigned and experimenters were blind to condition. The experimental conditions pulled words from both negative and positive age stereotype word banks. Following Levy (1996), negative age stereotype words included alzheimer’s, decline, dependent, senile, misplaces, dementia, dying, forgets, confused, decrepit, incompetent, and diseased. The positive age stereotype words included guidance, wise, alert, sage, accomplished, learned, improving, advise, creative, enlightened, insightful, and astute. Each experimental condition utilized a different proportion of negative versus positive words: the all negative condition (n = 15) included all 12 unique negative words; the mostly negative condition (n = 16) included all 12 negative words and two positive words; the mostly positive condition (n = 16) included all 12 positive words and two negative words; and the half positive/half negative condition (n = 16) contained seven of each type. Each experimental condition began with one of two cue words (old or senior); whichever word did not begin the block appeared in a random position in the rest of the list.
Four neutral words were included in each experimental condition, while the control condition (n = 17) contained entirely neutral words. However, different words were selected for this study due to some concern regarding the true neutrality of neutral words used in Levy’s original study (e.g., together can be interpreted in the positive context of relational togetherness) as well as the fact that the neutral words used in each of Levy’s experimental condition had extremely high frequency rates when compared with the positive or negative words. For this study, twenty words were selected based on neutral valence and low arousal ratings as provided by Warriner et al. (2013) and frequency ratings as provided by the Corpus of Contemporary American English (Davies 2008). Four were included in all experimental conditions on the basis of being comparable in both word length and frequency to the affectively laden words of the experimental conditions.
As in Levy (1996), each condition involved a block of 20 words total. Levy repeated two strong emotional words in each category. Rather than repeat words, in order to explore the impact of mixed stereotype messages we filled those word slots with terms of a competing valence such that alzheimer’s and senile appeared in the mostly positive condition, and wise and sage appeared in the mostly negative condition. The exception to this methodology was the all negative condition, which followed Levy’s approach of repeating alzheimer’s and senile.
Although the original aim of the priming task was to ensure “perception without awareness,” an error in the software specifications resulted in a patterned mask appearing too late to effectively prevent afterimages of the priming stimuli: the mask appeared after participants had responded to a prime, rather than immediately after stimulus presentation. All participants were exposed to the same priming intervention; however, all participants indicated at least some awareness of the primes, suggesting explicit rather than implicit priming. This awareness was generally reported by participants in practice trials used before stereotype priming to determine priming speed; as such, all participants were administered the fastest possible priming speed (approximately 17 ms). Hypotheses were reviewed upon discovery of this concern; however, given evidence for comparability of effects of implicit and explicit age stereotype priming (e.g., Meisner 2012), our prediction of higher DW with increasing exposure to negative stereotypes was unchanged.
Post-priming procedure
As in pre-priming, participants completed a brief memory battery including both immediate and delayed recall tasks. During the delay period, participants completed a modified version of the PANAS-X in which they were asked to consider their feelings over the past hour. Following the memory assessments, participants completed a packet containing the FADS and a demographics sheet. This packet also included the question “At what age do you believe ‘old age’ begins?” as a way of assessing self-relevance of aging stereotypes, consistent with previous assessments of this construct (e.g., Levy et al. 2012).
Results
Group equivalence
Experimental groups did not differ as a function of age, F(4, 75) = 1.09, p = .37. They also did not differ in terms of baseline positive affect F(4, 75) = 1.09, p = .37, or negative affect, F(4, 74) = 1.82, p = .13. Groups were also equivalent in terms of proportion of participants with a family history of dementia, X 2 (4, N = 78) = 7.67, p = .10. Groups did not differ in mean response time to priming stimuli, F(4, 75) = 0.98, p = .42, , or in accuracy of identifying where the stimulus occurred, F(4, 75) = 1.08, p = .37, . Average response time for each prime was 734.08 ms (SD = 182.16), with an overall accuracy of 97.6 %.
Dementia worry
Within this sample, scores on the FADS demonstrated excellent reliability (Cronbach’s α = 0.95). Mean FADS item response score, ranging from a possible 0 to 4, did not differ by gender, t(78) = 0.87, p = .39. Looking at the sample as a whole, the presence of a self-reported family history of dementia was not significantly related to FADS mean item response scores, t(76) = −0.99, p = .32. Mean FADS item response and standard deviations by condition and overall are presented in Table 2. The FADS correlated significantly with both pre-priming positive affect, r(78) = −0.25, p = .03, and pre-priming negative affect, r(78) = 0.23, p = .04. The FADS also correlated significantly with post-priming negative affect, r(77) = 0.31, p = .01, but the corresponding positive correlation was not significant, r(77) = −0.17, p = .13. This pattern of correlations suggests higher DW was associated with lower positive affect and higher negative affect. The FADS also correlated modestly but not significantly with age, r(78) = −0.20, p = .08, suggesting that increased age was possibly associated with lower levels of DW.
Table 2.
Mean response and standard deviations/errors for Fear of Alzheimer’s Disease Scale by exposure to aging stereotype content
| Control | Mostly positive | Half and half | Mostly negative | All negative | Overall | |
|---|---|---|---|---|---|---|
| FADS ANOVA | 1.06 (0.55) | 1.04 (0.65) | 1.02 (0.70) | 1.29 (0.71) | 1.61 (0.55) | 1.20 (0.66) |
| FADS ANCOVA | 0.98 (0.15) | 0.78 (0.18) | 1.20 (0.20) | 1.38 (0.21) | 1.76 (0.18) | 1.22 (0.08) |
FADS Fear of Alzheimer’s Disease Scale. Possible item responses range from 0 to 4. FADS ANOVA shows the mean item response and standard deviations by condition for the overall sample (N = 80); FADS ANCOVA shows the mean item response and standard errors by condition when self-relevance of aging stereotypes was added as a covariate (n = 51)
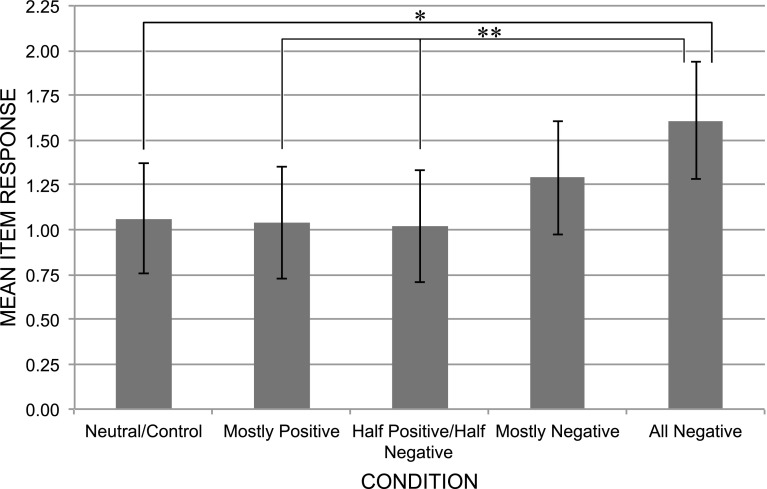
One-way ANOVA was used to test the hypothesis that increasing exposure to negative aging stereotype information would significantly impact DW as measured by the FADS. As predicted, there was a statistically significant effect of condition on FADS mean scores, F(4, 75) = 2.48, p = .05, . Using the Least Significant Difference (LSD) test of post hoc comparisons, FADS scores in the all negative condition (M = 1.61, SD = 0.55) were significantly higher as compared to the control condition (M = 1.06, SD = 0.55; p = .02), the mostly positive condition (M = 1.04, SD = 0.65; p = .01), and the half positive/half negative condition (M = 1.02, SD = 0.70; p = .01). Although FADS scores for the all negative condition were higher than those in the mostly negative condition (M = 1.29, SD = 0.71), this difference was not statistically significant (p = .16). These data are presented in Fig. 1.
Fig. 1.
Mean item responses with 95 % confidence intervals for Fear of Alzheimer’s Disease Scale (FADS) by condition. *p < .05, **p = .01 (see separate figure file)
Relevance of aging stereotypes
To assess aging stereotype relevance, participants’ estimates of when old age begins were subtracted from their actual ages. Narrative responses were converted to numbers (e.g., if a participant responded that old age begins “15 years from how old I am now” and they were 70, their response was entered as 85). Approximately one-third of the sample provided responses that could not be converted to numerical scores (e.g., “it’s relative”); therefore, the sample size for these analyses was reduced to 51. Results suggested that on average, respondents tended to estimate “old age” as beginning just over 10 years from their current age (M = −10.33, SD = 10.78). This variable was not impacted by experimental condition, F(4, 46) = 1.39, p = .25, .
Given that these results suggest aging stereotype words may not be considered high in self-relevance for a large portion of the sample—a possibility hypothesized to alter or neutralize the impact of stereotype priming (Levy et al. 2012)—analyses assessing the impact of experimental condition on DW were rerun, controlling for relevance of aging stereotypes as a covariate. Doing so resulted in more robust statistical significance and markedly increased effect size, F(4, 45) = 4.39, p = .004, . Pairwise comparisons using LSD methods revealed a pattern of increases in endorsed DW with increased exposure to negative aging stereotype content: when controlling for relevance of stereotypes, the mean FADS item response in the all negative condition (M = 1.76, SE = 0.18) was approximately one point higher (on a 5-point scale) than those of the mostly positive condition (M = 0.76, SE = 0.18; p < .001), with the control and experimental conditions falling in between the two. Mean item response by condition controlling for stereotype self-relevance is included in Table 2 and Fig. 2.
Fig. 2.
Mean item responses with 95 % confidence intervals for Fear of Alzheimer’s Disease Scale (FADS) by condition, controlling for self-relevance of aging stereotypes. *p < .05, **p < .01 (see separate figure file)
Positive and negative affect
Mixed ANOVAs were conducted for both positive and negative affect to explore how primes impact older adults’ self-reported affect. (One participant’s responses to the affect post-measure were not valid and therefore degrees of freedom are slightly different.) A main effect of time existed for both positive affect, F(4, 74) = 9.25, p < .01, , and negative affect, F(4, 74) = 9.26, p < .01, . This main effect represented a general flattening of reported affect from pre- to post-priming. There was no significant interaction of time and prime for either positive affect, F(4, 74) = 1.91, p = .12, , or negative affect, F(4, 74) = 1.68, p = .17, , suggesting that priming with aging stereotype words did not significantly change self-reported affect.
Discussion
The present study aimed to better understand how mixed positive and negative aging stereotype messages impact older adults’ levels of DW. Consistent with hypotheses, aging stereotypes exerted some effect on levels of DW as measured by the FADS. Although pairwise comparisons revealed that statistically significant differences existed only when comparing the all negative condition to conditions including half or fewer negative stereotype words, the impact of priming condition on FADS scores represented a medium to large effect size, suggesting a meaningful effect of aging stereotypes on DW. Furthermore, the general pattern was suggestive of increasing levels of DW associated with exposure to higher proportions of negative versus positive stereotype words, as predicted. Worth noting is that those in the mostly positive condition endorsed DW levels that were not significantly different from the control condition, whereas those in the mostly negative condition endorsed significantly higher DW as compared to control. This seems to provide further support for existing evidence of stronger impact of negative age stereotype priming in older adults as compared to positive (Meisner 2012).
Additionally, the pattern of results was made more robust when the relevance of aging stereotypes was controlled for, suggesting that the impact of aging stereotypes on DW is strengthened among those closer to self-identifying as an older adult. It should also be noted that although DW correlated with positive and negative affect, affect scores were unaffected by the priming task. This suggests that although DW relates to affect, priming-related change in affect does not explain the effect of aging stereotype content on DW.
These results provide important initial evidence on ways that DW, previously unexamined in an experimental context, may be influenced. Although DW levels were relatively low overall in our sample (similar to levels reported in French et al.’s initial validation study in 2012, M = 0.98), they were increased by a relatively small, time-limited manipulation of self-relevant aging stereotypes. Given the prevalence of everyday ageism—particularly stereotypes with pessimistic predictions for cognitive functioning—the finding that exposure to negative stereotype content may increase DW raises concerns for the types of self-fulfilling prophecies discussed in other literature on aging (e.g., Kornadt and Rothermund 2012; Levy and Leifheit-Limson 2009) and offers additional support for existing evidence that frequent exposure to dementia-related contexts is associated with higher DW (Kessler et al. 2014). The question of whether increased DW resulting from exposure to negative aging stereotypes could potentially translate into future declines in cognitive functioning is an empirical one that warrants future direct study. Yet literature indicative of anxiety’s deleterious effects on cognition (Sinoff and Werner 2003; Yochim et al. 2013; Zahodne et al. 2014) suggests that DW—like other health-related anxieties, such as cancer worry (Hay et al. 2005)—represents an important health concern to address. This study suggests that one way to do so might be to address and combat negative aging stereotypes that may inflate DW to maladaptive levels among older adults.
Study limitations and directions for future research
Although care was taken to ensure the integrity of this research design, important limitations must be noted. First, a lack of immediate afterimage masking resulted in a priming task in which participants were able to perceive at least some of the words. As mentioned previously, this likely resulted in an explicit rather than implicit priming task as originally planned for the study’s design. This required a reconceptualization of the study as one of explicit priming effects on DW. Consistent with evidence of comparable effects among implicit and explicit age-related stereotype priming (Meisner 2012), our hypotheses concerning priming’s impact on DW were supported even with participants’ partial awareness of priming content. Some previous research within the domain of memory has been suggestive of more reliable effects of implicit priming of aging stereotypes over explicit (e.g., Stein et al. 2002). It is possible that while implicit priming is more critical for effects on actual memory performance, feelings and thoughts about dementia as measured by the FADS are more vulnerable to stereotype content presented at the level of conscious awareness.
Another limitation is the composition of the study sample. Participants were recruited from a participant registry composed largely of higher functioning older adults. Additionally, women, Christians, and Whites were disproportionately represented within this sample. Although such a sample is comparable to similar previous studies (e.g., Levy 1996), results may not be generalizable to a more diverse sample. Inclusion in the study also required participants to meet a pre-specified cutoff score for memory performance in order to rule out cognitive impairment. While this represents a strength in that the sample was more tightly controlled than previous studies that did not screen for cognitive impairment (e.g., Levy 1996; Stein et al. 2002), the results may not be generalizable to those with cognitive impairment.
This study presents several opportunities for future research. A follow-up study using purely implicit priming interventions would provide more information on the impact of aging stereotypes on older adults’ DW, and could additionally be directly compared to this study’s findings to better understand the different effects elicited by implicit versus explicit priming. Such a study could also be expected to further elucidate the effects of mixed stereotype messages as opposed to strictly positive or negative stereotype content.
One exclusionary criterion for this study was age: participants were required to be at least 60 years of age. Particularly given the salience of concerns related to Alzheimer’s disease and other dementia processes for middle-aged adults—many of whom may be caring for aging parents—understanding how stereotypes associated with aging and old age impact middle-aged adults’ DW would be a fruitful area for future research. Caregiving for a parent with dementia has been identified as a possible risk factor for DW (Cutler and Hodgson 1996), and although family history of dementia was not associated with DW in the present study, this relationship may be different among middle-aged caregiving adults whose future cognitive functioning may seem more uncertain than that of older adults who may believe they have already aged past the threat of dementia.
To the best of our knowledge, this study represents the first experimental investigation of DW. Given the potential for positive consequences of DW (e.g., engagement in healthy behaviors to prevent dementia; Kessler et al. 2012), future research aimed at understanding how to manipulate DW in beneficial ways is warranted. In particular, identifying what levels of DW are adaptive versus maladaptive and establishing under what circumstances DW might predict adaptive health-related behaviors would offer valuable insight into this currently underexplored area. As older adults continue to make up an increasingly substantial proportion of the population (Aldwin and Gilmer 2013), achieving a more thorough knowledge of their concerns regarding dementia—including clearer understanding of what factors may lead to DW, what outcomes it may create, and how it may be influenced by internalized stereotype messages—becomes an important direction for continued research on healthy and successful aging.
Footnotes
Responsible editor: H.-W. Wahl.
References
- Aldwin C, Gilmer D. Health, illness, and optimal aging. New York: Springer; 2013. [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev Gen Psychol. 2001;5:323–370. doi: 10.1037/1089-2680.5.4.323. [DOI] [Google Scholar]
- Cuddy AJC, Norton MI, Fiske ST. This old stereotype: the pervasiveness and persistence of the elderly stereotype. J Soc Issues. 2005;61:267–285. doi: 10.1111/j.1540-4560.2005.00405.x. [DOI] [Google Scholar]
- Cutler SJ. Worries about getting Alzheimer’s: Who’s concerned? Am J Alzheimers Dis. 2015;30:591–598. doi: 10.1177/1533317514568889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler SJ, Brăgaru C. Long-term and short-term predictors of worries about getting Alzheimer’s disease. Eur J Ageing. 2015;12:341–351. doi: 10.1007/s10433-015-0350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler SJ, Hodgson LG. Anticipatory dementia: a link between memory appraisals and concerns about developing Alzheimer’s disease. The Gerontologist. 1996;36:657–664. doi: 10.1093/geront/36.5.657. [DOI] [PubMed] [Google Scholar]
- Cutler SJ, Hodgson LG. Correlates of personal concerns about developing Alzheimer’s disease among middle-aged persons. Am J Alzheimer’s Dis. 2001;16:335–343. doi: 10.1177/153331750101600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M (2008) The corpus of contemporary American English: 450 million words, 1990-present. http://corpus.byu.edu/coca
- French SL, Floyd M, Wilkins S, Osato S. The Fear of Alzheimer’s Disease Scale: a new measure designed to assess anticipatory dementia in older adults. Int J Geriatr Psychiatr. 2012;27:521–528. doi: 10.1002/gps.2747. [DOI] [PubMed] [Google Scholar]
- Hay JL, Buckley TR, Ostroff JS. The role of cancer worry in cancer screening: a theoretical and empirical review of the literature. Psycho-Oncology. 2005;14:517–534. doi: 10.1002/pon.864. [DOI] [PubMed] [Google Scholar]
- Hess TM, Hinson JT, Statham JA. Explicit and implicit stereotype activation effects on memory: Do age and awareness moderate the impact of priming? Psychol Aging. 2004;19:495–505. doi: 10.1037/0882-7974.19.3.495. [DOI] [PubMed] [Google Scholar]
- Hodgson LG, Cutler SJ. Anticipatory dementia and well-being. Am J Alzheimer’s Dis Other Dement. 1997;12:62–66. doi: 10.1177/153331759701200203. [DOI] [Google Scholar]
- Kessler E, Bowen CE, Baer M, Froelich L, Wahl H. Dementia worry: A psychological examination of an unexplored phenomenon. Eur J Ageing. 2012;9:275–284. doi: 10.1007/s10433-012-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler E, Tempel J, Wahl H. Concerns about one’s aging: the role of work context and psychological distress. GeroPsych J Gerontopsychol Geriatr Psychiatry. 2014;27:81–86. doi: 10.1024/1662-9647/a000105. [DOI] [Google Scholar]
- Kornadt AE, Rothermund K. Internalization of age stereotypes into the self-concept via future self-views: a general model and domain-specific differences. Psychol Aging. 2012;27:164–172. doi: 10.1037/a0025110. [DOI] [PubMed] [Google Scholar]
- Levy BR. Improving memory in old age through implicit self-stereotyping. J Pers Soc Psychol. 1996;71:1092–1107. doi: 10.1037/0022-3514.71.6.1092. [DOI] [PubMed] [Google Scholar]
- Levy BR. Mind matters: cognitive and physical effects of aging self-stereotypes. J Gerontol Ser B Psychol Sci Soc Sci. 2003;58:203–211. doi: 10.1093/geronb/58.4.p203. [DOI] [PubMed] [Google Scholar]
- Levy BR. Stereotype embodiment: a psychosocial approach to aging. Curr Dir Psychol Sci. 2009;18:332–336. doi: 10.1111/j.1467-8721.2009.01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Leifheit-Limson E. The stereotype-matching effect: greater influence on functioning when age stereotypes correspond to outcomes. Psychol Aging. 2009;24:230–233. doi: 10.1037/a0014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Zonderman AB, Slade MD, Ferrucci L. Memory shaped by age stereotypes over time. J Gerontol B-Psychol. 2012;67:432–436. doi: 10.1093/geronb/gbr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisner BA. A meta-analysis of positive and negative age stereotype priming effects on behavior among older adults. J Gerontol Ser B Psychol Sci Soc Sci. 2012;67B:13–17. doi: 10.1093/geronb/gbr062. [DOI] [PubMed] [Google Scholar]
- MetLife Foundation (2011) What America thinks. http://www.metlife.com/assets/cao/foundation/alzheimers-2011.pdf. Accessed 25 June 2015
- North MS, Fiske ST. An inconvenienced youth? Ageism and its potential intergenerational roots. Psychol Bull. 2012;138:982–997. doi: 10.1037/a0027843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. Anticipating response to predictive genetic testing for Alzheimer’s disease: a survey of first-degree relatives. Gerontologist. 2000;40:43–52. doi: 10.1093/geront/40.1.43. [DOI] [PubMed] [Google Scholar]
- Sinoff G, Werner P. Anxiety disorder and accompanying subjective memory loss in the elderly as a predictor of future cognitive decline. Int J Geriatr Psychiatr. 2003;18:951–959. doi: 10.1002/gps.1004. [DOI] [PubMed] [Google Scholar]
- Stein R, Blanchard-Fields F, Hertzog C. The effects of age-stereotype priming on the memory performance of older adults. Exp Aging Res. 2002;28:169–181. doi: 10.1080/03610730252800184. [DOI] [PubMed] [Google Scholar]
- Suhr JA, Kinkela JH. Perceived threat of Alzheimer disease (AD): the role of personal experience with AD. Alzheimer Dis Assoc Disord. 2007;21:225–231. doi: 10.1097/WAD.0b013e31813e6683. [DOI] [PubMed] [Google Scholar]
- Warriner AB, Kuperman V, Brysbaert M. Norms of valence, arousal, and dominance for 13,915 English lemmas. Behav Res Methods. 2013;45:1191–1207. doi: 10.3758/s13428-012-0314-x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA (1999) The PANAS-X: manual for the positive and negative affect schedule—expanded form. http://ir.uiowa.edu/psychology_pubs/11. Accessed 25 June 2015
- Wechsler D. Wechsler Memory Scale—fourth edition technical and interpretive manual. San Antonio TX: Pearson; 2009. [Google Scholar]
- Werner P. Assessing correlates of concern about developing Alzheimer’s dementia among adults with no family history of the disease. Am J Alzheimer’s Dis Other Dement. 2002;17:331–337. doi: 10.1177/153331750201700609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitbourne SK, Sneed JR. The paradox of well-being, identity processes, and stereotype threat: ageism and its potential relationships to the self in later life. In: Nelson T, editor. Ageism: stereotyping and prejudice against older persons. Cambridge: MIT; 2004. pp. 247–273. [Google Scholar]
- Yochim BP, Mueller AE, Segal DL. Late life anxiety is associated with decreased memory and executive functioning in community dwelling older adults. J Anxiety Disord. 2013;27:567–575. doi: 10.1016/j.janxdis.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Zahodne LB, Nowinski CJ, Gershon RC, Manly JJ. Which psychosocial factors best predict cognitive performance in older adults? J Int Neuropsychol Soc. 2014;20:487–495. doi: 10.1017/S1355617714000186. [DOI] [PMC free article] [PubMed] [Google Scholar]