Abstract
AIM
To evaluate the incidence, etiology, and predictors of mortality of severe hypoxic hepatitis.
METHODS
We used computerized patient records to identify consecutive cases of severe hypoxic hepatitis admitted to a tertiary hospital in Singapore over a one-year period. We defined severe hypoxic hepatitis as elevation of serum transaminases more than 100 times upper limit of normal in the clinical setting of cardiac, circulatory or respiratory failure after exclusion of other causes of hepatitis. We used multivariable regression analysis to determine predictors for mortality.
RESULTS
We identified 75 cases of severe hypoxic hepatitis out of 71380 hospital admissions over one year, providing an incidence of 1.05 cases per 1000 admissions. Median age was 65 years (range 19-88); 57.3% males. The most common etiologies of severe hypoxic hepatitis were acute myocardial infarction and sepsis. Fifty-three patients (71%) died during the hospitalization. The sole independent predictive factor for mortality was serum albumin measured at the onset of severe hypoxic hepatitis. Patients with low serum albumin of less than 28 g/L have more than five-fold increase risk of death (OR = 5.39, 95%CI: 1.85-15.71).
CONCLUSION
Severe hypoxic hepatitis is uncommon but has a high mortality rate. Patients with low serum albumin are at highest risk of death.
Keywords: Severe, Mortality, Albumin, Incidence, Hypoxic hepatitis, Predictors, Etiology, Prognosis
Core tip: Hypoxic hepatitis is an important cause of liver injury that is associated with a high mortality rate. We sought to evaluate the incidence, etiology and predictors of mortality of severe hypoxic hepatitis in a large tertiary-level hospital in Singapore. Our findings confirm that the prevalence and mortality rate of severe hypoxic hepatitis in Asians is consistent with previous studies. Importantly, the unique finding from our study is that low serum albumin level is an independent predictive factor for mortality in severe hypoxic hepatitis, with a five-fold increase in risk of death in patients with serum albumin less than 28 g/L.
INTRODUCTION
Hypoxic hepatitis - inflammation and necrosis of the liver due to hypoxia - can be a devastating disease. It is characterized by a substantial but transient increase in serum transaminase levels in the setting of cardiac, circulatory or respiratory failure, after exclusion of viral hepatitis and drug-induced liver injury[1]. Although initially referred to as “ischemic hepatitis”, the term hypoxic hepatitis is now preferred as it is recognized that ischemia is not the sole contributing factor[2-4]. The typical presentation of hypoxic hepatitis is a sudden massive increase in serum transaminases, typically above ten times the upper limit of normal, due to massive hepatocyte necrosis. Characteristically there is predominant elevation of aspartate transaminase (AST) over alanine transaminase (ALT) followed by their rapid decline.
Severe hypoxic hepatitis can occur when there is massive elevation of serum transaminases, more than 100 times the upper limit of normal. The incidence, causes and predictive factors of mortality in patients with severe hypoxic hepatitis have not been well described. In a previous study examining the clinical outcomes of patients with extreme elevations of serum transaminases (ALT and/or AST more than 3000 U/L), we observed a high rate of mortality in patients with low serum albumin and advanced age[5]. In this present study, we sought to determine the incidence and predictors of mortality in patients with severe hypoxic hepatitis.
MATERIALS AND METHODS
Identification of patients with severe hypoxic hepatitis
Consecutive cases of severe hypoxic hepatitis were identified from the computerized database of patient admissions to a large tertiary care hospital in Singapore over a one-year period. Cases of severe hypoxic hepatitis were defined by the presence of the following three factors: Massive elevation of serum transaminases (either ALT and/or AST values greater than 3000 U/L), with rapid decline over 5 d, a typical clinical setting of cardiac, circulatory or respiratory failure, and the exclusion of all other causes of liver necrosis, particularly viral or drug-related hepatitis[1].
The ethics committee of the hospital approved waiver of informed consent for this retrospective study, which was conducted in accordance with the Declaration of Helsinki. Case records of patients fulfilling the above criteria were retrieved and two independent reviewers (Chang PE and Goh BBG) systematically extracted the relevant patient demographic data, clinical details and hemodynamic data. Relevant laboratory data was analyzed, including liver function, renal function and cardiac function tests. Specifically, the date of elevation of serum transaminases to > 3000 U/L and the trend of resolution were analyzed. Baseline laboratory data was defined as the laboratory values performed on the day of onset of elevation of serum transaminases to > 3000 U/L.
Analysis of precipitating factors for severe hypoxic hepatitis
Patient records were analyzed in detail to identify precipitating factors for hypoxic hepatitis. In particular, episodes of hypotension, arrhythmia, bradycardia, hypoxia and acidosis in the 48 h prior to the development of severe hypoxic hepatitis were analyzed. A hypotensive episode was defined as documented systolic blood pressure < 90 mmHg on at least two separate readings. Arrhythmia was defined as an abnormally rapid heart rate > 120 beats per minute, accompanied by electrocardiogram evidence of an abnormal heart rhythm. Bradycardia was defined as documented heart rate less than 60 beats per minute or a lowering of the heart rate by more than 25% of the baseline heart rate. Hypoxic episodes were defined as arterial oxygen saturation < 90% on pulse oximetry and/or partial pressure of oxygen < 60 mmHg on arterial blood gas. Metabolic acidosis was defined as a pH < 7.4 on arterial blood gas associated with serum bicarbonate of < 20 mmol/L. The inpatient medication records and clinical drug history were carefully analyzed to identify any potential hepatotoxic medications taken prior to the onset of severe hypoxic hepatitis.
Clinical course of severe hypoxic hepatitis
The clinical evolution of consecutive patients with severe hypoxic hepatitis was recorded for each subject. This included admissions to the intensive care unit (ICU), length of stay in ICU and survival to discharge from hospital. For patients who died, the cause of death was based on the diagnosis stated on the death certificate. In cases where post-mortem examination was performed, the cause of death was based on the final coroner’s report. The etiology of hypoxic hepatitis was based on thorough review of clinical data, laboratory results, clinical evolution and autopsy details.
Study outcome
The data was analyzed to identify potential predictive factors for mortality, defined as death within the same admission as the episode of severe hypoxic hepatitis. Patients who survived to discharge were followed up for a further six months to determine the incidence of delayed mortality.
Statistical analysis
Clinical variables were compared between patients who died and those who survived to discharge. χ2 analysis was performed for comparisons of discrete variables and Students t-test was used for comparison of continuous variables. Multivariable regression analysis was performed to identify independent predictors of early mortality. Survival comparisons were performed using Kaplan Meier analysis and compared using log rank statistics. All statistical analyses were performed using SPPS version 21 (Chicago, IL, United States). A P value of < 0.05 was considered statistically significant. Values in the text are described as mean ± SD or number (percentage of total) unless specified otherwise.
RESULTS
Incidence of severe hypoxic hepatitis
Of a total of 71380 admissions to the Singapore General Hospital over the course of one year, 75 patients fulfilled the predefined criteria for severe hypoxic hepatitis, providing an incidence of 1.05 cases of severe hypoxic hepatitis per 1000 admissions.
Clinical characteristics of severe hypoxic hepatitis
The clinical characteristics of the 75 patients are summarized in Table 1. Median age was 65 years, of which 57% of were male. All patients were Asians, with a predominance of Chinese followed by Indians and Malays respectively, in keeping with the multi-ethnic nature of the Singapore population. A pre-existing history of ischemic heart disease and cardiac failure was present in 55% and 36% respectively. A precipitating hypotensive event in the 48 h preceding the rise in liver enzymes was documented in 73% of cases. Precipitating episodes of bradycardia and metabolic acidosis were identified in 14.7% and 57.3% respectively. As expected, AST levels were higher than ALT levels at the onset of hypoxic liver injury with mean ALT and AST levels of 2295 ± 1656 U/L and 4896 ± 2986 U/L respectively. The mean ratio of ALT to AST at onset (ALT/AST ratio_D1) was 0.70 ± 1.36. Peak ALT and AST levels reached 2834 ± 1938 U/L and 5894 ± 3149 U/L respectively, typically within the first 3 d. Normalization of serum transaminases occurred in 82% of the 22 patients who survived. Mean number of days to normalization of ALT was 43 ± 46 d and 29 ± 23 d for AST.
Table 1.
Clinical characteristics of patients with severe hypoxic hepatitis n (%)
| n = 75 | |
| Demographics | |
| Age | 61.9 ± 16.6 |
| Male gender | 43 (57.3) |
| Race (Chinese/Malay/Indian) | 51/20/4 (68/26.7/5.3) |
| Pre-existing co-morbidities | |
| Pre-existing ischemic heart disease | 41 (54.7) |
| Pre-existing cardiac failure | 27 (36.0) |
| Pre-existing renal failure | 27 (36.0) |
| Pre-existing chronic viral hepatitis | 5 (6.7) |
| Precipitating conditions | |
| Documented hypotension | 55 (73.3) |
| Documented bradycardia | 11 (14.7) |
| Documented metabolic acidosis | 43 (57.3) |
| Baseline laboratory parameters1 | |
| Albumin (g/L) | 25.3 ± 6.9 |
| Bilirubin (μmol/L) | 76.1 ± 82.6 |
| GGT (U/L) | 126 ± 90 |
| Creatinine (mmol/L) | 321 ± 211 |
| Prothrombin time (seconds) | 27.8 ± 12.2 |
| ALT (U/L) | 2295 ± 1656 |
| AST (U/L) | 4896 ± 2986 |
| ALT/AST ratio_D12 | 0.70 ± 1.36 |
| Peak ALT (U/L) | 2834 ± 1938 |
| Peak AST (U/L) | 5894 ± 3148 |
| Clinical outcome | |
| Admitted to ICU | 50 (66.7) |
| Died within same admission | 53 (70.7) |
Measured at onset of severe hypoxic hepatitis;
Ratio of the ALT to AST at the first documentation of either ALT or AST > 3000U/L. AST: Aspartate transaminase; ALT: Alanine transaminase; GGT: Gamma glutamyl transferase; ICU: Intensive care unit.
Fifty patients (66.7%) with severe hypoxic hepatitis required admission to the ICU. All patients required vasopressor support. Of these, 37 (74.0%) died. Mean duration of stay in ICU was significantly longer in those who died compared to those who survived (9.4 ± 12.1 d vs 3.2 ± 2.0 d, P = 0.02). All 13 patients who were discharged from ICU recovered and were safely discharged from hospital.
Etiology of severe hypoxic hepatitis
The underlying etiology of severe hypoxic hepatitis was due to acute myocardial infarction (AMI) in 36% and septicemic shock in 32% (Figure 1). Congestive cardiac failure, chronic respiratory failure and gastrointestinal hemorrhage accounted for the remaining cases of severe hypoxic hepatitis.
Figure 1.
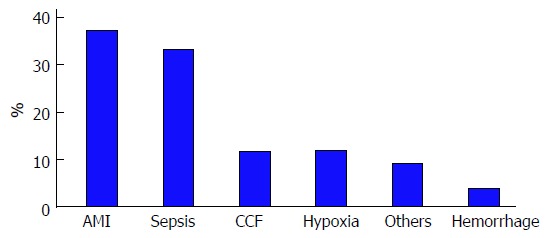
Etiology of severe hypoxic hepatitis. AMI: Acute myocardial infarction; CCF: Congestive cardiac failure.
Mortality in severe hypoxic hepatitis
Severe hypoxic hepatitis was associated with a high mortality rate, accounting for 53 (71%) deaths within the same admission. The main causes of death were AMI in 39.6%, sepsis in 30.2%, metastatic cancer in 13.2% and gastrointestinal hemorrhage in 5.7%. Amongst the survivors who were discharged, there were no cases of delayed mortality in the 6-mo follow-up period. The ability to recover from the acute hypoxic injury was thus associated with an excellent prognosis. The clinical characteristics of patients who survived and died within the same admission are compared in Table 2.
Table 2.
Comparison between patients with severe hypoxic hepatitis who died and survived n (%)
| Died (n = 53) | Survived (n = 22) | P value | |
| Demographics | |||
| Age | 64.3 ± 17.6 | 56.3 ± 12.8 | 0.059 |
| Male gender | 35 (66.0) | 8 (36.4) | 0.018 |
| Race (Chinese/Malay/Indian) | 36/13/4 | 15/7/0 | 0.373 |
| Pre-existing co-morbidities | |||
| Pre-existing ischemic heart disease | 27 (50.9) | 12 (54.5) | 0.793 |
| Pre-existing cardiac failure | 16 (30.2) | 11 (50.0) | 0.104 |
| Pre-existing renal failure | 19 (35.8) | 8 (36.4) | 0.966 |
| Precipitating conditions | |||
| Documented hypotension | 45 (84.9) | 14 (63.6) | 0.041 |
| Documented bradycardia | 9 (17.0) | 2 (9.1) | 0.379 |
| Documented metabolic acidosis | 33 (62.3) | 10 (45.5) | 0.180 |
| Baseline laboratory parameters1 | |||
| Albumin (g/L) | 23.7 ± 6.8 | 29.1 ± 5.8 | 0.001 |
| Bilirubin (μmol/L) | 79.2 ± 84.4 | 68.8 ± 79.7 | 0.625 |
| ALT (U/L) | 2051 ± 1601 | 2880 ± 1674 | 0.048 |
| AST (U/L) | 5093 ± 2943 | 4440 ± 3103 | 0.395 |
| ALT/AST ratio_D12 | 0.50 ± 0.80 | 1.18 ± 2.15 | 0.051 |
| GGT (U/L) | 111 ± 67 | 158 ± 123 | 0.161 |
| Creatine kinase | 2949 ± 6180 | 395 ± 419 | 0.006 |
| Creatine kinase MB | 37.1 ± 56.6 | 13.5 ± 20.7 | 0.015 |
| Troponin T (Trop T) | 2.02 ± 4.21 | 0.49 ± 1.42 | 0.031 |
| Lactate dehydrogenase | 2503 ± 2552 | 4035 ± 3671 | 0.229 |
| Creatinine (mmol/L) | 297.3 ± 172.9 | 376.6 ± 276.3 | 0.143 |
| Prothombin time (seconds) | 27.8 ± 12.5 | 27.7 ± 11.9 | 0.980 |
| Peak ALT (U/L) | 2572 ± 2026 | 3464 ± 1575 | 0.069 |
| Peak AST (U/L) | 6223 ± 3138 | 5131 ± 3110 | 0.176 |
Measured at onset of severe hypoxic hepatitis;
Ratio of the ALT to AST at the first documentation of either ALT or AST > 3000 U/L. AST: Aspartate transaminase; ALT: Alanine transaminase; GGT: Gamma glutamyl transferase.
On univariate analysis, four variables were found to be significantly different between cases of severe hypoxic hepatitis who survived and those who died within the same admission (Table 3). Mortality was more common in males and in those with a precipitating hypotensive event. Interestingly, baseline serum albumin level and ALT (but not AST) levels measured at onset of severe hypoxic hepatitis were significantly lower in patients who died. However, the peak ALT and AST levels did not have any discerning effect on mortality. Markers of cardiac infarction (CK, CKMB and troponin T) were significantly elevated in those who died whereas bilirubin and prothrombin time were not different in the two groups, suggesting that the underlying cause of death in severe hypoxic hepatitis is related to underlying cardiac ischemia and not to liver failure.
Table 3.
Univariate and multivariable analysis of predictive factors for mortality in severe hypoxic hepatitis
| Variable |
Univariate analysis |
Multivariable analysis |
||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age | 1.03 (0.99-1.06) | 0.069 | 1.07 (0.99-1.14) | 0.056 |
| Male gender | 0.29 (0.10-0.83) | 0.021 | 0.17 (0.03-1.19) | 0.074 |
| Precipitating hypotensive episode | 3.21 (1.02-10.14) | 0.046 | 4.40 (0.47-40.9) | 0.192 |
| Albumin | 0.88 (0.80-0.96) | 0.004 | 0.83 (0.71-0.96) | 0.015 |
| ALT | 1.00 (0.99-1.00) | 0.054 | 1.00 (1.00-1.00) | 0.609 |
| Creatine kinase | 1.00 (1.00-1.00) | 0.032 | 1.00 (1.00-1.00) | 0.068 |
| Creatine kinase MB | 1.02 (0.99-1.05) | 0.122 | 0.98 (0.94-1.02) | 0.330 |
| Troponin T | 1.39 (0.88-2.16) | 0.178 | 1.29 (0.74-2.27) | 0.374 |
| ALT/AST ratio_D11 | 0.65 (0.35-1.19) | 0.160 | 0.22 (0.04-13.1) | 0.465 |
Ratio of the ALT to AST at the first documentation of either ALT or AST > 3000 U/L. ALT: Alanine transaminase; AST: Aspartate transaminase.
On multivariable analysis (Table 3), the sole independent predictor of early inpatient mortality in severe hypoxic hepatitis was baseline serum albumin. Using area under receiving operator curve (AUROC) statistics (Figure 2), serum albumin was determined to be an accurate predictor of mortality in severe hypoxic hepatitis with an AUROC of 0.740 (P = 0.001, 95%CI: 0.617-0.862). Baseline serum albumin < 28 g/L was an accurate predictor of mortality with a sensitivity of 75%, specificity of 64% and positive predictive value of 83%. Using logistic regression analysis, a baseline serum albumin lower than 28 g/L was associated with a five-fold increased risk of early mortality (OR = 5.39, 95%CI: 1.85-15.71). Median survival was 85% lower in severe hypoxic hepatitis patients with baseline serum albumin less than 28 g/L compared to those with baseline albumin greater than 28 g/L (3.0 d vs 21.0 d, P = 0.015 by log-rank comparison, Figure 3).
Figure 2.
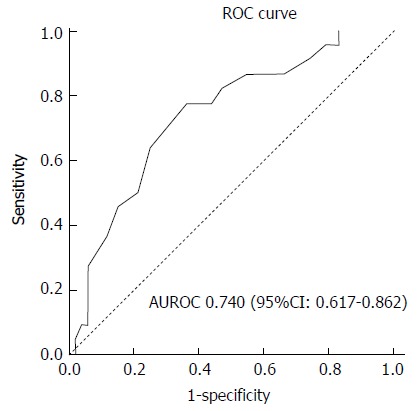
Receiver operating curve of baseline serum albumin to predict mortality in severe hypoxic hepatitis. AUROC: Area under receiving operator curve; ROC: Receiver operating curve.
Figure 3.
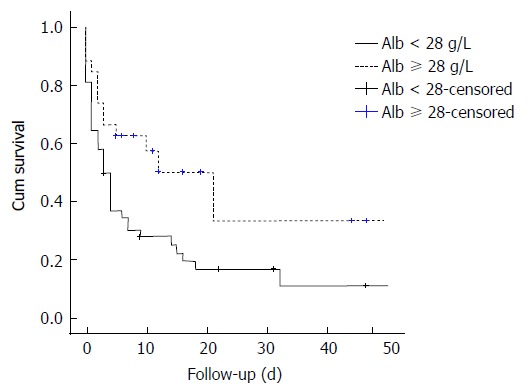
Kaplan-Meier comparison of survival between severe hypoxic hepatitis patients with baseline serum album < 28 g/L and ≥ 28 g/L. Alb: Albumin.
DISCUSSION
To our knowledge, this is the first study to describe the clinical course and outcome of Asian patients with severe hypoxic hepatitis. Several key findings are noted - firstly, severe hypoxic hepatitis occurs with an incidence of 1.05 in every 1000 admissions to a tertiary level general hospital in Singapore. The clinical presentation of severe hypoxic hepatitis in Asian patients is not different from that reported in Western studies[6-8]. Secondly, the clinical outcome of severe hypoxic hepatitis is poor with a high mortality rate of 71%. Thirdly, most deaths associated with severe hypoxic hepatitis are due to acute myocardial infarction and sepsis and not due to liver failure. Finally and most importantly, the unique finding in our study is that low serum albumin at baseline is an independent predictor of early mortality in patients with severe hypoxic hepatitis.
There is a wide variation in the reported incidence of hypoxic hepatitis ranging from 1 per 1000 admissions to 4 per 100 admissions[6,7]. The reason for this wide variation is the denominator, i.e., the population of patients studied. The incidence of hypoxic hepatitis is expectedly lower in studies including all general admissions compared to those focusing on admissions to intensive care units[8-10]. The denominator in our study was all admissions to a tertiary care hospital over a one-year period. The incidence of 1.05 cases of severe hypoxic hepatitis per 1000 admissions in our Asian center is consistent with the literature from studies performed in Western populations[11-14].
Our study demonstrates that the clinical profile of Asian patients with severe hypoxic hepatitis is similar to that reported in Western studies. A history of ischemic heart disease and cardiac failure are common and was present in 55% and 36% of our study cohort respectively. Pre-existing passive hepatic congestion has been proposed as a pre-requisite condition for the development of hypoxic hepatitis[15,16]. Patients with chronically elevated right heart pressures are prone to developing congestive hepatopathy resulting in decreased hepatic perfusion to hepatocytes. In such patients, a slight decrease in the hepatic arterial perfusion pressure may be sufficient to cause hypoxic hepatitis. Indeed, Seeto et al[17] have demonstrated that as little as 15 min of transient systemic hypotension is sufficient to produce massive hepatocyte necrosis in patients with pre-existing congestive hepatopathy. However, as demonstrated in our study, a precipitating episode of hypotension is not necessarily seen in all patients with hypoxic hepatitis[16].
Severe hypoxic hepatitis is associated with a high mortality rate[18]. More than two-thirds of patients in our study cohort died, which is at the higher end of the range of 30%-77% reported in similar studies[6,9,10,18]. The underlying etiology of severe hypoxic hepatitis is strongly associated with risk of early mortality. Ninety-three percent of patients with severe hypoxic hepatitis due to AMI died compared to only 58% of non-AMI-related etiologies. However, neither the severity of elevation of liver transaminases nor the rate of resolution of elevated transaminases was associated with mortality. The cause of death has not been reported in meta-analysis of studies on hypoxic hepatitis[7]. In our study, the main cause of death was due to cardiovascular failure in 40%, followed by septic shock with multi-organ failure in 30%. In both conditions, the underlying pathophysiological process is inadequate perfusion pressure to the essential organs. Importantly, liver failure is not the cause of death in patients with severe hypoxic hepatitis. This reinforces the point that the primary strategy in the management of patients with severe hypoxic hepatitis is to focus on maintaining systemic perfusion pressure and to correct the underlying cause of hypoperfusion be it due to primary cardiac pump failure, massive peripheral vasodilatation due to sepsis or hypovolemia from hemorrhage[6].
The novel aspect of this study is the finding that baseline serum albumin is an independent predictor of early mortality in patients with severe hypoxic hepatitis. Duration of hypoxic hepatitis, INR, presence of septic shock, SOFA score, jaundice, need for renal replacement and vasopressor therapy have previously been suggested to be factors that predict mortality in hypoxic hepatitis[9,18-21]. However, serum albumin has never been previously reported to be an independent predictor for mortality in hypoxic hepatitis.
Hypoalbuminemia is a well-known predictor of mortality in patients with acute illness[22,23]. Serum albumin on admission has been shown to be a strong predictor of inpatient mortality in internal medicine wards[24,25]. Furthermore, a progressive decrement of serum albumin concentration is associated with a 24%-56% increase in odds of death[26]. Decline in albumin levels occurs in acute illnesses due to increased catabolism, reduced hepatic synthesis and renal loss. Albumin is often viewed as a non-specific negative acute phase protein that reflects the severity of the underlying illness. It is thus conceivable that low serum albumin may be associated with increased mortality in patients with hypoxic hepatitis, as in any other systemic illness. However, our study provides evidence that albumin is an independent predictor for mortality in severe hypoxic hepatitis, with a strong odds ratio. This suggests that low serum albumin levels may play a direct role in causing cardiovascular-related mortality in patients with severe hypoxic hepatitis.
Low serum albumin levels are associated with increased risk of mortality in coronary disease[27] and cardiac failure[28,29]. This is attributed to slower coronary flow in patients with low albumin levels[30]. In a study investigating the relationship between serum albumin and coronary flow rate following primary percutaneous coronary intervention[31], baseline serum albumin levels were significantly lower in the group with no coronary reflow compared to normal coronary reflow and serum albumin was identified as an independent predictor of no-reflow. Low levels of serum albumin cause an increase in coronary blood viscosity and disruption of endothelial function due to increased concentrations of free lysophosphatidylcholine[32]. In addition, albumin is an important inhibitor of platelet aggregation through increases in production of antiaggregatory prostaglandin PGD2 from cyclic endoperoxidases[33]. Low levels of serum albumin thus increase risk of platelet aggregation in the coronary vasculature, hence increasing risk of AMI. The novel association observed between low serum albumin levels and increased mortality risk in severe hypoxic hepatitis can thus be potentially explained by the effects of low albumin on slowing coronary flow and increased platelet aggregation, thus increasing risk of acute myocardial infarction and early death. However, this postulation remains speculative and needs to be evaluated in future prospective studies.
Low serum albumin levels are also associated with increased mortality in patients with severe sepsis[34]. Serum albumin plays an important role in modulating innate immune responses to systemic inflammation and sepsis[35]. The ability of serum albumin to modulate inflammation and oxidative stress as well as inhibit neutrophil adhesion could provide some protection from endothelial dysfunction mediated by these factors[36,37]. Although several studies have demonstrated efficacy of albumin infusions in improving outcome in septic patients[38], others have not demonstrated any meaningful benefit[39,40]. It thus remains unclear whether low serum albumin is a direct cause of mortality or a surrogate for severity of illness in patients with sepsis.
Our findings are clinically important because it provides the clinician with a simple way to identify patients with severe hypoxic hepatitis who are at highest risk of death. Patients with severe hypoxic hepatitis with a baseline serum albumin level less than 28 g/L can be fast-tracked for ICU care and early vasopressor therapy to maintain adequate central perfusion. Of interest is whether timely intervention with intravenous albumin will result in a reduction of mortality in patients with severe hypoxic hepatitis.
There are several limitations to this study, including the retrospective study design. The study was limited to a single center, which may limit the generalizability of the findings. This study focused on patients with severe elevation of serum transaminases beyond 100 times the upper limit of normal. The findings of our study are thus limited to patients with severe hypoxic hepatitis and may not be applicable to milder degrees of hypoxic hepatitis.
In conclusion, severe hypoxic hepatitis is associated with high mortality. Most deaths are related to underlying cardiovascular failure and septic shock with multi-organ failure. Low serum albumin levels at the onset of severe hypoxic hepatitis is an independent predictor of mortality and is a useful clinical marker for early prognostication of patients at high risk of death.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr. Roy Soetikno and Foo Yang Yann for their assistance in reviewing and editing the manuscript.
COMMENTS
Background
Hypoxic hepatitis is an important cause of acute liver injury that is associated with a high mortality rate.
Research frontiers
The novel aspect of this study is the finding that baseline serum albumin is an independent predictor of early mortality in patients with severe hypoxic hepatitis.
Innovations and breakthroughs
This is the first study to describe the clinical course and outcome of Asian patients with severe hypoxic hepatitis. The unique finding in this study is that low serum albumin at baseline is an independent predictor of early mortality in patients with severe hypoxic hepatitis.
Applications
These findings are important because it provides the clinician with a simple way to identify patients with severe hypoxic hepatitis who are at the highest risk of death.
Peer-review
It was a nice retrospective study and very interesting to read. What I especially liked that you did not forget to state that mortality actually depends on the underlying disease and not the condition of the liver itself.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was approved by the ethics review board of Singapore General Hospital.
Informed consent statement: The ethics review board approved waiver of consent as the clinical data was anonymized prior to analysis and only aggregate data were reported.
Conflict-of-interest statement: None of the authors have any conflict of interests related to this study.
Data sharing statement: No additional data are available.
Peer-review started: January 20, 2017
First decision: March 13, 2017
Article in press: June 13, 2017
P- Reviewer: Vaitiekiene A S- Editor: Gong ZM L- Editor: A E- Editor: Li D
Contributor Information
Pik-Eu Chang, Department of Gastroenterology and Hepatology, Singapore General Hospital, Singapore 169856, Singapore; Duke-NUS Medical School, Singapore 169857, Singapore.
Boon-Bee George Goh, Department of Gastroenterology and Hepatology, Singapore General Hospital, Singapore 169856, Singapore; Duke-NUS Medical School, Singapore 169857, Singapore.
Victoria Ekstrom, Department of Gastroenterology and Hepatology, Singapore General Hospital, Singapore 169856, Singapore.
Ming-Liang Ong, Department of Gastroenterology and Hepatology, Singapore General Hospital, Singapore 169856, Singapore.
Chee-Kiat Tan, Department of Gastroenterology and Hepatology, Singapore General Hospital, Singapore 169856, Singapore; Duke-NUS Medical School, Singapore 169857, Singapore. tan.chee.kiat@singhealth.com.sg.
References
- 1.Henrion J, Schapira M, Luwaert R, Colin L, Delannoy A, Heller FR. Hypoxic hepatitis: clinical and hemodynamic study in 142 consecutive cases. Medicine (Baltimore) 2003;82:392–406. doi: 10.1097/01.md.0000101573.54295.bd. [DOI] [PubMed] [Google Scholar]
- 2.Bynum TE, Boitnott JK, Maddrey WC. Ischemic hepatitis. Dig Dis Sci. 1979;24:129–135. doi: 10.1007/BF01324740. [DOI] [PubMed] [Google Scholar]
- 3.Birgens HS, Henriksen J, Matzen P, Poulsen H. The shock liver. Clinical and biochemical findings in patients with centrilobular liver necrosis following cardiogenic shock. Acta Med Scand. 1978;204:417–421. [PubMed] [Google Scholar]
- 4.Ebert EC. Hypoxic liver injury. Mayo Clin Proc. 2006;81:1232–1236. doi: 10.4065/81.9.1232. [DOI] [PubMed] [Google Scholar]
- 5.Chang PE, Goh GB, Tan CK. Low serum albumin and advanced age predict early mortality in Asian patients with extreme elevations of serum aminotransferase. J Dig Dis. 2016;17:193–201. doi: 10.1111/1751-2980.12323. [DOI] [PubMed] [Google Scholar]
- 6.Henrion J. Hypoxic hepatitis. Liver Int. 2012;32:1039–1052. doi: 10.1111/j.1478-3231.2011.02655.x. [DOI] [PubMed] [Google Scholar]
- 7.Tapper EB, Sengupta N, Bonder A. The Incidence and Outcomes of Ischemic Hepatitis: A Systematic Review with Meta-analysis. Am J Med. 2015;128:1314–1321. doi: 10.1016/j.amjmed.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 8.Fuhrmann V, Kneidinger N, Herkner H, Heinz G, Nikfardjam M, Bojic A, Schellongowski P, Angermayr B, Schöniger-Hekele M, Madl C, et al. Impact of hypoxic hepatitis on mortality in the intensive care unit. Intensive Care Med. 2011;37:1302–1310. doi: 10.1007/s00134-011-2248-7. [DOI] [PubMed] [Google Scholar]
- 9.Raurich JM, Llompart-Pou JA, Ferreruela M, Colomar A, Molina M, Royo C, Ayestarán I, Ibáñez J. Hypoxic hepatitis in critically ill patients: incidence, etiology and risk factors for mortality. J Anesth. 2011;25:50–56. doi: 10.1007/s00540-010-1058-3. [DOI] [PubMed] [Google Scholar]
- 10.Taylor RM, Tujios S, Jinjuvadia K, Davern T, Shaikh OS, Han S, Chung RT, Lee WM, Fontana RJ. Short and long-term outcomes in patients with acute liver failure due to ischemic hepatitis. Dig Dis Sci. 2012;57:777–785. doi: 10.1007/s10620-011-1918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson RD, O’Connor ML, Kerr RM. Extreme serum elevations of aspartate aminotransferase. Am J Gastroenterol. 1995;90:1244–1245. [PubMed] [Google Scholar]
- 12.Whitehead MW, Hawkes ND, Hainsworth I, Kingham JG. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999;45:129–133. doi: 10.1136/gut.45.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruguera M, Barrera JM, Corradi F, Mas A. [Hypertransaminasemia greater than 400 U/l in adults seen at a tertiary hospital. Prospective study of etiology] Gastroenterol Hepatol. 2005;28:15–19. doi: 10.1157/13070378. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs S, Bogomolski-Yahalom V, Paltiel O, Ackerman Z. Ischemic hepatitis: clinical and laboratory observations of 34 patients. J Clin Gastroenterol. 1998;26:183–186. doi: 10.1097/00004836-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Henrion J. Hypoxic hepatitis: the point of view of the clinician. Acta Gastroenterol Belg. 2007;70:214–216. [PubMed] [Google Scholar]
- 16.Birrer R, Takuda Y, Takara T. Hypoxic hepatopathy: pathophysiology and prognosis. Intern Med. 2007;46:1063–1070. doi: 10.2169/internalmedicine.46.0059. [DOI] [PubMed] [Google Scholar]
- 17.Seeto RK, Fenn B, Rockey DC. Ischemic hepatitis: clinical presentation and pathogenesis. Am J Med. 2000;109:109–113. doi: 10.1016/s0002-9343(00)00461-7. [DOI] [PubMed] [Google Scholar]
- 18.Hickman PE, Potter JM. Mortality associated with ischaemic hepatitis. Aust N Z J Med. 1990;20:32–34. doi: 10.1111/j.1445-5994.1990.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 19.Fuhrmann V, Kneidinger N, Herkner H, Heinz G, Nikfardjam M, Bojic A, Schellongowski P, Angermayr B, Kitzberger R, Warszawska J, et al. Hypoxic hepatitis: underlying conditions and risk factors for mortality in critically ill patients. Intensive Care Med. 2009;35:1397–1405. doi: 10.1007/s00134-009-1508-2. [DOI] [PubMed] [Google Scholar]
- 20.Jäger B, Drolz A, Michl B, Schellongowski P, Bojic A, Nikfardjam M, Zauner C, Heinz G, Trauner M, Fuhrmann V. Jaundice increases the rate of complications and one-year mortality in patients with hypoxic hepatitis. Hepatology. 2012;56:2297–2304. doi: 10.1002/hep.25896. [DOI] [PubMed] [Google Scholar]
- 21.Horvatits T, Trauner M, Fuhrmann V. Hypoxic liver injury and cholestasis in critically ill patients. Curr Opin Crit Care. 2013;19:128–132. doi: 10.1097/MCC.0b013e32835ec9e6. [DOI] [PubMed] [Google Scholar]
- 22.Herrmann FR, Safran C, Levkoff SE, Minaker KL. Serum albumin level on admission as a predictor of death, length of stay, and readmission. Arch Intern Med. 1992;152:125–130. [PubMed] [Google Scholar]
- 23.Viasus D, Garcia-Vidal C, Simonetti A, Manresa F, Dorca J, Gudiol F, Carratalà J. Prognostic value of serum albumin levels in hospitalized adults with community-acquired pneumonia. J Infect. 2013;66:415–423. doi: 10.1016/j.jinf.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Barchel D, Almoznino-Sarafian D, Shteinshnaider M, Tzur I, Cohen N, Gorelik O. Clinical characteristics and prognostic significance of serum albumin changes in an internal medicine ward. Eur J Intern Med. 2013;24:772–778. doi: 10.1016/j.ejim.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Numeroso F, Barilli AL, Delsignore R. Prevalence and significance of hypoalbuminemia in an internal medicine department. Eur J Intern Med. 2008;19:587–591. doi: 10.1016/j.ejim.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 26.Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol. 1997;50:693–703. doi: 10.1016/s0895-4356(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 27.Djoussé L, Rothman KJ, Cupples LA, Levy D, Ellison RC. Serum albumin and risk of myocardial infarction and all-cause mortality in the Framingham Offspring Study. Circulation. 2002;106:2919–2924. doi: 10.1161/01.cir.0000042673.07632.76. [DOI] [PubMed] [Google Scholar]
- 28.Horwich TB, Kalantar-Zadeh K, MacLellan RW, Fonarow GC. Albumin levels predict survival in patients with systolic heart failure. Am Heart J. 2008;155:883–889. doi: 10.1016/j.ahj.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 29.Liu M, Chan CP, Yan BP, Zhang Q, Lam YY, Li RJ, Sanderson JE, Coats AJ, Sun JP, Yip GW, et al. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2012;14:39–44. doi: 10.1093/eurjhf/hfr154. [DOI] [PubMed] [Google Scholar]
- 30.Cetin M, Zencir C, Tasolar H, Baysal E, Balli M, Akturk E. The association of serum albumin with coronary slow flow. Wien Klin Wochenschr. 2014;126:468–473. doi: 10.1007/s00508-014-0559-8. [DOI] [PubMed] [Google Scholar]
- 31.Kurtul A, Ocek AH, Murat SN, Yarlioglues M, Demircelik MB, Duran M, Ergun G, Cay S. Serum albumin levels on admission are associated with angiographic no-reflow after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Angiology. 2015;66:278–285. doi: 10.1177/0003319714526035. [DOI] [PubMed] [Google Scholar]
- 32.Joles JA, Willekes-Koolschijn N, Koomans HA. Hypoalbuminemia causes high blood viscosity by increasing red cell lysophosphatidylcholine. Kidney Int. 1997;52:761–770. doi: 10.1038/ki.1997.393. [DOI] [PubMed] [Google Scholar]
- 33.Mikhailidis DP, Ganotakis ES. Plasma albumin and platelet function: relevance to atherogenesis and thrombosis. Platelets. 1996;7:125–137. doi: 10.3109/09537109609023571. [DOI] [PubMed] [Google Scholar]
- 34.Artero A, Zaragoza R, Camarena JJ, Sancho S, González R, Nogueira JM. Prognostic factors of mortality in patients with community-acquired bloodstream infection with severe sepsis and septic shock. J Crit Care. 2010;25:276–281. doi: 10.1016/j.jcrc.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Kragh-Hansen U. Molecular and practical aspects of the enzymatic properties of human serum albumin and of albumin-ligand complexes. Biochim Biophys Acta. 2013;1830:5535–5544. doi: 10.1016/j.bbagen.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Taverna M, Marie AL, Mira JP, Guidet B. Specific antioxidant properties of human serum albumin. Ann Intensive Care. 2013;3:4. doi: 10.1186/2110-5820-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunduz A, Turedi S, Mentese A, Karahan SC, Hos G, Tatli O, Turan I, Ucar U, Russell RM, Topbas M. Ischemia-modified albumin in the diagnosis of acute mesenteric ischemia: a preliminary study. Am J Emerg Med. 2008;26:202–205. doi: 10.1016/j.ajem.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 38.Delaney AP, Dan A, McCaffrey J, Finfer S. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med. 2011;39:386–391. doi: 10.1097/CCM.0b013e3181ffe217. [DOI] [PubMed] [Google Scholar]
- 39.Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, Fanizza C, Caspani L, Faenza S, Grasselli G, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412–1421. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 40.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]


