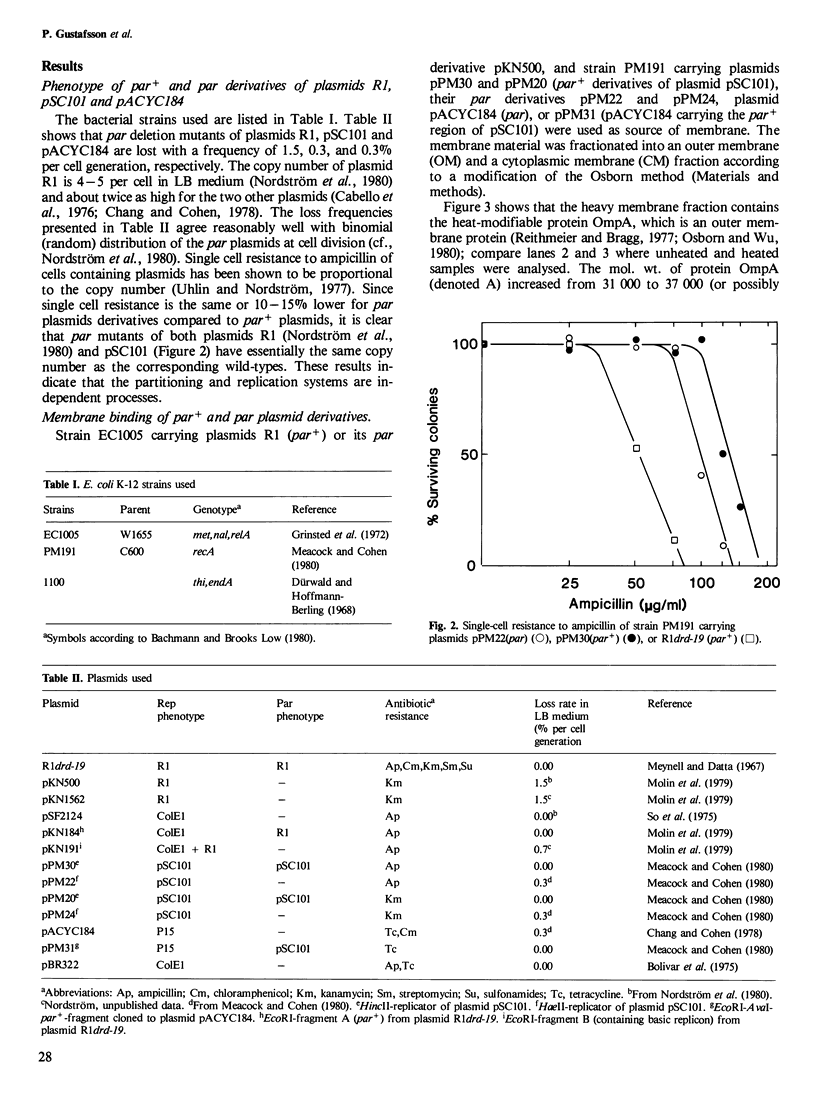
Abstract
The binding between par+ and par plasmid DNA to different membrane fractions of Escherichia coli was investigated. Membrane material from cells carrying different Par+ and Par- derivatives of plasmids R1 and pSC101 was isolated and fractionated into an outer and a cytoplasmic membrane fraction. The presence of plasmid DNA in the two membrane fractions was measured either by nick-translation of the membrane-bound DNA, followed by filter-hybridization to homologous DNA, or by filter-hybridization of the membrane-bound DNA to nick-translated homologous purified plasmid DNA. The DNA of par derivatives of plasmids R1 and pSC101 could be detected only in the cytoplasmic membrane fraction, whereas the corresponding par+ plasmid DNA also appeared in the outer membrane material, indicating a specific binding between the R1 and pSC101 partition loci and the bacterial outer membrane. The experiment was then modified by fractionation of the membrane material from cells carrying hybrids between the vector pSF2124 and the par region or the basic replicon region of plasmid R1. The DNA of the membrane fractions were filter-hybridized to nick-translated probes. Again, the par+ region caused hybridization to the outer membrane material. Therefore, we may conclude that controlled partitioning involves binding of DNA to membrane material that has the same density as the outer membrane of the host bacteria. This finding offers a biochemical 'assay' for studies of the molecular biology of plasmid partitioning.
Full text
PDF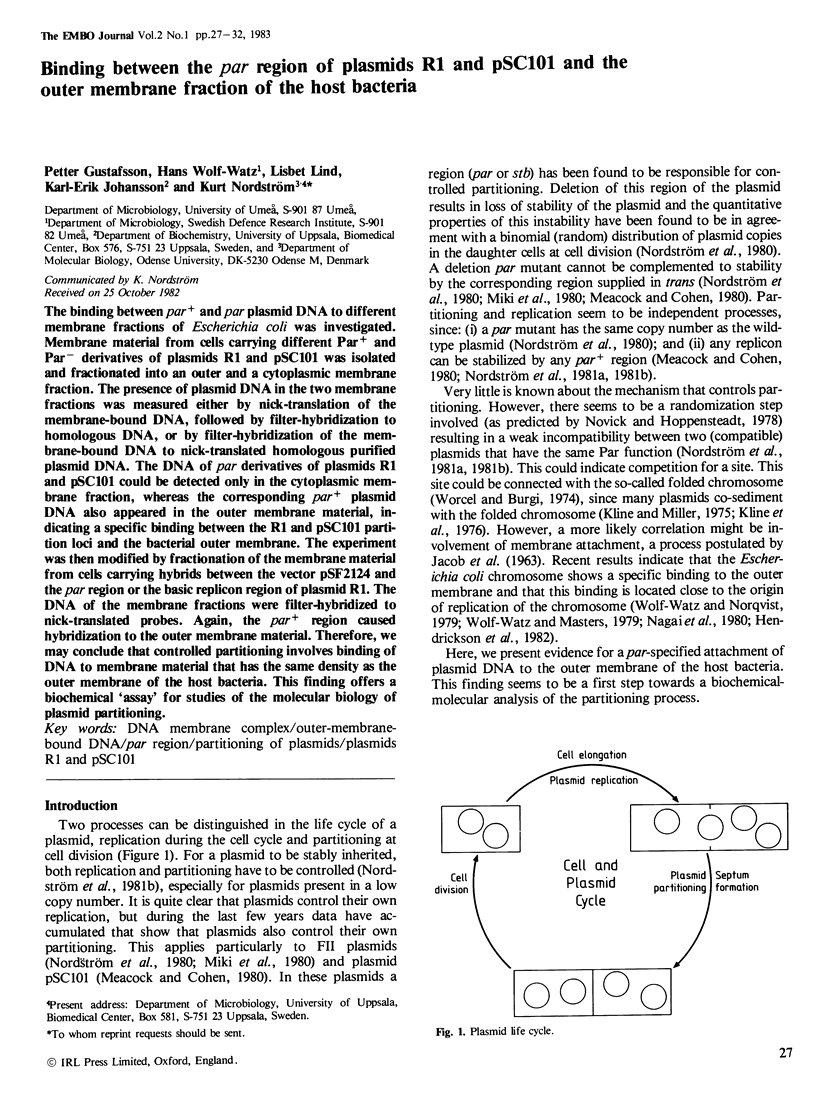




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Cabello F., Timmis K., Cohen S. N. Replication control in a composite plasmid constructed by in vitro linkage of two distinct replicons. Nature. 1976 Jan 29;259(5541):285–290. doi: 10.1038/259285a0. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Latta P., Bouanchaud D., Novick R. P. Partition kinetics and thermosensitive replication of pT169, a naturally occurring multicopy tetracycline resistance plasmid of Staphylococcus aureus. Plasmid. 1978 Jun;1(3):366–375. doi: 10.1016/0147-619x(78)90052-5. [DOI] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Donachie W. D. Control of cell division in Escherichia coli: experiments with thymine starvation. J Bacteriol. 1969 Oct;100(1):260–268. doi: 10.1128/jb.100.1.260-268.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürwald H., Hoffmann-Berling H. Endonuclease-I-deficient and ribonuclease I-deficient Escherichia coli mutants. J Mol Biol. 1968 Jul 14;34(2):331–346. doi: 10.1016/0022-2836(68)90257-x. [DOI] [PubMed] [Google Scholar]
- Engberg B., Hjalmarsson K., Nordström K. Inhibition of cell division in Escherichia coli K-12 by the R-factor R1 and copy mutants of R1. J Bacteriol. 1975 Nov;124(2):633–640. doi: 10.1128/jb.124.2.633-640.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson W. G., Kusano T., Yamaki H., Balakrishnan R., King M., Murchie J., Schaechter M. Binding of the origin of replication of Escherichia coli to the outer membrane. Cell. 1982 Oct;30(3):915–923. doi: 10.1016/0092-8674(82)90296-3. [DOI] [PubMed] [Google Scholar]
- Kline B. C., Miller J. R., Cress D. E., Wlodarczyk M., Manis J. J., Otten M. R. Nonintegrated plasmid-chromosome complexes in Escherichia coli. J Bacteriol. 1976 Aug;127(2):881–889. doi: 10.1128/jb.127.2.881-889.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline B. C., Miller J. R. Detection of nonintegrated plasmid deoxyribonucleic acid in the folded chromosome of Escherichia coli: physiochemical approach to studying the unit of segregation. J Bacteriol. 1975 Jan;121(1):165–172. doi: 10.1128/jb.121.1.165-172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen H. A., Donachie W. D. Intracellular localization and effects on cell division of a plasmid blocked in deoxyribonucleic acid replication. J Bacteriol. 1977 Nov;132(2):392–397. doi: 10.1128/jb.132.2.392-397.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacock P. A., Cohen S. N. Partitioning of bacterial plasmids during cell division: a cis-acting locus that accomplishes stable plasmid inheritance. Cell. 1980 Jun;20(2):529–542. doi: 10.1016/0092-8674(80)90639-x. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Miki T., Easton A. M., Rownd R. H. Cloning of replication, incompatibility, and stability functions of R plasmid NR1. J Bacteriol. 1980 Jan;141(1):87–99. doi: 10.1128/jb.141.1.87-99.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin S., Stougaard P., Uhlin B. E., Gustafsson P., Nordström K. Clustering of genes involved in replication, copy number control, incompatibility, and stable maintenance of the resistance plasmid R1drd-19. J Bacteriol. 1979 Apr;138(1):70–79. doi: 10.1128/jb.138.1.70-79.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Hendrickson W., Balakrishnan R., Yamaki H., Boyd D., Schaechter M. Isolation of a replication origin complex from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):262–266. doi: 10.1073/pnas.77.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Eriksson-Grennberg K. G., Boman H. G. Resistance of Escherichia coli to penicillins. 3. AmpB, a locus affecting episomally and chromosomally mediated resistance to ampicillin and chlorampheincol. Genet Res. 1968 Oct;12(2):157–168. doi: 10.1017/s0016672300011770. [DOI] [PubMed] [Google Scholar]
- Nordström K., Molin S., Aagaard-Hansen H. Partitioning of plasmid R1 in Escherichia coli. I. Kinetics of loss of plasmid derivatives deleted of the par region. Plasmid. 1980 Sep;4(2):215–227. doi: 10.1016/0147-619x(80)90011-6. [DOI] [PubMed] [Google Scholar]
- Nordström K., Molin S., Aagaard-Hansen H. Partitioning of plasmid R1 in Escherichia coli. II. Incompatibility properties of the partitioning system. Plasmid. 1980 Nov;4(3):332–339. doi: 10.1016/0147-619x(80)90071-2. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Hoppensteadt F. C. On plasmid incompatibility. Plasmid. 1978 Sep;1(4):421–434. doi: 10.1016/0147-619x(78)90001-x. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- Reithmeier R. A., Bragg P. D. Molecular characterization of a heat-modifiable protein from the outer membrane of Escherichia coli. Arch Biochem Biophys. 1977 Jan 30;178(2):527–534. doi: 10.1016/0003-9861(77)90223-5. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Rosner J. L., Kass L. R., Yarmolinsky M. B. Parallel behavior of F and Pl in causing indirect induction of lysogenic bacteria. Cold Spring Harb Symp Quant Biol. 1968;33:785–789. doi: 10.1101/sqb.1968.033.01.090. [DOI] [PubMed] [Google Scholar]
- So M., Gill R., Falkow S. The generation of a ColE1-Apr cloning vehicle which allows detection of inserted DNA. Mol Gen Genet. 1975 Dec 30;142(3):239–249. doi: 10.1007/BF00425649. [DOI] [PubMed] [Google Scholar]
- Som T., Sternberg N., Austin S. A nonsense mutation in bacteriophage P1 eliminates the synthesis of a protein required for normal plasmid maintenance. Plasmid. 1981 Mar;5(2):150–160. doi: 10.1016/0147-619x(81)90016-0. [DOI] [PubMed] [Google Scholar]
- Wolf-Watz H., Masters M. Deoxyribonucleic acid and outer membrane: strains diploid for the oriC region show elevated levels of deoxyribonucleic acid-binding protein and evidence for specific binding of the oriC region to outer membrane. J Bacteriol. 1979 Oct;140(1):50–58. doi: 10.1128/jb.140.1.50-58.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Norqvist A. Deoxyribonucleic acid and outer membrane: binding to outer membrane involves a specific protein. J Bacteriol. 1979 Oct;140(1):43–49. doi: 10.1128/jb.140.1.43-49.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Burgi E. Properties of a membrane-attached form of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):91–105. doi: 10.1016/0022-2836(74)90576-2. [DOI] [PubMed] [Google Scholar]



