Abstract
Background
Despite the growing prevalence of opioid use among offenders, pharmacotherapy remains an underused treatment approach in correctional settings. The aim of this 4-year trial is to assess the clinical utility, effectiveness, and cost implications of extended-release naltrexone (XR-NTX, Vivitrol®; Alkermes Inc) alone and in conjunction with patient navigation for jail inmates with opioid use disorder (OUD).
Methods
Opioid-dependent inmates will be randomly assigned to one of three treatment conditions before being released to the community to include: 1) XR-NTX only; 2) XR-NTX plus patient navigation (PN), and 3) enhanced treatment-as-usual (ETAU) with drug education and a community treatment referral. Before release from jail, participants in the XR-NTX and XR-NTX plus PN conditions will receive their first XR-NTX injection. Those in the XR-NTX plus PN condition also will meet with a patient navigator. Participants in both XR-NTX conditions will be scheduled for medical management sessions twice monthly for months 1–3, monthly medical management sessions for months 4–6, with monthly injections for 5 months post-release (which, given the pre-release injection, results in a 6-month medication phase). Follow-up data collection will occur at 1, 3, 6, and 12 months post release.
Results
We discuss the study’s rationale, aims, methods, and anticipated findings. The primary outcome is the presence of a DSM 5 OUD diagnosis 1 year after randomization (6 months after the end of the active treatment phase).
Discussion
We hypothesize that providing XR-NTX prior to release from jail will be particularly beneficial for this extremely high-risk population by reducing opioid use, associated criminal behavior, and injection-related disease risk.
Keywords: Opioid dependence, jail inmates, experimental, injectable naltrexone, extended release naltrexone
1. Introduction and Background
In a given year, a quarter of all people in the United States who have HIV, a third who have HCV infection, and more than 40% who have tuberculosis disease will pass through a correctional facility that same year [1,2]. Likewise, the risk of death among parolees during the first two weeks following release from prison is nearly 13 times greater than those of similar demographic background—with drug overdose being the leading cause [3,4]. As dire as this finding is, it may be an underestimate of the problem. A study of newly released prisoners in England and Wales found that mortality rates among males were 29 times higher than the general population during the first two weeks of release [5].
The efficacy of naltrexone in the treatment of opioid dependence has been well established. Naltrexone, an opioid receptor antagonist, blocks the euphoric effects of heroin and other opioids. This characteristic has fostered growing acceptance of naltrexone by correctional authorities who prefer not to provide opioid agonist treatment with medications such as methadone or buprenorphine [6]. However, it is typically taken orally on a daily basis, making adherence a problem among all but the most committed patients. Cornish et al. [7] randomly assigned federal probationers to a 6-month program of probation plus naltrexone and brief drug counseling or to probation plus counseling alone and found that opioid use was significantly lower in the naltrexone group, with the mean percent of opioid positive urine tests among the naltrexone subjects at 8%, versus 30% for control subjects (p < .05). Likewise, 56% of the controls and 26% of the naltrexone group (p <. 05) had their probation status revoked within the 6-month study period and were returned to prison. But treatment compliance was a problem, with only 52% of subjects in the naltrexone group continuing on medication for the 6-months duration of the study.
Still, the effectiveness of oral naltrexone is mitigated by poor compliance, hampering clinical utility in real-world settings. In one study of a prison-based naltrexone program, only 7% of the enrolled patients remained in treatment for six months [8]. To address the issue of poor adherence, extended-release naltrexone (XR-NTX, Vivitrol® with 380mg naltrexone delivered intramuscularly every four weeks; Alkermes, Inc.) was developed to provide long-acting pharmacotherapy for one month per dose. The purpose of this proposed study is to assess the relative effects and economic impact of this pharmacotherapy with and without a patient navigator (XR-NTX, XR-NTX plus PN) and compared to an enhanced treatment-as-usual (ETAU) condition consisting of drug eduction, overdose prevention information, and referral to community-based treatment for sentenced jail inmates.
This study is part of the Studies of Medications for Addiction Treatment in Correctional Settings (SOMATICS) project, a National Institute on Drug Abuse cooperative study that examines approaches to delivering FDA-approved pharmacotherapies to recently arrested adults with opioid dependence. The other two studies, focusing on interim methadone (Friends Research, Inc.) and XR-NTX (New York University), are described elsewhere in this issue.
2. Research Design and Study Population
2.1 Overview of Study Design
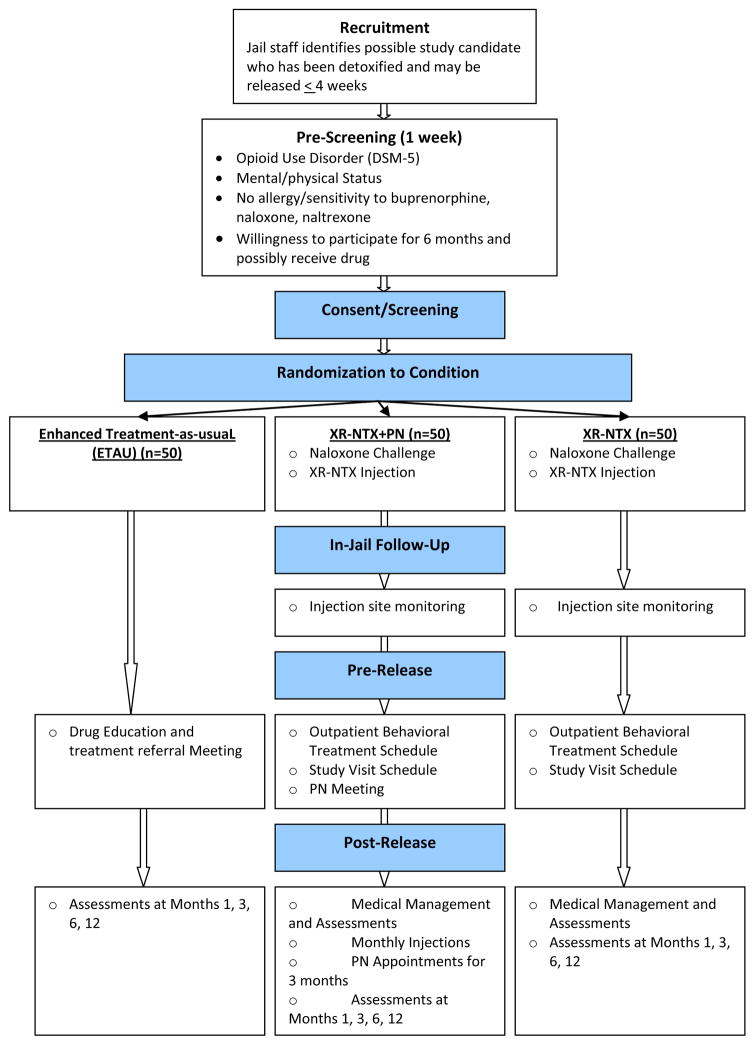
The proposed four-year study is a randomized, open-label trial that will examine feasibility, efficacy, and economic impact of a depot, extended-release medication for opioid use disorders, alone or in conjunction with PN, and an ETAU condition with drug and overdose prevention information and referral to community treatment receiving no medication. Participants in the XR-NTX and XR-NTX plus PN groups will receive standard medical management. Before discharge from jail, participants randomized to one of two medication conditions receive XR-NTX and then receive subsequent injections every four weeks for 5 additional months. Those in the XR-NTX plus PN condition will meet with a PN before discharge and regularly (weekly in the first month; biweekly in months 2–3) after release to discuss barriers to treatment, possible treatment program participation following release, and will address social support and other participant needs. Before discharge, the ETAU condition group will participate in one session designed to provide a presentation and discussion of drug-related issues and receive a referral to treatment in the community. Following release from jail, all participants in the medication conditions will receive phone calls from the study team to schedule medical management appointments to occur twice monthly for the first 3 months after release then monthly for the last 3 months of the intervention phase for medical management and to complete assessments (see Figure 1).
Figure 1.
Study Flow
Note: XR-NTX= Extended release naltrexone, PN = patient navigation, ETAU = enhanced treatment-as-usual,
2.2 Duration of Study and Clinic Visit Schedule
The duration of this study will include a projected 2–4 weeks for screening/baseline assessments and medication induction before release from jail, and a 24-week intervention phase to include, specific to assigned condition, XR-NTX, , PN, drug and overdose education and referral, assessments, and medical management (for participants in the XR-NTX conditions). The screening phase will differ in the length of time needed to complete eligibility assessments, random assignment, and to complete medication induction in the XR-NTX conditions. Induction will be scheduled to occur within the 4 weeks prior to discharge. Screening assessments will include the collection of laboratory samples and medical assessments to ensure participant safety, including confirmation of opioid-free status (urine drug screen [UDS] and naloxone challenge). Assessment visits at 1, 3, 6, and 12-months will take about 30–60 minutes, depending on the scheduled assessments. Medical management visits will last from 20–60 minutes and will include collection of urine specimens and other short measures of status and well-being. PN sessions are expected to take about 60 minutes. Drug education (DE) sessions will take about 20 minutes.
2.3 Study Population
Participants will be 150 males and females sentenced jail inmates meeting DSM-5 criteria for opioid use disorders who are 18 years and older, have been detoxified from opioids in the Bernalillo County Metropolitan Detention Center and meet eligibility criteria.
Inclusion Criteria
Study participants must:
Be at least 18 years of age or older,
Meet criteria for DSM-5 opioid use disorders,
Be detained for at least 48 hours,
Have an expected release date within one year,
Plan to reside in area after release,
Have at least one instance of relapse to opioid use after a period of abstinence.
Exclusion Criteria
Study participants must not:
Have a medical (e.g., liver failure, congestive heart failure) or psychiatric condition (e.g., suicidal ideation, psychosis) that would make participation unsafe in the judgment of the medical staff or the PI,
Have chronic pain and are currently or have plans to undergo pain treatment/therapy,
Have known sensitivity to naltrexone or naloxone,
Have participated in an investigational drug study within the past 30 days prior to screening,
Be a nursing or pregnant female, or not agree to use a medically acceptable form of birth control such as oral contraceptives, barrier (diaphragm or condom), levonorgestrel implant, intra-uterine progesterone contraceptives system, medroxyprogesterone acetate contraceptive injection, or complete abstinence. Females who become pregnant during the course of the study will be withdrawn from the study and, if requested, will be provided with referrals for drug treatment and/or medical care,
Have any pending legal action that could prohibit continued participation for the 24-week intervention period of the study, such as legal proceedings that could possibly result in incarceration,
Have a current pattern of alcohol, benzodiazepine, or other depressant or sedative hypnotic use, as determined by the study physician, which would preclude safe participation in the study.
Participant Recruitment
Recruitment will occur through a close collaboration with jail staff, combined with IRB-approved presentations and posted announcements in the jail facilities and will proceed until 150 participants are recruited, consented, and randomized. Given the potential recruitment pool of more than 80 individuals per month, it is expected that an average of 6–8 individuals will be randomized per month across the 20-month enrollment period. Based on prior evidence in similar trials, refusals and early (pre-randomization) dropouts will account for approximately 20% of individuals presenting for screening. We expected that a baseline sample of 150 participants with 50 participants per condition would yield a final aggregate sample of approximately 120 participants, assuming 10–20% dropout. (Our similar studies have had 85–90% follow-up rates.) Thus, a final evaluable sample size of 120 participants would permit detection of a medium-large effect size (~.60) between conditions for some of the outcome variables (at ~.70 power) and .5 effect size (at .80 power) for others.
Recruitment plans include careful attention to the issue of voluntariness. All jail and study staff will be extensively trained on this issue to ensure that their actions and words do not convey any level of coercion. Training will include a discussion of the fact that treatment and study compliance are optimized when participants have the opportunity to consider the study, ask questions, and provide voluntary consent to participate. Furthermore, all recruitment documents and the informed consent form (ICF) will emphasize that decisions whether or not to participate are solely up to the individuals; that the decision will not affect the treatment or possible treatment to which the individual is eligible; and that participation/non-participation will not affect the sentence, release, probation, or any other aspect of the individual’s incarceration.
The main method of recruitment will be announcements of the study made to jail inmates. An informational flyer will be provided to arrestees who are within 4 weeks of release and have been detoxified from opioid use. Individuals who convey interest in this study to jail staff will be referred to study staff. If still interested after receiving a description of this study, study candidates will complete a consent process that includes a detailed explanation of the study, risks and benefits, and that study participation is voluntary and will have no effect on a participant’s sentence, jail term, probation, parole, or release.
2.4 Treatment-as-usual Procedures for Jail Inmates
Inmates are detoxified (if necessary) under physician supervision within the jail setting. After this, they are moved to the general inmate population but have follow-up visits with the jail physicians. While in custody, they may opt to pursue participation in available onsite counseling programs. No formal system is in place for making community-based referrals upon release.
2.5 Study Sites
For this project, the primary sites for recruitment, data collection, and treatment services are the Bernalillo County Metropolitan Detention Center and the community-based University of New Mexico (UNM), Center on Alcoholism, Substance Abuse and Addictions Department of Psychiatry and Recovery Services.
Bernalillo County Metropolitan Detention Center (BCMDC)
Recruitment and induction will occur at the jail sites in the BCMDC System. The jail facility houses sentenced and unsentenced inmates. The types of inmates housed here are general population, violent/assaultive offenders, psychiatric, and inmates needing medical services. This facility is staffed with Sheriff Service Technicians, intake and release specialists, medical service staff contracted by the County, and clerical support staff. Bernalillo County opened the BCMDC facility in 2003. Currently, the BCMDC averages about 40,000 bookings per year, with an average daily population of 1,600. Males account for 87% of the inmate population. With regard to race/ethnicity, 54% are Hispanic, 23% White-Non-Hispanic, 12% American Indian, 9% African-American, 0.4% Asian, and 1.7% are categorized as “Other.” Currently the BCMDC detoxifies an average of 758 individuals monthly—412 from alcohol and 346 from opiates. The BCMDC provides methadone maintenance to inmates who were in community-based methadone maintenance treatment (MMT) prior to incarceration
The University of New Mexico Addiction Substance Abuse Program (ASAP), The University of New Mexico Center on Alcoholism, Substance Abuse, and Addictions (CASAA) and Recovery Services
These programs will be the locations for post-release medical management visits and follow-up assessment. ASAP offers a range of treatment services designed to help individuals coping with substance use disorders. Services include screening, crisis intervention, individual, group, and family counseling, HIV/Hepatitis education, assessment, and evaluation, referrals to community-based services, and outreach to the community. ASAP is staffed by the UNM Department of Psychiatry and UNM Hospital and is conveniently located adjacent to the UNM CASAA facility. CASAA is an addictions research center where investigators conduct clinical trials of innovative approaches to prevention and treatment. Recovery Services is a private addiction medicine treatment program operating both methadone programs and outpatient office based opioid treatments and counseling in New Mexico.
Although not research sites for this protocol, other outpatient treatment programs also providing psychosocial interventions are located throughout Bernalillo County. In particular, the Metropolitan Assessment and Treatment Services (MATS) program provides comprehensive detoxification, treatment, and transitional housing services for medically-indigent populations in the Albuquerque area. Operated by Bernalillo County, the MATS program has extensive partnerships with other community medical, mental health, alcohol/drug and homeless service agencies.
University of California, Los Angeles Integrated Substance Abuse Program (UCLA ISAP)
The leadership, data management, and analysis portions of the proposed project will occur at UCLA ISAP in Los Angeles.
2.6 Outcome Measures
The project emphasizes evaluation of the clinical utility of the XR-NTX with and without a PN condition (XR-NTX, XR-NTX plus PN), as compared to an ETAU condition. Descriptive statistics will provide an overall assessment of the implementation of the protocol.
Primary Outcome Measure
The primary outcomes are the presence of a current DSM-5 diagnosis of opioid use disorder 6 months after the intervention phase, and self-reported opioid use, collected using the Timeline Follow Back Interview (TLFB) (See Table 1 for a full list of study measures and citations).
Table 1.
Data Collection Time and Event Schedule
| Timepoints | Screening | Pre-release | Post-release | |
|---|---|---|---|---|
| Measures | Medical Mgmt | Assessments | ||
| Months 1, 3, 6, 12 | ||||
| Safety & Med. Measures | ||||
| Physical Exam/Medical History | X | |||
| Injection site inspection | X | X | ||
| Vitals | X | X | ||
| 12-lead ECG | X | |||
| Clinical Lab Tests | X | |||
| HIV Test | X | |||
| Pregnancy Test | X | X | ||
| Prior/Concomitant Medications | X | X | ||
| Adverse Events | X | X | ||
| Drug Use Measures | ||||
| DSM-5 Checklist | X | |||
| COWS (Withdrawal) [18] | X | |||
| SOWS (Withdrawal) [19] | X | |||
| Urine Drug Screen (UDS) | X | X | X | X |
| Substance Use Report (TLFB) [20] | X | X | X | X |
| VAS (Craving) [21] | X | X | X | X |
| RAB [22] | X | X | X | X |
| Crime and Recidivism | ||||
| Self-Report Arrest/Treatment History |
X | X | X | X |
| Arrest Records | X | |||
| Cost | ||||
| Economic Form 90 | X | X | X | X |
Note: DSM = Data and Statistical Manual, COWS= Clinical Opiate Withdrawal Scale, SOWS= Subjective Opiate Withdrawal Scale, AE= Adverse Events.
Secondary Outcome Measures
Additional outcome measures include: HIV risk behaviors (compared using repeated measures procedures to evaluate possible change in sexual and drug-related behaviors that may inhibit or promote HIV infection) measured (via face-to-face interview) by the HIV Risk Assessment Battery (RAB); self-reported number of days incarcerated during the intervention and follow-up phases; self-reported number of days of opioid and other drug use measured by TLFB, and objective measures of drug use by urine drug screens (UDS) at the 6-month post-release assessment (end of the intervention phase); self-reported days in drug abuse treatment; self-reported number of arrests; self-reported craving for opioids; self-reported number of overdoses; and self-reported motivation for treatment.
2.7 Study Procedures
Approvals
This study was reviewed and approved by the Medical Institutional Review Board (M-IRB) of the UCLA, and the IRB of the University of New Mexico. We also received a Federal Certificate of Confidentiality from the U.S. Department of Health & Human Services (DA-14-094). The protocol has also been reviewed and approved by the Office of Human Research Protections. The study was also registered on clinicaltrials.gov (Protocol ID: NCT02110264).
The Consent Process
All participants will voluntarily sign an informed consent prior to study participation. The informed consent process involves a detailed verbal description of the study and the data collection procedures. The participant will be encouraged to ask questions about the study procedures throughout the process. The risks of participating in this study will be detailed in the consent form. Staff will emphasize that participation is voluntary and that participants may withdraw consent at any time without prejudice, and that they will be given referrals to other local treatment programs.
After discussing the study procedures, potential risks and benefits, the voluntary nature of study participation, and that participation will have no effect on a participant’s sentence, jail term, probation, parole, or release, study candidates will answer a brief quiz to verify and document a thorough understanding of the research prior to signing the consent form. The study candidate will sign the consent form as witnessed by a study investigator or physician. Research staff will receive extensive training in the informed consent process. A copy of the signed consent form will be placed in the participants’ private lockbox (a storage container where inmates’ personal belongings are held during the term of incarceration).
Screening
The study team will provide a basic description of the study to interested individuals who respond to IRB-approved flyers and announcements. Study staff will verify pre-eligibility status of each study candidate with jail staff (e.g., has completed opioid detoxification, jail term and expected release date). Individuals determined as pre-eligible will be scheduled for a consent/screening appointment. A complete medical history and physical exam will occur after consent, including blood chemistries (including liver function tests) and electrocardiogram (ECG). Participants may be excluded from the study at this point if they do not meet medical inclusion/exclusion criteria. After medical clearance for the study, all other baseline assessment data will be collected. Participants will be randomized to study condition when results of all assessments, are obtained and eligibility is confirmed. Screening and baseline assessment will take ~4 hours, excluding review of lab results. This process may occur over multiple days.
Random Assignment
Eligible participants will be randomly assigned to study condition (XR-NTX, XR-NTX plus PN, ETAU) in a 1:1:1 strategy using an urn randomization procedure [9] to provide multivariate balance across two characteristics correlated with outcomes in addiction treatment trials: type of opioid (heroin or prescription drug) and gender.
Individuals who terminate participation before induction onto the assigned medication (or similar time-point for behavioral assignments) will be replaced. Analyses will follow an intent-to-treat scenario. [10]
XR-NTX Pre-administration and Injection Procedures
Individuals must be free from opioids for at least 7 days before receiving the XR-NTX injection, which is confirmed via self-report and facility records. To ensure opioid abstinence at time of induction, a naloxone challenge will occur followed by one day of oral naltrexone before XR-NTX injection, adhering to NIDA-approved and IRB-approved procedures. Subsequent XR-NTX injections administered every four weeks will require confirmation of opioid abstinence by either naloxone challenge or administration of oral naltrexone, as determined appropriate and necessary by the Study Medical team Physician.
The pre-induction strategy includes:
Step 1. Participants must self-report no clinically significant opioid use (i.e., at any level that could constitute a potential risk of precipitating opioid withdrawal upon naloxone administration in the next step) in the previous seven days.
Step 2. A urine drug screen will be administered shortly before the naloxone challenge and must be negative for opioids. Individuals who are opioid-negative will continue in the pre-induction process. Individuals may have an additional urine drug screen on a subsequent day if the study medical clinician determines that a second screen is appropriate.
Step 3. Completion of all pertinent psychosocial and medical screening and eligibility assessments.
Step 4. Absence of opioids in a urine screen is not absolute proof that a patient is entirely opioid-free; as such a naloxone challenge will be administered. Prior to subsequent XR-NTX injections every four weeks, the naloxone challenge can be performed at the discretion of the study physician to ensure continued suitability for the XR-NTX injection. An example of a naloxone challenge procedure may begin with intravenous (IV), intramuscular (IM), or subcutaneous delivery of 0.1mg naloxone. If no significant opioid withdrawal symptoms appear after a few minutes, a second dose of 0.3mg would then be administered followed by a brief observation period. With no observed discomfort, 0.8mg naloxone would then be administered as the final dose, followed by an observation period. A minimum 0.8mg bolus must be given before determining the outcome of the challenge. An alternative is to challenge with a single 0.8mg bolus. The determination as to whether the participant is eligible to continue on to IV induction will be made by the study medical clinician based on clinical judgment, including both objective and subjective assessments. Attention to individual symptoms and symptom changes should guide determination of eligibility. Signs of discomfort or an increase in withdrawal symptoms following naloxone administration should be taken as a positive result of the challenge, with induction delayed until a negative challenge result is achieved.
Participants who experience withdrawal symptoms following the naloxone challenge can be treated with ancillary medications if appropriate, observed until symptoms resolve, and given the opportunity to be re-challenged on a future date. Participants who are not interested in continuing to participate, or who fail a repeat naloxone challenge, will not be eligible to participate.
Extended-release Naltrexone (XR-NTX)
XR-NTX has naltrexone-containing microspheres that are delivered by injection every four weeks into the muscles of the buttock. Plasma concentrations of naltrexone and 6-beta naltrexol (its main metabolite) after a single XR-NTX injection are detectable for at least 30 days and must be re-administered to maintain its effect. Continued use of naltrexone is not associated with tolerance or physiological dependence and is generally well-tolerated [11].
XR-NTX will be provided to participants as a gluteal IM injection (380 mg) administered every four weeks. The injection shall be administered following the guidelines provided in the package insert, including the pre-induction procedures described above.
Well-developed precautionary procedures will be followed to avoid adverse events associated with induction. For example, body habitus will be assessed during the physical exam at screening to assure that needle length is adequate for IM administration as an inadvertent subcutaneous injection may increase the likelihood of injection site reactions. The needle provided in the XR-NTX package is a customized needle required for injection of medication. Individuals whose body habitus precludes a gluteal intramuscular injection of naltrexone using the required needle will be excluded from the study. (At the end of the six-month intervention period, participants expressing a desire to continue receiving injectable naltrexone will be referred to community care for re-assessment and continued treatment.)
Behavioral Treatments
Enhanced Treatment-as-usual (ETAU)
This condition includes standardized materials for drug education and overdose prevention education delivered to participants by the research assistant (RA) in the jail along with a referral to community-based drug abuse treatment. This occurs in a single session.
Patient Navigator (PN)
In addition to receiving XR-NTX, participants in the XR-NTX plus PN condition will be assigned a PN. The PN will be employed by the research grant, and trained in conjunction with his/her counterpart on a similar trial conducted by Friends Research, Inc. in Baltimore (part of the SOMATICS collaborative) to ensure consistency. The PN will provide one-on-one assistance to surmount barriers to entry and adherence with medical care for chronic disease. Originally designed to improve outcomes in oncology for disadvantaged female patients, the PN conceptual foundation is a strengths-based case management perspective to help patients keep their appointments (through scheduling, reminders, and accompanying the patients), improve communication between the patient and their providers, offer health education, provide assistance with personal barriers to treatment (e.g., transportation, health insurance, childcare), and offer emotional support [12]. Clinical trials have found that PN increased cancer screening and follow-up rates, improved entry and adherence to HIV treatment, and increased adherence to medical appointments and greater likelihood of achieving an undetectable viral load compared to controls. [13]
Following the in-person visit in jail, the PN will have contact (in person or by phone) with participants at least once per week. During the second and third months, frequency of contact may be lower for most participants but no less than once every other week. On average, it is anticipated that the PN will have contact with each participant eight times.
Medical Management
At each clinic visit, participants in the two XR-NTX conditions will meet with study physicians and other study personnel such as nurses, Physician’s Assistants, research assistants, and counselors, to review urine results, discuss adverse events, consider the study medication effects and side effects, and discuss other pertinent issues in keeping with sound medical practice. Participants’ engagement in self-help groups such as 12-step is permitted. Medical Management visits will occur twice-monthly during months 1–3, and monthly during months 4–6, and XR-NTX injections will be provided monthly to participants in the XR-NTX and the XR-NTX plus PN conditions.
“Rescue” Protocol
Participants who develop significant problems, who cannot tolerate naltrexone, or whose pre-existing condition worsens during the study may receive increased levels of care deemed necessary by the Study Physician, and they will be referred to other treatment resources.
Ancillary Medications
Ancillary medications may be provided by the study medical team for study medication-related side effects as clinically indicated. A range of prescription and over-the-counter ancillary medications may be used for anxiety, nausea, vomiting, diarrhea, muscle pain, and insomnia.
Concomitant Medications
Participants will be instructed to contact the study medical clinician before taking any non-study medications, including prescription drugs, over-the-counter preparations, or herbal supplements, during the course of the study. Participants reporting use of medications that may interact with naltrexone will be excluded or withdrawn based on clinical judgment.
Management of Study Medications
Appropriately qualified and trained medical personnel will maintain an accurate and current accounting of all study medication, which will be available for verification by study monitors. Drug-accountability records including perpetual inventory, will include the amount of study medication ordered, received, transferred between areas of the study site, and those dispensed to individual participants.
Study Medication Storage
Study medication will be stored in compliance with federal, state, and local laws and institutional policy. Study medication will be stored in a locked, secure, limited-access location under the conditions specified by the package insert; XR-NTX will be stored in a locked refrigerator.
Dispensing of Study Medications
All study medications shall be dispensed by an appropriate licensed physician/physician assistant appropriately trained and authorized to dispense study medications. XR-NTX injections will be administered at baseline (prior to release), and every four weeks for 24 weeks (total of 6 injections).
Participant Withdrawal
Aside from pregnancy, there are no formal criteria for investigator withdrawal of a participant, however, any participant for whom study participation is deemed potentially unsafe as determined by the medical clinician will be withdrawn from participation, even if the participant would like to continue. Also, participants who have difficulty complying with the study procedures may be withdrawn. Participants will be withdrawn from medication if it is clinically determined that continuation may be unsafe. For example, participants who develop uncontrolled hypertension will be discontinued and instructed to see their primary care provider, or will be provided with referrals for medical care. Women who are assigned to one of the XR-NTX conditions and become pregnant during the intervention period will be withdrawn from study medication, referred for medical care, and the pregnancy will be followed until an outcome is known. Participants who experience intolerable side effects or other physical or psychiatric conditions regardless of relationship to the study medication will also be withdrawn from further study medication administration. However, there are few known medications that interact with naltrexone, so this is not a major concern for this study.
The medical clinician may determine that a participant’s clinical condition has deteriorated during the course of the study. Examples of clinical deterioration that might trigger a decision to withdraw the participant from medication include the following:
The initiation or recurrence of risky behaviors that make further participation unsafe;
Overdose;
Emergence of psychosis, suicidal ideation, severe cognitive impairment or dangerous criminal behaviors;
Evidence of general medical deterioration; or
New onset of psychiatric or medical conditions that would require intervention that would preclude continued participation in the study protocol.
In the event the participant is withdrawn from further medication administration, referrals to treatment programs or recommendations for medical care will be provided. The study medical clinician, in collaboration with the principal investigator may consult with the study medical monitor in making this decision. If possible, these participants will continue in research follow-up. At any time, participants may decide that they no longer wish to continue to receive medication or to participate in the study.
Participants withdrawn early from the study will be referred to appropriate services. Participants in the XR-NTX and XR-NTX plus PN conditions will not require any medication taper. Participants who are re-incarcerated will remain in the study. Those in the XR-NTX conditions will be eligible for additional injections if (1) they are returned to the MDC, and (2) their new jail term coincides with a scheduled injection.
Subject Payments
Study participants will receive gift cards as compensation for time, travel, parking, and other costs borne by the participant. Participants will be provided with medical management at no cost, and those in the XR-NTX and XR-NTX plus PN conditions will receive no-cost medication.
Incentives will be provided for participating in screening, twice-monthly medical management visits in months 1–3 and monthly visits in months 4–6 during the intervention phase, and assessment visits at months 1, 3, 6, and 12. A $25 incentive will be provided for screening; $20 for each of 9 medical management visits ($180), $40 for each of 3 post-release assessment visits ($120), and $80 for a final visit at month 12. Each participant will be eligible to receive $405.
Study Assessments
The full list of measures and instruments to be used in this study is provided in Table 1. In summary, screening/baseline assessments will include a medical and psychiatric history, physical examination, clinical lab tests (blood chemistry, hematology, urinalysis and hepatitis screening), 12-lead electrocardiograph, vital signs, pregnancy test (for females), and urine toxicology screen. Assessments completed at months 1, 3, 6, and 12 include drug use, dosing and protocol compliance, and other measures of status and functioning. Safety of study participants and intervention tolerability will be assessed throughout the study by assessment of adverse events, suicidality, and measures of drug use. Participants who experience an adverse event deemed as compromising their safety will be discontinued from participation and provided referrals for medical care.
The primary outcomes include opioid use and DSM-5 diagnosis of opioid use disorder via modified CIDI-2 Substance Abuse Module at 6-months post-intervention. Opioid use will be computed from the Timeline Follow-Back (TLFB) as the number of days of self-reported opioid use in the past 30 days at the 6-month assessment. The cost analysis will represent the provider perspective and measure and value resources associated with XR-NTX, XR-NTX plus PN, and ETAU. The total provider cost for each of the interventions will be the sum of costs associated with medical personnel (physician/nurses), labs, supplies and educational materials, equipment, medications, building space, and any other miscellaneous resources used in the interventions. Results of the cost analyses will be summarized as total intervention costs and mean cost per participant. Cost-effectiveness analyses (CEA) will compare differences in provider costs and participant outcomes in the experimental conditions (XR-NTX, and XR-NTX plus PN) relative to ETAU and produce incremental cost-effectiveness ratios. Three measures of effectiveness will be explored in the CEA: percentage of days abstinent from opioid use; percentage of days not incarcerated; and proportion of participants not engaging in HIV risk behaviors.
The full, cross-site analysis plan is described in the Chandler et al. paper included in this issue.
3. Results
Study recruitment began on July 15, 2015. As of April 6, 2016, 48 inmates have been screened, 44 have been consented, and 16 have been randomized. We expect a monthly accrual rate of eight participants per month, now that the logistical issues have been resolved. The primary reason for losing consented participants is early release (N=24). The remaining four did not pass the medical screening.
4. Discussion
Effectiveness of medications for chronic health disorders is compromised when patients do not adhere to their prescribed medication. Adherence to long-term regimens requires a combination of information about the treatment, counseling about the importance of adhering to the regimen, and reminders of scheduled appointments in order to counter non-compliance with medication protocols, which is the major element of failed therapy [14]. A meta-analysis of 33 medication adherence studies found that the “full benefits of medications cannot be realized at currently achievable levels of adherence; therefore more studies of innovative approaches to assist patients to follow prescriptions for medication are needed” [15; p. 2868]. Long-lasting or extended-release depot medications represent a strong innovation that could greatly increase medication adherence and improve pharmacotherapy practices and outcomes in correctional populations, who have consistently low rates of engagement in continuing care for substance use disorders. But much remains to be learned regarding adherence to XR-NTX. Springer and colleagues [16], for example, recently reported that HIV+ jail inmates with alcohol or opioid use disorder were less likely to continue XR-NTX treatment if they also had a positive drug screen for cocaine prior to release. The present study will improve our understanding of the predictors of adherence, as well as the extent to which adjunctive care, such as patient navigation, can enhance treatment participation and outcomes.
Providing XR-NTX before release from jail is expected to be a particularly beneficial condition to assist individuals in avoiding return to drug use once returned to the community. In spite of this, a national survey of correctional systems revealed that use of pharmacotherapies for SUD during re-entry is minimal [17]. Findings from the present study—and those of the SOMATICS cooperative—are needed to overcome the resistance to pharmacotherapy that remains common in U.S. correctional settings. A description of the overall collaboration can be found in Chandler et al. (this volume).
Acknowledgments
Funding for this study is provided by NIDA through a cooperative agreement (U01DA034743; PI: David Farabee, PI). The other collaborating sites under this cooperative are New York University (U01DA033336; PI: Josh Lee) and Friends Research Inc, (U01DA013636; PI: Robert Schwartz). The NIDA Science Partner is Redonna Chandler.
Footnotes
ClinicalTrials.Gov: NCT02110264
5. Conflicts of Interest
Drs. Farabee and Ling have received in-kind support in the form of study medications from Alkermes. Dr. McCrady has no conflicts of interest. Dr. Condon has received in-kind support in the form of study medication from Alkermes.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hammett TM, Harmon MP, Rhodes W. The burden of infectious disease among inmates of and releasees from US correctional facilities, 1997. Am J Public Health. 2002;92:189–194. doi: 10.2105/ajph.92.11.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among inmates of and releasees from US correctional facilities, 2006: declining share of epidemic but persistent public health opportunity. PloS one. 2009;4(11):e7558. doi: 10.1371/journal.pone.0007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, Koepsell TD. Release from prison—A high risk of death for former inmates. The New England Journal of Medicine. 2007;356(2):157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binswanger IA, Blatchford PJ, Mueller SR, Stern MF. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Annals of Internal Medicine. 2013;159(9):592–600. doi: 10.7326/0003-4819-159-9-201311050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merrall LC, Kariminia A, Binswanger IA, Hobbs MS, Farrell M, Marsden J, Hutchinson SJ, Bird SM. Meta-analysis of drug-related deaths soon after release from prison. Addiction. 2010;105:1545–54. doi: 10.1111/j.1360-0443.2010.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farabee D. Naltrexone as negative reinforcement: Comments on “A behavioral analysis of coercion in substance abuse treatment. Journal of Substance Abuse Treatment. 2006;31(2):141–142. doi: 10.1016/j.jsat.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Cornish JW, Metzger D, Woody GE, Wilson D, McLellan AT, Vandergrift B, O’Brien CP. Naltrexone pharmacotherapy for opioid dependent federal probationers. J Subst Abuse Treat. 1997;14:529–534. doi: 10.1016/s0740-5472(97)00020-2. [DOI] [PubMed] [Google Scholar]
- 8.Shearer J, Wodak AD, Dolan KA. Evaluation of a prison-based naltrexone program. International Journal of Prisoner Health. 2007;3(3):214–224. [Google Scholar]
- 9.Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol. 1994;(Suppl 12):70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- 10.Gupta SK. Intention-to-treat concept: a review. Perspectives in Clinical Research. 2011;2(3):109. doi: 10.4103/2229-3485.83221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. The Lancet. 2011;377(9776):1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- 12.Robinson-White S, Conroy B, Slavish KH, Rosenzweig M. Patient navigation in breast cancer: a systematic review. Cancer Nursing. 2010;33(2):127–140. doi: 10.1097/NCC.0b013e3181c40401. [DOI] [PubMed] [Google Scholar]
- 13.Battaglia TA, Roloff K, Posner MA, Freund KM. Improving follow-up to abnormal breast cancer screening in an urban population. Cancer. 2007;109(S2):359–367. doi: 10.1002/cncr.22354. [DOI] [PubMed] [Google Scholar]
- 14.Haynes RB, Montague P, Oliver T, McKibbon KA, Brouwers MC, Kanani R. Interventions for helping patients follow prescriptions for medications. Vol. 1. Oxford: The Cochrane Library; 2002. [DOI] [PubMed] [Google Scholar]
- 15.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 16.Springer SA, Brown SE, Di Paola A. Correlates of retention on extended-release naltrexone among persons living with HIV infection transitioning to the community from the criminal justice system. Drug and Alcohol Dependence. 2015;157:158–165. doi: 10.1016/j.drugalcdep.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedmann PD, Hoskinson R, Jr, Gordon M, Schwartz R, Kinlock T, Knight K, Flynn PM, Welsh WN, Stein LA, Sacks S, O’Connell DJ. Medication-assisted treatment in criminal justice agencies affiliated with the criminal justice-drug abuse treatment studies (CJ-DATS): availability, barriers, and intentions. Substance Abuse. 2012;33(1):9–18. doi: 10.1080/08897077.2011.611460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metzger DS. The risk assessment battery (RAB): Validity and reliability. Presented at Sixth Annual Meeting of the National Cooperative Vaccine Development Groups for AIDS; Alexandria, VA. 1993. [Google Scholar]
- 19.Gossop M. The development of a short opiate withdrawal scale (SOWS) Addictive Behaviors. 1990;15(5):487–490. doi: 10.1016/0306-4603(90)90036-w. [DOI] [PubMed] [Google Scholar]
- 20.Sobell LC, Sobell MB. Measuring alcohol consumption. Humana Press; 1992. Timeline follow-back; pp. 41–72. [Google Scholar]
- 21.Bond A, Lader M. The use of analogue scales in rating subjective feelings. British Journal of Medical Psychology. 1974;47(3):211–218. [Google Scholar]
- 22.Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS) Journal of Psychoactive Drugs. 2003;35(2):253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]