Abstract
Objective
The Tumor Board (TB) allows for an interdisciplinary approach to cancer treatment designed to encourage evidence-based treatment. However, its role in facilitating clinical trial participation has not been reported. We aimed to determine whether a prospective TB is an effective strategy for trial recruitment and to identify steps within the TB process that facilitate discussion of trial eligibility and optimize accrual.
Methods
We conducted a retrospective cross-sectional analysis of women presented to Gynecologic Oncology TB between March and December 2008. Patient demographics, TB recommendations, and post-TB patient discussions were abstracted. These were compared to data derived from the Department of Oncology Research to determine research team awareness of eligible patients and confirm trial enrollment(s). Data analysis was completed with Chi-square test; risk ratios and confidence intervals were calculated as summary measures.
Results
We reviewed 1213 case presentations involving 916 women. Overall, 358 TB recommendations (30%) identified eligible patients, of which enrollment consisted of 87 (24%) trials (6% therapeutic trials and 18% non-therapeutic trials). Compared to other types of TB recommendations, those involving trials were discussed less frequently at post-TB patient visits (79% vs. 44%). Documentation of trial discussion at the post-TB visit was more likely to result in trial participation, versus solely relying on the research staff to communicate enrollment eligibility with the treating team (RR 2.5, p = 0.006).
Conclusions
Patients identified by the TB were 2.5-times as likely to enroll in a clinical trial, but trials were mentioned only 44% of the time. Interventions that facilitate trial discussions during post-TB meetings are needed to improve trial participation.
Keywords: Tumor board, Clinical trials, Gynecologic oncology, Ovarian cancer, Endometrial cancer, Cervical cancer
Introduction
The Tumor Board (TB) is a multidisciplinary conference that incorporates radiologists, pathologists, surgeons, radiation oncologists, and medical oncologists where individual patient cases are reviewed with the aim to provide evidence-based management recommendations. Currently the American College of Surgeons (ACOS) Commission on Cancer (CoC) requires multidisciplinary TB conferences at each of its approved hospital cancer programs and establishes standards to ensure quality control [1]. The CoC currently requires that 10% of an institution's annual caseload be discussed each year and at least 75% of the cases be presented prospectively to ensure an effective role in patient management. Hospital TB conferences have been an accepted part of cancer care for well over 50 years [2], with the benefits of a multidisciplinary discussion extending to patient care [3–5], changes in surgical management [6], staff education [7–9], collaboration among physicians [7], limitation of liability [8], and enrollment in protocols [8,10].
Unfortunately, with regards to the latter, only 2–4% of adult cancer patients in the United States participate in National Cancer Institute (NCI)-sponsored treatment clinical trials, a rate that has not improved in almost 2 decades [11,12]. With recommended target participation rates between 10% and 15% [13], low patient accrual to trials has prompted a wide spectrum of efforts, including legislation reform (e.g. enactment of the National Institutes of Health Revitalization Act in 1993 to ensure inclusion of women and minorities in clinical research) and increased NCI budget for research funding. Potential physician, patient, and system barriers to cancer clinical trial enrollment have previously been characterized [12,14–16]. Some factors specific to gynecological oncology include the relatively low incidence of some diseases (e.g. vulvar cancer) and strict clinical trial inclusion criteria for disease stage severity [17].
Few studies have investigated the impact of multidisciplinary TB conferences on patient accrual in cancer clinical trials, and even fewer have been conducted within the field of gynecologic oncology [5,10]. Therefore, we sought to address the impact of the TB on clinical trial accrual by examining the impact of the multidisciplinary Gynecologic Oncology TB on clinical research at our academic women's oncology program. The Program of Women's Oncology is the state's largest cancer center dedicated to women, receiving referrals from local clinics and private offices across southern New England. Off-site serviced areas include Fall River and South County Commons, both within Rhode Island as well as Cape Cod, Massachusetts and New London, Connecticut. The Gynecologic Oncology TB includes participation from all nine board-certified/eligible gynecologic oncologists and three medical oncologists working alongside two radiation oncology groups and pathology and radiology divisions at Women & Infants' Hospital specialized in women's health. Over 90% of all newly diagnosed gynecologic oncology patients are presented at the Tumor Board. It is a full member of the Gynecologic Oncology Group (GOG), the NCI funded cooperative group specializing in clinical research in gynecologic oncology.
We hypothesized that patients were appropriately receiving TB recommendations regarding trial availability, but were subsequently less likely to have a post-TB discussion with their physician regarding trial eligibility compared to other TB recommendations.
Materials and methods
Prior to the initiation of the study, all procedures were reviewed and approved by the Institutional Review Board. Data for this study were derived from patients who were presented at the Program of Women's Oncology weekly, prospective, and multidisciplinary TB at Women & Infants' Hospital, Providence, Rhode Island. If a woman was presented to TB more than once, (i.e., with a different/new diagnosis of cancer), we captured this data under separate study ID's where each presentation would be considered as an individual TB presentation, though still considered as referring to one patient.
Specific data collected included demographics and tumor-specific information (histologic diagnosis, stage, surgical outcome), number of TB presentation(s) (1st vs. 2nd vs. multiple), and specific recommendations made. TB recommendations were classified as surgery, chemotherapy, radiation therapy, endocrine treatment, clinical trial eligibility, referral to the Cancer Risk Assessment & Prevention Program for genetic counseling, referral to the Clinic for Sexuality, Intimacy and Fertility, no treatment and/or further workup. We also collected TB recommendations that included referral to specialty consultation, TB re-presentation, or were otherwise unspecified.
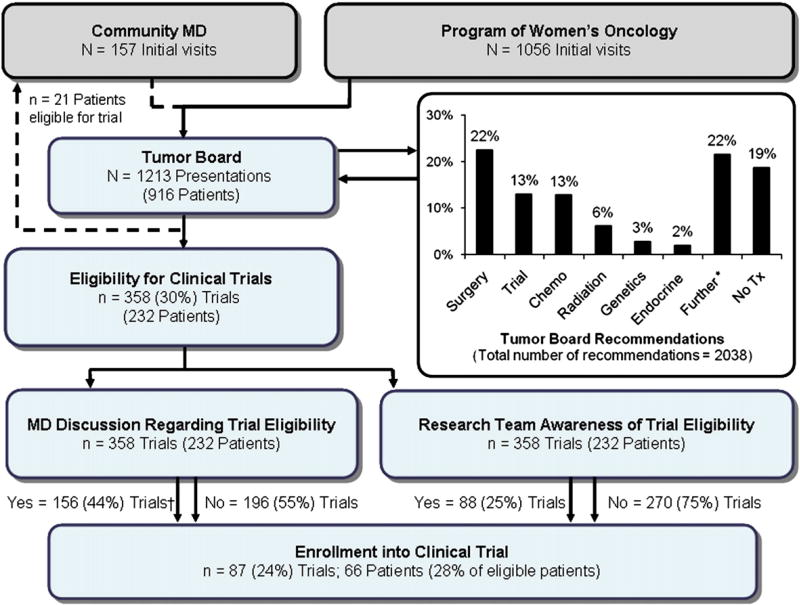
Patient charts and dictated notes were reviewed to assess whether TB recommendations were discussed with patients and if they were subsequently implemented. Data on patient enrollment into clinical trials were retrieved from physician notes and confirmed by cross-referencing each patient with the Department of Research enrollment list. Cases presented by community physicians and those not followed at the Program in Women's Oncology were excluded from subsequent analysis. A flow chart of the TB process, from initial presentation to final recommendation(s) and subsequent physician discussion is shown in Fig. 1. As part of this process, patients identified by TB for trial participation are tracked by research staff who regardless of physician follow-up at the post-TB discussion, also approach the treating physician(s) to make them aware of pertinent eligible trials.
Fig. 1.
Flowchart of the Program of Women's Oncology multidisciplinary Gynecologic Oncology Tumor Board process at Women and Infants' Hospital. Abbreviations: MD—Physician; Trial—Eligibility for clinical trial; Chemo—Chemotherapy; Genetics—Referral to the Cancer Risk & Prevention Program; Endocrine—Hormonal/Endocrine therapy; Further—Further work-up; Tx—Treatment. *Further work-up included: imaging, biopsy, dilatation and curettage, tumor markers, specialty consultations, exam under anesthesia, unspecified, Tumor Board re-presentation, pap smear, colposcopy, cystoscopy, hysteroscopy, proctoscopy, serial beta hcg, human papillomavirus (HPV) testing, HPV vaccine, pain clinic, urinalysis, urine cytology, laparoscopy, drainage, and laser ablation. †Missing/unknown discussions (Total 6).
To determine the impact of TB recommendations on trial accrual, we specifically restricted our analysis to only those patients who received a TB recommendation for clinical trial eligibility. The trial enrollment rate was calculated by dividing the total number of trial enrollments by the total number of TB recommendations. In addition, we specifically calculated the percentage of patients enrolled in therapeutic trials among all cancer patients during our study period, again excluding all referrals from community physicians.
Variables were compared by Chi-square test or McNemar's test. Risk ratios (RR), difference in proportions, and their corresponding 95% confidence intervals (CIs) were calculated as summary measures. Two-tailed p-values were reported with p<0.05 considered statistically significant. Data analysis was performed with STATA 9.0 (StataCorp, College Station, TX).
Results
We reviewed 1213 presentations from 40 TB conferences conducted between March 18, 2008 and December 30, 2008. There were 794 (65%) first presentations, 365 (30%) second presentations, and 54 (4%) multiple presentations, which involved 916 patients. Table 1 provides a summary of our patient demographics. Median age of patients presented was 57 (range, 16 to 95). Ninety percent were white, 3% African American, 1% Asian, and 6% unspecified/other race. Table 2 describes the composition of diagnoses by disease site that were reviewed at TB. Endometrial cancer was the most common diagnosis (40%), followed by ovarian (23%), and cervical cancers (15%).
Table 1.
Characteristics of case presentations that were reviewed by the Multidisciplinary Gynecologic Oncology Tumor Board between March 18, 2008 and December 30, 2008.
| Characteristics | N (%) |
|---|---|
| Agea | |
| 16–34 | 87 (9) |
| 35–49 | 193 (21) |
| 50–64 | 336 (37) |
| ≥ 65 | 302 (33) |
| Racea,b | |
| White | 785 (90) |
| African American | 22 (3) |
| Asian | 11 (1) |
| Unspecified/other | 52 (6) |
| Referrals to Tumor Board | 1213 |
| Program of Women's Oncology | 1042 (86) |
| Community physician | 171 (14) |
| Tumor Board presentations | 1213 |
| 1 | 794 (66) |
| 2 | 365 (30) |
| ≥ 3 | 54 (4) |
Data based on information provided by each patient reviewed, not per case presentation, as one patient may be presented multiple times.
Missing data.
Table 2.
Diagnosis by organ site per Tumor Board presentation.
| Diagnosis/stagea | N = 1290; n (%) | Diagnosis/stagea | N = 1290; n (%) |
|---|---|---|---|
| Cervix | 197 (15) | Uterus | 504 (40) |
| Benign | 3 | Benign | 46 |
| LGSIL/HGSIL | 5/5 | CAH | 10 |
| ASCUS/AGUS | 12/6 | I | 145 |
| Dysplasia | 31 | II | 19 |
| In situ | 31 | III | 42 |
| I | 56 | IV | 16 |
| II | 13 | Incompletely Staged | 40 |
| III | 12 | Unstaged | 186 |
| IV | 9 | Vagina | 16 (1) |
| Melanoma | 2 | Benign | 1 |
| Unstaged | 12 | Dysplasia | 7 |
| Fallopian tube | 22 (2) | II | 1 |
| I | 9 | Melanoma | 1 |
| III | 4 | Unstaged | 6 |
| Incompletely staged | 2 | Vulva | 90 (7) |
| Unstaged | 7 | Benign | 4 |
| GTD | 7 (1) | Dysplasia | 15 |
| Partial mole | 3 | In situ | 3 |
| Complete mole | 1 | I | 13 |
| Choriocarcinoma | II | 7 | |
| Stage 3 | 1 | III | 3 |
| Unstaged | 2 | IV | 3 |
| Ovary | 294 (23) | Incompletely staged | 12 |
| Benign | 65 | Unstaged | 17 |
| I | 42 | Paget's disease | 3 |
| II | 12 | Basal cell carcinoma | 9 |
| III | 54 | Melanoma | 1 |
| IV | 10 | Other | 138 (11) |
| Incompletely staged | 7 | Solid tumors | 31 |
| Unstaged | 104 | Unknown pelvic mass | 62 |
| Primary Peritoneal | 22 (2) | Unknown primary | 29 |
| III | 17 | Benign disease | 16 |
| IV | 1 | Total malignant disease | 612 (47) |
| Unstaged | 4 |
Abbreviations: GTD-Gestational trophoblastic disease; CAH- complex atypical hyperplasia.
FIGO system as defined by the International Federation of Gynecology and Obstetrics.
Thirteen percent of all TB recommendations noted patient eligibility for a clinical trial(s). Compared to other recommendations, it was the fourth most commonly cited (Table 3, Fig. 1). Other TB recommendations, in descending order, were as follows: surgery (22%), further work-up (22%), no treatment (19%), chemotherapy (13%), radiation treatment (6%), referral to the Cancer Risk & Prevention Program (3%), and endocrine/hormonal therapy (2%). Patients were identified for inclusion in diagnostic studies in 65% of cases; therapeutic studies were identified for 32% of patients presented. Regarding organ-specificity, the majority of patients were eligible for either an endometrial study (39%) or ovarian cancer trial (29%). Table 4 summarizes the overall distribution of eligible patients for specific tumor sites and for specific trial types (diagnostic, therapeutic or prognostic).
Table 3.
Summary of tumor board recommendations.
| Recommendations | Tumor Board presentations, N (%) |
|---|---|
| Surgery | 458 (22) |
| Chemotherapy | 260 (13) |
| Radiation therapy | 129 (6) |
| Endocrine treatment | 39 (2) |
| Eligibility for clinical trial(s)a | 270 (13) |
| Cancer Risk & Prevention Program | 56 (3) |
| Further work up | 446 (22) |
| Imaging | 150 (7) |
| Biopsy | 63 (3) |
| Dilatation and curettage | 35 (2) |
| Tumor markers | 53 (3) |
| Specialty consultations | 76 (4) |
| Otherb | 69 (3) |
| No treatment | 380 (19) |
| Total | 2038 |
N represents the sum of tumor board letters that stated patient eligibility for clinical trial, regardless of the number of trials recommended.
Other: exam under anesthesia (12), unspecified (10), tumor board re-presentation (9), pap smear (7), colposcopy (6), cystoscopy (5), hysteroscopy (4), proctoscopy (3), serial beta hcg (2), HPV testing (2), HPV vaccine (2), pain clinic (2), urinalysis (1), urine cytology (1), laparoscopy (1), drainage (1), and laser ablation (1).
Table 4.
Distribution of clinical trials by cancer site comparing post-Tumor Board physician discussion and research team awareness of patient trial eligibility.
| Cancer site | Trial(s) | Eligible total N | MD, n (%) | Research, n (%) | Both, n (%) | p-valuea | Differenceb (95% CI) |
|---|---|---|---|---|---|---|---|
| Cervix | 4 | 22 | 11 (50) | 6 (27) | 4 (18) | 0.2 | 23% (−7 to 52%) |
| Diagnostic | 3 | 8 | 2 (25) | 2 (25) | 1 (13) | ||
| Therapeutic | 2 | 14 | 9 (64) | 4 (29) | 3 (21) | ||
| Prognostic | – | – | – | – | – | ||
| Ovarian | 14 | 105 | 38 (36) | 16 (15) | 10 (26) | 0.0002 | 21% (10–32%) |
| Diagnostic | 3 | 56 | 13 (23) | 10 (18) | 5 (9) | ||
| Therapeutic | 10 | 45 | 22 (49)c | 6 (13) | 5 (11) | ||
| Prognostic | 1 | 4 | 3 (75) | 0 | 0 | ||
| Fallopian tube | 7 | 12 | 3 (25) | 1 (8) | 0 | 0.6 | 17% (−23 to 56%) |
| Diagnostic | – | – | – | – | – | ||
| Therapeutic | 5 | 5 | 3 (60) | 0 | 0 | ||
| Prognostic | 2 | 7 | 0 | 1 (14) | 0 | ||
| Primary peritoneal | 8 | 10 | 4 (40) | 2 (20) | 2 (20) | 0.5 | 20% (−15% to 55%) |
| Diagnostic | – | – | – | – | – | ||
| Therapeutic | 7 | 10 | 4 (40)* | 2 (20) | 2 (20) | ||
| Prognostic | 1 | 0 | 0 | 0 | 0 | ||
| Uterus | 6 | 141 | 75 (53) | 43 (30) | 34 (24) | 0.0001 | 28% (19–36%) |
| Diagnostic | 4 | 113 | 54 (48) | 37 (33) | 29 (26) | ||
| Therapeutic | 2 | 28 | 21 (75) | 6 (21) | 5 (18) | ||
| Prognostic | – | – | – | – | – | ||
| Vulva | 4 | 20 | 10 (50) | 3 (15) | 2 (10) | 0.04 | 35% (5–65%) |
| Diagnostic | 2 | 8 | 3 (38) | 0 | 0 | ||
| Therapeutic | 2 | 12 | 7 (58) | 3 (25) | 2 (17) | ||
| Prognostic | – | – | – | – | – | ||
| Solid tumor/pelvic mass | 4 | 48 | 15 (31) | 17 (35) | 7 (15) | 0.8 | −4% (−24% to 15%) |
| Diagnostic | 3 | 46 | 15 (33)* | 17 (37) | 7 (15) | ||
| Therapeutic | – | – | – | – | – | ||
| Prognostic | 1 | 2 | 0* | 0 | 0 | ||
| Total | 48 | 358 | 156 (44) | 88 (25) | 59 (16) | <0.0001 | 19% (13–25%) |
Abbreviations: Eligible—number of potential trial enrollments from Tumor Board recommendations; MD—post-Tumor Board discussion with physician; Research—Research team awareness; Both—Patient who received both physician discussion and were also known to the research team.
p-value by McNemar's test.
Difference in proportion of total MD discussed vs. research team aware (95% CI).
Missing/unknown data (total 6).
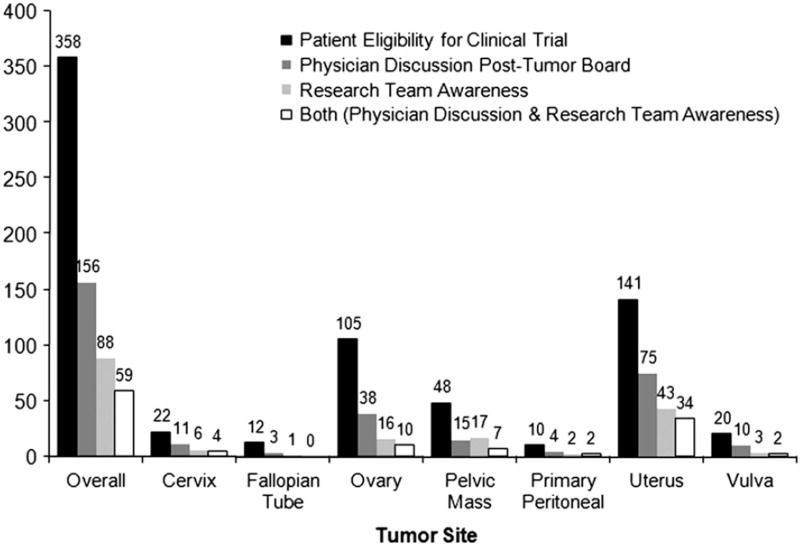
Restricting subsequent analyses to those patients who received care within the Program of Women's Oncology, we found documentation of a formal post-TB discussion between physician and patient in 67% of cases. However, for cases in which the TB recommended trial consideration, we could find discussion relevant to trials in only 44% of post-TB discussions, which is significantly lower compared to documented discussion on other TB recommendations made (RR 0.55, p<0.0001, 95% CI 0.59–0.63). Interestingly, we found that having physicians discuss trial recommendations stemming from the TB was associated with a significantly higher rate of recruitment to trials when compared to the sole reliance on research staff for clinical trial accrual (44% vs. 25%, p<0.001) (Fig. 2).
Fig. 2.
Distribution of clinical trial by tumor site comparing patient eligibility, post-Tumor Board physician discussion and research team awareness of patient trial eligibility. (n/a).
Of the 358 TB recommendations stating patient eligibility for a clinical trial, 87 patients were ultimately enrolled, for an overall accrual rate of 28%. When this analysis was restricted to enrollment in therapeutic trials, the accrual rate was 6%. Again, compared to reliance solely on research staff for recruitment, patients were 2.5 times more likely to participate in a clinical trial(s) if post-TB discussion of trials was documented (RR 2.5, p = 0.006). Those patients who had a post-TB discussion with their physician and were also tracked by the research team were significantly more likely to be enrolled in a clinical trial than either group alone (physician discussion—RR 1.4; p = 0.02; research team—RR = 3.5; p<0.0001).
Of note, there were 11 patients whose physicians appropriately identified them to be eligible for a clinical trial and discussed this option despite lack of documentation in the TB recommendations. Two of the 11 patients subsequently enrolled in a clinical trial.
Discussion
Low patient accrual to clinical trials is an important public health concern because results from such studies have potential to positively impact clinical management and outcomes. For example, clinical trials expand the number of treatment options available to cancer patients, providing access to new therapeutic approaches that may otherwise not be available on the market. They also help to further elucidate the natural history of malignant disease, allowing for more sensitive/specific diagnostic tools and targeted interventions. Therefore, to optimally manage cancer patients, it is critical to address specific issues related to clinical trial availability and low enrollment rates [11,12].
In our review of 1213 GOTB presentations from March to December 2008, 358 recommendations included patient eligibility for a clinical trial(s). This led to an overall accrual rate of 28%. Six percent of the 419 cancer patients participated in a therapeutic clinical trial, which is slightly increased from the 2–4% annual enrollment rate of NCI-sponsored treatment trials. Whether this national statistic is an optimal rate for comparison or the 10–15% goal previously established in the literature is a different question altogether and remains unanswered.
Post-TB discussions between physicians and patients were 45% less likely to include trial eligibility than any other recommendations. This not only highlights an area within the TB process where targeted interventions may help increase trial accrual, but it also raises other important considerations regarding optimal trial accrual. Specifically, what decision-making processes do physicians consider when deciding whether or not to offer a clinical trial to their patients? Mannel et al. [18] investigated physician trial enrollment at a single institution and found that senior faculty had a higher rate of enrollment than junior faculty (71% vs. 31%) and similarly those faculty who were principal investigators were more likely to enroll. Availability of patients, patient variances, support staff, and institutional commitment were deemed secondary to physician factors in regards to successful enrollment on clinical trials. These results also warrant further quality assurance studies to evaluate whether enrollment rates reflect appropriate referrals to clinical trial rather than individual physician referral habits.
Although our findings are specific to the TB process, they reiterate important patient, physician, and system barriers to clinical trial accrual that have previously been published [19,16]. Grunfeld et al. [14] conducted a qualitative study of the perspective of clinical research associates suggesting system factors have the greatest impact on ability to accrue. Specific barriers described included the following: trial and pharmaceutical company requirements (i.e. documentation), as well as busier clinics which imposed conflict between work demands, and time taken out to accrue patients. In contrast, they also studied facilitators to clinical trial enrollment in clinical trials and reported that patients seem more knowledgeable about trials than they have in the past. Even more encouraging is the consistency of this finding with other studies that show patients are willing to learn about clinical trials as well as participate in them if approached appropriately by a physician [20,21].
Other facilitators to enrollment in cancer clinical trials have been characterized by Sateren et al. [22] who reported the number of oncologists (p = 0.04) and the presence of approved cancer programs (p<0.0001) were both significantly associated with increased enrollment rates. Whether these associations can be attributed to the multidisciplinary TB process remains unanswered. This limitation in conjunction with previously reported system barriers to trial accrual highlights the value of our study. Not only do we elucidate the potential for multidisciplinary TBs to optimize clinical trial enrollment, but we also sought to address specific areas within the TB process where interventions are most needed.
Our study has several limitations. Given our 42-week study period, we were not adequately able to compare our patient accrual to that of the annual rate of NCI-treatment trials nationwide. In addition, we were unable to determine if a discussion regarding clinical trials took place in the absence of documentation. However, given that this limitation only applied to 6 cases, we do not feel that this significantly alters our results. Further, no data were available on patients who were directly referred to TB by a community physician, nor did we assess physician and patient barriers to enrollment. Finally, the majority of presentations concerned first presentations of women with suspected or newly diagnosed cancers. Thus, the opportunities for identification of women with advanced or metastatic disease for trials by the TB process is not adequately addressed in the scope of this study.
Cancer clinical trials are an important cornerstone to bridging our knowledge of the natural history of malignant disease and clinical practice. Not only do they provide an effective means of evaluating new diagnostic, therapeutic, and prognostic agents, but some studies, although controversial, have shown participation in trials is not harmful [23] and has potential to improve clinical outcomes [24]. Djubegovic et al. [25] found that 30% of trials had statistically significant results, 80% of which new interventions were superior to the standard of care. This study also confirmed a pattern of trial successes that has become more stable overtime, estimating that 25% to 50% of new cancer treatments that reach phase 3 randomized clinical trials will prove successful. Therefore, it is important to make appropriate referrals to clinical trials to optimize the benefits for individual study participants without compromising the power or generalizability of results to the target population at whole.
Future efforts should focus on interventions that aim to: (1) improve post-TB discussions between physicians and patients to incorporate all recommendations, especially eligibility for clinical trials; (2) identify and improve system, physician, and patient barriers to trial enrollment; (3) use clinical research assistants in the TB process to optimize accrual; and (4) further investigate the role of community physicians within the TB process. We propose such interventions with the hope of improving the TB process, optimizing cancer clinical trial accrual, and ultimately enhancing patient satisfaction of their care.
Footnotes
This work was originally presented at the International Gynecologic Cancer Society Meeting in Bangkok, Thailand, October 2008.
Conflict of interest satement
The authors declare that there are no conflicts of interest.
References
- 1.The American College of Surgeons Commission on Cancer: Cancer Program Standards 2009 Revised Edition. [accessed April 30, 2009]; Available from: URL: www.facs.org/cancer/coc/cocprogramstandards.pdf.
- 2.Henson DE, Frelick RW, Ford LG, et al. Results of a national survey of characteristics of hospital tumor conferences. Surg Gynecol Obstet. 1990 Jan;170(1):1–6. [PubMed] [Google Scholar]
- 3.Petty JK, Vetto JT. Beyond doughnuts: tumor board recommendations influence patient care. J Cancer Educ. 2002 Summer;17(2):97–100. doi: 10.1080/08858190209528807. [DOI] [PubMed] [Google Scholar]
- 4.Chang JH, Vines E, Bertsch H, et al. The impact of a multidisciplinary breast cancer center on recommendations for patient management: the University of Pennsylvania experience. Cancer. 2001 Apr 1;91(7):1231–7. doi: 10.1002/1097-0142(20010401)91:7<1231::aid-cncr1123>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Santoso JT, Schwertner B, Coleman RL, Hannigan EV. Tumor board in gynecologic oncology. Int J Gynecol Cancer. 2004 Mar-Apr;14(2):206–9. doi: 10.1111/j.1048-891X.2004.014200.x. [DOI] [PubMed] [Google Scholar]
- 6.Newman EA, Guest AB, Helvie MA, et al. Changes in surgical management resulting from case review at a breast cancer multidisciplinary tumor board. Cancer. 2006 Nov 15;107(10):2346–51. doi: 10.1002/cncr.22266. [DOI] [PubMed] [Google Scholar]
- 7.Bakemeier RF, Beck S, Murphy JR. Educational and consultative functions, topics, and methods of hospital general tumor conferences. J Cancer Educ. 1995 Winter;9(4):217–25. [PubMed] [Google Scholar]
- 8.Gross GE. The role of the tumor board in a community hospital. CA Cancer J Clin. 1987 Mar-Apr;37(2):88–92. doi: 10.3322/canjclin.37.2.88. [DOI] [PubMed] [Google Scholar]
- 9.Radecki SE, Nyquist JG, Gates JD, Abrahamson S, Henson DE. Educational characteristics of tumor conferences in teaching and non-teaching hospitals. J Cancer Educ. 1995 Winter;9(4):204–16. [PubMed] [Google Scholar]
- 10.Chekerov R, Denkert C, Boehmer D, et al. Online tumor conference in the clinical management of gynecological cancer: experience from a pilot study in Germany. Int J Gynecol Cancer. 2008 Jan-Feb;18(1):1–7. doi: 10.1111/j.1525-1438.2007.00971.x. [DOI] [PubMed] [Google Scholar]
- 11.Tejeda HA, Green SB, Trimble EL, et al. Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst. 1996 Jun 19;88(12):812–6. doi: 10.1093/jnci/88.12.812. [DOI] [PubMed] [Google Scholar]
- 12.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001 Mar 15;19(6):1728–33. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 13.Nott L, Yeend S, Pirc L, Pittman K, Patterson K, Price TJ. Successfully improving access and accrual to oncology clinical trials. Cancer. 2007 Apr 15;109(8):1451–3. doi: 10.1002/cncr.22571. [DOI] [PubMed] [Google Scholar]
- 14.Grunfeld E, Zitzelsberger L, Coristine M, Aspelund F. Barriers and facilitators to enrollment in cancer clinical trials: qualitative study of the perspectives of clinical research associates. Cancer. 2002 Oct 1;95(7):1577–83. doi: 10.1002/cncr.10862. [DOI] [PubMed] [Google Scholar]
- 15.Markman M, Petersen J, Montgomery R. Influence of tumor type, disease status, and patient age on self-reported interest regarding participation in cancer clinical trials. Cancer. 2006 Aug 15;107(4):849–53. doi: 10.1002/cncr.21997. [DOI] [PubMed] [Google Scholar]
- 16.Siminoff LA, Zhang A, Colabianchi N, Sturm CM, Shen Q. Factors that predict the referral of breast cancer patients onto clinical trials by their surgeons and medical oncologists. J Clin Oncol. 2000 Mar;18(6):1203–11. doi: 10.1200/JCO.2000.18.6.1203. [DOI] [PubMed] [Google Scholar]
- 17.Harrison JD, Carter J, Young JM, Solomon MJ. Difficult clinical decisions in gynecological oncology: identifying priorities for future clinical research. Int J Gynecol Cancer. 2006 Jan-Feb;16(1):1–7. doi: 10.1111/j.1525-1438.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 18.Mannel R, Walker J, Gould N, et al. Impact of individual physician on enrollment of patients into clinical trials. Am J Clin Oncol (CCT) 2003;26(2):171–3. doi: 10.1097/00000421-200304000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Patel A, Wilke HJ, II, Mingay D, Ellis JE. Patient attitudes toward granting consent to participate in perioperative randomized clinical trials. J Clin Anesth. 2004 Sep;16(6):426–34. doi: 10.1016/j.jclinane.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Markman M. Providing research participants with findings from completed cancer-related clinical trials: not quite as simple as it sounds. Cancer. 2006 Apr 1;106(7):1421–4. doi: 10.1002/cncr.21757. [DOI] [PubMed] [Google Scholar]
- 21.Comis RL, Miller JD, Aldige CR, Krebs L, Stoval E. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003 Mar 1;21(5):830–5. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- 22.Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002 Apr 15;20(8):2109–17. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 23.Vist GE, Hagen KB, Devereaux PJ, Bryant D, Kristoffersen DT, Oxman AD. Systematic review to determine whether participation in a trial influences outcome. BMJ. 2005 May 21;330(7501):1175. doi: 10.1136/bmj.330.7501.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stiller CA. Centralised treatment, entry to trials and survival. Br J Cancer. 1994 Aug;70(2):352–62. doi: 10.1038/bjc.1994.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Djulbegovic B, Kumar A, Soares HP, et al. Treatment success in cancer: new cancer treatment successes identified in phase 3 randomized controlled trials conducted by the National Cancer Institute-sponsored cooperative oncology groups, 1955 to 2006. Arch Intern Med. 2008 Mar 24;168(6):632–42. doi: 10.1001/archinte.168.6.632. [DOI] [PMC free article] [PubMed] [Google Scholar]