Abstract
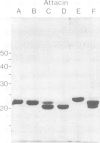
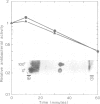
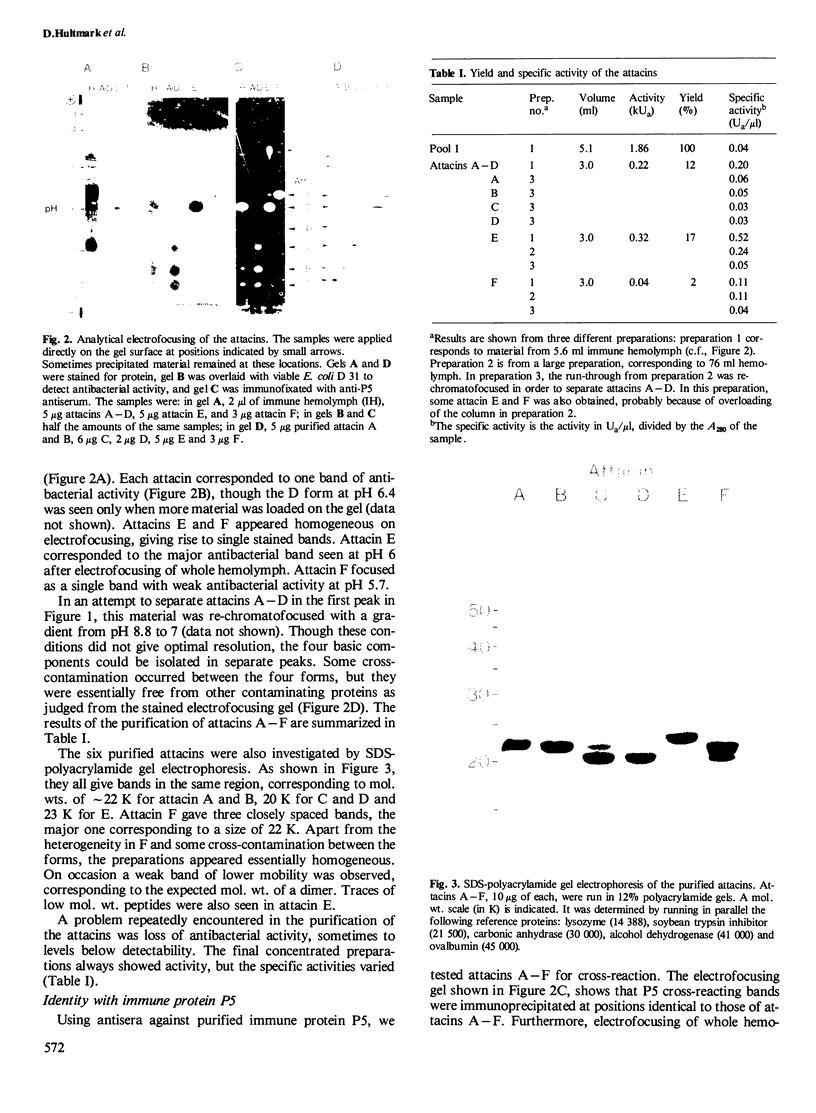
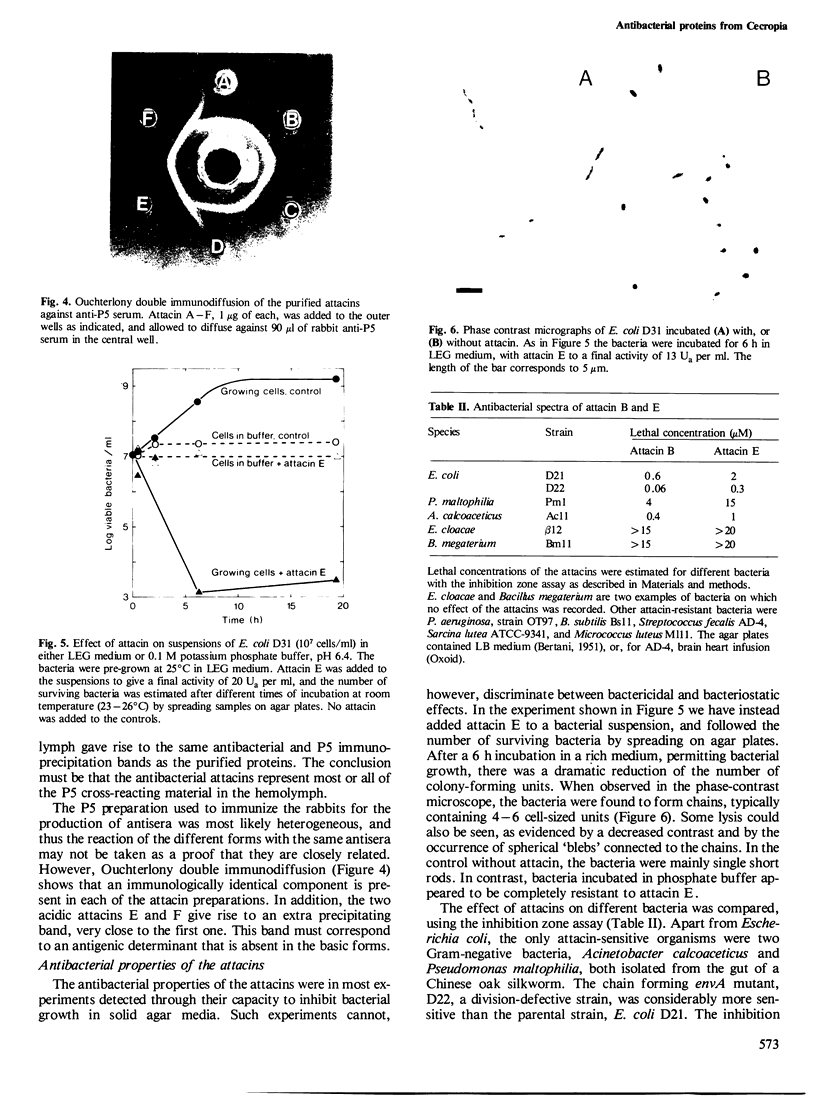
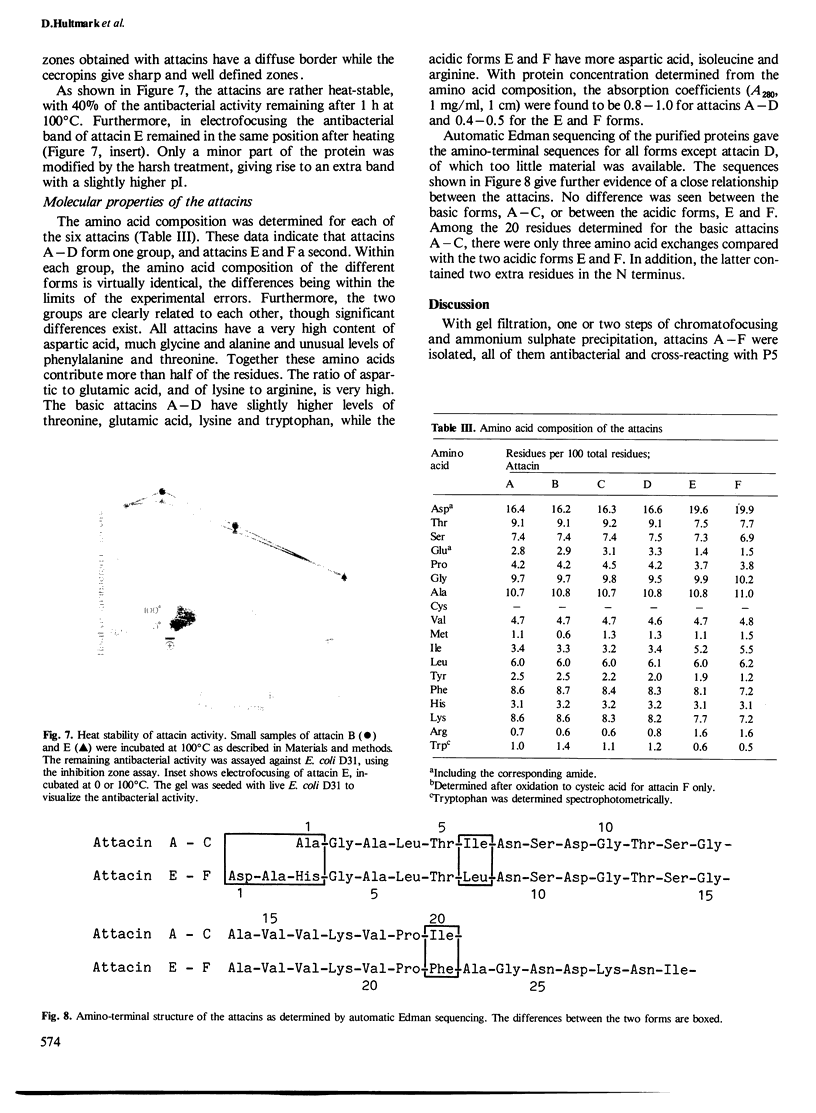
Six closely related antibacterial proteins, attacins A-F, were isolated from the hemolymph of immunized pupae of the Cecropia moth, Hyalophora cecropia. Chromatofocusing separated attacins A-F, with isoelectric points between 5.7 and 8.3. Immunological experiments show that the attacins constitute antibacterially active forms of the previously isolated inducible immune protein P5. Their mol. wts., 20-23 K, are similar to that of protein P5, but significantly lower than 28 K found for preP5 synthesized in vitro (see accompanying paper). The six attacins can be divided into two groups according to their amino acid composition and amino-terminal sequences, attacins A-D constitute a basic group and attacins E and F an acidic one. Within each group the forms are very similar. The attacins efficiently killed Escherichia coli and two other Gram-negative bacteria isolated from the gut of a silk worm but they did not act on other Gram-positive and Gram-negative bacteria tested. Only growing cells of E. coli were attacked; cells suspended in phosphate buffer were inert. Besides the cecropins and lysozyme, the attacins represent a third class of antibacterial proteins in the humoral immune system of H. cecropia.
Full text
PDF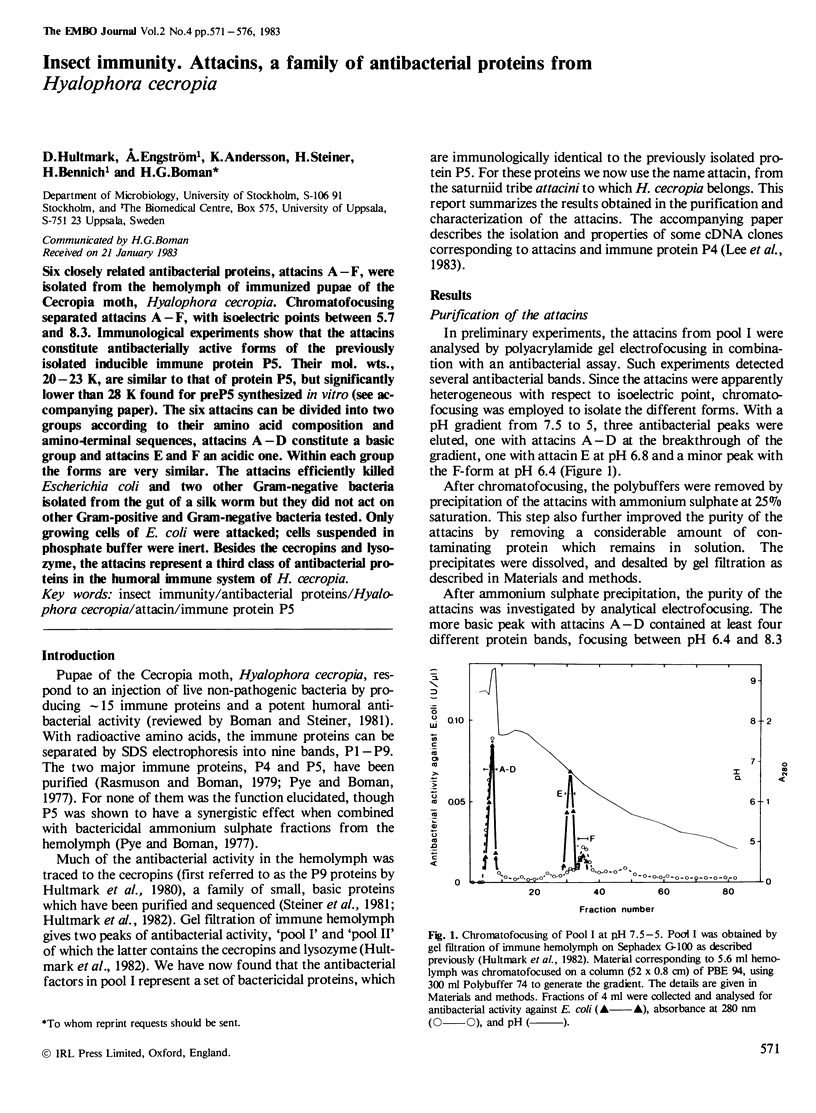


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G., Jonsson S., Monner D., Normark S., Bloom G. D. Cell-surface alterations in Escherichia coli K-12 with chromosmal mutations changing ampicillin resistance. Ann N Y Acad Sci. 1971 Jun 11;182:342–357. doi: 10.1111/j.1749-6632.1971.tb30670.x. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Steiner H. Humoral immunity in Cecropia pupae. Curr Top Microbiol Immunol. 1981;94-95:75–91. doi: 10.1007/978-3-642-68120-2_2. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Engström A., Bennich H., Kapur R., Boman H. G. Insect immunity: isolation and structure of cecropin D and four minor antibacterial components from Cecropia pupae. Eur J Biochem. 1982 Sep;127(1):207–217. doi: 10.1111/j.1432-1033.1982.tb06857.x. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Steiner H., Rasmuson T., Boman H. G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem. 1980 May;106(1):7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Edlund T., Ny T., Faye I., Boman H. G. Insect immunity. Isolation of cDNA clones corresponding to attacins and immune protein P4 from Hyalophora cecropia. EMBO J. 1983;2(4):577–581. doi: 10.1002/j.1460-2075.1983.tb01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meienhofer J., Maeda H., Glaser C. B., Czombos J., Kuromizu K. Primary structure of neocarzinostatin, an antitumor protein. Science. 1972 Nov 24;178(4063):875–876. doi: 10.1126/science.178.4063.875. [DOI] [PubMed] [Google Scholar]
- Napier M. A., Holmquist B., Strydom D. J., Goldberg I. H. Neocarzinostatin chromophore: purification of the major active form and characterization of its spectral and biological properties. Biochemistry. 1981 Sep 15;20(19):5602–5608. doi: 10.1021/bi00522a038. [DOI] [PubMed] [Google Scholar]
- Pye A. E., Boman H. G. Insect immunity. III. Purification and partial characterization of immune protein P5 from hemolymph of Hyalophora cecropia pupae. Infect Immun. 1977 Aug;17(2):408–414. doi: 10.1128/iai.17.2.408-414.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H., Hultmark D., Engström A., Bennich H., Boman H. G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981 Jul 16;292(5820):246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]