Abstract
AIM
To assess differences in clinical outcomes of isolated renal failure (RF) compared to other forms of organ failure (OF) in patients with severe acute pancreatitis (SAP).
METHODS
Using a prospectively maintained database of patients with acute pancreatitis admitted to a tertiary medical center between 2003 and 2016, those with evidence of persistent OF were classified to renal, respiratory, cardiovascular, or multi-organ (2 or more organs). Data regarding demographics, comorbidities, etiology of acute pancreatitis, and clinical outcomes were prospectively recorded. Differences in clinical outcomes after development of isolated RF in comparison to other forms of OF were determined using independent t and Mann-Whitney U tests for continues variables, and χ2 test for discrete variables.
RESULTS
Among 500 patients with acute pancreatitis, 111 patients developed persistent OF: mean age was 54 years, and 75 (67.6%) were male. Forty-three patients had isolated OF: 17 (15.3%) renal, 25 (21.6%) respiratory, and 1 (0.9%) patient with cardiovascular failure. No differences in demographics, etiology of acute pancreatitis, systemic inflammatory response syndrome scores, or development of pancreatic necrosis were seen between patients with isolated RF vs isolated respiratory failure. Patients with isolated RF were less likely to require nutritional support (76.5% vs 96%, P = 0.001), ICU admission (58.8% vs 100%, P = 0.001), and had shorter mean ICU stay (2.4 d vs 15.7 d, P < 0.001), compared to isolated respiratory failure. None of the patients with isolated RF or isolated respiratory failure died.
CONCLUSION
Among patients with SAP per the Revised Atlanta Classification, approximately 15% develop isolated RF. This subgroup seems to have a less protracted clinical course compared to other forms of OF. Isolated RF might be weighed less than isolated respiratory failure in risk predictive modeling of acute pancreatitis.
Keywords: Renal failure, Respiratory failure, Organ failure, Acute pancreatitis, Clinical outcomes
Core tip: In a large prospective observational study, we show that approximately 15% of patients with severe acute pancreatitis develop isolated renal failure. This subgroup has an overall better prognosis and less protracted clinical course compared to patients with other isolated or multiple organ failure. These results can be useful in allocating healthcare resources and counseling patients. We propose that isolated renal failure be weighed less in risk predictive modeling of acute pancreatitis.
INTRODUCTION
Acute pancreatitis (AP) is a leading cause of gastrointestinal-related hospital admissions, and its incidence continues to increase[1]. While the majority of patients have a mild and self-limiting clinical course, about 20% progress to develop end-organ failure with or without local complications including pancreatic necrosis. Overall, 1%-2% of patients die from AP-related complications[2].
The Revised Atlanta Classification, based on an international consensus, stratifies AP according to disease severity into 3 categories: mild [no organ failure (OF) and no local complications], moderate (transient OF resolving in less than 48 h and/or local complications), and severe (persistent OF lasting at least 48 h with/without local complications)[3].
Within the spectrum of severe AP (SAP) according to the Revised Atlanta Classification, persistent OF encompasses isolated OF or multiple OF. The three main organ systems that are affected in SAP are renal, respiratory, and/or cardiovascular. Patients with persistent OF frequently have local complications[4]. Variability of the impact of organ-specific failure on the clinical course of AP may be important to appropriately triage healthcare resources, guide management, predict long-term outcomes, and allow the comparison of different therapeutic interventions[5].
Renal failure (RF) in SAP can be isolated, but more commonly is part of multiple OF. Acute RF in the setting of multiple OF has been shown to be associated with worse clinical outcomes including prolonged hospital stay, admission to the intensive care unit (ICU), and higher mortality[6]. One study reported a 10-fold increase in mortality when acute RF complicates SAP[7]. However, the specific clinical outcomes of the SAP patient subgroup with isolated RF have not been well characterized. The aim of this study was to assess the incidence of isolated RF in patients with SAP, and analyze differences in clinical outcomes compared to other forms of OF (isolated and multiple).
MATERIALS AND METHODS
Study population
Pancreatitis-associated risk of organ failure (PROOF) is an ongoing observational study conducted at the University of Pittsburgh Medical Center and approved by the institutional review board (protocol ID PRO08010374). Patients who meet at least 2 of 3 criteria for diagnosis of AP are considered eligible for enrollment: presence of abdominal pain characteristic of AP, serum lipase level ≥ 3 times the upper limit of normal, and imaging findings consistent with AP. Patients with imaging and/or clinical findings suggestive of chronic pancreatitis or pancreatic cancer are excluded. After obtaining informed consent, each patient is enrolled into the study following admission or transfer to our center, and is prospectively followed until hospital discharge. The study has been conducted in three separate time periods, with consecutive enrollment of patients starting in June 2003 until January 2016.
Pertinent demographic, laboratory, and radiological data were prospectively recorded. Outside hospital medical records of transferred patients were reviewed. Diagnosis and etiology of AP, development of local complications, type and duration of OF, and other clinical outcomes including need for nutritional support, admission to the ICU, and length of stay were recorded. Need for nutritional support refers to inability to tolerate oral feeding and includes enteral tube feedings and/or parenteral nutrition. Length of stay refers to overall length of stay from the time of admission to discharge, which includes duration of stay in outside hospitals for transferred patients. For patients with RF, fractional excretion of sodium (FENa) was calculated as [(Urine Na/Serum Na)/(Urine Cr/Serum Cr) × 100], when these values were available. Need for and duration of hemodialysis, and development of chronic kidney disease at 3 mo were recorded. For patients with respiratory failure, need for and duration of intubation, and need for tracheostomy were recorded.
Definition and classification of organ failure
Patients who developed OF were classified into: isolated RF, respiratory failure, cardiovascular failure, or multiple OF (2 or more organs). OF was defined as a score of 2 or more for one of these organ systems using the modified Marshall scoring system. RF is defined by a serum creatinine of 1.9 mg/dL or higher, respiratory failure is defined by a PaO2/FiO2 of 201 or less, and cardiovascular failure is defined by a systolic blood pressure < 90 mmHg that is not fluid responsive[8].
Statistical analysis
Data regarding demographics, comorbidities, etiology of AP, and clinical outcomes were stratified and compared with respect to development and type of OF. Initially, patients with isolated OF were compared with those with no OF and those with multiple OF. Then, patients with isolated RF were compared to those with other types of isolated OF. Next, we attempted a multivariate logistic regression model to determine predictors of organ failure subtypes (which was coded as a dichotomous variable: patients with isolated renal failure were coded “0” and those with isolated pulmonary failure “1”). Patient characteristics with a P-value < 0.25 in univariate analysis were included in multivariate model. A stepwise forward and backwards elimination method were attempted to find a final parsimonious model. Additionally, absence of collinearity was confirmed using pre-specified variance inflation factor of 5. Normality in continuous data was evaluated utilizing Kolmogorov-Smirnov test. Mean ± SD or median with interquartile range were used for continuous variables and counts with percentages for categorical variables. Differences in clinical outcomes following development of isolated RF in comparison to other forms of OF, were determined using t-test and Mann-Whitney U tests for continues variables, and Pearson’s chi-square test for categorical variables. All tests were two-tailed and a P-value ≤ 0.05 was considered statistically significant. Analyses were performed using SPSS (IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp), and Stata (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP). The statistical review of the study was performed by a biomedical statistician.
RESULTS
A total of 500 patients with AP were studied. Figure 1 shows the patient cohort distribution according to development of OF, duration, and specific organ(s) involved.. One hundred and eleven patients developed persistent OF: 43 (38.7%) developed isolated OF and 68 (61.3%) developed multiple OF. The mean age for patients with persistent OF was 54.2 years, and 75 (67.6%) were male.
Figure 1.
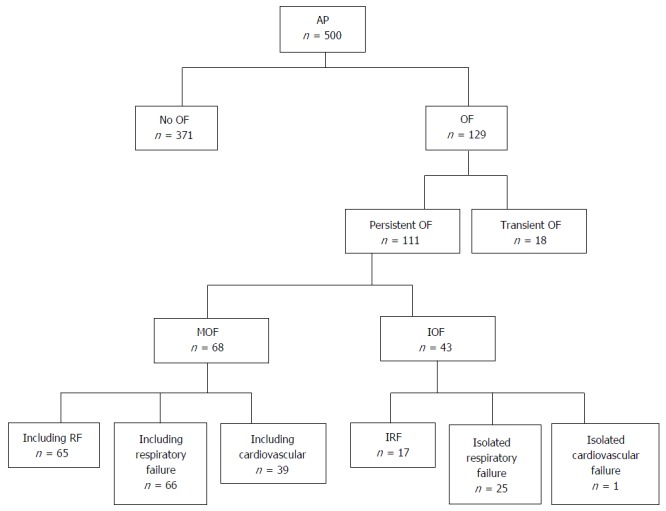
Patient cohort flowchart based on development of organ failure, duration, and specific organ(s) involved.
Table 1 summarizes the characteristics of all AP patients, comparing patients with isolated, multiple, and no persistent OF. Overall, the three groups were comparable in age, gender, race, etiology of AP, and pre-existing chronic illnesses. Table 2 shows the differences in severity predictors, imaging findings, interventions, and clinical outcomes among AP patients with isolated, multiple, and no persistent OF. As expected, SIRS criteria were more common in patients with OF than those with no persistent OF. Need for admission to the ICU (100% vs 83.7%, P = 0.001), and median length of ICU stay (18.5 d vs 6 d, P < 0.001), were higher in patients with multiple OF than isolated OF. Patients with multiple OF were more likely to have infected pancreatic necrosis (38.9%), extra-pancreatic infections (44.1%), and longer median total hospital stay (37 d), than those with isolated OF. Twenty two of 68 patients with multiple OF (32.4%) died, while none of the patients with isolated OF or no persistent OF died (P < 0.001).
Table 1.
Characteristics among patients with isolated organ failure, multiple organ failure, and no persistent organ failure
| IOF | MOF | P value | No OF | P value | |
| n = 43 | n = 68 | n = 389 | |||
| Mean age ± SD | 50.7 (18.5) | 56.4 (15.4) | 0.08 | 50.5 ± 18.9 | 0.97 |
| Male | 29 (67.4) | 46 (67.6) | 0.98 | 178 (45.8) | 0.007 |
| Caucasian | 39 (90.7) | 61 (89.7) | 0.87 | 344 (88.4) | 0.66 |
| Median BMI (IQR) | 28.8 (25.7-32.9) | 32.2 (28.7-36.4) | 0.009 | 27.5 (24.2-32.9) | 0.27 |
| Transfers | 35 (81.4) | 61 (89.7) | 0.21 | 168 (43.2) | < 0.001 |
| First episode of AP | 35 (81.4) | 58 (85.3) | 0.59 | 233 (59.9) | 0.006 |
| Etiology | |||||
| Biliary | 17 (39.5) | 35 (51.5) | 0.33 | 144 (37) | 0.03 |
| Alcoholic | 12 (27.9) | 8 (11.8) | 51 (13.1) | ||
| Idiopathic | 4 (9.3) | 9 (13.2) | 77 (19.8) | ||
| Hypertriglyceridemia | 4 (9.3) | 9 (13.2) | 26 (6.7) | ||
| Post-ERCP | 2 (4.7) | 3 (4.4) | 63 (16.2) | ||
| Other | 4 (9.3) | 4 (5.9) | 28 (7.2) | ||
| Comorbidities | |||||
| Pre-existing heart failure | 0 | 2 (2.9) | 0.26 | 9 (2.3) | 0.31 |
| Pre-existing renal failure | 1 (2.3) | 4 (5.9) | 0.38 | 8 (2.1) | 0.9 |
| Pre-existing respiratory disorder | 2 (4.7) | 1 (1.5) | 0.31 | 16 (4.1) | 0.87 |
Values presented as mean ± SD or n (%). P values: 1st column reflects comparison of IOF to MOF, and 2nd column reflects comparison of IOF to no OF. IOF: Isolated organ failure; MOF: Multiple organ failure; OF: Organ failure; AP: Acute pancreatitis; BMI: Body mass index; IQR: Interquartile range; ERCP: Endoscopic retrograde cholangiopancreatography.
Table 2.
Severity predictors, imaging findings, and clinical outcomes among acute pancreatitis patients with isolated organ failure, multi-organ failure, and no persistent organ failure n (%)
| IOF | MOF | P value | No OF | P value | |
| n = 43 | n = 68 | n = 389 | |||
| SIRS score on admission ≥ 2 | 23 (53.5) | 45 (66.2) | 0.18 | 107 (27.5) | < 0.001 |
| SIRS score at 48 h of admission ≥ 2 | 26 (60.5) | 52 (76.5) | 0.07 | 84 (21.6) | < 0.001 |
| Mean IVF the first 24 h in liters (SD) | 4.38 (1.98) | 5.04 (1.79) | 0.41 | 3.79 (1.5) | 0.23 |
| ICU admission | 36 (83.7) | 68 (100) | 0.001 | 50 (12.9) | < 0.001 |
| Median ICU LOS in days (IQR) | 6 (2-14.4) | 18.5 (10-35) | < 0.001 | 0 | < 0.001 |
| Need for nutritional support | 38 (88.4) | 58 (85.3) | 0.64 | 80 (20.6) | < 0.001 |
| Pancreatic necrosis1 | 28/39 (71.8) | 45/54 (83.3) | 0.18 | 80/251 (31.9) | < 0.001 |
| Infected necrosis1 | 5/39 (12.8) | 21/54 (38.9) | 0.006 | 11/164 (6.7) | 0.2 |
| Need for drainage or debridement | 13 (30.2) | 38 (55.9) | 0.008 | 31 (8) | < 0.001 |
| Extra-pancreatic infections | 8 (18.6) | 30 (44.1) | 0.006 | 27 (6.9) | 0.008 |
| Median LOS in days (IQR) | 20 (16-32) | 37 (25-53) | < 0.001 | 6 (4-9) | < 0.001 |
| Mortality | 0 | 22 (32.4) | < 0.001 | 0 |
Denominators reflect number of patients with available contrast-enhanced computed tomography scan. P values: 1st column reflects comparison of IOF to MOF, and 2nd column reflects comparison of IOF to OF. AP: Acute pancreatitis; IOF: Isolated organ failure; MOF: Multi-organ failure; OF: Organ failure; SIRS: Systemic inflammatory response syndrome; IVF: Intravenous fluids; ICU: Intensive care unit; LOS: Length of stay; IQR: Interquartile range.
Isolated renal failure compared to renal failure as part of multiple organ failure
In total, 17 patients developed isolated RF while 65 patients had RF as part of multiple OF. The majority of patients with multiple OF had RF (96%). Among those with isolated RF, only 2 (11.8%) patients required the initiation of hemodialysis compared to 36 (55.4%) of patients with RF as part of multiple OF (P = 0.001). The FENa, when available, was less than 2% for most patients in both groups (66.7% of isolated RF and 88.9% of RF as part of multiple OF, P = 0.17). Length of stay was significantly shorter in patients with isolated RF compared to those with multiple OF (19 d vs 38 d, P = 0.001). There was no difference in the development of chronic kidney diseases between both groups (16.7% of isolated RF patients vs 26.2% of multiple OF patients, P = 0.496). None of the patients with isolated RF required prolonged hemodialysis after discharge, while 55.2% of those with RF as part of multiple OF did (P = 0.001). None of the patients with IRF died, while 22 (33.9%) of those with RF as part of multiple OF died (P = 0.005).
Isolated renal failure compared to isolated respiratory failure
Overall, 43 developed isolated OF: 17 (15.3%) RF, 25 (21.6%) had respiratory failure, and 1 (0.9%) patient had cardiovascular failure. Thus, cardiovascular failure was not included in further analysis. No differences in demographics, etiology of AP, systemic inflammatory response syndrome scores, or development of pancreatic necrosis were present between isolated RF and isolated respiratory failure. Patients with isolated RF were less likely to require nutritional support (76.5% vs 96%, P = 0.01), had lower requirement for ICU admission (58.8% vs 100%, P = 0.001), and had shorter mean ICU stay (2.4 d vs 15.7 d, P = 0.001), compared to isolated respiratory failure. There were no statistically significant differences in the development of pancreatic necrosis, need for pancreatic drainage or debridement, or overall length of hospital stay between the two groups. None of the patients with isolated RF or isolated respiratory failure died (Table 3). In a multivariate logistic regression model after controlling for other factors, length of ICU stay was statistically shorter in patients with isolated RF compared to those with isolated pulmonary failure (P = 0.032). Table 4 summarizes the specific clinical parameters of each of the 17 patients with isolated RF.
Table 3.
Demographics and clinical outcomes in severe acute pancreatitis patients with isolated renal failure and isolated respiratory failure n (%)
| Isolated renal failure | Isolated respiratory failure | P value | |
| n = 17 | n = 25 | ||
| Male | 13 (76.5) | 16 (64) | 0.39 |
| Mean ± SD | 56.1 (18.2) | 47.1 (18.6) | 0.13 |
| Mean BMI (SD) | 32 (7.3) | 28.5 (4.8) | 0.06 |
| Biliary etiology | 6 (35.3) | 11 (44) | 0.57 |
| ICU admission | 10 (58.8) | 25 (100) | < 0.001 |
| Mean ICU LOS in days (SD) | 2.4 (3.1) | 15.7 (12.7) | < 0.001 |
| Need for nutritional support | 13 (76.5) | 24 (96) | 0.01 |
| Pancreatic necrosis | 11 (64.7) | 16 (64) | 0.8 |
| Need for drainage or debridement | 5 (29.4) | 7 (28) | 0.92 |
| Median LOS (IQR) | 19 (15-28) | 22 (16-35) | 0.31 |
| Mortality | 0 | 0 |
SD: Standard deviation; BMI: Body mass index; ICU: Intensive care unit; LOS: Length of stay; IQR: interquartile range.
Table 4.
Specific clinical parameters of patients with isolated renal failure
| Patient | Age | Gender | Etiology | Comorbidity | PNec | Peak Cr | Dialysis | Dialysis at DC | Dialysis indication | ICU | LOS |
| 1 | 50 | M | Post-ERCP | Cirrhosis | No | 2.9 | No | NA | NA | No | 19 |
| 2 | 54 | M | HTG | None | No | 2.6 | No | NA | NA | No | 18 |
| 3 | 68 | M | Idiopathic | Diabetes | Yes | 2 | No | NA | NA | Yes | 19 |
| 4 | 64 | M | Biliary | Diabetes | Yes | 3.8 | No | NA | NA | No | 84 |
| 5 | 40 | M | Alcohol | Cirrhosis | Yes | 4.1 | No | NA | NA | Yes | 12 |
| 6 | 81 | F | Biliary | None | Yes | 2.2 | No | NA | NA | No | 15 |
| 7 | 32 | M | Alcohol | None | Yes | 3.6 | No | NA | NA | No | 21 |
| 8 | 78 | F | Biliary | Diabetes | Yes | 1.9 | No | NA | NA | Yes | 28 |
| 9 | 58 | M | Other | None | Yes | 2 | No | NA | NA | No | 51 |
| 10 | 60 | F | Alcohol | None | No | 2.5 | No | NA | NA | Yes | 26 |
| 11 | 81 | M | Biliary | None | No | 4 | No | NA | NA | Yes | 17 |
| 12 | 29 | M | Other | None | Yes | 7.8 | Yes | No | Hyperkalemia, volume overload | Yes | 30 |
| 13 | 41 | M | HTG | Diabetes | Yes | 6.6 | No | NA | NA | Yes | 18 |
| 14 | 77 | M | Biliary | COPD, Diabetes | No | 3.3 | No | NA | NA | Yes | 12 |
| 15 | 62 | F | Post-ERCP | Cholangiocarcinoma | No | 2.9 | No | NA | NA | No | 6 |
| 16 | 54 | M | Biliary | None | Yes | 14.6 | Yes | No | Hyperkalemia | Yes | 33 |
| 17 | 24 | M | HTG | COPD | Yes | 4.1 | No | NA | NA | Yes | 11 |
M: Male; F: Female; ERCP: Endoscopic retrograde cholangiopancreatography; HTG: Hypertriglyceridemia; COPD: Chronic obstructive pulmonary disease; PNec: Pancreatic necrosis; Cr: Creatinine; DC: Discharge; NA: Not applicable; ICU: Intensive care unit; LOS: Length of stay.
DISCUSSION
Prior studies have reported the clinical outcomes including hospital stay and mortality for SAP patients who developed acute RF[6,7,9,10]. Comparison across those studies has been difficult due to heterogeneity of the patient populations, varying definitions of acute RF and AP severity, and different end-points. However, no prior study has focused on AP patients with isolated RF and the clinical outcomes of this subgroup are not known.
One retrospective study from Europe of 563 patients with AP found that 79 (14%) developed acute RF, and that this group had an overall 10-fold increase in mortality (75% vs 7%, P < 0.001). However, the majority of patients with RF (96%) actually had multiple OF, and outcomes of AP patients with isolated RF were not reported[7]. Another cross-sectional study from China included 228 patients with SAP and found that the overall incidence of acute RF is 18%. This study showed that underlying chronic kidney disease, hypoxemia, and abdominal compartment syndrome, were independent factors for development of acute RF in those patients[6]. Again, the unique characteristics of patients with isolated RF were not evaluated.
To our knowledge, this is the first study to evaluate the clinical outcomes of SAP patients with isolated RF. In our prospectively enrolled cohort of AP patients admitted or transferred to a major tertiary medical center, the overall incidence of acute RF in patients with SAP was 74% but only 15% developed isolated RF. The majority of patients with RF, whether isolated or as part of multiple OF, had a FENa of less than 2% which is consistent with a pre-renal etiology of kidney injury. When RF is part of multiple OF, namely respiratory and/or cardiovascular failure, there is a statistically significant association with increased need for hemodialysis (55%), median length of hospital stay (38 d), and death (34%). In patients with isolated RF, only 12% required hemodialysis, their median length of hospital stay was 19 days, and importantly none of those patients died.
As only 1 patient developed isolated cardiovascular failure in our cohort, the isolated OF comparison focused on RF vs respiratory failure. Despite absence of differences in baseline characteristics between both groups, patients with isolated RF were significantly less likely to require nutritional support (77% vs 96%), require ICU admission (59% vs 100%), and had shorter mean ICU stay (2.4 d vs 15.7 d), compared to isolated respiratory failure. The explanation for the excess in need for ICU care in patients with isolated respiratory failure is mostly related to the need for mechanical ventilation which none of the isolated RF would have necessarily required. There were no significant differences in the development of pancreatic necrosis, need for pancreatic drainage or debridement, or overall length of hospital stay between the two groups.
The mechanisms of acute RF development in AP patients are not well studied and the resulting renal injury is likely multifactorial. Proposed mechanisms include hypoxemia-driven injury to the renal tubular epithelial cells[11], impairment of renal microcirculation due to released pancreatic amylase[12], release of apoptotic molecules including cytokines from the inflamed pancreas leading to renal cellular injury[13], and abdominal compartment syndrome that may develop in patients with SAP and lead to decreased renal perfusion pressure causing ischemic injury[14]. Accordingly, the favorable outcomes of patients with isolated RF, as shown in this study, could be a reflection of milder renal injury due to absence of hypoxemia when there is no associated respiratory failure. Furthermore, an intact cardiovascular response likely minimizes the degree of renal hypoperfusion that one would otherwise expect when renal injury and cardiovascular collapse are present simultaneously. Moreover, in contrast to isolated respiratory failure, isolated RF in the setting of AP could represent a milder reversible form of organ failure. This may have implications on the amount and timing of intravenous fluid resuscitation as excess fluid may lead to pulmonary edema and respiratory failure, while adequate properly timed hydration may correct RF.
The main limitation of our study is the observational design and therefore inability to determine the mechanistic roles of isolated RF on outcomes of SAP patients. Also, as the study is conducted in a tertiary referral center, our findings may not be representative of the community setting of all comers with AP, but rather a “sicker” group of patients. The major strengths of the study are the prospective method of patient enrollment, large sample size, and inclusion of all AP patients regardless of disease etiology.
In summary, our study shows that approximately 15% of patients with SAP develop isolated RF. This subgroup seems to have an overall better prognosis and less protracted clinical course compared to patients with isolated respiratory failure and multiple OF. These results can be useful in allocating healthcare resources and counseling patients. We propose that isolated RF might be weighed less in risk predictive modeling of AP.
COMMENTS
Background
About 20% of patients with acute pancreatitis have severe disease as evident by the development of persistent organ failure, which could be isolated or involve multiple organ systems.
Research frontiers
Acute renal failure in the setting of multiple organ failure has been shown to be associated with worse clinical outcomes in patient with severe acute pancreatitis. However, the specific clinical outcomes of patients who develop isolated renal failure are not well characterized.
Innovations and breakthroughs
This study shows that approximately 15% of patients with severe acute pancreatitis develop isolated renal failure. This subgroup seems to have an overall better prognosis and less protracted clinical course compared to patients with isolated respiratory failure and multiple organ failure.
Applications
Understanding the relatively less negative impact of isolated renal failure on clinical outcomes of patients with severe acute pancreatitis is important to allocate healthcare resources and counsel patients. They also propose that isolated renal failure might be weighed less in risk predictive modeling of acute pancreatitis.
Peer-review
This interesting article studies the association between subtypes of organ failure outcomes after acute pancreatitis in a large cohort of patients from a tertiary hospital. The study is well written and performed.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of the University of Pittsburgh.
Informed consent statement: All study participants, or their legal guardian, provided written consent prior to study enrollment.
Conflict-of-interest statement: The authors of this manuscript have no conflicts of interest to disclose.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at papachri@pitt.edu. Participants gave informed consent for data sharing.
Peer-review started: March 28, 2017
First decision: April 20, 2017
Article in press: July 4, 2017
P- Reviewer: Ikeura T, Stocco G S- Editor: Qi Y L- Editor: A E- Editor: Li D
References
- 1.Peery AF, Crockett SD, Barritt AS, Dellon ES, Eluri S, Gangarosa LM, Jensen ET, Lund JL, Pasricha S, Runge T, et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology. 2015;149:1731–1741.e3. doi: 10.1053/j.gastro.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 4.Mounzer R, Langmead CJ, Wu BU, Evans AC, Bishehsari F, Muddana V, Singh VK, Slivka A, Whitcomb DC, Yadav D, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142:1476–1482; quiz e15-e16. doi: 10.1053/j.gastro.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Nawaz H, Mounzer R, Yadav D, Yabes JG, Slivka A, Whitcomb DC, Papachristou GI. Revised Atlanta and determinant-based classification: application in a prospective cohort of acute pancreatitis patients. Am J Gastroenterol. 2013;108:1911–1917. doi: 10.1038/ajg.2013.348. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Qian Z, Liu Z, Liu X, Han X, Kang H. Risk factors and outcome of acute renal failure in patients with severe acute pancreatitis. J Crit Care. 2010;25:225–229. doi: 10.1016/j.jcrc.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Kes P, Vucicević Z, Ratković-Gusić I, Fotivec A. Acute renal failure complicating severe acute pancreatitis. Ren Fail. 1996;18:621–628. doi: 10.3109/08860229609047686. [DOI] [PubMed] [Google Scholar]
- 8.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Herrera Gutiérrez ME, Seller Pérez G, de La Rubia De Gracia C, Chaparro Sánchez MJ, Nacle López B. [Acute renal failure profile and prognostic value in severe acute pancreatitis] Med Clin (Barc) 2000;115:721–725. doi: 10.1016/s0025-7753(00)71674-5. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Li Y, Tang Y, Liu F, Yu S, Zhang L, Zeng X, Zhao Y, Fu P. Effect of acute kidney injury on mortality and hospital stay in patient with severe acute pancreatitis. Nephrology (Carlton) 2015;20:485–491. doi: 10.1111/nep.12439. [DOI] [PubMed] [Google Scholar]
- 11.Basnakian AG, Kaushal GP, Shah SV. Apoptotic pathways of oxidative damage to renal tubular epithelial cells. Antioxid Redox Signal. 2002;4:915–924. doi: 10.1089/152308602762197452. [DOI] [PubMed] [Google Scholar]
- 12.Nishiwaki H, Ko I, Hiura A, Ha SS, Satake K, Sowa M. Renal microcirculation in experimental acute pancreatitis of dogs. Ren Fail. 1993;15:27–31. doi: 10.3109/08860229309065568. [DOI] [PubMed] [Google Scholar]
- 13.Malmstrøm ML, Hansen MB, Andersen AM, Ersbøll AK, Nielsen OH, Jørgensen LN, Novovic S. Cytokines and organ failure in acute pancreatitis: inflammatory response in acute pancreatitis. Pancreas. 2012;41:271–277. doi: 10.1097/MPA.0b013e3182240552. [DOI] [PubMed] [Google Scholar]
- 14.Al-Bahrani AZ, Abid GH, Holt A, McCloy RF, Benson J, Eddleston J, Ammori BJ. Clinical relevance of intra-abdominal hypertension in patients with severe acute pancreatitis. Pancreas. 2008;36:39–43. doi: 10.1097/mpa.0b013e318149f5bf. [DOI] [PubMed] [Google Scholar]


