Abstract
Prostate cancer (PCa) remains the most common cancer in American men. African-American (AA) men continue to have higher PCa prevalence and mortality rates compared to men in other populations. In addition to socioeconomic factors and lifestyle differences, molecular alterations contribute to this discrepancy. We summarize molecular genetics research results interrelated with the biology of PCa racial disparity. Androgen and androgen receptor (AR) pathways have long been associated with prostate growth. Racial differences have also been found among variants of genes of the enzymes involved in androgen biosynthesis and metabolism. Growth factors and their receptors are a potential cause of the disparity in PCa. Recent molecular and biotechnological approaches in the field of proteomics and genomics will greatly aid the advancement of translational research on racial disparity in PCa, which may help, in finding new prognostic markers and novel therapeutic approaches for the treatment of PCa in AA.
Keywords: Prostate Cancer, Androgen Receptors (AR), Racial Disparity
2. INTRODUCTION
Prostate cancer (PCa) is the second most common cause of death from malignancy in American men. The incidence of PCa varies widely between ethnic populations and countries. In 2015, approximately 220,800 cases of prostate cancer was newly diagnosed; 27,540 PCa deaths were estimated in the United States. PCa is the most commonly diagnosed cancer among African American (AA) men. In AA, there is a higher incidence of PCa at a younger age with poor prognosis than men of other ethnicities (1–3). It is estimated that one in five AA men will be diagnosed with prostate cancer in their lifetime (4). Along with positive family history and older age, African ancestry has long been recognized as an important risk factor for PCa (5, 6). The underlying reasons for this disparity are not well understood, although existing evidence implicates important genetic components. While it has been argued that racial variation may be largely due to lifestyle, dietary, socioeconomic (7, 8), or clinical factors, these cannot fully explain the discrepancy (1–3, 9) or the results of migration studies, and consequently, genetic parameters may be important. Studies of the pathology and recurrence of tumors in AA and Caucasian American (CA) men have suggested that racial differences in the biology of PCa tumors may explain observed differences in outcome (10, 11).
Although there are some effective treatment approaches for clinically localized PCa through surgery and radiotherapy, however, the metastatic PCa remains incurable. The metastatic potential of tumor cells and its possible dissemination to secondary sites are critical factors related to its mortality rates. In spite of the high incidence and mortality rates, the molecular mechanisms involved in oncogenesis and progression to PCa are still poorly understood, especially related to the progression to the metastatic form. PCa etiology remains obscure and its tumors vary from indolent forms with low progression rates to extremely aggressive with rapid growth rates. Several methods have been used for the study of oncogenes expression, characterization, chromosomal aberrations and heredity loci in this neoplastic disease (12, 13). In the current review, we are highlighting recent research on differential androgen levels in prostate cancer development in ethnic population.
3. DIFFERENTIAL ANDROGEN LEVELS IN PROSTATE CANCER DEVELOPMENT IN ETHNIC POPULATION
Androgens and the androgen receptor pathway constitute the most intensely studied field in PCa. Several aspects of the pathway are related to the racial disparity of PCa.
3.1. Androgen receptors (AR)
Prostate cells require androgen stimulation in the form of testosterone and 5α-dihydrotestosterone (DHT-A) for normal growth and maintenance. The AR is the key receiver of androgen signal. After formation of the complex AR- androgen, it translocated to the nucleus and acts in the transcription of androgen response genes (14). AR- androgen complex regulates the expression of genes necessary for the growth and development of both normal and malignant prostate tissue. Immunohistochemical studies of malignant and benign prostate tissue from AA and Caucasian American (CA) men who underwent radical prostatectomy for PCa, showed expression of AR protein was 22% higher in the benign prostate and 81% higher in PCa of AA patients (15). This suggests that differences in androgenic stimulation may play an important role in racial disparity.
The AR gene is over 90 kb in length, located within chromosome Xq11–12 and has eight exons. Exon 1 encodes the N-terminal (transactivation) domain, which controls transcriptional activation of the receptor as well as two polymorphic trinucleotide repeats (CAG and GGC), which code for polyglutamine and polyglycine tracts, respectively, in the N-terminal domain. Studies have shown that CAG repeat varies in length from 11 to 31 repeats in normal men (16), and an inverse relationship has been demonstrated between CAG repeat length and AR transcriptional activation ability (17). Short CAG and GGC repeat lengths are associated with an increased risk of developing PCa (18–20), specifically individuals with CAG repeat length less than 20 and GGC repeat length less than 16 (18, 20–22). AA men populations have significantly shorter repeat length than CA men (17, 23, 24).
Prostate cancer may occur at a younger age and progress more rapidly in AA than CA because of racial differences in androgenic stimulation of the prostate. CWR-22 xenograft model of androgen regulated genes include CDK1 and CDK2, cyclin A and B1 (25); α-enolase, α-tubulin, IκBα, IGFBP-5 (26), PSA, hK-2, Nkx3.1., ARA-70 (27); EF-1α, tomoregulin, TRX-R1, and Mxi-1 are expressed at higher levels in recurrent and androgen-dependent tumors with higher proliferation rate in PCa (27, 28).
3.2. Serum androgen levels
Although there is no clear relationship between circulating androgen levels and PCa (29–31), high levels of androgens have long been considered as risk factor (29, 32). Racial differences in maternal androgens during gestation, serum androgens and time of puberty during adolescence may imprint the AA prostate to respond more to similar levels of androgenic stimulation in adulthood. AA men (aged 31 to 50) have higher mean serum testosterone level (15%) (33) than CA men (34). However, elevated testosterone and dihydrotestosterone (DHT) have not been persuasively shown to increase the risk of PCa (35).
3.3. Tissue androgens
Testosterone is the major circulating androgen, and DHT is 1/10 the concentration of testosterone in serum. DHT is the major intra-prostatic androgen. In prostate tissues 5α-reductase converts testosterone to DHT. DHT is a preferred ligand for the AR, since AR–DHT complex is more stable than the AR–testosterone complex. Also androgen levels are measured for radical prostatectomy from clinically localized PCa (37).
Steroid hormones analysis from snap frozen tissue obtained intra-operatively from radical prostatectomy specimens from AA and CA showed similar levels of testosterone, DHT, dehydroepiandrosterone, dehydroepiandrosterone sulfate, and PSA. However, AA had higher androstenedione (ASD) and sex hormone-binding globulin (SHBG) levels. Age, BMI, PSA, and pathologic gleason sum and stage (nonparametric rank analysis) make the racial differences between ASD and SHBG. A higher ASD tissue level in AA is not the result of higher levels of testosterone whereas higher serum levels of SHBG have been postulated to be protective against prostate cancer. SHBG decreases bioavailable testosterone for androgen receptor ligand activation. Although AA has been reported to have higher serum levels of SHBG than CA (36), but SHBG serum levels have not correlated significantly with risk of prostate cancer (37, 38).
More recently, SHBG has been demonstrated to be produced in the human PCa cell lines (LNCaP, DU-145, and PC3), and in cultured human prostate epithelial and stromal cells (27). SHBG binds to a membrane receptor in prostate (39), which was reported to initiate an intracellular signal that increased cAMP levels and modulated androgen action in the prostate (40). Immunostaining revealed heterogeneous expression of SHGB protein, primarily in the epithelium of both benign prostate (27) and prostate cancer (41). Also in situ hybridization of adjacent sections confirmed local synthesis of SHBG (27). Higher tissue levels of SHBG may enhance androgen action in prostate tissue (42) of AA through cAMP-dependent pathways.
4. GENES INVOLVED IN ANDROGEN BIOSYNTHESIS
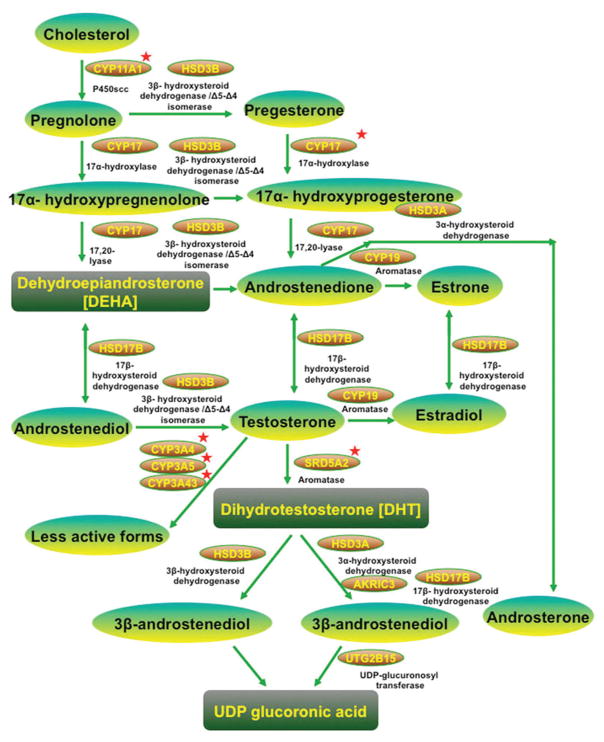
Variants in the genes involved in androgen biosynthesis and metabolism are compelling candidates for susceptibility factors in PCa pathogenesis. Many of these genes have been found to harbor genetic polymorphisms. These polymorphisms can potentially change androgen levels in prostate tissue. Genetic polymorphisms in these pathways are summarized in Figure 1.
Figure 1.
Gene involved in Androgen biosynthesis and metabolism in prostate cancer. Genetic polymorphisms in genes associated with androgen biosynthesis/metabolism and AR, which show differential racial frequencies and potential association with prostate cancer. Red star indicates the genes, which has racial frequency difference.
4.1. CYP11A1
CYP11A1 gene is the first rate-limiting step for biosynthesis of both testosterone and estrogen, located on 15q23–q24 chromosome encodes for the enzyme P450scc, and has pentanucleotide (TAAAA) n repeat range (4 to 10-repeat sequences) at 5′-untranslated region (UTR) (60), which catalyzes cholesterol to pregnolone. Population based studies have found higher prevalence of a 6-repeat allele in Japanese populations compared with the higher prevalence of a 4-repeat allele in European and African populations (43, 44). Interestingly, Japanese PCa patients without the 4-repeat allele have an increased risk of metastatic tiPCa compared to those with the 4-repeat allele (44). However, a positive association with PCa risk was not identified in the European populations (45).
4.2. CYP17
CYP17 gene is located on chromosome 10, encodes the cytochrome P450c17a enzyme (46), which mediates both 17α-hydroxylase and 17,20-lyase activities in testosterone biosynthesis in the gonads and adrenals (46). The 5′-untranslated promoter region of CYP17 contains a polymorphic T-to-C substitution that gives rise to A1 (T) and A2 (C) alleles (47). Previous studies reported that the A2 allele may be associated with an increased risk of PCa (48–53); however, other results have either been inconclusive (54, 55) or showed a possible increased risk from the A1 allele (56, 57). The results of a meta-analysis suggest that CYP17 polymorphisms may have a role in PCa susceptibility in AA but not CA men (58). The A2 allele was slightly less frequent in AA versus CA men, but a different study had the opposite finding (59). Ultimately, there may be little difference in A2 frequency and a null effect of the CYP17 polymorphism on androgen levels.
4.3. SRD5A2
Intra-prostatic DHT levels may be integral to racial variations in risk (60). Steroid 5α-reductase irreversibly converts testosterone into DHT. Two forms of steroid 5α-reductase exist, steroid 5α-reductase type1 (SRD5A1) and steroid 5α-reductase type 2 (SRD5A2). SRD5A1 is expressed more abundantly in extra-prostatic tissues (e.g., skin), whereas SRD5A2 is exclusively expressed in the prostate (61). Activity of 5α-reductase was reported lower in Asian than in white and black men (60, 62). This gene is more polymorphic in AA men than previously assumed (63), though SRD5A2 TA repeats alleles are only present in high-risk AA men and not in lower risk CA and Asian men (60, 62). Thus, it has been proposed that certain steroid 5α-reductase enzyme variants encoded by SRD5A2 genes marked by particular TA repeat alleles may result in an elevation of enzyme activity, leading to an increased prostatic level of DHT, which may increase the risk for developing PCa. Furthermore, the V89L and A49T variants of SRD5A2 gene have been shown to alter the conversion of testosterone to DHT (64, 65). While the V89L polymorphism is decreased the production of DHT (66) and A49T variant increases its production, particularly in AA and Hispanic men (67)
4.4. HSD3B family
The HSD3B1 and HSD3B2 genes are located on 1p13.1., and encode 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase 1 and 2 isoenzymes (3β-HSD types 1 and 2). The proteins are bifunctional enzymes that catalyze androstendione production in steroidogenic tissues and convert the active DHT into inactive metabolites in steroid target tissues (68). A N367T (AAC>ACC, rs1047303) polymorphism in HSD3B1 has been reported to present at a high frequency in Caucasian (31%), inter-medium frequency in AA (11.7.%) and low frequency in Asian men (8.5.%), although the variant has a similar activity to the wild type (69). A complex (TG)n (TA)n(CA)n dinucleotide repeat polymorphism is found in intron 3 of the HSD3B2 gene (70). The common alleles occurred at variable frequencies in different racial populations. The longer allele formed more stable hairpin structures with faster degradation rate of DHT. Longer alleles commonly found in Asian men (71, 72), whereas short alleles have been found to be associated with an increased PCa risk in Caucasian but not in AA men (71). There are two SNPs in HSD3B2, rs1819698 and rs1538989 reported which is more common in AA than CA and increased the risk of PCa in AA but not CA (77). The interaction between HSD3B1 and HSD3B2 polymorphisms has also been investigated. Although the N367T polymorphism in HSD3B1 is weakly associated with PCa risk, but the combination with HSD3B2 rs1819698 is greatly enhanced the association (73).
4.5. CYP19A1
The CYP19A1 gene is located on chromosome 15q21.1., and encodes the enzyme aromatase, which catalyzes the irreversible conversion of C19 androgens, androstenedione and testosterone, to the C18 estrogens, estrone and estradiol respectively. More than 30 SNPs have been detected in different populations. Several SNPs (rs2470152, rs749292, rs727479) are confirmed to be associated with serum estradiol of men (74, 75). The polymorphisms of CYP19 gene in Caucasian and AA men (rs2470152, rs12439137, rs3751592, rs2470164) are associated with PCa risk (77). Particularly, the rs2470164 was reported to increase PCa risk in Caucasian men with different frequency among healthy Caucasian (50%) and AA men (5.6.%). The tetranucleotide repeat (TTTA) n is located in intron 4 of CYP19A1. The TTTA repeat numbers range from 7 to 13 and are designated as A1 to A7 according to the repeat number. Most studies for this polymorphism are among Asian men. In Asian men, A1 is found more frequently (~50% of the population) than all other alleles (76, 77). Several studies have shown that TTTA repeat length is associated with PCa risk (57, 80, 81, 82), however, TTTA repeat length is not associated with the American population (80). The polymorphism Arg264Cys substitution (rs700519) is found at a higher frequency in Indian men (27%) (78) in comparison to AA (16.8.%) and Caucasian men (4–8.1.%) (79–81). Studies among Caucasian and Indian men showed a tendency for this polymorphism to increase risk of PCa (78, 81), but large scale population studies failed to confirmed the results in Caucasian men (75, 79, 80).
4.6. CYP3A family
Cytochrome P450 3A (CYP3A) enzymes hydroxylate testosterone and dehydroepiandrosterone to less active metabolites. The CYP3A locus consists of four genes in humans, CYP3A4, CYP3A5, CYP3A7 and CYP3A43, all of which reside in a 231 kb region of chromosome 7q21–22.1 (82) (see details in Table 1).
Table 1.
CYP3A family genes and their allelic functions
| Gene | Characteristic | Reference |
|---|---|---|
| CYP3A4 |
|
(167–169) (170, 171) (172) |
| CYP3A5 |
|
(173) (170, 174) (175, 176) |
| CYP3A43 |
|
(177, 178) (179) |
4.7. HSD17B family
The 17β-hydroxysteroid dehydrogenases (17β-HSDs) are involved in regulation of estrogens and androgens by catalyzing the reduction of 17-ketosteroids or the oxidation of 17β-hydroxysteroids. 17β-HSD1 is encoded by HSD17B1 gene located on 17q21 and involved in estrogen and testosterone biosynthesis. The polymorphism of HSD17B1 (Ser313Gly, rs605059) is detected in Caucasian men (40%), and associated with either familial or sporadic cases of PCa (83). In studies from multi-ethnic groups (The Breast and Prostate Cancer Cohort Consortium, BPC3), four common SNPs (rs676387, rs605059, rs598126, rs2010750) were detected, although none were found to be associated with PCa risk, however, only varying frequencies of haplotypes between different races. The haplotype CAAC is commonly found in AA men and CAGC is more prevalent in white and black than Asian men, whereas inverse association with PCa risk in Latino and Japanese American but not in AA, Native Hawaiian, or white men (83).
17β-HSD2 is encoded by HSD17B2 and located on 16q24. 17β-HSD2 is involved in the conversion of active androgens into their less active forms. SNPs in HSD17B2 (rs1424151) are significantly associated with plasma testosterone level in Caucasian men (84), but no association with PCa was detected (84, 85).
17β-HSD3 is encoded by HSD17B3 and located on 9q22, catalyzes androstenedione to testosterone. The frequency of the G289S polymorphism (rs2066479) of HSD17B3, was 4.3.–7.3.% in Caucasian men and was reported to significantly increase PCa risk in Italian men (86), but studies in Finnish and Swedish men found no positive associations (87).
HSD17B4 gene is located on 5q21 and encodes androgen/estrogen inactivating enzyme 17β-HSD4. It was reported to be associated with the outcome of PCa patients (88, 89). 17β-HSD5 belongs to the aldo-keto reductase (AKR) superfamily and is formally known as AKR1C3 encoded by the AKR1C3 gene located on 10p14-p15, It catalyzes the conversion of androstenedione to testosterone and DHT to androstanediol. An A to G substitution was identified in exon 2 that confers a Glu77 Gly (rs41306308) change, and occurred in 4.8.% of Caucasian men but was completely absent in Asian men, and the Glu77Gly polymorphism was associated with lower testosterone levels in serum (90). Moreover, promoter polymorphism (A to G, rs3763676) of AKR1C3 is more prevalent in Caucasian than Asian men (90), whereas men with the A allele have significantly lower risk of PCa (91).
4.8. UGT2B15
UGT2B15 is a member of UDP glucuronosyltransferases (UGTs) family, which glucuronidate steroids and other endogenous molecules, encoded by the UGT2B15 gene and located on 14q13–q21.1. It has a high capacity to glucuronidate 3α- and rostenediol and a moderate capacity for DHT. A nonsense mutation in codon 85 (aspartate>tyrosine, D85Y, Asp85Tyr) has been identified in the UGT2B15 gene. The 85Y variant associates with a 2-fold increase in activity for 3α-androstenediol and DHT, it is likely to lead to lower androgen exposure compared with 85D. A study found that Asians have higher 85D allele frequency than Caucasians (92), however, other studies are inconclusive (93, 94).
5. OTHER FACTORS INVOLVE IN PROSTATE CANCER DEVELOPMENT
5.1. Sex hormone binding globulin
Sex hormone-binding globulin (SHBG) gene is located on 17p12-p13 and encodes a steroid binding protein, and act as regulator of free plasma androgens. It also mediates androgen and estrogen signaling at the cell membrane via cyclic adenosine monophosphate. Most of the studies are reported that black men have higher PCa risk with higher plasma SHBG levels in prostate tissue than white and Asian men (95). Interestingly, the higher risk population have a higher SHBG level, A collaborative analysis of 18 prospective studies found the fifth highest serum SHBG levels had a relative PCa risk reduction of 14% when compared with the fifth lowest (96). A common polymorphism in the SHBG, and D356N gene encodes for an additional N-glycosylation consensus site, which may reduce its clearance from circulation and alter its binding to membrane receptors (97). In the multicenter study the SHBG-D356N heterozygotic polymorphism had a higher frequency in white men (17%) than black men (7.8.%) (129). The D356N heterozygote is associated with increasing PCa risk in non-Hispanic white but not in black men. Studies carried out in British and US men reported no association found between PCa and SHBG polymorphisms (98).
5.2. Growth factors and receptors
In most studies growth factor receptors concerning the racial disparity of PCa are EGFR and EPHB2 (Table 2). In addition, AA men have been found to have higher IGF-1 and lower IGFB-3 levels, which may cause higher tumor growth with lower anti-tumor activity (99).
Table 2.
Growth receptors and their characteristics in the prostate cancer heath disparities
| Growth receptors | Characteristics | References |
|---|---|---|
| EGFR (Epidermal growth factor receptor) |
|
(180–186) |
| EPHB2 (Ephrin type-B receptor 2) |
|
(187–189) |
| VDR (Vitamin D receptor) |
|
(190–192) |
5.3. Differences in apoptotic genes in relation to prostate cancer racial disparity
5.3.1. Anti-apoptotic BCL-2
Studies show that altered expression of the BCL-2 gene may be an important factor underlying the greater aggressiveness of PCa in AA men (100). BCL-2 has a central role in preventing cancer cells from death, via its anti-apoptotic effect, and its up-regulation in AA men may be responsible for PCa cell survival and resistance to therapies. Thus, a positive connection between over-expression of BCL-2 and increased in prostate tumors proliferation in AA but not in CA men, and suggest BCL-2 dependent aggressive behavior of PCa in AA men (100).
5.3.2. MDM2
In response to stress, cells activate a complex pathway involving tumor suppressor gene p53that is responsible for cell cycle arrest, DNA repair, and apoptosis as protection from the deleterious effects of mutation (101). MDM2 is a key negative regulator of tumor suppressor p53, by targeting p53 for proteasomal degradation (102–104). Several reports suggested that MDM2 overexpression was significantly associated with advanced stage PCa (105–107). Another studies have also shown that inhibiting MDM2 expression enhances the effects of radiation and chemotherapy on PCa cells (108–110). A single nucleotide polymorphism in the MDM2 promoter, SNP309, enhances transcriptional activation of MDM2 and has been associated with early onset of several types of cancer (111–115). Interestingly, MDM2, protein expression was significantly higher in CA(78%) than AA(45%) patients (116), and also MDM2 and AA ethnicity have both been associated with poor prognosis. However, the relationship between these two variables was neither causative nor correlative.
5.4. Genetics variations between AA and CA prostate cancer
PCa is also caused by multiple genes through complex interaction including environmental factors (85, 117–119). There may be ethnic variation in the frequency of alleles that may be associated with PCa risk and/or progression. Although the incidence and mortality for PCa may differ among different racial groups, the increased risk for PCa attributed to family history of this disease is consistent across different racial backgrounds, supporting the possibility of a common genetic basis of disease (120). The analysis of genetic alterations in PCa is challenging because PCa often has genetic and morphological heterogeneity, multifocality and the presence of more than one lesion of independent origin (121–124). Linkage studies, determine the susceptibility loci for PCa on several chromosomes and several candidate genes if the tumors in AA men are different from those in CA men (125).
5.4.1. Chromosome 8
The short arm of chromosome 8 (8p22–23) has been proposed as a potential location for one or more genes important in the development of PCa (126, 127). The short arm of chromosome 8 is frequently deleted in both adenocarcinomas and PINs (128, 129), which has lead to the assumption that the inactivation of an unidentified tumor suppressor gene on 8p is involved in prostate tumor initiation (130–132). Other studies on the loss of chromosome 8p in AA and CA men have generated conflict findings (133), however, the racial differences in the association between PCa recurrence and several prognostic factors of cancer progression, including Gleason score, surgical margin, and TNM stage are positively correlated.
5.4.2. miRNA
MicroRNAs (miRNAs) are a class of small, endogenous, non-coding RNAs that regulate gene expression at the levels of transcription and post-transcription (134, 135). miRNA inhibits translation of target genes involved in a variety of fundamental cellular processes including organ development, differentiation, and cancer formation (136–139). Functional studies of individual miRNAs have shown that miRNAs can act as oncogenes or tumor suppressor genes (140). miRNAs are differently dysregulated in uterine leiomyomas in both AA and CA women, indicating that miRNAs expression are associated with the racial disparity in cancer (145). In the prostate, the expression of 5 miRNAs, miR- 30c, miR-301, miR-219, miR-261, and miR- 1b1, were reported racially different in benign prostate tissue (146). Expression of commonly dysregulated miRNAs in PCa revealed racial differences in the expression of let-7c and miR30c in AA prostate tissue (147). Thus, distinct gene expression and genome-wide copy number variation between AA and CA prostate cancer might play a role in the racial disparity of PCa.
5.5. PSA levels in AA and CA prostate cancer patients
FDA approved the PSA test in the year 1986 for monitoring the status of disease and diagnosis (1992)., The test is performed on symptomatic and asymptomatic men in an effort to diagnosed PCa early and to monitor disease recurrence and progression (148). Past surveys of urologists revealed significant variation in the use of the PSA test (149), including racial disparities in PSA surveillance, with AA men half as likely as CA men to receive annual monitoring (150). A recent study concluded that PSA testing is probably not able to explain current racial differences in PCa mortality rates (151). Interestingly, recently a relationship was reported between serum PSA levels and polymorphisms in the PSA and AR genes (152). Specifically, serum PSA levels increased by 7% with each decreasing AR CAG repeats allele size among individuals homozygous for a single nucleotide polymorphism in the PSA gene promoter. A recent study of the ERDA1 locus revealed that large CAG repeats are more common among Asian populations, less common in populations of European ancestry, and least common in African populations (153). This pattern showed similarity with the AR trinucleotide repeats studies.
5.6. Familial prostate cancer susceptibility genes
Variation in genetic susceptibility also play a key role in the incidence of, and mortality from, prostate cancer in AA and CA men (154). Although the etiology of PCa is complex, the study on family and twin cases suggested that the inherited genetic susceptibility is a critical risk factor in PCa. PCa susceptibility genes have been mapped to 1q24–25 (155), Xq27–28 (156), and 8p22–23 (126), and other loci including PCaP and CaPB (157), HPC20 (158), and HPC2/ELAC (159), have been examined. The frequency of these genes and an understanding of their importance in hereditary prostate cancer cases, sporadic white American cases, and unaffected controls continues to evolve, but almost all reported studies contain few AA. Recently, more groups have begun to examine affected AA families. Familial aggregation rates were similar in AA and white Americans men (85). Race-specific penetrance estimates for the carriers of deleterious genotypes, which were similar in AA and white Americans, but low in Asian Americans (160). The higher incidences of prostate cancer in AA may not be caused by a higher prevalence of germline mutations predisposing to the disease in AA. Men with more than six family members with prostate cancer had a higher chance of presenting with lymph node metastatic or widely metastatic prostate cancer than men with only four to six family members affected (161). Linkage analysis of 33 AA prostate cancer families from two independent research groups provided some evidence for clustering at HPC1. Increased evidence of linkage was found in families with prostate cancer diagnosis younger than age 65 years, and male-to-male transmission (162). These studies, though very preliminary, suggest that genetics could contribute to the increased aggressiveness of prostate cancer in AA.
5.7. Other genes in prostate cancer
5.7.1. MSR1
Recently, the macrophage scavenger receptor 1 (MSR1) gene has been proposed as a link between germline alterations in 8p and PCa (163, 164). Both common sequence variants and rare germline mutations have been suggested as potential PCa susceptibility factors. Several rare germline mutations of the MSR1 gene were found to co-segregate with PCa, and one of the germline mutations was associated with an increased risk of PCa among AA men (163). In a subsequent study of CA men, the same authors examined five common sequence variants of MSR1 and reported significantly different allele frequencies for each of the variants among men with PCa compared with unaffected men (164), with each, except INDEL7, associated with an elevated risk for PCa. An ensuing study examined each of these five common MSR1 sequence variants in AA men (118). They found that the Asp174Tyr mutation is nearly twice as common among PCa patients compared with controls; however, after adjusting for age, none of the sequence variants were associated with a significantly increased risk of PCa, providing limited support for an association in AA men.
5.7.2. Caveolin-1
Caveolin-1 is a structural protein found in caveolae that support cholesterol transport and signal transduction. Caveolin-1 suppresses c-myc-mediated apoptosis, and is overexpressed in murine and human PCa. Caveolin-1 is expressed higher levels in PCa in AA than CA (165), and PCa grows more rapidly in AA due to reduced rates of apoptosis (165). Therefore, radiation and androgen-deprivation therapy are less effective for AA PCa patients (166).
6. CONCLUSIONS
Due to the complex nature of the AR signaling pathway, there are different ways that genetic polymorphisms contribute to the deregulation of this pathway and increase PCa risk. Future detailed studies need to include an integrated analysis of the combined effect of these polymorphisms on the AR pathway as well as androgen metabolism/biosynthesis in the development of PCa. Analysis of these complex polymorphisms in androgen synthesis and AR signaling pathway with racial disparities are causing difficult situation at clinical front to achieve functional confirmation to establish their contribution to PCa development. Recent molecular and biotechnological approaches in the field of proteomics and genomics will greatly aid the advancement of translational research on racial disparity in PCa, which may help, in finding new prognostic markers and novel therapeutic approaches for the treatment of PCa in AA.
Acknowledgments
This study was supported in part by the funds 5G12MD007602 from NIH and CA193758 from NCI.
References
- 1.Cotter MP, Gern RW, Ho GY, Chang RY, Burk RD. Role of family history and ethnicity on the mode and age of prostate cancer presentation. Prostate. 2002;50(4):216–21. doi: 10.1002/pros.10051. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman RM, Gilliland FD, Eley JW, Harlan LC, Stephenson RA, Stanford JL, Albertson PC, Hamilton AS, Hunt WC, Potosky AL. Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2001;93(5):388–95. doi: 10.1093/jnci/93.5.388. [DOI] [PubMed] [Google Scholar]
- 3.Platz EA, Rimm EB, Willett WC, Kantoff PW, Giovannucci E. Racial variation in prostate cancer incidence and in hormonal system markers among male health professionals. J Natl Cancer Inst. 2000;92(24):2009–17. doi: 10.1093/jnci/92.24.2009. [DOI] [PubMed] [Google Scholar]
- 4.Desantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin. 2013 doi: 10.3322/caac.21173. [DOI] [PubMed] [Google Scholar]
- 5.Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361(9360):859–64. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 6.Schaid DJ. The complex genetic epidemiology of prostate cancer. Hum Mol Genet. 2004;13(Spec No 1):R103–21. doi: 10.1093/hmg/ddh072. [DOI] [PubMed] [Google Scholar]
- 7.Du XL, Fang S, Coker AL, Sanderson M, Aragaki C, Cormier JN, Xing Y, Gor BJ, Chan W. Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma: findings from a large community-based cohort. Cancer. 2006;106(6):1276–85. doi: 10.1002/cncr.21732. [DOI] [PubMed] [Google Scholar]
- 8.Reddy S, Shapiro M, Morton R, Jr, Brawley OW. Prostate cancer in black and white Americans. Cancer Metastasis Rev. 2003;22(1):83–86. doi: 10.1023/A:1022216119066. [DOI] [PubMed] [Google Scholar]
- 9.Robbins AS, Whittemore AS, Thom DH. Differences in socioeconomic status and survival among white and black men with prostate cancer. Am J Epidemiol. 2000;151(4):409–16. doi: 10.1093/oxfordjournals.aje.a010221. [DOI] [PubMed] [Google Scholar]
- 10.Powell IJ. Prostate cancer in the African American: is this a different disease? Semin Urol Oncol. 1998;16(4):221–6. [PubMed] [Google Scholar]
- 11.Robbins AS, Whittemore AS, Van Den Eeden SK. Race, prostate cancer survival, and membership in a large health maintenance organization. J Natl Cancer Inst. 1998;90(13):986–90. doi: 10.1093/jnci/90.13.986. [DOI] [PubMed] [Google Scholar]
- 12.Li PE, Nelson PS. Prostate cancer genomics. Curr Urol Rep. 2001;2(1):70–8. doi: 10.1007/s11934-001-0028-6. [DOI] [PubMed] [Google Scholar]
- 13.Karan D, Lin MF, Johansson SL, Batra SK. Current status of the molecular genetics of human prostatic adenocarcinomas. Int J Cancer. 2003;103(3):285–93. doi: 10.1002/ijc.10813. [DOI] [PubMed] [Google Scholar]
- 14.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 15.Gaston KE, Kim D, Singh S, Ford OH, 3rd, Mohler JL. Racial differences in androgen receptor protein expression in men with clinically localized prostate cancer. J Urol. 2003;170(3):990–3. doi: 10.1097/01.ju.0000079761.56154.e5. [DOI] [PubMed] [Google Scholar]
- 16.Edwards A, Hammond HA, Jin L, Caskey CT, Chakraborty R. Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups. Genomics. 1992;12(2):241–53. doi: 10.1016/0888-7543(92)90371-X. [DOI] [PubMed] [Google Scholar]
- 17.Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994;22(15):3181–6. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovannucci E, Stampfer MJ, Krithivas K, Brown M, Dahl D, Brufsky A, Talcott J, Hennekens CH, Kantoff PW. The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci U S A. 1997;94(7):3320–3. doi: 10.1073/pnas.94.7.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy DO, Scher HI, Bogenreider T, Sabbatini P, Zhang ZF, Nanus DM, Catterall JF. Androgen receptor CAG repeat lengths in prostate cancer: correlation with age of onset. J Clin Endocrinol Metab. 1996;81(12):4400–5. doi: 10.1210/jcem.81.12.8954049. [DOI] [PubMed] [Google Scholar]
- 20.Platz EA, Giovannucci E, Dahl DM, Krithivas K, Hennekens CH, Brown M, Stampfer MJ, Kantoff PW. The androgen receptor gene GGN microsatellite and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7(5):379–84. [PubMed] [Google Scholar]
- 21.Ingles SA, Ross RK, Yu MC, Irvine RA, La Pera G, Haile RW, Coetzee GA. Association of prostate cancer risk with genetic polymorphisms in vitamin D receptor and androgen receptor. J Natl Cancer Inst. 1997;89(2):166–70. doi: 10.1093/jnci/89.2.166. [DOI] [PubMed] [Google Scholar]
- 22.Stanford JL, Just JJ, Gibbs M, Wicklund KG, Neal CL, Blumenstein BA, Ostrander EA. Polymorphic repeats in the androgen receptor gene: molecular markers of prostate cancer risk. Cancer Res. 1997;57(6):1194–8. [PubMed] [Google Scholar]
- 23.Sartor O, Zheng Q, Eastham JA. Androgen receptor gene CAG repeat length varies in a race-specific fashion in men without prostate cancer. Urology. 1999;53(2):378–80. doi: 10.1016/S0090-4295(98)00481-6. [DOI] [PubMed] [Google Scholar]
- 24.Irvine RA, Yu MC, Ross RK, Coetzee GA. The CAG and GGC microsatellites of the androgen receptor gene are in linkage disequilibrium in men with prostate cancer. Cancer Res. 1995;55(9):1937–40. [PubMed] [Google Scholar]
- 25.Gregory CW, Johnson RT, Jr, Presnell SC, Mohler JL, French FS. Androgen receptor regulation of G1 cyclin and cyclin-dependent kinase function in the CWR22 human prostate cancer xenograft. J Androl. 2001;22(4):537–48. [PubMed] [Google Scholar]
- 26.Gregory CW, Kim D, Ye P, D’Ercole AJ, Pretlow TG, Mohler JL, French FS. Androgen receptor up-regulates insulin-like growth factor binding protein-5 (IGFBP-5) expression in a human prostate cancer xenograft. Endocrinology. 1999;140(5):2372–81. doi: 10.1210/en.140.5.2372. [DOI] [PubMed] [Google Scholar]
- 27.Hryb DJ, Nakhla AM, Kahn SM, St George J, Levy NC, Romas NA, Rosner W. Sex hormone-binding globulin in the human prostate is locally synthesized and may act as an autocrine/paracrine effector. J Biol Chem. 2002;277(29):26618–22. doi: 10.1074/jbc.M202495200. [DOI] [PubMed] [Google Scholar]
- 28.Mohler JL, Morris TL, Ford OH, 3rd, Alvey RF, Sakamoto C, Gregory CW. Identification of differentially expressed genes associated with androgen-independent growth of prostate cancer. Prostate. 2002;51(4):247–55. doi: 10.1002/pros.10086. [DOI] [PubMed] [Google Scholar]
- 29.Wolk A, Andersson SO, Bergstrom R. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1997;89(11):820. doi: 10.1093/jnci/89.11.820. [DOI] [PubMed] [Google Scholar]
- 30.Pienta KJ, Goodson JA, Esper PS. Epidemiology of prostate cancer: molecular and environmental clues. Urology. 1996;48(5):676–83. doi: 10.1016/S0090-4295(96)00219-1. [DOI] [PubMed] [Google Scholar]
- 31.Meikle AW, Smith JA., Jr Epidemiology of prostate cancer. Urol Clin North Am. 1990;17(4):709–18. [PubMed] [Google Scholar]
- 32.Makridakis NM, Reichardt JK. Molecular epidemiology of hormone-metabolic loci in prostate cancer. Epidemiol Rev. 2001;23(1):24–9. doi: 10.1093/oxfordjournals.epirev.a000791. [DOI] [PubMed] [Google Scholar]
- 33.Ellis L, Nyborg H. Racial/ethnic variations in male testosterone levels: a probable contributor to group differences in health. Steroids. 1992;57(2):72–5. doi: 10.1016/0039-128X(92)90032-5. [DOI] [PubMed] [Google Scholar]
- 34.Ross R, Bernstein L, Judd H, Hanisch R, Pike M, Henderson B. Serum testosterone levels in healthy young black and white men. J Natl Cancer Inst. 1986;76(1):45–8. [PubMed] [Google Scholar]
- 35.Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6 Suppl 1):3–12. doi: 10.1016/j.urology.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Wu AH, Whittemore AS, Kolonel LN, John EM, Gallagher RP, West DW, Hankin J, Teh CZ, Dreon DM, Paffenbarger RS., Jr Serum androgens and sex hormone-binding globulins in relation to lifestyle factors in older African-American, white, and Asian men in the United States and Canada. Cancer Epidemiol Biomarkers Prev. 1995;4(7):735–41. [PubMed] [Google Scholar]
- 37.Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118–26. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- 38.Heikkila R, Aho K, Heliovaara M, Hakama M, Marniemi J, Reunanen A, Knekt P. Serum testosterone and sex hormone-binding globulin concentrations and the risk of prostate carcinoma: a longitudinal study. Cancer. 1999;86(2):312–5. doi: 10.1002/(SICI)1097-0142(19990715)86:2<312:AID-CNCR15>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 39.Ding VD, Moller DE, Feeney WP, Didolkar V, Nakhla AM, Rhodes L, Rosner W, Smith RG. Sex hormone-binding globulin mediates prostate androgen receptor action via a novel signaling pathway. Endocrinology. 1998;139(1):213–8. doi: 10.1210/endo.139.1.5681. [DOI] [PubMed] [Google Scholar]
- 40.Hryb DJ, Khan MS, Romas NA, Rosner W. The control of the interaction of sex hormone-binding globulin with its receptor by steroid hormones. J Biol Chem. 1990;265(11):6048–54. [PubMed] [Google Scholar]
- 41.Usui T, Ishibe T, Nakatsu H, Nakahara M, Nihira H, Miyachi Y. Immunohistochemical localization of testosterone-estradiol-binding globulin in a clinically hormone-independent prostatic carcinoma. Urol Int. 1983;38(1):16–8. doi: 10.1159/000280854. [DOI] [PubMed] [Google Scholar]
- 42.Kahn SM, Hryb DJ, Nakhla AM, Romas NA, Rosner W. Sex hormone-binding globulin is synthesized in target cells. J Endocrinol. 2002;175(1):113–20. doi: 10.1677/joe.0.1750113. [DOI] [PubMed] [Google Scholar]
- 43.Piras I, Falchi A, Moral P, Melis A, Giovannoni L, Paoli G, Calo C, Vona G, Varesi L. Frequencies of promoter pentanucleotide (TTTTA)n of CYP11A gene in European and North African populations. Genet Test. 2008;12(1):93–6. doi: 10.1089/gte.2007.0060. [DOI] [PubMed] [Google Scholar]
- 44.Kumazawa T, Tsuchiya N, Wang L, Sato K, Kamoto T, Ogawa O, Nakamura A, Kato T, Habuchi T. Microsatellite polymorphism of steroid hormone synthesis gene CYP11A1 is associated with advanced prostate cancer. Int J Cancer. 2004;110(1):140–4. doi: 10.1002/ijc.20070. [DOI] [PubMed] [Google Scholar]
- 45.Tang L, Yao S, Till C, Goodman PJ, Tangen CM, Wu Y, Kristal AR, Platz EA, Neuhouser ML, Stanczyk FZ, Reichardt JK, Santella RM, Hsing A, Hoque A, Lippman SM, Thompson IM, Ambrosone CB. Repeat polymorphisms in estrogen metabolism genes and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Carcinogenesis. 2011;32(10):1500–6. doi: 10.1093/carcin/bgr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Picado-Leonard J, Miller WL. Cloning and sequence of the human gene for P450c17 (steroid 17 alpha-hydroxylase/17,20 lyase): similarity with the gene for P450c21. DNA. 1987;6(5):439–48. doi: 10.1089/dna.1987.6.439. [DOI] [PubMed] [Google Scholar]
- 47.Carey AH, Waterworth D, Patel K, White D, Little J, Novelli P, Franks S, Williamson R. Polycystic ovaries and premature male pattern baldness are associated with one allele of the steroid metabolism gene CYP17. Hum Mol Genet. 1994;3(10):1873–6. doi: 10.1093/hmg/3.10.1873. [DOI] [PubMed] [Google Scholar]
- 48.Lunn RM, Bell DA, Mohler JL, Taylor JA. Prostate cancer risk and polymorphism in 17 hydroxylase (CYP17) and steroid reductase (SRD5A2) Carcinogenesis. 1999;20(9):1727–31. doi: 10.1093/carcin/20.9.1727. [DOI] [PubMed] [Google Scholar]
- 49.Gsur A, Bernhofer G, Hinteregger S, Haidinger G, Schatzl G, Madersbacher S, Marberger M, Vutuc C, Micksche M. A polymorphism in the CYP17 gene is associated with prostate cancer risk. Int J Cancer. 2000;87(3):434–7. doi: 10.1002/1097-0215(20000801)87:3<434:AID-IJC19>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 50.Yamada Y, Watanabe M, Murata M, Yamanaka M, Kubota Y, Ito H, Katoh T, Kawamura J, Yatani R, Shiraishi T. Impact of genetic polymorphisms of 17-hydroxylase cytochrome P-450 (CYP17) and steroid 5alpha-reductase type II (SRD5A2) genes on prostate-cancer risk among the Japanese population. Int J Cancer. 2001;92(5):683–6. doi: 10.1002/1097-0215(20010601)92:5<683:AID-IJC1255>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 51.Haiman CA, Stampfer MJ, Giovannucci E, Ma J, Decalo NE, Kantoff PW, Hunter DJ. The relationship between a polymorphism in CYP17 with plasma hormone levels and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2001;10(7):743–8. [PubMed] [Google Scholar]
- 52.Kittles RA, Panguluri RK, Chen W, Massac A, Ahaghotu C, Jackson A, Ukoli F, Adams-Campbell L, Isaacs W, Dunston GM. Cyp17 promoter variant associated with prostate cancer aggressiveness in African Americans. Cancer Epidemiol Biomarkers Prev. 2001;10(9):943–7. [PubMed] [Google Scholar]
- 53.Stanford JL, Noonan EA, Iwasaki L, Kolb S, Chadwick RB, Feng Z, Ostrander EA. A polymorphism in the CYP17 gene and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2002;11(3):243–7. [PubMed] [Google Scholar]
- 54.Chang B, Zheng SL, Isaacs SD, Wiley KE, Carpten JD, Hawkins GA, Bleecker ER, Walsh PC, Trent JM, Meyers DA, Isaacs WB, Xu J. Linkage and association of CYP17 gene in hereditary and sporadic prostate cancer. Int J Cancer. 2001;95(6):354–9. doi: 10.1002/1097-0215(20011120)95:6<354:AID-IJC1062>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 55.Latil AG, Azzouzi R, Cancel GS, Guillaume EC, Cochan-Priollet B, Berthon PL, Cussenot O. Prostate carcinoma risk and allelic variants of genes involved in androgen biosynthesis and metabolism pathways. Cancer. 2001;92(5):1130–7. doi: 10.1002/1097-0142(20010901)92:5<1130::AID-CNCR1430>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 56.Wadelius M, Andersson AO, Johansson JE, Wadelius C, Rane E. Prostate cancer associated with CYP17 genotype. Pharmacogenetics. 1999;9(5):635–9. doi: 10.1097/00008571-199910000-00010. [DOI] [PubMed] [Google Scholar]
- 57.Habuchi T, Liqing Z, Suzuki T, Sasaki R, Tsuchiya N, Tachiki H, Shimoda N, Satoh S, Sato K, Kakehi Y, Kamoto T, Ogawa O, Kato T. Increased risk of prostate cancer and benign prostatic hyperplasia associated with a CYP17 gene polymorphism with a gene dosage effect. Cancer Res. 2000;60(20):5710–3. [PubMed] [Google Scholar]
- 58.Ntais C, Polycarpou A, Ioannidis JP. Association of the CYP17 gene polymorphism with the risk of prostate cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2003;12(2):120–6. [PubMed] [Google Scholar]
- 59.Feigelson HS, McKean-Cowdin R, Pike MC, Coetzee GA, Kolonel LN, Nomura AM, Le Marchand L, Henderson BE. Cytochrome P450c17alpha gene (CYP17) polymorphism predicts use of hormone replacement therapy. Cancer Res. 1999;59(16):3908–10. [PubMed] [Google Scholar]
- 60.Ross RK, Bernstein L, Lobo RA, Shimizu H, Stanczyk FZ, Pike MC, Henderson BE. 5-alpha-reductase activity and risk of prostate cancer among Japanese and US white and black males. Lancet. 1992;339(8798):887–9. doi: 10.1016/0140-6736(92)90927-U. [DOI] [PubMed] [Google Scholar]
- 61.Thigpen AE, Silver RI, Guileyardo JM, Casey ML, McConnell JD, Russell DW. Tissue distribution and ontogeny of steroid 5 alpha-reductase isozyme expression. J Clin Invest. 1993;92(2):903–10. doi: 10.1172/JCI116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lookingbill DP, Demers LM, Wang C, Leung A, Rittmaster RS, Santen RJ. Clinical and biochemical parameters of androgen action in normal healthy Caucasian versus Chinese subjects. J Clin Endocrinol Metab. 1991;72(6):1242–8. doi: 10.1210/jcem-72-6-1242. [DOI] [PubMed] [Google Scholar]
- 63.Reichardt JK, Makridakis N, Henderson BE, Yu MC, Pike MC, Ross RK. Genetic variability of the human SRD5A2 gene: implications for prostate cancer risk. Cancer Res. 1995;55(18):3973–5. [PubMed] [Google Scholar]
- 64.Montie JE, Pienta KJ. Review of the role of androgenic hormones in the epidemiology of benign prostatic hyperplasia and prostate cancer. Urology. 1994;43(6):892–9. doi: 10.1016/0090-4295(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 65.Anwar R, Gilbey SG, New JP, Markham AF. Male pseudohermaphroditism resulting from a novel mutation in the human steroid 5 alpha-reductase type 2 gene (SRD5A2) Mol Pathol. 1997;50(1):51–2. doi: 10.1136/mp.50.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Makridakis N, Ross RK, Pike MC, Chang L, Stanczyk FZ, Kolonel LN, Shi CY, Yu MC, Henderson BE, Reichardt JK. A prevalent missense substitution that modulates activity of prostatic steroid 5alpha-reductase. Cancer Res. 1997;57(6):1020–2. [PubMed] [Google Scholar]
- 67.Makridakis NM, Ross RK, Pike MC, Crocitto LE, Kolonel LN, Pearce CL, Henderson BE, Reichardt JK. Association of missense substitution in SRD5A2 gene with prostate cancer in African-American and Hispanic men in Los Angeles, USA. Lancet. 1999;354(9183):975–8. doi: 10.1016/S0140-6736(98)11282-5. [DOI] [PubMed] [Google Scholar]
- 68.Simard J, Durocher F, Mebarki F, Turgeon C, Sanchez R, Labrie Y, Couet J, Trudel C, Rheaume E, Morel Y, Luu-The V, Labrie F. Molecular biology and genetics of the 3 beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. J Endocrinol. 1996;150(Suppl):S189–207. [PubMed] [Google Scholar]
- 69.Wang L, Salavaggione E, Pelleymounter L, Eckloff B, Wieben E, Weinshilboum R. Human 3beta-hydroxysteroid dehydrogenase types 1 and 2: Gene sequence variation and functional genomics. J Steroid Biochem Mol Biol. 2007;107(1–2):88–99. doi: 10.1016/j.jsbmb.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verreault H, Dufort I, Simard J, Labrie F, Luu-The V. Dinucleotide repeat polymorphisms in the HSD3B2 gene. Hum Mol Genet. 1994;3(2):384. doi: 10.1093/hmg/3.2.384. [DOI] [PubMed] [Google Scholar]
- 71.Neslund-Dudas C, Bock CH, Monaghan K, Nock NL, Yang JJ, Rundle A, Tang D, Rybicki BA. SRD5A2 and HSD3B2 polymorphisms are associated with prostate cancer risk and aggressiveness. Prostate. 2007;67(15):1654–63. doi: 10.1002/pros.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Devgan SA, Henderson BE, Yu MC, Shi CY, Pike MC, Ross RK, Reichardt JK. Genetic variation of 3 beta-hydroxysteroid dehydrogenase type II in three racial/ethnic groups: implications for prostate cancer risk. Prostate. 1997;33(1):9–12. doi: 10.1002/(SICI)1097-0045(19970915)33:1<9:AID-PROS2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 73.Chang BL, Zheng SL, Hawkins GA, Isaacs SD, Wiley KE, Turner A, Carpten JD, Bleecker ER, Walsh PC, Trent JM, Meyers DA, Isaacs WB, Xu J. Joint effect of HSD3B1 and HSD3B2 genes is associated with hereditary and sporadic prostate cancer susceptibility. Cancer Res. 2002;62(6):1784–9. [PubMed] [Google Scholar]
- 74.Eriksson AL, Lorentzon M, Vandenput L, Labrie F, Lindersson M, Syvanen AC, Orwoll ES, Cummings SR, Zmuda JM, Ljunggren O, Karlsson MK, Mellstrom D, Ohlsson C. Genetic variations in sex steroid-related genes as predictors of serum estrogen levels in men. J Clin Endocrinol Metab. 2009;94(3):1033–41. doi: 10.1210/jc.2008-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Travis RC, Schumacher F, Hirschhorn JN, Kraft P, Allen NE, Albanes D, Berglund G, Berndt SI, Boeing H, Bueno-de-Mesquita HB, Calle EE, Chanock S, Dunning AM, Hayes R, Feigelson HS, Gaziano JM, Giovannucci E, Haiman CA, Henderson BE, Kaaks R, Kolonel LN, Ma J, Rodriguez L, Riboli E, Stampfer M, Stram DO, Thun MJ, Tjonneland A, Trichopoulos D, Vineis P, Virtamo J, Le Marchand L, Hunter DJ. CYP19A1 genetic variation in relation to prostate cancer risk and circulating sex hormone concentrations in men from the Breast and Prostate Cancer Cohort Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2734–44. doi: 10.1158/1055-9965.EPI-09-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sonoda T, Suzuki H, Mori M, Tsukamoto T, Yokomizo A, Naito S, Fujimoto K, Hirao Y, Miyanaga N, Akaza H. Polymorphisms in estrogen related genes may modify the protective effect of isoflavones against prostate cancer risk in Japanese men. Eur J Cancer Prev. 2010;19(2):131–7. doi: 10.1097/CEJ.0b013e328333fbe2. [DOI] [PubMed] [Google Scholar]
- 77.Huang YC, Chen M, Lin MW, Chung MY, Chang YH, Huang WJ, Wu TT, Hsu JM, Yang S, Chen YM. CYP19 TCT trinucleotide Del/Del genotype is a susceptibility marker for prostate cancer in a Taiwanese population. Urology. 2007;69(5):996–1000. doi: 10.1016/j.urology.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 78.Onsory K, Sobti RC, Al-Badran AI, Watanabe M, Shiraishi T, Krishan A, Mohan H, Kaur P. Hormone receptor-related gene polymorphisms and prostate cancer risk in North Indian population. Mol Cell Biochem. 2008;314(1–2):25–35. doi: 10.1007/s11010-008-9761-1. [DOI] [PubMed] [Google Scholar]
- 79.Mononen N, Seppala EH, Duggal P, Autio V, Ikonen T, Ellonen P, Saharinen J, Saarela J, Vihinen M, Tammela TL, Kallioniemi O, Bailey-Wilson JE, Schleutker J. Profiling genetic variation along the androgen biosynthesis and metabolism pathways implicates several single nucleotide polymorphisms and their combinations as prostate cancer risk factors. Cancer Res. 2006;66(2):743–7. doi: 10.1158/0008-5472.CAN-05-1723. [DOI] [PubMed] [Google Scholar]
- 80.Beuten J, Gelfond JA, Franke JL, Weldon KS, Crandall AC, Johnson-Pais TL, Thompson IM, Leach RJ. Single and multigenic analysis of the association between variants in 12 steroid hormone metabolism genes and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1869–80. doi: 10.1158/1055-9965.EPI-09-0076. [DOI] [PubMed] [Google Scholar]
- 81.Modugno F, Weissfeld JL, Trump DL, Zmuda JM, Shea P, Cauley JA, Ferrell RE. Allelic variants of aromatase and the androgen and estrogen receptors: toward a multigenic model of prostate cancer risk. Clin Cancer Res. 2001;7(10):3092–6. [PubMed] [Google Scholar]
- 82.Gellner K, Eiselt R, Hustert E, Arnold H, Koch I, Haberl M, Deglmann CJ, Burk O, Buntefuss D, Escher S, Bishop C, Koebe HG, Brinkmann U, Klenk HP, Kleine K, Meyer UA, Wojnowski L. Genomic organization of the human CYP3A locus: identification of a new, inducible CYP3A gene. Pharmacogenetics. 2001;11(2):111–21. doi: 10.1097/00008571-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 83.Kraft P, Pharoah P, Chanock SJ, Albanes D, Kolonel LN, Hayes RB, Altshuler D, Andriole G, Berg C, Boeing H, Burtt NP, Bueno-de-Mesquita B, Calle EE, Cann H, Canzian F, Chen YC, Crawford DE, Dunning AM, Feigelson HS, Freedman ML, Gaziano JM, Giovannucci E, Gonzalez CA, Haiman CA, Hallmans G, Henderson BE, Hirschhorn JN, Hunter DJ, Kaaks R, Key T, Le Marchand L, Ma J, Overvad K, Palli D, Pike MC, Riboli E, Rodriguez C, Setiawan WV, Stampfer MJ, Stram DO, Thomas G, Thun MJ, Travis R, Trichopoulou A, Virtamo J, Wacholder S. Genetic variation in the HSD17B1 gene and risk of prostate cancer. PLoS Genet. 2005;1(5):e68. doi: 10.1371/journal.pgen.0010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun T, Oh WK, Jacobus S, Regan M, Pomerantz M, Freedman ML, Lee GS, Kantoff PW. The impact of common genetic variations in genes of the sex hormone metabolic pathways on steroid hormone levels and prostate cancer aggressiveness. Cancer Prev Res (Phila) 2011;4(12):2044–50. doi: 10.1158/1940-6207.CAPR-11-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cunningham GR, Ashton CM, Annegers JF, Souchek J, Klima M, Miles B. Familial aggregation of prostate cancer in African-Americans and white Americans. Prostate. 2003;56(4):256–62. doi: 10.1002/pros.10252. [DOI] [PubMed] [Google Scholar]
- 86.Margiotti K, Kim E, Pearce CL, Spera E, Novelli G, Reichardt JK. Association of the G289S single nucleotide polymorphism in the HSD17B3 gene with prostate cancer in Italian men. Prostate. 2002;53(1):65–8. doi: 10.1002/pros.10134. [DOI] [PubMed] [Google Scholar]
- 87.Lindstrom S, Zheng SL, Wiklund F, Jonsson BA, Adami HO, Balter KA, Brookes AJ, Sun J, Chang BL, Liu W, Li G, Isaacs WB, Adolfsson J, Gronberg H, Xu J. Systematic replication study of reported genetic associations in prostate cancer: Strong support for genetic variation in the androgen pathway. Prostate. 2006;66(16):1729–43. doi: 10.1002/pros.20489. [DOI] [PubMed] [Google Scholar]
- 88.Rasiah KK, Gardiner-Garden M, Padilla EJ, Moller G, Kench JG, Alles MC, Eggleton SA, Stricker PD, Adamski J, Sutherland RL, Henshall SM, Hayes VM. HSD17B4 overexpression, an independent biomarker of poor patient outcome in prostate cancer. Mol Cell Endocrinol. 2009;301(1–2):89–96. doi: 10.1016/j.mce.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 89.Ross RW, Oh WK, Xie W, Pomerantz M, Nakabayashi M, Sartor O, Taplin ME, Regan MM, Kantoff PW, Freedman M. Inherited variation in the androgen pathway is associated with the efficacy of androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2008;26(6):842–7. doi: 10.1200/JCO.2007.13.6804. [DOI] [PubMed] [Google Scholar]
- 90.Jakobsson J, Palonek E, Lorentzon M, Ohlsson C, Rane A, Ekstrom L. A novel polymorphism in the 17beta-hydroxysteroid dehydrogenase type 5 (aldo-keto reductase 1C3) gene is associated with lower serum testosterone levels in caucasian men. Pharmacogenomics J. 2007;7(4):282–9. doi: 10.1038/sj.tpj.6500419. [DOI] [PubMed] [Google Scholar]
- 91.Schulze JJ, Karypidis H, Ekstrom L. Basal and Regulatory Promoter Studies of the AKR1C3 Gene in Relation to Prostate Cancer. Front Pharmacol. 2012;3:151. doi: 10.3389/fphar.2012.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lampe JW, Bigler J, Bush AC, Potter JD. Prevalence of polymorphisms in the human UDP-glucuronosyltransferase 2B family: UGT2B4(D458E), UGT2B7(H268Y), and UGT2B15(D85Y) Cancer Epidemiol Biomarkers Prev. 2000;9(3):329–33. [PubMed] [Google Scholar]
- 93.Okugi H, Nakazato H, Matsui H, Ohtake N, Nakata S, Suzuki K. Association of the polymorphisms of genes involved in androgen metabolism and signaling pathways with familial prostate cancer risk in a Japanese population. Cancer Detect Prev. 2006;30(3):262–8. doi: 10.1016/j.cdp.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 94.Gsur A, Preyer M, Haidinger G, Schatzl G, Madersbacher S, Marberger M, Vutuc C, Micksche M. A polymorphism in the UDP-Glucuronosyltransferase 2B15 gene (D85Y) is not associated with prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11(5):497–8. [PubMed] [Google Scholar]
- 95.Heald AH, Ivison F, Anderson SG, Cruickshank K, Laing I, Gibson JM. Significant ethnic variation in total and free testosterone concentration. Clin Endocrinol (Oxf) 2003;58(3):262–6. doi: 10.1046/j.1365-2265.2003.01653.x. [DOI] [PubMed] [Google Scholar]
- 96.Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100(3):170–83. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Power SG, Bocchinfuso WP, Pallesen M, Warmels-Rodenhiser S, Van Baelen H, Hammond GL. Molecular analyses of a human sex hormone-binding globulin variant: evidence for an additional carbohydrate chain. J Clin Endocrinol Metab. 1992;75(4):1066–70. doi: 10.1210/jc.75.4.1066. [DOI] [PubMed] [Google Scholar]
- 98.Low YL, Taylor JI, Grace PB, Mulligan AA, Welch AA, Scollen S, Dunning AM, Luben RN, Khaw KT, Day NE, Wareham NJ, Bingham SA. Phytoestrogen exposure, polymorphisms in COMT, CYP19, ESR1, and SHBG genes, and their associations with prostate cancer risk. Nutr Cancer. 2006;56(1):31–9. doi: 10.1207/s15327914nc5601_5. [DOI] [PubMed] [Google Scholar]
- 99.Winter DL, Hanlon AL, Raysor SL, Watkins-Bruner D, Pinover WH, Hanks GE, Tricoli JV. Plasma levels of IGF-1, IGF-2, and IGFBP-3 in white and African-American men at increased risk of prostate cancer. Urology. 2001;58(4):614–8. doi: 10.1016/S0090-4295(01)01273-0. [DOI] [PubMed] [Google Scholar]
- 100.deVere White RW, Deitch AD, Jackson AG, Gandour-Edwards R, Marshalleck J, Soares SE, Toscano SN, Lunetta JM, Stewart SL. Racial differences in clinically localized prostate cancers of black and white men. J Urol. 1998;159(6):1979–82. doi: 10.1016/S0022-5347(01)63216-6. discussion 1982–3. [DOI] [PubMed] [Google Scholar]
- 101.Pietsch EC, Humbey O, Murphy ME. Polymorphisms in the p53 pathway. Oncogene. 2006;25(11):1602–11. doi: 10.1038/sj.onc.1209367. [DOI] [PubMed] [Google Scholar]
- 102.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387(6630):296–9. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 103.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387(6630):299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 104.Bond GL, Hu W, Levine AJ. MDM2 is a central node in the p53 pathway: 12 years and counting. Curr Cancer Drug Targets. 2005;5(1):3–8. doi: 10.2174/1568009053332627. [DOI] [PubMed] [Google Scholar]
- 105.Osman I, Drobnjak M, Fazzari M, Ferrara J, Scher HI, Cordon-Cardo C. Inactivation of the p53 pathway in prostate cancer: impact on tumor progression. Clin Cancer Res. 1999;5(8):2082–8. [PubMed] [Google Scholar]
- 106.Leite KR, Franco MF, Srougi M, Nesrallah LJ, Nesrallah A, Bevilacqua RG, Darini E, Carvalho CM, Meirelles MI, Santana I, Camara-Lopes LH. Abnormal expression of MDM2 in prostate carcinoma. Mod Pathol. 2001;14(5):428–36. doi: 10.1038/modpathol.3880330. [DOI] [PubMed] [Google Scholar]
- 107.Khor LY, Desilvio M, Al-Saleem T, Hammond ME, Grignon DJ, Sause W, Pilepich M, Okunieff P, Sandler H, Pollack A. MDM2 as a predictor of prostate carcinoma outcome: an analysis of Radiation Therapy Oncology Group Protocol 8610. Cancer. 2005;104(5):962–7. doi: 10.1002/cncr.21261. [DOI] [PubMed] [Google Scholar]
- 108.Bianco R, Caputo R, Caputo R, Damiano V, De Placido S, Ficorella C, Agrawal S, Bianco AR, Ciardiello F, Tortora G. Combined targeting of epidermal growth factor receptor and MDM2 by gefitinib and antisense MDM2 cooperatively inhibit hormone-independent prostate cancer. Clin Cancer Res. 2004;10(14):4858–64. doi: 10.1158/1078-0432.CCR-03-0497. [DOI] [PubMed] [Google Scholar]
- 109.Wang H, Yu D, Agrawal S, Zhang R. Experimental therapy of human prostate cancer by inhibiting MDM2 expression with novel mixed-backbone antisense oligonucleotides: in vitro and in vivo activities and mechanisms. Prostate. 2003;54(3):194–205. doi: 10.1002/pros.10187. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Z, Li M, Wang H, Agrawal S, Zhang R. Antisense therapy targeting MDM2 oncogene in prostate cancer: Effects on proliferation, apoptosis, multiple gene expression, and chemotherapy. Proc Natl Acad Sci U S A. 2003;100(20):11636–41. doi: 10.1073/pnas.1934692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bond GL, Menin C, Bertorelle R, Alhopuro P, Aaltonen LA, Levine AJ. MDM2 SNP309 accelerates colorectal tumour formation in women. J Med Genet. 2006;43(12):950–2. doi: 10.1136/jmg.2006.043539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lind H, Zienolddiny S, Ekstrom PO, Skaug V, Haugen A. Association of a functional polymorphism in the promoter of the MDM2 gene with risk of nonsmall cell lung cancer. Int J Cancer. 2006;119(3):718–21. doi: 10.1002/ijc.21872. [DOI] [PubMed] [Google Scholar]
- 113.Menin C, Scaini MC, De Salvo GL, Biscuola M, Quaggio M, Esposito G, Belluco C, Montagna M, Agata S, D’Andrea E, Nitti D, Amadori A, Bertorelle R. Association between MDM2-SNP309 and age at colorectal cancer diagnosis according to p53 mutation status. J Natl Cancer Inst. 2006;98(4):285–8. doi: 10.1093/jnci/djj054. [DOI] [PubMed] [Google Scholar]
- 114.Alhopuro P, Ylisaukko-Oja SK, Koskinen WJ, Bono P, Arola J, Jarvinen HJ, Mecklin JP, Atula T, Kontio R, Makitie AA, Suominen S, Leivo I, Vahteristo P, Aaltonen LM, Aaltonen LA. The MDM2 promoter polymorphism SNP309T-->G and the risk of uterine leiomyosarcoma, colorectal cancer, and squamous cell carcinoma of the head and neck. J Med Genet. 2005;42(9):694–8. doi: 10.1136/jmg.2005.031260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yarden RI, Friedman E, Metsuyanim S, Olender T, Ben-Asher E, Papa MZ. MDM2 SNP309 accelerates breast and ovarian carcinogenesis in BRCA1 and BRCA2 carriers of Jewish-Ashkenazi descent. Breast Cancer Res Treat. 2008;111(3):497–504. doi: 10.1007/s10549-007-9797-z. [DOI] [PubMed] [Google Scholar]
- 116.Wang G, Firoz EF, Rose A, Blochin E, Christos P, Pollens D, Mazumdar M, Gerald W, Oddoux C, Lee P, Osman I. MDM2 expression and regulation in prostate cancer racial disparity. Int J Clin Exp Pathol. 2009;2(4):353–60. [PMC free article] [PubMed] [Google Scholar]
- 117.Zeigler-Johnson CM, Walker AH, Mancke B, Spangler E, Jalloh M, McBride S, Deitz A, Malkowicz SB, Ofori-Adjei D, Gueye SM, Rebbeck TR. Ethnic differences in the frequency of prostate cancer susceptibility alleles at SRD5A2 and CYP3A4. Hum Hered. 2002;54(1):13–21. doi: 10.1159/000066695. [DOI] [PubMed] [Google Scholar]
- 118.Miller DC, Zheng SL, Dunn RL, Sarma AV, Montie JE, Lange EM, Meyers DA, Xu J, Cooney KA. Germ-line mutations of the macrophage scavenger receptor 1 gene: association with prostate cancer risk in African-American men. Cancer Res. 2003;63(13):3486–9. [PubMed] [Google Scholar]
- 119.Oakley-Girvan I, Feldman D, Eccleshall TR, Gallagher RP, Wu AH, Kolonel LN, Halpern J, Balise RR, West DW, Paffenbarger RS, Jr, Whittemore AS. Risk of early-onset prostate cancer in relation to germ line polymorphisms of the vitamin D receptor. Cancer Epidemiol Biomarkers Prev. 2004;13(8):1325–30. [PubMed] [Google Scholar]
- 120.Monroe KR, Yu MC, Kolonel LN, Coetzee GA, Wilkens LR, Ross RK, Henderson BE. Evidence of an X-linked or recessive genetic component to prostate cancer risk. Nat Med. 1995;1(8):827–9. doi: 10.1038/nm0895-827. [DOI] [PubMed] [Google Scholar]
- 121.Bastacky SI, Wojno KJ, Walsh PC, Carmichael MJ, Epstein JI. Pathological features of hereditary prostate cancer. J Urol. 1995;153(3 Pt 2):987–92. [PubMed] [Google Scholar]
- 122.Mirchandani D, Zheng J, Miller GJ, Ghosh AK, Shibata DK, Cote RJ, Roy-Burman P. Heterogeneity in intratumor distribution of p53 mutations in human prostate cancer. Am J Pathol. 1995;147(1):92–101. [PMC free article] [PubMed] [Google Scholar]
- 123.Qian J, Bostwick DG, Takahashi S, Borell TJ, Herath JF, Lieber MM, Jenkins RB. Chromosomal anomalies in prostatic intraepithelial neoplasia and carcinoma detected by fluorescence in situ hybridization. Cancer Res. 1995;55(22):5408–14. [PubMed] [Google Scholar]
- 124.Sakr WA, Grignon DJ, Crissman JD, Heilbrun LK, Cassin BJ, Pontes JJ, Haas GP. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20–69: an autopsy study of 249 cases. In vivo. 1994;8(3):439–43. [PubMed] [Google Scholar]
- 125.Rebbeck TR. Genetics, disparities, and prostate cancer. LDI Issue Brief. 2005;10(7):1–4. [PubMed] [Google Scholar]
- 126.Xu J, Zheng SL, Hawkins GA, Faith DA, Kelly B, Isaacs SD, Wiley KE, Chang B, Ewing CM, Bujnovszky P, Carpten JD, Bleecker ER, Walsh PC, Trent JM, Meyers DA, Isaacs WB. Linkage and association studies of prostate cancer susceptibility: evidence for linkage at 8p22–23. Am J Hum Genet. 2001;69(2):341–50. doi: 10.1086/321967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gibbs M, Stanford JL, Jarvik GP, Janer M, Badzioch M, Peters MA, Goode EL, Kolb S, Chakrabarti L, Shook M, Basom R, Ostrander EA, Hood L. A genomic scan of families with prostate cancer identifies multiple regions of interest. Am J Hum Genet. 2000;67(1):100–9. doi: 10.1086/302969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Emmert-Buck MR, Vocke CD, Pozzatti RO, Duray PH, Jennings SB, Florence CD, Zhuang Z, Bostwick DG, Liotta LA, Linehan WM. Allelic loss on chromosome 8p12–21 in microdissected prostatic intraepithelial neoplasia. Cancer Res. 1995;55(14):2959–62. [PubMed] [Google Scholar]
- 129.Vocke CD, Pozzatti RO, Bostwick DG, Florence CD, Jennings SB, Strup SE, Duray PH, Liotta LA, Emmert-Buck MR, Linehan WM. Analysis of 99 microdissected prostate carcinomas reveals a high frequency of allelic loss on chromosome 8p12–21. Cancer Res. 1996;56(10):2411–6. [PubMed] [Google Scholar]
- 130.Zitzelsberger H, Engert D, Walch A, Kulka U, Aubele M, Hofler H, Bauchinger M, Werner M. Chromosomal changes during development and progression of prostate adenocarcinomas. Br J Cancer. 2001;84(2):202–8. doi: 10.1054/bjoc.2000.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Verma RS, Manikal M, Conte RA, Godec CJ. Chromosomal basis of adenocarcinoma of the prostate. Cancer Invest. 1999;17(6):441–7. doi: 10.3109/07357909909021436. [DOI] [PubMed] [Google Scholar]
- 132.Bookstein R. Tumor suppressor genes in prostatic oncogenesis. J Cell Biochem Suppl. 1994;19:217–23. [PubMed] [Google Scholar]
- 133.Kalapurakal JA, Jacob AN, Kim PY, Najjar DD, Hsieh YC, Ginsberg P, Daskal I, Asbell SO, Kandpal RP. Racial differences in prostate cancer related to loss of heterozygosity on chromosome 8p12–23. Int J Radiat Oncol Biol Phys. 1999;45(4):835–40. doi: 10.1016/S0360-3016(99)00283-7. [DOI] [PubMed] [Google Scholar]
- 134.BarTel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 135.Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432(7014):231–5. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 136.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101(9):2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 138.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, Menard S, Palazzo JP, Rosenberg A, Musiani P, Volinia S, Nenci I, Calin GA, Querzoli P, Negrini M, Croce CM. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65(16):7065–70. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 139.Liu CG, Calin GA, Meloon B, Gamliel N, Sevignani C, Ferracin M, Dumitru CD, Shimizu M, Zupo S, Dono M, Alder H, Bullrich F, Negrini M, Croce CM. An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proc Natl Acad Sci U S A. 2004;101(26):9740–4. doi: 10.1073/pnas.0403293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Akao Y, Nakagawa Y, Naoe T. MicroRNAs 143 and 145 are possible common onco-microRNAs in human cancers. Oncol Rep. 2006;16(4):845–50. doi: 10.3892/or.16.4.845. [DOI] [PubMed] [Google Scholar]
- 141.Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y, Takahashi T. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65(21):9628–32. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 142.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–33. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Voorhoeve PM, le Sage C, Schrier M, Gillis AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A, Zlotorynski E, Yabuta N, De Vita G, Nojima H, Looijenga LH, Agami R. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell. 2006;124(6):1169–81. doi: 10.1016/j.cell.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 144.Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26(19):2799–803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 145.Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, Mittal K, Soteropoulos P, Wei JJ. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer. 2007;46(4):336–47. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]
- 146.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 147.Rahman KM, Sakr WA. The therapeutic value of natural agents to treat miRNA targeted breast cancer in African-American and Caucasian-American women. Curr Drug Targets. 2012;13(14):1917–25. doi: 10.2174/138945012804545461. [DOI] [PubMed] [Google Scholar]
- 148.Polascik TJ, Oesterling JE, Partin AW. Prostate specific antigen: a decade of discovery--what we have learned and where we are going. J Urol. 1999;162(2):293–306. doi: 10.1016/S0022-5347(05)68543-6. [DOI] [PubMed] [Google Scholar]
- 149.Oh J, Colberg JW, Ornstein DK, Johnson ET, Chan D, Virgo KS, Johnson FE. Current followup strategies after radical prostatectomy: a survey of American Urological Association urologists. J Urol. 1999;161(2):520–3. doi: 10.1016/S0022-5347(01)61939-6. [DOI] [PubMed] [Google Scholar]
- 150.Zeliadt SB, Penson DF, Albertsen PC, Concato J, Etzioni RD. Race independently predicts prostate specific antigen testing frequency following a prostate carcinoma diagnosis. Cancer. 2003;98(3):496–503. doi: 10.1002/cncr.11492. [DOI] [PubMed] [Google Scholar]
- 151.Mariotto AB, Etzioni R, Krapcho M, Feuer EJ. Reconstructing PSA testing patterns between black and white men in the US from Medicare claims and the National Health Interview Survey. Cancer. 2007;109(9):1877–86. doi: 10.1002/cncr.22607. [DOI] [PubMed] [Google Scholar]
- 152.Xue WM, Coetzee GA, Ross RK, Irvine R, Kolonel L, Henderson BE, Ingles SA. Genetic determinants of serum prostate-specific antigen levels in healthy men from a multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2001;10(6):575–9. [PubMed] [Google Scholar]
- 153.Deka R, Guangyun S, Wiest J, Smelser D, Chunhua S, Zhong Y, Chakraborty R. Patterns of instability of expanded CAG repeats at the ERDA1 locus in general populations. Am J Hum Genet. 1999;65(1):192–8. doi: 10.1086/302453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shibata A, Whittemore AS. Genetic predisposition to prostate cancer: possible explanations for ethnic differences in risk. Prostate. 1997;32(1):65–72. doi: 10.1002/(SICI)1097-0045(19970615)32:1<65:AID-PROS9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 155.Smith JR, Freije D, Carpten JD, Gronberg H, Xu J, Isaacs SD, Brownstein MJ, Bova GS, Guo H, Bujnovszky P, Nusskern DR, Damber JE, Bergh A, Emanuelsson M, Kallioniemi OP, Walker-Daniels J, Bailey-Wilson JE, Beaty TH, Meyers DA, Walsh PC, Collins FS, Trent JM, Isaacs WB. Major susceptibility locus for prostate cancer on chromosome 1 suggested by a genome-wide search. Science. 1996;274(5291):1371–4. doi: 10.1126/science.274.5291.1371. [DOI] [PubMed] [Google Scholar]
- 156.Xu J, Meyers D, Freije D, Isaacs S, Wiley K, Nusskern D, Ewing C, Wilkens E, Bujnovszky P, Bova GS, Walsh P, Isaacs W, Schleutker J, Matikainen M, Tammela T, Visakorpi T, Kallioniemi OP, Berry R, Schaid D, French A, McDonnell S, Schroeder J, Blute M, Thibodeau S, Gronberg H, Emanuelsson M, Damber JE, Bergh A, Jonsson BA, Smith J, Bailey-Wilson J, Carpten J, Stephan D, Gillanders E, Amundson I, Kainu T, Freas-Lutz D, Baffoe-Bonnie A, Van Aucken A, Sood R, Collins F, Brownstein M, Trent J. Evidence for a prostate cancer susceptibility locus on the X chromosome. Nat Genet. 1998;20(2):175–9. doi: 10.1038/2477. [DOI] [PubMed] [Google Scholar]
- 157.Xu J, Zheng SL, Chang B, Smith JR, Carpten JD, Stine OC, Isaacs SD, Wiley KE, Henning L, Ewing C, Bujnovszky P, Bleeker ER, Walsh PC, Trent JM, Meyers DA, Isaacs WB. Linkage of prostate cancer susceptibility loci to chromosome 1. Hum Genet. 2001;108(4):335–45. doi: 10.1007/s004390100488. [DOI] [PubMed] [Google Scholar]
- 158.Zheng SL, Xu J, Isaacs SD, Wiley K, Chang B, Bleecker ER, Walsh PC, Trent JM, Meyers DA, Isaacs WB. Evidence for a prostate cancer linkage to chromosome 20 in 159 hereditary prostate cancer families. Hum Genet. 2001;108(5):430–5. doi: 10.1007/s004390100513. [DOI] [PubMed] [Google Scholar]
- 159.Xu J, Zheng SL, Carpten JD, Nupponen NN, Robbins CM, Mestre J, Moses TY, Faith DA, Kelly BD, Isaacs SD, Wiley KE, Ewing CM, Bujnovszky P, Chang B, Bailey-Wilson J, Bleecker ER, Walsh PC, Trent JM, Meyers DA, Isaacs WB. Evaluation of linkage and association of HPC2/ELAC2 in patients with familial or sporadic prostate cancer. Am J Hum Genet. 2001;68(4):901–11. doi: 10.1086/319513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gong G, Oakley-Girvan I, Wu AH, Kolonel LN, John EM, West DW, Felberg A, Gallagher RP, Whittemore AS. Segregation analysis of prostate cancer in 1,719 white, African-American and Asian-American families in the United States and Canada. Cancer Causes Control. 2002;13(5):471–82. doi: 10.1023/A:1015755219674. [DOI] [PubMed] [Google Scholar]
- 161.Ahaghotu C, Baffoe-Bonnie A, Kittles R, Pettaway C, Powell I, Royal C, Wang H, Vijayakumar S, Bennett J, Hoke G, Mason T, Bailey-Wilson J, Boykin W, Berg K, Carpten J, Weinrich S, Trent J, Dunston G, Collins F. Clinical characteristics of African-American men with hereditary prostate cancer: the AAHPC Study. Prostate Cancer Prostatic Dis. 2004;7(2):165–9. doi: 10.1038/sj.pcan.4500719. [DOI] [PubMed] [Google Scholar]
- 162.Brown WM, Lange EM, Chen H, Zheng SL, Chang B, Wiley KE, Isaacs SD, Walsh PC, Isaacs WB, Xu J, Cooney KA. Hereditary prostate cancer in African American families: linkage analysis using markers that map to five candidate susceptibility loci. Br J Cancer. 2004;90(2):510–4. doi: 10.1038/sj.bjc.6601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu J, Zheng SL, Komiya A, Mychaleckyj JC, Isaacs SD, Hu JJ, Sterling D, Lange EM, Hawkins GA, Turner A, Ewing CM, Faith DA, Johnson JR, Suzuki H, Bujnovszky P, Wiley KE, DeMarzo AM, Bova GS, Chang B, Hall MC, McCullough DL, Partin AW, Kassabian VS, Carpten JD, Bailey-Wilson JE, Trent JM, Ohar J, Bleecker ER, Walsh PC, Isaacs WB, Meyers DA. Germline mutations and sequence variants of the macrophage scavenger receptor 1 gene are associated with prostate cancer risk. Nat Genet. 2002;32(2):321–5. doi: 10.1038/ng994. [DOI] [PubMed] [Google Scholar]
- 164.Xu J, Zheng SL, Komiya A, Mychaleckyj JC, Isaacs SD, Chang B, Turner AR, Ewing CM, Wiley KE, Hawkins GA, Bleecker ER, Walsh PC, Meyers DA, Isaacs WB. Common sequence variants of the macrophage scavenger receptor 1 gene are associated with prostate cancer risk. Am J Hum Genet. 2003;72(1):208–12. doi: 10.1086/345802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yang G, Addai J, Ittmann M, Wheeler TM, Thompson TC. Elevated caveolin-1 levels in African-American versus white-American prostate cancer. Clin Cancer Res. 2000;6(9):3430–3. [PubMed] [Google Scholar]
- 166.Mouraviev V, Li L, Tahir SA, Yang G, Timme TM, Goltsov A, Ren C, Satoh T, Wheeler TM, Ittmann MM, Miles BJ, Amato RJ, Kadmon D, Thompson TC. The role of caveolin-1 in androgen insensitive prostate cancer. J Urol. 2002;168(4 Pt 1):1589–96. doi: 10.1016/S0022-5347(05)64526-0. [DOI] [PubMed] [Google Scholar]
- 167.Waxman DJ, Attisano C, Guengerich FP, Lapenson DP. Human liver microsomal steroid metabolism: identification of the major microsomal steroid hormone 6 beta-hydroxylase cytochrome P-450 enzyme. Arch Biochem Biophys. 1988;263(2):424–36. doi: 10.1016/0003-9861(88)90655-8. [DOI] [PubMed] [Google Scholar]
- 168.Waxman DJ, Lapenson DP, Aoyama T, Gelboin HV, Gonzalez FJ, Korzekwa K. Steroid hormone hydroxylase specificities of eleven cDNA-expressed human cytochrome P450s. Arch Biochem Biophys. 1991;290(1):160–6. doi: 10.1016/0003-9861(91)90602-F. [DOI] [PubMed] [Google Scholar]
- 169.Ozdemir V, Kalow W, Tang BK, Paterson AD, Walker SE, Endrenyi L, Kashuba AD. Evaluation of the genetic component of variability in CYP3A4 activity: a repeated drug administration method. Pharmacogenetics. 2000;10(5):373–88. doi: 10.1097/00008571-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 170.Plummer SJ, Conti DV, Paris PL, Curran AP, Casey G, Witte JS. CYP3A4 and CYP3A5 genotypes, haplotypes, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003;12(9):928–32. [PubMed] [Google Scholar]
- 171.Loukola A, Chadha M, Penn SG, Rank D, Conti DV, Thompson D, Cicek M, Love B, Bivolarevic V, Yang Q, Jiang Y, Hanzel DK, Dains K, Paris PL, Casey G, Witte JS. Comprehensive evaluation of the association between prostate cancer and genotypes/haplotypes in CYP17A1, CYP3A4, and SRD5A2. Eur J Hum Genet. 2004;12(4):321–32. doi: 10.1038/sj.ejhg.5201101. [DOI] [PubMed] [Google Scholar]
- 172.Powell IJ, Zhou J, Sun Y, Sakr WA, Patel NP, Heilbrun LK, Everson RB. CYP3A4 genetic variant and disease-free survival among white and black men after radical prostatectomy. J Urol. 2004;172(5 Pt 1):1848–52. doi: 10.1097/01.ju.0000142779.76603.be. [DOI] [PubMed] [Google Scholar]
- 173.Leskela S, Honrado E, Montero-Conde C, Landa I, Cascon A, Leton R, Talavera P, Cozar JM, Concha A, Robledo M, Rodriguez-Antona C. Cytochrome P450 3A5 is highly expressed in normal prostate cells but absent in prostate cancer. Endocr Relat Cancer. 2007;14(3):645–54. doi: 10.1677/ERC-07-0078. [DOI] [PubMed] [Google Scholar]
- 174.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27(4):383–91. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 175.Zhenhua L, Tsuchiya N, Narita S, Inoue T, Horikawa Y, Kakinuma H, Kato T, Ogawa O, Habuchi T. CYP3A5 gene polymorphism and risk of prostate cancer in a Japanese population. Cancer Lett. 2005;225(2):237–43. doi: 10.1016/j.canlet.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 176.Vaarala MH, Mattila H, Ohtonen P, Tammela TL, Paavonen TK, Schleutker J. The interaction of CYP3A5 polymorphisms along the androgen metabolism pathway in prostate cancer. Int J Cancer. 2008;122(11):2511–6. doi: 10.1002/ijc.23425. [DOI] [PubMed] [Google Scholar]
- 177.Domanski TL, Finta C, Halpert JR, Zaphiropoulos PG. cDNA cloning and initial characterization of CYP3A43, a novel human cytochrome P450. Mol Pharmacol. 2001;59(2):386–92. doi: 10.1124/mol.59.2.386. [DOI] [PubMed] [Google Scholar]
- 178.Stone A, Ratnasinghe LD, Emerson GL, Modali R, Lehman T, Runnells G, Carroll A, Carter W, Barnhart S, Rasheed AA, Greene G, Johnson DE, Ambrosone CB, Kadlubar FF, Lang NP. CYP3A43 Pro(340) Ala polymorphism and prostate cancer risk in African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1257–61. doi: 10.1158/1055-9965.EPI-04-0534. [DOI] [PubMed] [Google Scholar]
- 179.Siemes C, Visser LE, de Jong FH, Coebergh JW, Uitterlinden AG, Hofman A, Stricker BH, van Schaik RH. Cytochrome P450 3A gene variation, steroid hormone serum levels and prostate cancer--The Rotterdam Study. Steroids. 2010;75(12):1024–32. doi: 10.1016/j.steroids.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 180.Ratan HL, Gescher A, Steward WP, Mellon JK. ErbB receptors: possible therapeutic targets in prostate cancer? BJU Int. 2003;92(9):890–5. doi: 10.1111/j.1464-410x.2003.04503.x. [DOI] [PubMed] [Google Scholar]
- 181.Sirotnak FM, Zakowski MF, Miller VA, Scher HI, Kris MG. Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res. 2000;6(12):4885–92. [PubMed] [Google Scholar]
- 182.Sirotnak FM, She Y, Lee F, Chen J, Scher HI. Studies with CWR22 xenografts in nude mice suggest that ZD1839 may have a role in the treatment of both androgen-dependent and androgen-independent human prostate cancer. Clin Cancer Res. 2002;8(12):3870–6. [PubMed] [Google Scholar]
- 183.Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, Scher HI, Sliwkowski MX. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2(2):127–37. doi: 10.1016/S1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 184.Di Lorenzo G, Tortora G, D’Armiento FP, De Rosa G, Staibano S, Autorino R, D’Armiento M, De Laurentiis M, De Placido S, Catalano G, Bianco AR, Ciardiello F. Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin Cancer Res. 2002;8(11):3438–44. [PubMed] [Google Scholar]
- 185.Shuch B, Mikhail M, Satagopan J, Lee P, Yee H, Chang C, Cordon-Cardo C, Taneja SS, Osman I. Racial disparity of epidermal growth factor receptor expression in prostate cancer. J Clin Oncol. 2004;22(23):4725–9. doi: 10.1200/JCO.2004.06.134. [DOI] [PubMed] [Google Scholar]
- 186.Douglas DA, Zhong H, Ro JY, Oddoux C, Berger AD, Pincus MR, Satagopan JM, Gerald WL, Scher HI, Lee P, Osman I. Novel mutations of epidermal growth factor receptor in localized prostate cancer. Front Biosci. 2006;11:2518–25. doi: 10.2741/1986. [DOI] [PubMed] [Google Scholar]
- 187.Gibbs M, Stanford JL, McIndoe RA, Jarvik GP, Kolb S, Goode EL, Chakrabarti L, Schuster EF, Buckley VA, Miller EL, Brandzel S, Li S, Hood L, Ostrander EA. Evidence for a rare prostate cancer-susceptibility locus at chromosome 1p36. Am J Hum Genet. 1999;64(3):776–87. doi: 10.1086/302287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Matsui H, Suzuki K, Ohtake N, Nakata S, Takeuchi T, Yamanaka H, Inoue I. Genomewide linkage analysis of familial prostate cancer in the Japanese population. J Hum Genet. 2004;49(1):9–15. doi: 10.1007/s10038-003-0099-y. [DOI] [PubMed] [Google Scholar]
- 189.Kittles RA, Baffoe-Bonnie AB, Moses TY, Robbins CM, Ahaghotu C, Huusko P, Pettaway C, Vijayakumar S, Bennett J, Hoke G, Mason T, Weinrich S, Trent JM, Collins FS, Mousses S, Bailey-Wilson J, Furbert-Harris P, Dunston G, Powell IJ, Carpten JD. A common nonsense mutation in EphB2 is associated with prostate cancer risk in African American men with a positive family history. J Med Genet. 2006;43(6):507–11. doi: 10.1136/jmg.2005.035790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Williams H, Powell IJ, Land SJ, Sakr WA, Hughes MR, Patel NP, Heilbrun LK, Everson RB. Vitamin D receptor gene polymorphisms and disease free survival after radical prostatectomy. Prostate. 2004;61(3):267–75. doi: 10.1002/pros.20103. [DOI] [PubMed] [Google Scholar]
- 191.Shui IM, Mucci LA, Kraft P, Tamimi RM, Lindstrom S, Penney KL, Nimptsch K, Hollis BW, Dupre N, Platz EA, Stampfer MJ, Giovannucci E. Vitamin D-related genetic variation, plasma vitamin D, and risk of lethal prostate cancer: a prospective nested case-control study. J Natl Cancer Inst. 2012;104(9):690–9. doi: 10.1093/jnci/djs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Signorello LB, Williams SM, Zheng W, Smith JR, Long J, Cai Q, Hargreaves MK, Hollis BW, Blot WJ. Blood vitamin d levels in relation to genetic estimation of African ancestry. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2325–31. doi: 10.1158/1055-9965.EPI-10-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]