Abstract
Gastrointestinal cancer is a group of tumors that affect multiple sites of the digestive system, including the stomach, liver, colon and pancreas. These cancers are very aggressive and rapidly metastasize, thus identifying effective targets is crucial for treatment. Galectin-1 (Gal-1) belongs to a family of glycan-binding proteins, or lectins, with the ability to cross-link specific glycoconjugates. A variety of biological activities have been attributed to Gal-1 at different steps of tumor progression. Herein, we summarize the current literature regarding the roles of Gal-1 in gastrointestinal malignancies. Accumulating evidence shows that Gal-1 is drastically up-regulated in human gastric cancer, hepatocellular carcinoma, colorectal cancer and pancreatic ductal adenocarcinoma tissues, both in tumor epithelial and tumor-associated stromal cells. Moreover, Gal-1 makes a crucial contribution to the pathogenesis of gastrointestinal malignancies, favoring tumor development, aggressiveness, metastasis, immunosuppression and angiogenesis. We also highlight that alterations in Gal-1-specific glycoepitopes may be relevant for gastrointestinal cancer progression. Despite the findings obtained so far, further functional studies are still required. Elucidating the precise molecular mechanisms modulated by Gal-1 underlying gastrointestinal tumor progression, might lead to the development of novel Gal-1-based diagnostic methods and/or therapies.
Keywords: Galectin-1; Gastric cancer; Hepatocellular carcinoma; Colorectal carcinoma; Pancreatic cancer; β1,6-N-acetylglucosaminyltransferase V
Core tip: Gastrointestinal cancer is a group of tumors that affect multiple sites of the digestive system, including the stomach, liver, colon and pancreas. Galectin-1 (Gal-1) is a β-galactoside-binding protein with the ability to cross-link specific glycoconjugates. Accumulating evidence shows that Gal-1 in human gastric cancer, hepatocellular carcinoma, colorectal cancer and pancreatic ductal adenocarcinoma tissues is up-regulated. Moreover, high levels of this galectin correlate with tumor development, aggressiveness, metastasis, immunosupression and angiogenesis. We also highlight that alterations in Gal-1-specific glycoepitopes may be relevant for gastrointestinal cancer progression.
INTRODUCTION
Gastrointestinal cancer is the most common group of malignancies. It refers to tumors developed at multiple sites of the gastrointestinal tract, including stomach, liver, colon and pancreas. During the past decade, advances in molecular medicine have led to improve prevention, diagnosis and therapy. Nevertheless, most of these cancers are very aggressive and rapidly metastasize and therefore, they are among the most deadly[1]. Thus, the identification of new molecular targets is crucial to develop more effective therapeutic strategies.
Galectins are proteins that decode and interpret the glycome. This is a family of glycan-binding proteins (lectins) with affinity for β-galactoside-containing N-glycans[2]. This affinity for N-glycans increases in proportion to β1-6 branching, which is mediated by the β1,6-N-acetylglucosaminyltransferase V (GnTV)[3] and extension with poly-N-acetyllactosamine[4]. Interestingly, the altered expression of galectins is considered a hallmark of cancer and mounting evidence illustrates their fundamental roles in tumor biology[5,6]. Besides, important changes in glycosylation occur during tumorigenesis and metastasis, including the increase in N-glycan size in several cancer-associated glycoproteins[7].
One member of this family is galectin-1 (Gal-1), which possesses specific structural features. It is secreted from cells and is able to bind and cross-link glycoconjugates on cell surfaces, including different integrins and glycoproteins of the extracellular matrix (ECM)[8]. Consequently, Gal-1 modulates cell adhesion, migration, survival and signaling[9]. Intracellularly, Gal-1 interacts with the RAS binding domain of RAF effectors and increases H-RAS nanoclustering driving tumor transformation[10]. Gal-1 expression is frequently increased in tumor tissues[11,12]. Elevated levels of Gal-1 secreted to the extracellular space of tumor and tumor-associated stromal cells, often correlate with tumor aggressiveness and acquisition of a metastatic phenotype[13]. This lectin also plays fundamental roles in tumor angiogenesis by modulating endothelial cell biology[14-16] and contributes to tumor immunoevasive programs[17]. Given these tumor-promoting activities, Gal-1 is considered a potential target for cancer therapy[13].
Important roles of some members of the galectin family have been described in gastrointestinal tumors[18-22]. In this review, we summarize the current knowledge regarding the expression and roles of Gal-1 in gastrointestinal malignancies, focusing on gastric cancer, hepatocellular carcinoma, colorectal carcinoma and pancreatic cancer. We also highlight that alterations in Gal-1-specific glycoepitopes may be relevant for gastrointestinal cancer progression. Our aim is to encourage researchers to continue exploring the complex roles of Gal-1 in gastrointestinal tumors. We are confident that basic and translational research in this field will lead to the development of Gal-1-targeted therapies for gastrointestinal cancer patients in the future.
ROLES OF GAL-1 IN GASTRIC CANCER
Gastric cancer (GC) ranks second for cancer deaths[1]. Most of these cancers are adenocarcinomas, which are subdivided into intestinal or diffuse types. In intestinal-type cancer, tumor cells exhibit intercellular adhesion and are arranged in tubular structures[23]. By contrast, in the diffuse type, tumor cells are poorly cohesive and infiltrate the stroma as small subgroups[23,24]. Although these subtypes are biological, clinical and epidemiologically different, in clinical practice this classification is not useful for predicting treatment response or survival[23]. Besides, according to the anatomical location, adenocarcinomas are classified as proximal and distal.
Risk factors for proximal GC include increased body weight, gastro-esophageal reflux disease and Barrett’s esophagus, while chronic infection with Helicobacter pylori (H. pylori) is the strongest risk factor for distal GC[25,26]. Besides, gene amplification of human epithelial growth factor receptor 2 (HER2), a proto-oncogene, is found in approximately 17% of GC samples[27].
GC is often diagnosed at advanced stages and therapy includes surgery with adjuvant chemotherapy or chemoradiation. Advances in the understanding of the molecular profiling of GC led to the development of trastuzumab and ramucirumab, humanized monoclonal antibodies anti-HER2 and anti-vascular endothelial growth factor receptor 2 (VEGFR-2), respectively[28]. However, the median survival at advanced stage is less than 1 year, pointing out the need to identify new molecular targets and biomarkers[29].
In this section we summarize the available knowledge of Gal-1 expression in GC and its contribution to progression.
Gal-1 expression in human GC tissues, correlation with clinicopathological parameters and prognostic significance
One of the main features of intestinal-type GC is chronic inflammation, which leads to precancerous lesions. High Gal-1 immunostaining was observed in premalignant chronic gastritis, gastric ulcer and intestinal metaplasia lesions, both in epithelium and stroma, suggesting Gal-1 involvement in gastric carcinogenesis[30,31]. In addition, Gal-1 overexpression in gastric adenocarcinoma tissues vs normal mucosa was reported[30-34]. Gal-1 was highly expressed in tumor-associated stromal cells and weakly expressed in cancer epithelial cells. Interestingly, Gal-1 staining intensity in tumor-associated stroma significantly correlated with tumor location, invasion, differentiation, tumor stage and lymph node metastasis[32,33,35-37]. Moreover, patients with high stromal Gal-1 expression exhibited a lower five-year survival rate respect to patients with low expression[32,33,37].
Although low Gal-1 expression in cancer epithelial cells in intestinal-type GC was observed, this galectin was detected in tumor cells of signet ring cell carcinoma (SRCC), a diffuse type of gastric carcinoma[38]. These differences could be explained by the specific SRCC oncogenesis which differs from that of tubular gastric adenocarcinoma[39].
A crucial step during cancer progression is epithelial-mesenchymal transition (EMT), which involves changes in cell-cell and cell-matrix interactions and cell motility, allowing tissue epithelial cancers to invade and metastasize[40]. During EMT, tumor cells change their epithelial cell morphology toward a fibroblastoid phenotype, being this process favored by the downregulation of epithelial cell markers and the up-regulation of mesenchymal markers. In GC, EMT is regulated through activation of glioma-associated oncogene 1 (Gli1), a key member of the Hedgehog (Hh) signaling pathway. The adherens protein E-cadherin is an epithelial marker which expression and localization are frequently deregulated in GC. It was found that Gal-1 expression in GC tissues was positively correlated with Gli-1 and vimentin (a mesenchymal cell marker) expression but negatively associated with E-cadherin expression[36,37].
Furthermore, Gal-1 was positively associated with VEGF, suggesting that both molecules can serve as indicators of poor prognosis for GC patients[32,41].
Cancer-associated fibroblasts (CAFs), one of the major components of tumor stroma, contribute to cancer cell proliferation, invasion and metastasis. A potent inducer of fibroblast transformation into CAFs is transforming growth factor beta 1 (TGF-β1), a cytokine elevated in tumor microenvironments. Interestingly, increased levels of Gal-1 were observed in CAFs isolated from GC patient tissues compared to fibroblasts from adjacent normal mucosa (NFs)[33,34,41]. Also, a positive correlation between Gal-1 and TGF-β1 in GC tissues was described[33]. Moreover, Gal-1 expression in CAFs correlated with β1 integrin expression in GC tissues[34]. This integrin is a cell adhesion receptor with various functions in cancer biology, such as proliferation, invasion and migration. Staining intensities of both β1 integrin and Gal-1 were associated with lymph node and distant metastasis and tumor/node/metastasis (TNM) stage of clinicopathological parameters. Strong intensity of double positive samples was associated with poor prognostic and low survival[34].
Therefore, elevated Gal-1 levels in GC tissues, mainly in stromal cells, are associated with tumor progression (Figure 1). Besides, Gal-1 expression may be related to EMT and metastasis. Further studies are required to confirm the usefulness of Gal-1 as a prognostic factor for GC.
Figure 1.
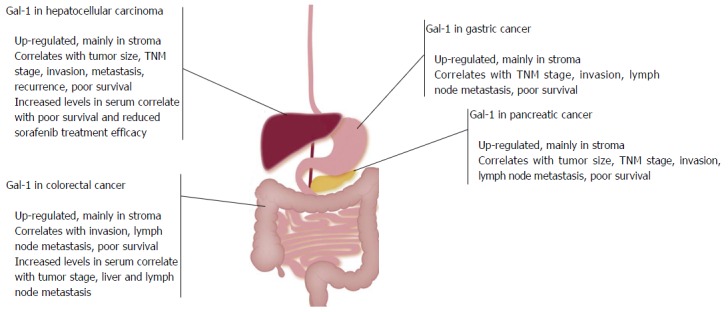
Galectin-1 expression in gastrointestinal tumors and correlation with clinicopathological parameters. Galectin-1 is drastically up-regulated in human gastric cancer, hepatocellular carcinoma, colorectal cancer and pancreatic ductal adenocarcinoma tissues, mainly in stroma. High levels of Gal-1 correlate with tumor aggressiveness, the acquisition of a metastatic phenotype and patient poor survival. TNM: Tumor/node/metastasis; Gal-1: Galectin-1.
Gal-1 contribution to GC development and angiogenesis in experimental models
The administration of N-methyl-N´-nitro-N-nitrosoguanidine (MNNG), a carcinogen that destroys stomach mucous barrier, constitutes a rat GC model in which animals develop benign and malignant lesions with an inflammatory cell-enriched stroma[42]. Enteric nervous system regulates immune and inflammatory processes thus, chemical myenteric denervation reduces MNNG-induced GC incidence[43]. Remarkably, Gal-1 was highly expressed in nondenervated-rat stomachs, whereas chemical myenteric denervation downregulated Gal-1 expression[42]. Thus, the decrease in Gal-1 levels had a protective role in this model, associated with a reduced density of inflammatory cells.
Further, the role of CAF-derived Gal-1 in tumor growth and angiogenesis was studied using the chick embryo chorioallantoic membrane (CAM) assay[41]. NFs or CAFs obtained from human samples were co-cultured with SGC7901 GC cells and injected into CAMs. Also, Gal-1 was overexpressed or silenced in CAFs. Both tumor volume and number of blood vessels were significantly higher with Gal-1-overexpressing CAFs respect to control CAFs and NFs[41].
These results suggest that Gal-1 participates in the development of experimental GC and that CAF-derived Gal-1 promotes gastric tumor growth and angiogenesis (Figure 2).
Figure 2.

Proposed models of Galectin-1 contribution to the progression of gastric cancer, hepatocellular carcinoma, colorectal cancer and pancreatic ductal adenocarcinoma. Galectin-1 (Gal-1) has key functions in tumor and tumor-associated stromal cells, favoring tumor cell proliferation and dissemination, angiogenesis and immunosuppression. A high exposure of Gal-1 glycan partners also contribute to tumor progression. ECM: Extracellular matrix; EMT: Epithelial-mesenchymal transition; GnTV: β1,6-N-acetylglucosaminyltransferase V; HSC: Hepatic stellate cell; PSC: Pancreatic stellate cell; TGF-β1: Transforming growth factor beta 1.
Molecular mechanisms associated with GC progression induced by Gal-1
Studies in human tissues suggest an association between Gal-1 expression and EMT in GC. Interestingly, the expression of cell adhesion-related genes in H. pylori-infected AGS GC cells was analysed by cDNA microarray. It was found that Gal-1 and α5 integrin genes were up-regulated, whereas E-cadherin gene was downregulated, compared to cells without infection[44]. Furthermore, Gal-1, β-catenin, vimentin, α6 and β4 integrin expression was elevated in high metastatic GC cell lines compared with low metastatic cells[36,45]. Besides, Gal-1 overexpression in MKN-74 low invasive cells decreased E-cadherin and increased Gli1 levels, activating Hh pathway and favoring cell invasion[36].
Further, the role of Gal-1 in the interaction between GC and stromal cells was studied. TGF-β1 secreted by MKN7 GC cells up-regulated Gal-1 in NFs in a phosphoinositide 3-kinase (PI3K)/AKT-dependent manner. Interestingly, Gal-1 induced the expression of α-smooth muscle actin (α-SMA, a myofibroblast marker) favoring transformation from NFs to CAFs[33]. Besides, CAF-derived Gal-1 inhibited apoptosis and promoted EMT, migration and invasion of several GC cell lines, decreasing E-cadherin expression but enhancing vimentin, Gli1[37], matrix metallopeptidase (MMP) 9[33], β1 integrin[34] and VEGF[41] expression. Moreover, silencing β1 integrin in GC cells prevented the up-regulation of Gli1[37] and the invasion-promoting effect of CAF-derived Gal-1[34]. These results suggest an essential role of β1 integrin in the Gal-1-induced EMT and metastasis in GC. Moreover, high expression of Gal-1 in gastric CAFs increased human umbilical vein endothelial cell proliferation, migration, tube formation and VEGFR-2 phosphorylation[41].
The immunosuppressive transcription factor FoxP3 was found to be expressed not only in regulatory T cells (Tregs) but also in SRCC tissues. Remarkably, Gal-1 expression was downregulated when FoxP3 was silenced in SRCC-derived OCUM-2M cells[38]. These findings suggest that FoxP3 expressed both in Tregs and SRCC tumor tissues may be important for Gal-1 tumor expression, which may subsequently induce immunosuppression.
As Gal-1 is a glycan-binding protein, alterations in the glycosylation pattern of its ligands within tumor microenvironment may affect galectin functions. For example, GnTV deficiency prevented Gal-1 binding to endothelial cells and decreased tumor growth by attenuating aberrant vascularization[46]. Particularly in GC, diverse alterations in cell glycosylation pattern modulate tumor cell behavior. GnTV overexpression in GC contributes to cell invasion and metastasis[47-49]. Interestingly, GnTV up-regulation in MKN45 GC cells induced cell migration mediated by α3β1 integrin glycosylation[50] and EMT involving E-cadherin glycosylation[51]. Thus, not only an alteration in Gal-1 protein levels but also the aberrant glycosylation pattern of its ligands, such as integrins, may contribute to GC progression.
Thus, Gal-1 has a relevant role in the crosstalk between GC and stromal cells, favoring tumor cell proliferation, EMT and dissemination and angiogenesis (Figure 2). Altogether, these findings point that Gal-1 regulates key steps during GC progression.
ROLES OF GAL-1 IN HEPATOCELLULAR CARCINOMA
Hepatocellular carcinoma (HCC), the most common type of liver cancer, is the third cause of cancer-related deaths. Risk factors include chronic hepatitis B or C virus infection, alcohol abuse and non-alcoholic fatty liver disease[52]. HCC usually develops in the background of chronic liver inflammation, advanced fibrosis and cirrhosis[53]. Thus, it arises not only as a consequence of the molecular changes that occur in transformed hepatocytes, but also due to the crosstalk between diverse cells and molecules within tumor microenvironment[54].
During the past decade new advances led to an earlier detection of HCC[55]. Additionally, current therapies such as, resection, transplantation and chemoembolization, benefit patients diagnosed at early HCC stages improving and extending their survival[56,57]. However, most patients are diagnosed at advanced stages and therefore, they are not amenable to surgery. Even after resection or transplantation, the prognosis remains unsatisfactory due to recurrence, metastasis and the development of new primary tumors[58,59].
Recent progress toward a better understanding of HCC has shed light on new molecular targeted and systemic therapies. Sorafenib, a multikinase inhibitor targeting VEGF, platelet-derived growth factor and RAF signaling pathways, prolongs survival in patients with advanced unresectable HCC[60,61]. Despite the modest increase in survival[60], undoubtedly the approval of oral administration of sorafenib highlights the importance of elucidating the molecular mechanisms underlying HCC progression for the development of novel therapies.
Here we review the accumulating evidence that points towards a remarkable role of Gal-1 in HCC progression, aggressiveness and metastasis.
Gal-1 expression in human HCC tissues, correlation with clinicopathological parameters and prognostic value
While in human normal or adjacent non-tumor liver tissues Gal-1 is expressed at low levels, in HCC its expression is dramatically up-regulated[62-66]. The transcription start site of Gal-1 gene promoter was frequently methylated in non-tumor liver, whereas it was hypomethylated in HCC tissues[63]. Besides, Gal-1 was significantly increased in HCC samples from patients with metastasis compared to those harboring a non-metastatic primary tumor and it was found accumulated in the stroma surrounding tumor hepatocytes[64]. Accordingly, Gal-1 was highly expressed in stromal α-SMA-positive cells in HCC, indicating that Gal-1 is overexpressed in hepatic stellate cells (HSCs)[66].
The prognostic value of Gal-1 in HCC patients was recently evaluated. Elevated Gal-1 expression in HCC was significantly associated with tumor aggressiveness (vascular invasion, incomplete encapsulation, poor differentiation and large tumor size), dissemination and enhanced risk of post-operative recurrence[65]. Additionally, high stromal Gal-1 expression in HCC was correlated with tumor size, TNM stage and distant metastasis and negatively correlated with patient prognosis[66]. Moreover, in HCC samples, an inverse correlation between miR-22 and Gal-1 expression was found[66]. miR-22 is downregulated in HCC[67] and Gal-1 has been predicted to be a target of miR-22.
Furthermore, a positive correlation was observed between Gal-1 expression and tumor-infiltrating FoxP3+ Tregs in HCC tissues[65]. Since Gal-1 is a key regulator of CD4+CD25+ Tregs, which in turn play an essential role in suppression of anticancer immunity[68], the interaction between Gal-1 and Tregs might play a role in immunosuppression against HCC. Recently, it was shown that patients with advanced HCC had significantly higher serum Gal-1 levels than healthy volunteers[69]. These elevated serum levels correlated with poor treatment efficacy of sorafenib and were an independent factor associated with poor progress-free survival and overall survival[69].
These findings demonstrate that Gal-1 overexpression in HCC tissues correlate with tumor aggressiveness and support the potential use of Gal-1 as a novel predictive and prognostic biomarker of HCC (Figure 1).
Gal-1 contribution to tumorigenesis, metastasis and chemoresistance in HCC experimental models
Gal-1 role in HCC tumor growth and metastasis in vivo was evidenced. We found an increase in tumor volume and in the number of draining-tumor lymph nodes in athymic mice injected with Gal-1-overexpressing human HepG2 HCC cells, respect to control mice[70]. Recently, Gal-1 involvement in HCC chemoresistance was also demonstrated. Cisplatin anti-tumor activity was enhanced with the administration of the galectin inhibitor thiodigalactoside in a mouse hepatoma model[71].
Interestingly, a dual role of Gal-1 in liver inflammation and tumorigenesis has been dissected in multidrug resistant knock-out mice[72]. This is an experimental model of inflammation-induced chronic cholestatic hepatitis at an early age and HCC at a later age, which together mimic the evolution of human disease[73]. Gal-1 showed a strain-dependent protective anti-inflammatory function at early stages delaying HCC development. Remarkably, Gal-1 deficiency in knock-out mice resulted in a significant retardation of liver regeneration, evidenced by a delay in hepatocyte proliferation, liver mass restoration and macrophage recruitment and a decrease in transient liver post-partial hepatectomy steatosis[74].
These findings revealed that Gal-1 controls hepatocarcinogenesis. It may act as a protective anti-inflammatory agent at early stages of the chronic liver pathology, but as a pro-tumorigenic agent at late stages, contributing to HCC growth and metastasis (Figure 2).
Functional studies associated with HCC progression, dissemination and chemoresistance induced by Gal-1
Gal-1 overexpression observed in HCC human tissues was confirmed in cell lines[63,64]. In fact, Gal-1 mRNA levels were higher in more invasive and undifferentiated human HCC cell lines as compared to well-differentiated HCC cells and normal liver cells. Interestingly, a methylation-sensitive factor was essential for Gal-1 gene activation in HCC cells[63].
Furthermore, the correlation between increased expression of Gal-1 in HCC and the presence of metastasis was validated by in vitro functional studies. Notably, Gal-1 overexpression increased human HuH-7 HCC cell migration and invasion through Sky receptor tyrosine kinase phosphorylation[64]. Next, our studies provided evidence of Gal-1 as a glycan-dependent modulator of HepG2 cell adhesion and polarization[70]. The pro-adhesive effect of Gal-1 was mediated by α1, α2, α3, αv and β1 integrins and PI3K and/or extracellular signal-regulated kinase (ERK) 1/2 signaling pathways[70]. Recently, we also demonstrated that increased levels of Gal-1 induced EMT in HCC cells, involving PI3K/AKT and WNT/β-catenin proliferative signaling pathways[75]. Gal-1 up-regulation in HepG2 cells induced E-cadherin downregulation and increased levels of the transcription factor Snail, one of the main inducers of EMT in HCC. Moreover, enhanced Gal-1 expression favored the transition from an epithelial cell morphology toward a fibroblastoid phenotype, vimentin up-regulation and resistance to anoikis (programmed cell death induced upon cell detachment from extracellular matrix)[75].
The glycosylation pattern of HCC cells also changes during EMT. Particularly, an increase of GnTV-mediated β1-6 N-glycan branching and a decrease of α2,6 sialylation (structure capable of precluding Gal-1 binding) were observed in HuH-7 cells undergoing EMT[76]. Interestingly, GnTV was increased in high metastatic HCC cells as compared to low metastatic cells[77], in the liver of a murine model of hepatocarcinogenesis as well as in regenerative liver[78]. Further, tetra-antennary glycans and GnTV expression were increased in HCC samples and positively correlated with TNM stage and impaired clinical outcome in patients[79,80]. Thus, these findings suggest that Gal-1-specific glycan epitopes are more exposed during HCC cell dissemination.
Regarding HCC cell growth and/or proliferation, extracellular Gal-1 (recombinant protein) attenuated cell cycle progression and induced apoptosis of HepG2 cells via interaction with α5β1 integrin[81,82]. Remarkably, during the early stages of HCC, TGF-β1 acts as a tumor suppressor; however in advanced stages, HCC cells lose their cytostatic response to this cytokine and undergo EMT. Recently, we demonstrated that TGF-β1 induced Gal-1 expression and secretion by HepG2 and HuH-7 cells. At the same time, intracellular Gal-1 modulated HepG2 cell proliferation and sensitivity to TGF-β1-induced growth inhibition[83]. Therefore, Gal-1 and TGF-β1 might function coordinately within the HCC microenvironment to regulate tumor growth, invasion and metastasis.
Concerning the interaction between tumor hepatocytes and stromal cells, we demonstrated that Gal-1 secreted from HCC cells promoted human SK-HEP-1 liver sinusoidal endothelial cell (LSEC) proliferation and migration and glycan-dependent HCC cell adhesion to LSECs[83]. Also, Gal-1 was augmented in activated compared to quiescent rat and human HSCs[66,84] and stimulated HSC proliferation and migration through carbohydrate-dependent mechanisms via ERK1/2 signaling pathway[85]. Recently, it was shown that HSC-derived Gal-1 promoted CD3+ T-cell apoptosis and Th1/Th2 cytokine balance skewing[66]. Remarkably, miR-22 inhibited Gal-1 expression in HSCs and its immunosuppressive function. Furthermore, HSCs isolated from HCC tissues exhibited higher Gal-1 expression and more powerful immunosuppression than HSCs derived from normal liver samples[66].
As for the role in HCC chemoresistance, Gal-1 expression and secretion increased in sorafenib-resistant HuH-7 cells (HuH-7-R) through the AKT/mammalian target of rapamycin (mTOR)/hypoxia inducible factor (HIF)-1α pathway. Interestingly, Gal-1 downregulation suppressed migratory and invasive abilities of HuH-7-R cells and restored sorafenib sensitivity[69]. Besides, Gal-1 induced chemoresistance to cisplatin in HepG2 and HuH-7 cells in a glycan-dependent manner, inhibiting AKT/mTOR signaling and triggering autophagy[71].
Thus, high levels of Gal-1 in tumor microenvironment induce HCC cell proliferation, EMT and dissemination, angiogenesis, immunosuppression and chemoresistance (Figure 2). Collectively, these findings reveal key roles of Gal-1 in HCC progression. Furthermore, Gal-1 represents a promising target to confront HCC chemoresistance.
ROLES OF GAL-1 IN COLORECTAL CANCER
Colorectal cancer (CRC) is the fourth cause of cancer-related deaths[1]. Nowadays, various techniques are available to detect CRC as precursor lesions[86]. Patients early diagnosed have a 5-year survival rate of 90%-95% following surgical resection. However, metastasis are present in approximately 20% of patients at initial diagnosis[87] and half patients will develop metastatic disease[88,89]. When diagnosed at advanced stages, treatment consists of a combination of surgery, chemotherapy and radiotherapy and the 5-year survival rate is only 5%-10%[90].
The greatest risk factor for CRC is increasing age[89]. Sporadic CRC, due to somatic mutations, account for about 70% of all cases. Familial CRC, a group of diseases harboring familial predisposition to develop CRC, is about 10%-30%, whereas hereditary diseases are 5%-7%[91]. CRC develops through a gradual accumulation of genetic and epigenetic changes, leading to the transformation of normal colonic mucosa into invasive cancer[86,89]. Some typical activating gene mutations arise on members of WNT signaling pathway and KRAS oncogene and inactivating mutations occur on the TP53 tumor suppressor gene[92].
Despite the recent achievements in the understanding of colorectal carcinogenesis, the incidence and mortality rates highlight the need for new diagnostic markers of early disease, an improved understanding of tumor progression and the identification of new genes and/or pathways contributing to malignancy.
In this section we analyze the current knowledge on the roles of Gal-1 in CRC progression and its potential use as therapeutic target.
Gal-1 expression in human CRC tissues, correlation with clinical features and potential use as biomarker
Positive Gal-1 expression was detected in CRC tissues many years ago, by immunohistochemistry and Western blot[93,94]. Gal-1 levels were low in normal and neoplastic epithelial cells, but the stroma showed a progressive Gal-1 overexpression from normal mucosa to adenomas and carcinomas[95]. Gal-1 increase in CRC tissues was further confirmed by several studies, but discrepancies on Gal-1 localization were found[96-101]. Some studies reported marked Gal-1 expression both in epithelial and stromal compartments, while others emphasized Gal-1 strong expression mainly in stroma. In addition, Gal-1 staining was faint in endothelial cells of normal tissues but strong in CRC-associated endothelium, especially in proliferating endothelial cells[15].
Concerning the correlation with patient clinical features, contradictory results were obtained. Epithelial Gal-1 levels in early stage samples were associated with short survival[97]. Furthermore, Gal-1 expression was related to tumor invasion and lymph node involvement, but not to tumor differentiation[98]. However, no correlation was found between stromal Gal-1 expression and tumor size, stage, differentiation, nor with patient survival or relapse-free probability[95]. Hence, increasing the number of cases analysed is crucial to reach a more reliable conclusion. Gal-1 expression was also higher in biopsies from radiotherapy-responder patients with rectal cancer than in those from nonresponders[102]. Thus, Gal-1 gene expression profiling was suggested to be useful in predicting the response to radiotherapy.
Some studies focused on the evaluation of circulating Gal-1 levels as possible biomarkers or prognosis predictors. An increase of plasma Gal-1 levels in CRC patients respect to healthy controls was detected. These levels were already increased in CRC early stages and importantly, were found decreased after patient surgery[101]. Also, high serum Gal-1 positively correlated with tumor stage and lymph node metastasis[19,103].
The expression of accessible binding sites specific for Gal-1 (determined by biotinylated Gal-1) increased with dysplastic and malignant progression[96]. Moreover, the increase in β1,6-branched structures in CRC human samples was associated with lymph node metastasis and decreased survival rates in CRC patients[104]. Accordingly, GnTV overexpressed in CRC tissues correlated with metastasis[105]. Remarkably, the up-regulation of a novel glycosiltransferase, β3GnT8, which can extend polylactosamine on N-glycans, was also described in CRC tissues[106].
Thus, overexpression of Gal-1 and a high exposure of its glycan partners in CRC tissues correlate with tumor progression. Further studies are required to confirm its potential use as a biomarker for CRC (Figure 1).
Molecular mechanisms associated with CRC cell adhesion, migration and growth/proliferation modulated by Gal-1
Gal-1 expression was analysed in many cell lines. Gal-1 mRNA was detected in normal colon and CRC cells, whereas Western blot analysis showed variable Gal-1 expression[96,107-109]. Further, when global protein expression was analysed in human poor and high metastatic CRC cell lines, Gal-1 was identified as a protein differentially expressed[110]. Mechanisms accounting for gene regulation were also studied. Gal-1 expression was induced by sodium butyrate, a differentiation-inducing agent and by the hypoxia-related transcription factor HIF-1α in CRC cells[98,108,111,112]. Interestingly, epigenetic regulation of Gal-1 expression was also observed. It was revealed that promoter methylation contributes to silencing Gal-1 gene transcription in CRC cells[108].
The role of Gal-1 and its cell surface glycan partners in CRC cell adhesion was evaluated. The inhibitor of N-acetyllactosamine synthesis, 2-Acetamido-l,3,6-tri-O-acetyl-4-deoxy-4-fluoro-a-D-glycopyranose, impaired HT-29 cell adhesion to Gal-1. This decreased attachment correlated with reduced levels of cell surface lysosome-associated membrane proteins (LAMP)[113]. Moreover, LAMP-1, LAMP-2 and carcinoembryonic antigen were identified as the major Gal-1-binding glycoproteins in CRC cells[112]. Gal-1 was capable as well of inducing Colo201 CRC cell adhesion to ECM glycoproteins[114]. Moreover, Gal-1 pro-adhesive effect was glycan-dependent with the involvement of PI3K and mitogen-activated protein kinase signaling pathways[114]. Of note, GnTV overexpression in CRC cell lines induced resistance to anoikis and WNT signaling activation[115].
Other Gal-1 roles in CRC cells seem to depend on whether the lectin is localized intra- or extracellularly and the cell line analysed. A Gal-1-enriched ECM decreased HCT-15, LoVo and CoLo201 cell motility[96], thus extracellular Gal-1 reduces CRC cell migration. Instead, contradictory effects were observed modifying intracellular Gal-1 expression. Silencing Gal-1 inhibited SW620 cell invasion and migration induced by hypoxia[98]. In contrast, Gal-1 up-regulation in LS-180 cells significantly decreased cell migration and invasion[108].
Regarding cell viability, Gal-1 overexpression induced Colo201 and LS-180 cell apoptosis[108,114], but extracellular Gal-1 had no effect on HCT-15, LoVo, DLD-1, HT-29, Caco-2 and Colo201 cell growth[81,96,114]. Interestingly, α5 integrin ectopic expression sensitized HT-29 and Caco-2 cells to exogenous Gal-1-induced growth inhibition, indicating that Gal-1-α5 integrin interaction attenuated cell cycle progression[81]. Further, Gal-1 overexpression induced LS-180 cell cycle arrest and apoptosis with concomitant downregulation of WNT and nuclear factor kappa B (NF-κB) signaling pathways[108]. Conversely, Gal-1 interaction with protocadherin-24 (a non-classical cadherin downregulated in CRC cells) enhanced Gal-1 retention in HCT116 CRC cell membranes. This interaction abolished Gal-1-mediated PI3K activation and decreased nuclear β-catenin in cells[116], suggesting that protocadherin-24 loss and Gal-1 up-regulation may lead to proliferative WNT signaling activation in these cells.
These findings show the relevance of Gal-1-glycan interaction in CRC cell adhesion and the complexity of Gal-1 function in CRC cell migration and growth (Figure 2). Therefore, further studies are required to decipher the mechanisms by which proliferative signaling pathways are regulated in CRC cells expressing endogenous Gal-1.
In vivo studies of Gal-1 role in CRC
Nonsteroidal anti-inflammatory drugs such as indomethacin (IN) exert anti-CRC activity via a cyclooxygenase-independent mechanism. Oral administration of IN significantly suppressed CRC growth of HCT116 xenografts in BALB/c-nude mice[117]. Of note, by using proteomic tools, it was demonstrated that IN downregulated Gal-1 expression in CRC, suggesting that IN-anti-proliferative activity against this tumor might be related to Gal-1[118]. However, the role of Gal-1 in CRC tumor growth still remains uncertain.
Alternatively, GnTV involvement in colon tumorigenesis was already investigated. Interestingly, GnTV levels in CRC cells injected into nude mice were positively associated with tumor growth. Moreover, using a CRC murine model (Apcmin/-) with different GnTV backgrounds, it was shown that GnTV knock-out resulted in reduced tumor size, increased animal survival and a decrease in colon cancer stem cell population. Furthermore, GnTV regulated WNT/β-catenin signaling pathway[115].
Strikingly, although much research focused on Gal-1 expression and function in human CRC tissues and cell lines, no confirmation of Gal-1 roles on CRC progression was obtained through experimental models. Even though extensive studies are required to evaluate Gal-1 function in vivo, we can speculate that Gal-1 and GnTV may act coordinately to influence CRC progression.
ROLES OF GAL-1 IN PANCREATIC CANCER
Pancreatic cancer (PC) is a highly lethal disease, for which incidence matches mortality. Pancreatic ductal adenocarcinoma (PDAC), the most common type of PC, is one of the most aggressive tumors. The overall 5-year survival rate for this malignancy remains the lowest of any cancer (7%), due to limited diagnostic methods, disease aggressiveness and lack of targeted therapeutic interventions[119].
Major risk factor for PC is chronic pancreatitis. Tumors often develop from microscopic non-invasive epithelial proliferations within the pancreatic ducts, referred to as pancreatic intraepithelial neoplasias (PanINs)[120]. Mutations in KRAS, CDKN2A and TP53 oncogenes and in tumor suppressor gene SMAD4 usually drive to PDAC[121]. Emerging data have highlighted the important contribution of tumor stroma in PC maintenance, progression and also, in chemotherapy resistance[122].
Treatment options have improved throughout the last decades. However, surgery remains the primary therapy and candidates to this intervention represent a very low percentage[121]. Furthermore, efficacy of conventional chemoradiotherapy for PDAC patients is limited[123]. Hence, a better understanding of the mechanisms underlying PC development and progression is needed.
In this section we provide an overview of the relevant contribution of Gal-1 to PC progression.
Gal-1 expression in human PC tissues, correlation with clinicopathological parameters and prognostic significance
Proteomic approaches were applied to identify proteins differentially expressed in normal and pathological human pancreas. While Gal-1 was undetectable in normal pancreas, its expression was up-regulated in PC[124-129]. These results, validated by Western blot and immunohistochemistry, showed that Gal-1 protein expression and staining intensity increased gradually from normal pancreas to chronic pancreatitis, PanIN lessions and PDAC[125,130-133]. Moreover, Gal-1 expression was significantly higher in poorly differentiated PDAC tumors compared to well/moderately differentiated ones[133,134]. Gal-1 expression was essentially restricted to the peritumoral stroma, with low expression within the neoplastic epithelium[125,126,131-133]. Concomitantly, stromal Gal-1 expression correlated with pancreatic fibrosis, a process that can develop cancer[135].
Within tumor stroma, Gal-1 was detected in α-SMA positive regions (activated pancreatic stellate cells, PSCs). Gal-1-expressing PSCs were scattered in chronic pancreatitis tissues, but they formed a tight fibrotic barrier surrounding the tumor in PC samples. Interestingly, CD3+ T cells were found to surround malignant cells in PDAC tissues with few infiltrating T cells in the tumor parenchyma. These results suggested that Gal-1-expressing PSCs can prevent CD3+ T cells from infiltrating the pancreatic tumor[132].
Notably, stromal Gal-1 expression in PC tissues was associated with an increase in tumor size, lymph node metastasis, perineural invasion and differentiation and served as an indicator of poor survival[130,134]. Accordingly, after quantitative proteomic profiling analysis of PDAC tissues, Gal-1 was suggested as a negative predictor of PC survival[131].
Thus, high levels of Gal-1, mainly in stroma, play fundamental roles early in PC tumorigenesis and progression (Figure 1).
Gal-1 involvement in PC progression in experimental models
Transgenic mice overexpressing the oncogene Myc under the control of elastase promoter (Ela-myc) constitute a well-characterized model of PC[136,137]. In tumors from Ela-myc mice, strong Gal-1 expression was detected in both epithelial and stromal cells[138,139]. Interestingly, Gal-1 depletion in these mice resulted in tumor microenvironment remodeling, rendering a decrease in tumor proliferation, angiogenesis and fibroblasts activation and an increase in T-cell and neutrophil infiltration and animal survival. Furthermore, levels of transcription factor Gli and Hh receptor Patched were reduced in Gal-1-depleted mice[138]. Hh-Gli pathway is involved in the initiation and progression of PDAC. Thus, these findings evidence a critical role of Gal-1 in PC development and progression through the Hh-Gli pathway.
Moreover, xenografts of wild-type or Gal-1-depleted PC cells injected in nude mice showed no differences in tumor progression[138]. Accordingly, implanted PC cells produced small tumors, which progressed slowly; whereas addition of PSCs to the implanted cancer cells resulted in large tumors with quick progression[130]. Also, cancer cells implanted with PC-associated PSCs exhibiting high levels of Gal-1 developed larger tumors, than those that were implanted with PSCs from normal pancreas[130]. These results point out the crucial contribution of tumor microenvironment in Gal-1-induced effects in pancreatic carcinogenesis.
The field of nano-oncology offers the possibility to use glycan-based galectin inhibitors or glycan ligand-based functionalized nanoparticles for diagnostic, prognosis and therapeutic purposes[7]. In this regard, taking advantage of Gal-1 up-regulation in PC, the delivery of magnetic nanoparticles using Gal-1 as a target receptor to PC tissues was described[140]. As targeting moieties, glycosylated peptides derived from tissue plasminogen activator (tPA) were covalently attached onto the nanoparticle surfaces[140]. tPA is a multifunctional protein overexpressed in PDAC and was described as a ligand of Gal-1 in pancreatic tissues[139,141]. Through magnetic resonance imaging of mouse PANC-1 xenografts, an increased uptake of targeted nanoparticles vs non-targeted nanoparticles was shown[140]. This result suggests that Gal-1 may serve as a strong candidate for PC therapy and opens up a window of theranostics (integrated diagnostic imaging and therapy) for this disease.
Functional studies associated with PC progression induced by Gal-1
Gal-1 expression was detected in several PC cell lines. During CFPAC-1 cell migration, Gal-1 localized at cell membranes, at the migration front[139]. There, it co-localized with its ligand tPA[139,141]. Gal-1 downregulation in CFPAC-1 cells markedly decreased tPA-induced ERK1/2 activation, cell proliferation, migration and invasion[139]. These findings support a new molecular mechanism by which Gal-1 interaction with tPA contributes to PDAC progression. In this interaction, tPA glycosylation was required. Interestingly, the modulation of glycosylation via galectin binding, with functional implications, was reported[142-144]. The tumor suppressor p16INK4a induced Gal-1 up-regulation and increased expression of α5β51 integrin at cell surfaces. Besides, p16INK4a changed cell glycosylation pattern, reducing glycoprotein α2,6 sialylation. As a consequence, the susceptibility to carbohydrate-dependent induction of anoikis was increased through Gal-1-α5β51 integrin interaction[142-144]. Moreover, the downregulation of MUC16, a heavily glycosylated type-I transmembrane mucin overexpressed in PDAC decreased PC cell adhesion to Gal-1[145].
Global analysis of differentially expressed genes in control and Gal-1-silenced PANC-1 cells revealed that Gal-1 levels correlate with genes involved in cell migration, adhesion and malignant transformation and with genes of the Hh-Gli axis[138]. Gal-1 downregulation reduced PANC-1 cell migration, motility and resistance to anoikis and increased the expression of fibronectin-1, integrin-α5, thrombospondin-1 and E-cadherin[138]. Moreover, Gal-1 silencing decreased the expression of upstream and downstream effectors of Hh-Gli axis both in PC cells and PSCs. On the contrary, Gal-1 overexpression in PDAC cells led to increased Gli transcriptional activity[138].
Interestingly, several studies demonstrated that activated PSCs express and secrete high levels of Gal-1. The expression of this galectin was higher in PSCs isolated from PDAC samples (CaPSCs) than those isolated from normal pancreatic tissues (NPSCs)[130,132,134,146-149]. Remarkably, Gal-1 knock-down in human PSCs reduced cell migration and invasion. Besides, exogenous Gal-1 was capable of inducing proliferation, collagen synthesis and chemokine production in rat PSCs, involving MEK1/2-ERK1/2 and c-Jun N-terminal kinase signaling pathways and transcription factors NF-κB and activator protein-1 activation[148,149]. Further, Gal-1 expression in PSCs was induced by conditioned media from CFPAC-1 PC cells[146]. Besides, CFPAC-1 cells co-cultured with PSCs showed an increase in MMP-2 and MMP-9 expression and a stronger ability to proliferate and invade than their counterparts alone. These effects were more pronounced in cancer cells co-cultured with CaPSCs than those ones with NPSCs and were partially blocked in the presence of lactose, a galectin inhibitor[130,146]. Furthermore, PSC-derived Gal-1 involvement in tumor immunosuppression was reported. Gal-1-overexpression in activated PSCs decreased CD3+ T cell viability and skewed the cytokine secretion balance towards a Th2 immune response[132,134].
Altogether, the results discussed herein reveal that a coordinated regulation of Gal-1/Gal-1 counter-receptor expression and glycosylation may lead to PC progression. Besides, Gal-1 might mediate the crosstalk between PC and the surrounding stroma by modulating Hh-Gli signaling pathway, favoring EMT, migration and immunosuppression (Figure 2).
CONCLUSION
The evidence we summarized here reveals the crucial contribution of Gal-1 to the pathogenesis of gastrointestinal malignancies. Remarkably, increased levels of Gal-1 in gastrointestinal tumor microenvironment favor progression, aggressiveness and metastasis. We also highlighted that alterations in Gal-1-specific glycoepitopes may be relevant for gastrointestinal cancer development. Undoubtedly, further functional studies are required. Yet, we are hopeful that researchers will get inspired by the findings obtained so far and will join efforts to elucidate the fascinating roles of Gal-1 and its glycan-partners, in these diseases. Thus, Gal-1-targeted therapies could be implemented in future interventions in gastrointestinal tumors.
ACKNOWLEDGMENTS
We apologize to the many authors whose papers could not be cited owing to space limitations.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare no conflict of interest related to this publication.
Peer-review started: February 14, 2017
First decision: April 21, 2017
Article in press: June 19, 2017
P- Reviewer: Bener Sadik R, Gazouli M, Sperti C S- Editor: Ma YJ L- Editor: A E- Editor: Li D
References
- 1.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, Hamadeh RR, Moore A, Werdecker A, Gessner BD, Te Ao B, McMahon B, Karimkhani C, Yu C, Cooke GS, Schwebel DC, Carpenter DO, Pereira DM, Nash D, Kazi DS, De Leo D, Plass D, Ukwaja KN, Thurston GD, Yun Jin K, Simard EP, Mills E, Park EK, Catalá-López F, deVeber G, Gotay C, Khan G, Hosgood HD 3rd, Santos IS, Leasher JL, Singh J, Leigh J, Jonas JB, Sanabria J, Beardsley J, Jacobsen KH, Takahashi K, Franklin RC, Ronfani L, Montico M, Naldi L, Tonelli M, Geleijnse J, Petzold M, Shrime MG, Younis M, Yonemoto N, Breitborde N, Yip P, Pourmalek F, Lotufo PA, Esteghamati A, Hankey GJ, Ali R, Lunevicius R, Malekzadeh R, Dellavalle R, Weintraub R, Lucas R, Hay R, Rojas-Rueda D, Westerman R, Sepanlou SG, Nolte S, Patten S, Weichenthal S, Abera SF, Fereshtehnejad SM, Shiue I, Driscoll T, Vasankari T, Alsharif U, Rahimi-Movaghar V, Vlassov VV, Marcenes WS, Mekonnen W, Melaku YA, Yano Y, Artaman A, Campos I, MacLachlan J, Mueller U, Kim D, Trillini M, Eshrati B, Williams HC, Shibuya K, Dandona R, Murthy K, Cowie B, Amare AT, Antonio CA, Castañeda-Orjuela C, van Gool CH, Violante F, Oh IH, Deribe K, Soreide K, Knibbs L, Kereselidze M, Green M, Cardenas R, Roy N, Tillmann T, Li Y, Krueger H, Monasta L, Dey S, Sheikhbahaei S, Hafezi-Nejad N, Kumar GA, Sreeramareddy CT, Dandona L, Wang H, Vollset SE, Mokdad A, Salomon JA, Lozano R, Vos T, Forouzanfar M, Lopez A, Murray C, Naghavi M. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 3.Lau KS, Dennis JW. N-Glycans in cancer progression. Glycobiology. 2008;18:750–760. doi: 10.1093/glycob/cwn071. [DOI] [PubMed] [Google Scholar]
- 4.Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, Urashima T, Oka T, Futai M, Muller WE, et al. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta. 2002;1572:232–254. doi: 10.1016/s0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 5.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 6.Compagno D, Laderach DJ, Gentilini L, Jaworski FM, Rabinovich GA. Delineating the “galectin signature” of the tumor microenvironment. Oncoimmunology. 2013;2:e23565. doi: 10.4161/onci.23565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hockl PF, Wolosiuk A, Pérez-Sáez JM, Bordoni AV, Croci DO, Toum-Terrones Y, Soler-Illia GJ, Rabinovich GA. Glyco-nano-oncology: Novel therapeutic opportunities by combining small and sweet. Pharmacol Res. 2016;109:45–54. doi: 10.1016/j.phrs.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 9.Elola MT, Wolfenstein-Todel C, Troncoso MF, Vasta GR, Rabinovich GA. Galectins: matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol Life Sci. 2007;64:1679–1700. doi: 10.1007/s00018-007-7044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaževitš O, Mideksa YG, Šolman M, Ligabue A, Ariotti N, Nakhaeizadeh H, Fansa EK, Papageorgiou AC, Wittinghofer A, Ahmadian MR, et al. Galectin-1 dimers can scaffold Raf-effectors to increase H-ras nanoclustering. Sci Rep. 2016;6:24165. doi: 10.1038/srep24165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thijssen VL, Heusschen R, Caers J, Griffioen AW. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim Biophys Acta. 2015;1855:235–247. doi: 10.1016/j.bbcan.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Demydenko D, Berest I. Expression of galectin-1 in malignant tumors. Exp Oncol. 2009;31:74–79. [PubMed] [Google Scholar]
- 13.Astorgues-Xerri L, Riveiro ME, Tijeras-Raballand A, Serova M, Neuzillet C, Albert S, Raymond E, Faivre S. Unraveling galectin-1 as a novel therapeutic target for cancer. Cancer Treat Rev. 2014;40:307–319. doi: 10.1016/j.ctrv.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Thijssen VL, Barkan B, Shoji H, Aries IM, Mathieu V, Deltour L, Hackeng TM, Kiss R, Kloog Y, Poirier F, et al. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer Res. 2010;70:6216–6224. doi: 10.1158/0008-5472.CAN-09-4150. [DOI] [PubMed] [Google Scholar]
- 15.Thijssen VL, Postel R, Brandwijk RJ, Dings RP, Nesmelova I, Satijn S, Verhofstad N, Nakabeppu Y, Baum LG, Bakkers J, et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci USA. 2006;103:15975–15980. doi: 10.1073/pnas.0603883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croci DO, Salatino M, Rubinstein N, Cerliani JP, Cavallin LE, Leung HJ, Ouyang J, Ilarregui JM, Toscano MA, Domaica CI, et al. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi’s sarcoma. J Exp Med. 2012;209:1985–2000. doi: 10.1084/jem.20111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabinovich GA, Conejo-García JR. Shaping the Immune Landscape in Cancer by Galectin-Driven Regulatory Pathways. J Mol Biol. 2016;428:3266–3281. doi: 10.1016/j.jmb.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Bacigalupo ML, Manzi M, Rabinovich GA, Troncoso MF. Hierarchical and selective roles of galectins in hepatocarcinogenesis, liver fibrosis and inflammation of hepatocellular carcinoma. World J Gastroenterol. 2013;19:8831–8849. doi: 10.3748/wjg.v19.i47.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrow H, Guo X, Wandall HH, Pedersen JW, Fu B, Zhao Q, Chen C, Rhodes JM, Yu LG. Serum galectin-2, -4, and -8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clin Cancer Res. 2011;17:7035–7046. doi: 10.1158/1078-0432.CCR-11-1462. [DOI] [PubMed] [Google Scholar]
- 20.Jung JH, Kim HJ, Yeom J, Yoo C, Shin J, Yoo J, Kang CS, Lee C. Lowered expression of galectin-2 is associated with lymph node metastasis in gastric cancer. J Gastroenterol. 2012;47:37–48. doi: 10.1007/s00535-011-0463-1. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Sun J, Ma C, Gao W, Song B, Xue H, Chen W, Chen X, Zhang Y, Shao Q, et al. Reduced Expression of Galectin-9 Contributes to a Poor Outcome in Colon Cancer by Inhibiting NK Cell Chemotaxis Partially through the Rho/ROCK1 Signaling Pathway. PLoS One. 2016;11:e0152599. doi: 10.1371/journal.pone.0152599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu KL, Huang EY, Jhu EW, Huang YH, Su WH, Chuang PC, Yang KD. Overexpression of galectin-3 enhances migration of colon cancer cells related to activation of the K-Ras-Raf-Erk1/2 pathway. J Gastroenterol. 2013;48:350–359. doi: 10.1007/s00535-012-0663-3. [DOI] [PubMed] [Google Scholar]
- 23.Lauren P. The Two Histological Main Types Of Gastric Carcinoma: Diffuse And So-Called Intestinal-Type Carcinoma. An Attempt At A Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 24.Berlth F, Bollschweiler E, Drebber U, Hoelscher AH, Moenig S. Pathohistological classification systems in gastric cancer: diagnostic relevance and prognostic value. World J Gastroenterol. 2014;20:5679–5684. doi: 10.3748/wjg.v20.i19.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–490. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 26.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688–694. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- 27.Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3:251–261. doi: 10.3978/j.issn.2078-6891.2012.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jou E, Rajdev L. Current and emerging therapies in unresectable and recurrent gastric cancer. World J Gastroenterol. 2016;22:4812–4823. doi: 10.3748/wjg.v22.i20.4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satolli MA, Buffoni L, Spadi R, Roato I. Gastric cancer: The times they are a-changin’. World J Gastrointest Oncol. 2015;7:303–316. doi: 10.4251/wjgo.v7.i11.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jorge YC, Mataruco MM, Araújo LP, Rossi AF, de Oliveira JG, Valsechi MC, Caetano A, Miyazaki K, Fazzio CS, Thomé JA, et al. Expression of annexin-A1 and galectin-1 anti-inflammatory proteins and mRNA in chronic gastritis and gastric cancer. Mediators Inflamm. 2013;2013:152860. doi: 10.1155/2013/152860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossi AF, Duarte MC, Poltronieri AB, Valsechi MC, Jorge YC, de-Santi Neto D, Rahal P, Oliani SM, Silva AE. Deregulation of annexin-A1 and galectin-1 expression in precancerous gastric lesions: intestinal metaplasia and gastric ulcer. Mediators Inflamm. 2014;2014:478138. doi: 10.1155/2014/478138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Tang D, Wang S, Li QG, Zhang JR, Li P, Lu Q, Niu G, Gao J, Ye NY, et al. High expressions of galectin-1 and VEGF are associated with poor prognosis in gastric cancer patients. Tumour Biol. 2014;35:2513–2519. doi: 10.1007/s13277-013-1332-8. [DOI] [PubMed] [Google Scholar]
- 33.Zheng L, Xu C, Guan Z, Su X, Xu Z, Cao J, Teng L. Galectin-1 mediates TGF-β-induced transformation from normal fibroblasts into carcinoma-associated fibroblasts and promotes tumor progression in gastric cancer. Am J Transl Res. 2016;8:1641–1658. [PMC free article] [PubMed] [Google Scholar]
- 34.He XJ, Tao HQ, Hu ZM, Ma YY, Xu J, Wang HJ, Xia YJ, Li L, Fei BY, Li YQ, et al. Expression of galectin-1 in carcinoma-associated fibroblasts promotes gastric cancer cell invasion through upregulation of integrin β1. Cancer Sci. 2014;105:1402–1410. doi: 10.1111/cas.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bektas S, Bahadir B, Ucan BH, Ozdamar SO. CD24 and galectin-1 expressions in gastric adenocarcinoma and clinicopathologic significance. Pathol Oncol Res. 2010;16:569–577. doi: 10.1007/s12253-010-9248-8. [DOI] [PubMed] [Google Scholar]
- 36.Chong Y, Tang D, Gao J, Jiang X, Xu C, Xiong Q, Huang Y, Wang J, Zhou H, Shi Y, et al. Galectin-1 induces invasion and the epithelial-mesenchymal transition in human gastric cancer cells via non-canonical activation of the hedgehog signaling pathway. Oncotarget. 2016;7:83611–83626. doi: 10.18632/oncotarget.13201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chong Y, Tang D, Xiong Q, Jiang X, Xu C, Huang Y, Wang J, Zhou H, Shi Y, Wu X, et al. Galectin-1 from cancer-associated fibroblasts induces epithelial-mesenchymal transition through β1 integrin-mediated upregulation of Gli1 in gastric cancer. J Exp Clin Cancer Res. 2016;35:175. doi: 10.1186/s13046-016-0449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshii M, Tanaka H, Ohira M, Muguruma K, Iwauchi T, Lee T, Sakurai K, Kubo N, Yashiro M, Sawada T, et al. Expression of Forkhead box P3 in tumour cells causes immunoregulatory function of signet ring cell carcinoma of the stomach. Br J Cancer. 2012;106:1668–1674. doi: 10.1038/bjc.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J Gastroenterol. 2015;21:11428–11438. doi: 10.3748/wjg.v21.i40.11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–376. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- 41.Tang D, Gao J, Wang S, Ye N, Chong Y, Huang Y, Wang J, Li B, Yin W, Wang D. Cancer-associated fibroblasts promote angiogenesis in gastric cancer through galectin-1 expression. Tumour Biol. 2016;37:1889–1899. doi: 10.1007/s13277-015-3942-9. [DOI] [PubMed] [Google Scholar]
- 42.Estofolete CF, Zucoloto S, Oliani SM, Polli-Lopes AC, Gil CD. Myenteric denervation downregulates galectin-1 and -3 expression in gastric carcinogenesis. Dig Dis Sci. 2011;56:1637–1644. doi: 10.1007/s10620-010-1516-7. [DOI] [PubMed] [Google Scholar]
- 43.Polli-Lopes AC, Zucoloto S, de Queirós Cunha F, da Silva Figueiredo LA, Garcia SB. Myenteric denervation reduces the incidence of gastric tumors in rats. Cancer Lett. 2003;190:45–50. doi: 10.1016/s0304-3835(02)00584-0. [DOI] [PubMed] [Google Scholar]
- 44.Lim JW, Kim H, Kim KH. Cell adhesion-related gene expression by Helicobacter pylori in gastric epithelial AGS cells. Int J Biochem Cell Biol. 2003;35:1284–1296. doi: 10.1016/s1357-2725(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 45.Chen YR, Juan HF, Huang HC, Huang HH, Lee YJ, Liao MY, Tseng CW, Lin LL, Chen JY, Wang MJ, et al. Quantitative proteomic and genomic profiling reveals metastasis-related protein expression patterns in gastric cancer cells. J Proteome Res. 2006;5:2727–2742. doi: 10.1021/pr060212g. [DOI] [PubMed] [Google Scholar]
- 46.Croci DO, Cerliani JP, Dalotto-Moreno T, Méndez-Huergo SP, Mascanfroni ID, Dergan-Dylon S, Toscano MA, Caramelo JJ, García-Vallejo JJ, Ouyang J, et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell. 2014;156:744–758. doi: 10.1016/j.cell.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 47.Tian H, Miyoshi E, Kawaguchi N, Shaker M, Ito Y, Taniguchi N, Tsujimoto M, Matsuura N. The implication of N-acetylglucosaminyltransferase V expression in gastric cancer. Pathobiology. 2008;75:288–294. doi: 10.1159/000151709. [DOI] [PubMed] [Google Scholar]
- 48.Granovsky M, Fata J, Pawling J, Muller WJ, Khokha R, Dennis JW. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat Med. 2000;6:306–312. doi: 10.1038/73163. [DOI] [PubMed] [Google Scholar]
- 49.Taniguchi N, Korekane H. Branched N-glycans and their implications for cell adhesion, signaling and clinical applications for cancer biomarkers and in therapeutics. BMB Rep. 2011;44:772–781. doi: 10.5483/bmbrep.2011.44.12.772. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Y, Nakagawa T, Itoh S, Inamori K, Isaji T, Kariya Y, Kondo A, Miyoshi E, Miyazaki K, Kawasaki N, et al. N-acetylglucosaminyltransferase III antagonizes the effect of N-acetylglucosaminyltransferase V on alpha3beta1 integrin-mediated cell migration. J Biol Chem. 2006;281:32122–32130. doi: 10.1074/jbc.M607274200. [DOI] [PubMed] [Google Scholar]
- 51.Pinho SS, Figueiredo J, Cabral J, Carvalho S, Dourado J, Magalhães A, Gärtner F, Mendonfa AM, Isaji T, Gu J, et al. E-cadherin and adherens-junctions stability in gastric carcinoma: functional implications of glycosyltransferases involving N-glycan branching biosynthesis, N-acetylglucosaminyltransferases III and V. Biochim Biophys Acta. 2013;1830:2690–2700. doi: 10.1016/j.bbagen.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 52.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 53.Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56:769–775. doi: 10.1002/hep.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512–527. doi: 10.1053/j.gastro.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol. 2013;10:34–42. doi: 10.1038/nrgastro.2012.199. [DOI] [PubMed] [Google Scholar]
- 56.de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56:S75–S87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- 57.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 58.Ercolani G, Grazi GL, Ravaioli M, Del Gaudio M, Gardini A, Cescon M, Varotti G, Cetta F, Cavallari A. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536–543. doi: 10.1097/01.SLA.0000059988.22416.F2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vivarelli M, Risaliti A. Liver transplantation for hepatocellular carcinoma on cirrhosis: strategies to avoid tumor recurrence. World J Gastroenterol. 2011;17:4741–4746. doi: 10.3748/wjg.v17.i43.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 61.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 62.Chung EJ, Sung YK, Farooq M, Kim Y, Im S, Tak WY, Hwang YJ, Kim YI, Han HS, Kim JC, et al. Gene expression profile analysis in human hepatocellular carcinoma by cDNA microarray. Mol Cells. 2002;14:382–387. [PubMed] [Google Scholar]
- 63.Kondoh N, Hada A, Ryo A, Shuda M, Arai M, Matsubara O, Kimura F, Wakatsuki T, Yamamoto M. Activation of Galectin-1 gene in human hepatocellular carcinoma involves methylation-sensitive complex formations at the transcriptional upstream and downstream elements. Int J Oncol. 2003;23:1575–1583. [PubMed] [Google Scholar]
- 64.Spano D, Russo R, Di Maso V, Rosso N, Terracciano LM, Roncalli M, Tornillo L, Capasso M, Tiribelli C, Iolascon A. Galectin-1 and its involvement in hepatocellular carcinoma aggressiveness. Mol Med. 2010;16:102–115. doi: 10.2119/molmed.2009.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu H, Chen P, Liao R, Li YW, Yi Y, Wang JX, Sun TW, Zhou J, Shi YH, Yang XR, et al. Overexpression of galectin-1 is associated with poor prognosis in human hepatocellular carcinoma following resection. J Gastroenterol Hepatol. 2012;27:1312–1319. doi: 10.1111/j.1440-1746.2012.07130.x. [DOI] [PubMed] [Google Scholar]
- 66.You Y, Tan JX, Dai HS, Chen HW, Xu XJ, Yang AG, Zhang YJ, Bai LH, Bie P. MiRNA-22 inhibits oncogene galectin-1 in hepatocellular carcinoma. Oncotarget. 2016;7:57099–57116. doi: 10.18632/oncotarget.10981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J, Yang Y, Yang T, Liu Y, Li A, Fu S, Wu M, Pan Z, Zhou W. microRNA-22, downregulated in hepatocellular carcinoma and correlated with prognosis, suppresses cell proliferation and tumourigenicity. Br J Cancer. 2010;103:1215–1220. doi: 10.1038/sj.bjc.6605895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Orentas RJ, Kohler ME, Johnson BD. Suppression of anti-cancer immunity by regulatory T cells: back to the future. Semin Cancer Biol. 2006;16:137–149. doi: 10.1016/j.semcancer.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 69.Yeh CC, Hsu CH, Shao YY, Ho WC, Tsai MH, Feng WC, Chow LP. Integrated Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC) and Isobaric Tags for Relative and Absolute Quantitation (iTRAQ) Quantitative Proteomic Analysis Identifies Galectin-1 as a Potential Biomarker for Predicting Sorafenib Resistance in Liver Cancer. Mol Cell Proteomics. 2015;14:1527–1545. doi: 10.1074/mcp.M114.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Espelt MV, Croci DO, Bacigalupo ML, Carabias P, Manzi M, Elola MT, Muñoz MC, Dominici FP, Wolfenstein-Todel C, Rabinovich GA, et al. Novel roles of galectin-1 in hepatocellular carcinoma cell adhesion, polarization, and in vivo tumor growth. Hepatology. 2011;53:2097–2106. doi: 10.1002/hep.24294. [DOI] [PubMed] [Google Scholar]
- 71.Su YC, Davuluri GV, Chen CH, Shiau DC, Chen CC, Chen CL, Lin YS, Chang CP. Galectin-1-Induced Autophagy Facilitates Cisplatin Resistance of Hepatocellular Carcinoma. PLoS One. 2016;11:e0148408. doi: 10.1371/journal.pone.0148408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Potikha T, Stoyanov E, Pappo O, Frolov A, Mizrahi L, Olam D, Shnitzer-Perlman T, Weiss I, Barashi N, Peled A, et al. Interstrain differences in chronic hepatitis and tumor development in a murine model of inflammation-mediated hepatocarcinogenesis. Hepatology. 2013;58:192–204. doi: 10.1002/hep.26335. [DOI] [PubMed] [Google Scholar]
- 73.Mauad TH, van Nieuwkerk CM, Dingemans KP, Smit JJ, Schinkel AH, Notenboom RG, van den Bergh Weerman MA, Verkruisen RP, Groen AK, Oude Elferink RP, et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237–1245. [PMC free article] [PubMed] [Google Scholar]
- 74.Potikha T, Ella E, Cerliani JP, Mizrahi L, Pappo O, Rabinovich GA, Galun E, Goldenberg DS. Galectin-1 is essential for efficient liver regeneration following hepatectomy. Oncotarget. 2016;7:31738–31754. doi: 10.18632/oncotarget.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bacigalupo ML, Manzi M, Espelt MV, Gentilini LD, Compagno D, Laderach DJ, Wolfenstein-Todel C, Rabinovich GA, Troncoso MF. Galectin-1 triggers epithelial-mesenchymal transition in human hepatocellular carcinoma cells. J Cell Physiol. 2015;230:1298–1309. doi: 10.1002/jcp.24865. [DOI] [PubMed] [Google Scholar]
- 76.Li S, Mo C, Peng Q, Kang X, Sun C, Jiang K, Huang L, Lu Y, Sui J, Qin X, et al. Cell surface glycan alterations in epithelial mesenchymal transition process of Huh7 hepatocellular carcinoma cell. PLoS One. 2013;8:e71273. doi: 10.1371/journal.pone.0071273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo R, Cheng L, Zhao Y, Zhang J, Liu C, Zhou H, Jia L. Glycogenes mediate the invasive properties and chemosensitivity of human hepatocarcinoma cells. Int J Biochem Cell Biol. 2013;45:347–358. doi: 10.1016/j.biocel.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 78.Miyoshi E, Nishikawa A, Ihara Y, Gu J, Sugiyama T, Hayashi N, Fusamoto H, Kamada T, Taniguchi N. N-acetylglucosaminyltransferase III and V messenger RNA levels in LEC rats during hepatocarcinogenesis. Cancer Res. 1993;53:3899–3902. [PubMed] [Google Scholar]
- 79.Liu H, Wu Q, Liu Y, Liu W, Zhang W, Pan D, Xu J. Prognostic significance of β1,6-N-acetylglucosaminyltransferase V expression in patients with hepatocellular carcinoma. Jpn J Clin Oncol. 2015;45:844–853. doi: 10.1093/jjco/hyv080. [DOI] [PubMed] [Google Scholar]
- 80.Yao M, Zhou DP, Jiang SM, Wang QH, Zhou XD, Tang ZY, Gu JX. Elevated activity of N-acetylglucosaminyltransferase V in human hepatocellular carcinoma. J Cancer Res Clin Oncol. 1998;124:27–30. doi: 10.1007/s004320050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fischer C, Sanchez-Ruderisch H, Welzel M, Wiedenmann B, Sakai T, André S, Gabius HJ, Khachigian L, Detjen KM, Rosewicz S. Galectin-1 interacts with the {alpha}5{beta}1 fibronectin receptor to restrict carcinoma cell growth via induction of p21 and p27. J Biol Chem. 2005;280:37266–37277. doi: 10.1074/jbc.M411580200. [DOI] [PubMed] [Google Scholar]
- 82.Sanchez-Ruderisch H, Detjen KM, Welzel M, André S, Fischer C, Gabius HJ, Rosewicz S. Galectin-1 sensitizes carcinoma cells to anoikis via the fibronectin receptor α5β1-integrin. Cell Death Differ. 2011;18:806–816. doi: 10.1038/cdd.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manzi M, Bacigalupo ML, Carabias P, Elola MT, Wolfenstein-Todel C, Rabinovich GA, Espelt MV, Troncoso MF. Galectin-1 Controls the Proliferation and Migration of Liver Sinusoidal Endothelial Cells and Their Interaction With Hepatocarcinoma Cells. J Cell Physiol. 2016;231:1522–1533. doi: 10.1002/jcp.25244. [DOI] [PubMed] [Google Scholar]
- 84.Kristensen DB, Kawada N, Imamura K, Miyamoto Y, Tateno C, Seki S, Kuroki T, Yoshizato K. Proteome analysis of rat hepatic stellate cells. Hepatology. 2000;32:268–277. doi: 10.1053/jhep.2000.9322. [DOI] [PubMed] [Google Scholar]
- 85.Maeda N, Kawada N, Seki S, Arakawa T, Ikeda K, Iwao H, Okuyama H, Hirabayashi J, Kasai K, Yoshizato K. Stimulation of proliferation of rat hepatic stellate cells by galectin-1 and galectin-3 through different intracellular signaling pathways. J Biol Chem. 2003;278:18938–18944. doi: 10.1074/jbc.M209673200. [DOI] [PubMed] [Google Scholar]
- 86.Binefa G, Rodríguez-Moranta F, Teule A, Medina-Hayas M. Colorectal cancer: from prevention to personalized medicine. World J Gastroenterol. 2014;20:6786–6808. doi: 10.3748/wjg.v20.i22.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leufkens AM, van den Bosch MA, van Leeuwen MS, Siersema PD. Diagnostic accuracy of computed tomography for colon cancer staging: a systematic review. Scand J Gastroenterol. 2011;46:887–894. doi: 10.3109/00365521.2011.574732. [DOI] [PubMed] [Google Scholar]
- 88.Van Cutsem E, Oliveira J; ESMO Guidelines Working Group. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 Suppl 4:61–63. doi: 10.1093/annonc/mdp130. [DOI] [PubMed] [Google Scholar]
- 89.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 90.Fung KY, Nice E, Priebe I, Belobrajdic D, Phatak A, Purins L, Tabor B, Pompeia C, Lockett T, Adams TE, et al. Colorectal cancer biomarkers: to be or not to be? Cautionary tales from a road well travelled. World J Gastroenterol. 2014;20:888–898. doi: 10.3748/wjg.v20.i4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burt RW. Colon cancer screening. Gastroenterology. 2000;119:837–853. doi: 10.1053/gast.2000.16508. [DOI] [PubMed] [Google Scholar]
- 92.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 93.Lotan R, Matsushita Y, Ohannesian D, Carralero D, Ota DM, Cleary KR, Nicolson GL, Irimura T. Lactose-binding lectin expression in human colorectal carcinomas. Relation to tumor progression. Carbohydr Res. 1991;213:47–57. doi: 10.1016/s0008-6215(00)90597-4. [DOI] [PubMed] [Google Scholar]
- 94.Irimura T, Matsushita Y, Sutton RC, Carralero D, Ohannesian DW, Cleary KR, Ota DM, Nicolson GL, Lotan R. Increased content of an endogenous lactose-binding lectin in human colorectal carcinoma progressed to metastatic stages. Cancer Res. 1991;51:387–393. [PubMed] [Google Scholar]
- 95.Sanjuán X, Fernández PL, Castells A, Castronovo V, van den Brule F, Liu FT, Cardesa A, Campo E. Differential expression of galectin 3 and galectin 1 in colorectal cancer progression. Gastroenterology. 1997;113:1906–1915. doi: 10.1016/s0016-5085(97)70010-6. [DOI] [PubMed] [Google Scholar]
- 96.Hittelet A, Legendre H, Nagy N, Bronckart Y, Pector JC, Salmon I, Yeaton P, Gabius HJ, Kiss R, Camby I. Upregulation of galectins-1 and -3 in human colon cancer and their role in regulating cell migration. Int J Cancer. 2003;103:370–379. doi: 10.1002/ijc.10843. [DOI] [PubMed] [Google Scholar]
- 97.Nagy N, Legendre H, Engels O, André S, Kaltner H, Wasano K, Zick Y, Pector JC, Decaestecker C, Gabius HJ, et al. Refined prognostic evaluation in colon carcinoma using immunohistochemical galectin fingerprinting. Cancer. 2003;97:1849–1858. doi: 10.1002/cncr.11268. [DOI] [PubMed] [Google Scholar]
- 98.Zhao XY, Chen TT, Xia L, Guo M, Xu Y, Yue F, Jiang Y, Chen GQ, Zhao KW. Hypoxia inducible factor-1 mediates expression of galectin-1: the potential role in migration/invasion of colorectal cancer cells. Carcinogenesis. 2010;31:1367–1375. doi: 10.1093/carcin/bgq116. [DOI] [PubMed] [Google Scholar]
- 99.Gopalan V, Saremi N, Sullivan E, Kabir S, Lu CT, Salajegheh A, Leung M, Smith RA, Lam AK. The expression profiles of the galectin gene family in colorectal adenocarcinomas. Hum Pathol. 2016;53:105–113. doi: 10.1016/j.humpath.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 100.Watanabe M, Takemasa I, Kawaguchi N, Miyake M, Nishimura N, Matsubara T, Matsuo E, Sekimoto M, Nagai K, Matsuura N, et al. An application of the 2-nitrobenzenesulfenyl method to proteomic profiling of human colorectal carcinoma: A novel approach for biomarker discovery. Proteomics Clin Appl. 2008;2:925–935. doi: 10.1002/prca.200780111. [DOI] [PubMed] [Google Scholar]
- 101.Watanabe M, Takemasa I, Kaneko N, Yokoyama Y, Matsuo E, Iwasa S, Mori M, Matsuura N, Monden M, Nishimura O. Clinical significance of circulating galectins as colorectal cancer markers. Oncol Rep. 2011;25:1217–1226. doi: 10.3892/or.2011.1198. [DOI] [PubMed] [Google Scholar]
- 102.Watanabe T, Komuro Y, Kiyomatsu T, Kanazawa T, Kazama Y, Tanaka J, Tanaka T, Yamamoto Y, Shirane M, Muto T, et al. Prediction of sensitivity of rectal cancer cells in response to preoperative radiotherapy by DNA microarray analysis of gene expression profiles. Cancer Res. 2006;66:3370–3374. doi: 10.1158/0008-5472.CAN-05-3834. [DOI] [PubMed] [Google Scholar]
- 103.Wu KL, Chen HH, Pen CT, Yeh WL, Huang EY, Hsiao CC, Yang KD. Circulating Galectin-1 and 90K/Mac-2BP Correlated with the Tumor Stages of Patients with Colorectal Cancer. Biomed Res Int. 2015;2015:306964. doi: 10.1155/2015/306964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seelentag WK, Li WP, Schmitz SF, Metzger U, Aeberhard P, Heitz PU, Roth J. Prognostic value of beta1,6-branched oligosaccharides in human colorectal carcinoma. Cancer Res. 1998;58:5559–5564. [PubMed] [Google Scholar]
- 105.Murata K, Miyoshi E, Kameyama M, Ishikawa O, Kabuto T, Sasaki Y, Hiratsuka M, Ohigashi H, Ishiguro S, Ito S, et al. Expression of N-acetylglucosaminyltransferase V in colorectal cancer correlates with metastasis and poor prognosis. Clin Cancer Res. 2000;6:1772–1777. [PubMed] [Google Scholar]
- 106.Ishida H, Togayachi A, Sakai T, Iwai T, Hiruma T, Sato T, Okubo R, Inaba N, Kudo T, Gotoh M, et al. A novel beta1,3-N-acetylglucosaminyltransferase (beta3Gn-T8), which synthesizes poly-N-acetyllactosamine, is dramatically upregulated in colon cancer. FEBS Lett. 2005;579:71–78. doi: 10.1016/j.febslet.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 107.Lahm H, André S, Hoeflich A, Fischer JR, Sordat B, Kaltner H, Wolf E, Gabius HJ. Comprehensive galectin fingerprinting in a panel of 61 human tumor cell lines by RT-PCR and its implications for diagnostic and therapeutic procedures. J Cancer Res Clin Oncol. 2001;127:375–386. doi: 10.1007/s004320000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Satelli A, Rao US. Galectin-1 is silenced by promoter hypermethylation and its re-expression induces apoptosis in human colorectal cancer cells. Cancer Lett. 2011;301:38–46. doi: 10.1016/j.canlet.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Satelli A, Rao PS, Gupta PK, Lockman PR, Srivenugopal KS, Rao US. Varied expression and localization of multiple galectins in different cancer cell lines. Oncol Rep. 2008;19:587–594. [PubMed] [Google Scholar]
- 110.Liang L, Qu L, Ding Y. Protein and mRNA characterization in human colorectal carcinoma cell lines with different metastatic potentials. Cancer Invest. 2007;25:427–434. doi: 10.1080/07357900701512258. [DOI] [PubMed] [Google Scholar]
- 111.Lotan R, Lotan D, Carralero DM. Modulation of galactoside-binding lectins in tumor cells by differentiation-inducing agents. Cancer Lett. 1989;48:115–122. doi: 10.1016/0304-3835(89)90046-3. [DOI] [PubMed] [Google Scholar]
- 112.Ohannesian DW, Lotan D, Lotan R. Concomitant increases in galectin-1 and its glycoconjugate ligands (carcinoembryonic antigen, lamp-1, and lamp-2) in cultured human colon carcinoma cells by sodium butyrate. Cancer Res. 1994;54:5992–6000. [PubMed] [Google Scholar]
- 113.Woynarowska B, Dimitroff CJ, Sharma M, Matta KL, Bernacki RJ. Inhibition of human HT-29 colon carcinoma cell adhesion by a 4-fluoro-glucosamine analogue. Glycoconj J. 1996;13:663–674. doi: 10.1007/BF00731455. [DOI] [PubMed] [Google Scholar]
- 114.Horiguchi N, Arimoto K, Mizutani A, Endo-Ichikawa Y, Nakada H, Taketani S. Galectin-1 induces cell adhesion to the extracellular matrix and apoptosis of non-adherent human colon cancer Colo201 cells. J Biochem. 2003;134:869–874. doi: 10.1093/jb/mvg213. [DOI] [PubMed] [Google Scholar]
- 115.Guo H, Nagy T, Pierce M. Post-translational glycoprotein modifications regulate colon cancer stem cells and colon adenoma progression in Apc(min/+) mice through altered Wnt receptor signaling. J Biol Chem. 2014;289:31534–31549. doi: 10.1074/jbc.M114.602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ose R, Oharaa O, Nagase T. Galectin-1 and Galectin-3 Mediate Protocadherin-24-Dependent Membrane Localization of β-catenin in Colon Cancer Cell Line HCT116. Curr Chem Genomics. 2012;6:18–26. doi: 10.2174/1875397301206010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang HM, Zhang GY. Indomethacin suppresses growth of colon cancer via inhibition of angiogenesis in vivo. World J Gastroenterol. 2005;11:340–343. doi: 10.3748/wjg.v11.i3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang YJ, Zhang GY, Xiao ZQ, Wang HM, Chen ZC. Preliminary proteomic analysis of indomethacin’s effect on tumor transplanted with colorectal cancer cell in nude mice. J Biochem Mol Biol. 2006;39:171–177. doi: 10.5483/bmbrep.2006.39.2.171. [DOI] [PubMed] [Google Scholar]
- 119.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 120.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Klöppel G, Longnecker DS, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 121.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 122.Wilson JS, Pirola RC, Apte MV. Stars and stripes in pancreatic cancer: role of stellate cells and stroma in cancer progression. Front Physiol. 2014;5:52. doi: 10.3389/fphys.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu CP, Shi J, Chen YX, Xie WF, Lin Y. Gemcitabine in the chemoradiotherapy for locally advanced pancreatic cancer: a meta-analysis. Radiother Oncol. 2011;99:108–113. doi: 10.1016/j.radonc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 124.Iacobuzio-Donahue CA, Ashfaq R, Maitra A, Adsay NV, Shen-Ong GL, Berg K, Hollingsworth MA, Cameron JL, Yeo CJ, Kern SE, et al. Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63:8614–8622. [PubMed] [Google Scholar]
- 125.Pan S, Chen R, Reimel BA, Crispin DA, Mirzaei H, Cooke K, Coleman JF, Lane Z, Bronner MP, Goodlett DR, et al. Quantitative proteomics investigation of pancreatic intraepithelial neoplasia. Electrophoresis. 2009;30:1132–1144. doi: 10.1002/elps.200800752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shen J, Person MD, Zhu J, Abbruzzese JL, Li D. Protein expression profiles in pancreatic adenocarcinoma compared with normal pancreatic tissue and tissue affected by pancreatitis as detected by two-dimensional gel electrophoresis and mass spectrometry. Cancer Res. 2004;64:9018–9026. doi: 10.1158/0008-5472.CAN-04-3262. [DOI] [PubMed] [Google Scholar]
- 127.Grützmann R, Pilarsky C, Ammerpohl O, Lüttges J, Böhme A, Sipos B, Foerder M, Alldinger I, Jahnke B, Schackert HK, et al. Gene expression profiling of microdissected pancreatic ductal carcinomas using high-density DNA microarrays. Neoplasia. 2004;6:611–622. doi: 10.1593/neo.04295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chung JC, Oh MJ, Choi SH, Bae CD. Proteomic analysis to identify biomarker proteins in pancreatic ductal adenocarcinoma. ANZ J Surg. 2008;78:245–251. doi: 10.1111/j.1445-2197.2008.04429.x. [DOI] [PubMed] [Google Scholar]
- 129.Iuga C, Seicean A, Iancu C, Buiga R, Sappa PK, Völker U, Hammer E. Proteomic identification of potential prognostic biomarkers in resectable pancreatic ductal adenocarcinoma. Proteomics. 2014;14:945–955. doi: 10.1002/pmic.201300402. [DOI] [PubMed] [Google Scholar]
- 130.Tang D, Zhang J, Yuan Z, Gao J, Wang S, Ye N, Li P, Gao S, Miao Y, Wang D, et al. Pancreatic satellite cells derived galectin-1 increase the progression and less survival of pancreatic ductal adenocarcinoma. PLoS One. 2014;9:e90476. doi: 10.1371/journal.pone.0090476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen R, Pan S, Ottenhof NA, de Wilde RF, Wolfgang CL, Lane Z, Post J, Bronner MP, Willmann JK, Maitra A, et al. Stromal galectin-1 expression is associated with long-term survival in resectable pancreatic ductal adenocarcinoma. Cancer Biol Ther. 2012;13:899–907. doi: 10.4161/cbt.20842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tang D, Yuan Z, Xue X, Lu Z, Zhang Y, Wang H, Chen M, An Y, Wei J, Zhu Y, et al. High expression of Galectin-1 in pancreatic stellate cells plays a role in the development and maintenance of an immunosuppressive microenvironment in pancreatic cancer. Int J Cancer. 2012;130:2337–2348. doi: 10.1002/ijc.26290. [DOI] [PubMed] [Google Scholar]
- 133.Berberat PO, Friess H, Wang L, Zhu Z, Bley T, Frigeri L, Zimmermann A, Büchler MW. Comparative analysis of galectins in primary tumors and tumor metastasis in human pancreatic cancer. J Histochem Cytochem. 2001;49:539–549. doi: 10.1177/002215540104900414. [DOI] [PubMed] [Google Scholar]
- 134.Tang D, Gao J, Wang S, Yuan Z, Ye N, Chong Y, Xu C, Jiang X, Li B, Yin W, et al. Apoptosis and anergy of T cell induced by pancreatic stellate cells-derived galectin-1 in pancreatic cancer. Tumour Biol. 2015;36:5617–5626. doi: 10.1007/s13277-015-3233-5. [DOI] [PubMed] [Google Scholar]
- 135.Wang L, Friess H, Zhu Z, Frigeri L, Zimmermann A, Korc M, Berberat PO, Büchler MW. Galectin-1 and galectin-3 in chronic pancreatitis. Lab Invest. 2000;80:1233–1241. doi: 10.1038/labinvest.3780131. [DOI] [PubMed] [Google Scholar]
- 136.Sandgren EP, Quaife CJ, Paulovich AG, Palmiter RD, Brinster RL. Pancreatic tumor pathogenesis reflects the causative genetic lesion. Proc Natl Acad Sci USA. 1991;88:93–97. doi: 10.1073/pnas.88.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Grippo PJ, Sandgren EP. Acinar-to-ductal metaplasia accompanies c-myc-induced exocrine pancreatic cancer progression in transgenic rodents. Int J Cancer. 2012;131:1243–1248. doi: 10.1002/ijc.27322. [DOI] [PubMed] [Google Scholar]
- 138.Martínez-Bosch N, Fernández-Barrena MG, Moreno M, Ortiz-Zapater E, Munné-Collado J, Iglesias M, André S, Gabius HJ, Hwang RF, Poirier F, et al. Galectin-1 drives pancreatic carcinogenesis through stroma remodeling and Hedgehog signaling activation. Cancer Res. 2014;74:3512–3524. doi: 10.1158/0008-5472.CAN-13-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roda O, Ortiz-Zapater E, Martínez-Bosch N, Gutiérrez-Gallego R, Vila-Perelló M, Ampurdanés C, Gabius HJ, André S, Andreu D, Real FX, et al. Galectin-1 is a novel functional receptor for tissue plasminogen activator in pancreatic cancer. Gastroenterology. 2009;136:1379–1390, e1-e5. doi: 10.1053/j.gastro.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 140.Rosenberger I, Strauss A, Dobiasch S, Weis C, Szanyi S, Gil-Iceta L, Alonso E, González Esparza M, Gómez-Vallejo V, Szczupak B, et al. Targeted diagnostic magnetic nanoparticles for medical imaging of pancreatic cancer. J Control Release. 2015;214:76–84. doi: 10.1016/j.jconrel.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 141.Roda O, Chiva C, Espuña G, Gabius HJ, Real FX, Navarro P, Andreu D. A proteomic approach to the identification of new tPA receptors in pancreatic cancer cells. Proteomics. 2006;6:S36–S41. doi: 10.1002/pmic.200500376. [DOI] [PubMed] [Google Scholar]
- 142.André S, Sanchez-Ruderisch H, Nakagawa H, Buchholz M, Kopitz J, Forberich P, Kemmner W, Böck C, Deguchi K, Detjen KM, et al. Tumor suppressor p16INK4a--modulator of glycomic profile and galectin-1 expression to increase susceptibility to carbohydrate-dependent induction of anoikis in pancreatic carcinoma cells. FEBS J. 2007;274:3233–3256. doi: 10.1111/j.1742-4658.2007.05851.x. [DOI] [PubMed] [Google Scholar]
- 143.Sanchez-Ruderisch H, Fischer C, Detjen KM, Welzel M, Wimmel A, Manning JC, André S, Gabius HJ. Tumor suppressor p16 INK4a: Downregulation of galectin-3, an endogenous competitor of the pro-anoikis effector galectin-1, in a pancreatic carcinoma model. FEBS J. 2010;277:3552–3563. doi: 10.1111/j.1742-4658.2010.07764.x. [DOI] [PubMed] [Google Scholar]
- 144.Amano M, Eriksson H, Manning JC, Detjen KM, André S, Nishimura S, Lehtiö J, Gabius HJ. Tumour suppressor p16(INK4a) - anoikis-favouring decrease in N/O-glycan/cell surface sialylation by down-regulation of enzymes in sialic acid biosynthesis in tandem in a pancreatic carcinoma model. FEBS J. 2012;279:4062–4080. doi: 10.1111/febs.12001. [DOI] [PubMed] [Google Scholar]
- 145.Muniyan S, Haridas D, Chugh S, Rachagani S, Lakshmanan I, Gupta S, Seshacharyulu P, Smith LM, Ponnusamy MP, Batra SK. MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism. Genes Cancer. 2016;7:110–124. doi: 10.18632/genesandcancer.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xue X, Lu Z, Tang D, Yao J, An Y, Wu J, Li Q, Gao W, Xu Z, Qian Z, et al. Galectin-1 secreted by activated stellate cells in pancreatic ductal adenocarcinoma stroma promotes proliferation and invasion of pancreatic cancer cells: an in vitro study on the microenvironment of pancreatic ductal adenocarcinoma. Pancreas. 2011;40:832–839. doi: 10.1097/MPA.0b013e318217945e. [DOI] [PubMed] [Google Scholar]
- 147.Chen R, Dawson DW, Pan S, Ottenhof NA, de Wilde RF, Wolfgang CL, May DH, Crispin DA, Lai LA, Lay AR, et al. Proteins associated with pancreatic cancer survival in patients with resectable pancreatic ductal adenocarcinoma. Lab Invest. 2015;95:43–55. doi: 10.1038/labinvest.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Masamune A, Satoh M, Hirabayashi J, Kasai K, Satoh K, Shimosegawa T. Galectin-1 induces chemokine production and proliferation in pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G729–G736. doi: 10.1152/ajpgi.00511.2005. [DOI] [PubMed] [Google Scholar]
- 149.Fitzner B, Walzel H, Sparmann G, Emmrich J, Liebe S, Jaster R. Galectin-1 is an inductor of pancreatic stellate cell activation. Cell Signal. 2005;17:1240–1247. doi: 10.1016/j.cellsig.2004.12.012. [DOI] [PubMed] [Google Scholar]


