Abstract
Background
Repetitive transcranial magnetic stimulation (rTMS) was approved in 2008 in the United States, and there are relatively few studies describing its use in regular clinical practice since approval.
Methods
From April 2011 to October 2014, ten sites within the National Network of Depression Centers (NNDC) provided data on 62 evaluable patients with a depressive episode. Treatment was determined naturalistically. Response was assessed by the Quick Inventory of Depressive Symptoms, Self-Report (QIDS-SR) as the primary outcome, and the Patient Health Questionnaire-9 (PHQ-9) and the clinician-rated Clinical Global Impression (CGI) as secondary depression measures.
Results
Enrolled patients exhibited significant treatment resistance, with 70.2% reporting more than 4 prior depressive episodes. Most patients received treatment with standard parameters (10 Hz over the left dorsolateral prefrontal cortex), although 22.6 % of the patients received 1 or 5 Hz stimulation at some point. Over 6 weeks of treatment, response and remission rates were 29.4% and 5.9%, respectively, for the QIDS-SR; 39.2% and 15.7%, respectively, for the PHQ-9; and 50.9% and 17.9%, respectively, for the CGI. Moderator analyses revealed no effect of prior depressive episodes, history of ECT or gender, although early life stress predicted a better response to rTMS therapy.
Limitations
The study was an open-label, registry trial, with relatively coarse clinical data, reflecting practice only in academic, depression-specialty centers. Because of the relatively small size and heterogeneity of the sample, type 2 errors are possible and positive findings are in need of replication.
Conclusion
rTMS demonstrates effectiveness in clinical practice within the NNDC, although remission rates appear slightly lower in comparison with other recent naturalistic studies.
Keywords: dorsolateral prefrontal cortex, neuromodulation, registry, brain
1. Introduction
Major depressive disorder (MDD) is one of the most common psychiatric disorders, with an annual incidence of around 7% (Kessler et al., 2005). Repetitive transcranial magnetic stimulation (rTMS) has been studied as a treatment for MDD for more than 20 years, and meta-analytic studies have demonstrated superiority for rTMS over sham stimulation in randomized controlled trials (Allan et al., 2011; Berlim et al., 2014; Gross et al., 2007; Herrmann and Ebmeier, 2006; Kozel and George, 2002; Lam et al., 2008; Schutter, 2010; Slotema et al., 2010; Xie et al., 2013), identifying effect sizes in the range of 0.4 – 0.6. The FDA cleared the first rTMS device for the treatment of depression in 2008, and three more under the 510(k) mechanism since 2008. In controlled clinical trials, response and remission rates have ranged around 25–50% and 12 – 35%, respectively (Allan et al., 2011; Berlim et al., 2014; Gross et al., 2007; Herrmann and Ebmeier, 2006; Kozel and George, 2002; Lam et al., 2008; Schutter, 2010; Slotema et al., 2010; Xie et al., 2013). However, since the beginning of rTMS research, the length and intensity of treatment have increased, which has been associated with a more robust therapeutic effect (Gross et al., 2007). Since initial FDA clearance, only a few studies have examined therapeutic response in actual clinical settings using the parameters under which the treatment was approved (>3000 pulses/session, minimum of 20 sessions, goal of 120% motor threshold). One study of 85 patients from a single academic center reported response and remission rates of 50.6% and 24.7%, respectively (Connolly et al., 2012), while a multi-site, naturalistic study of 307 patients (Carpenter et al., 2012), mostly in non-academic settings, found clinician-rated response and remission rates of 58% and 37%, respectively. However, with limited data, it remains an open question as to how rTMS is faring in regular clinical practice.
Although randomized, sham-controlled studies are necessary to establish efficacy for a therapy, the results of controlled trials may not generalize to clinical practice, which includes greater variation in patient characteristics, medications and rTMS treatment parameters than clinical trials. For patients considering whether or not to undergo treatment with rTMS, response rates in open-label trials, which combine both intrinsic treatment effects as well as placebo expectations, are the best guide to determine anticipated response rates. Therefore, to meet the critical need for naturalistic studies that reflect the evolving state of treatment in practice, the National Network of Depression Centers (NNDC) sponsored a registry study of rTMS therapy for depression. The NNDC is a 22-site consortium of centers focused on depressive disorders, all located in academic medical centers. Ten centers with rTMS operations participated in the registry. The goals of the study were three-fold: 1) characterize the variation in rTMS therapy in regular clinical practice, 2) measure response and remission rates, and 3) explore moderators of treatment response in a naturalistic, clinical setting.
2. Methods
2.1 Subjects
From April 2011 through October 2014, 81 subjects consented to participate in the registry study. Patients were recruited from amongst those who had sought clinical treatment with TMS in one of the participating centers. Eligibility criteria were minimal to encourage a sample representative of standard clinical practice in a tertiary care center: 1) receiving rTMS for their depressive disorder, as determined by their attending psychiatrist; 2) at least 18 years of age; 3) literate in English; 4) without a diagnosis of schizophrenia or schizoffective disorder; and 5) no contraindications to receiving rTMS. rTMS parameters and length of treatment were determined by the treating clinician, working with the patient. The cost of rTMS treatment was born by patients or their insurance companies. Other treatments, such as pharmacotherapy and psychotherapy, varied naturalistically. All subjects signed an informed consent document, describing the risks and benefits of participating in the registry, as approved by the local Institutional Review Board of each site.
2.2 Outcome measures
Standard measures were used to track outcome, including the following self-report measures: 1) Quick Inventory of Depressive Symptomatology - Self-Report (QIDS-SR): a 16-item patient self report measure which assesses the 9 DSM-IV symptom criteria for a major depressive episode (Rush et al., 2003); 2) Patient Health Questionnaire-9 (PHQ-9): a 9-item depression symptom severity scale (Gilbody et al., 2007); 3) Work and Social Adjustment Scale (WSAS): a 5-item scale assessing the impact of the patient’s symptoms on work and home life (Mundt et al., 2002); 4) Generalized Anxiety Disorder Assessment (GAD-7): a 7-item scale assessing the degree of anxiety experienced by the patient (Spitzer et al., 2006); 5) Adverse Childhood Experiences (ACE): a 10-item self-report scale assessing potentially traumatic experiences during childhood (Chapman et al., 2004). Patients also completed a 10-point pain intensity rating, and the Columbia Suicide Severity Rating Scale (Posner et al., 2007), adapted for self-report. Clinicians completed the Clinical Global Impression scale (CGI; severity, CGI-S, and change, CGI-I), single-item global ratings of severity and improvement with treatment (Guy and Bonato, 1970).
The registry was designed to acquire ratings at the initiation of rTMS treatment, and then for every week thereafter for the course of treatment. The QIDS-SR was the primary outcome measure, and the other measures were treated as secondary outcomes. Information about diagnosis, prior treatment and treatment response was based on the clinician’s interview and best judgment.
2.3 Analysis
Analysis included categorical and continuous measures of change on the primary and secondary outcome measures. Change was measured in the first 6 weeks of treatment, the standard endpoint for other rTMS studies in depression (Carpenter et al., 2012; Connolly et al., 2012; O’Reardon et al., 2007). Some patients had no treatments after baseline measurement; others had only 2 or 3 weeks of treatment, but a majority had at least 4 weeks of treatment and assessments. We excluded subjects from this analysis who had less than 4 weeks of assessments after baseline for reasons clearly not related to treatment, such as becoming lost to follow-up, moving away or withdrawing consent to participate in the registry. The remaining patients were included in an intent-to-treat analysis for QIDS-SR and PHQ-9 scores, including patients with less than 4 weeks of treatment, but at least one assessment after baseline. All-cause discontinuation of therapy, relevant to delivering the therapy, before 4 weeks includes important information, such as treatment non-response, logistical burden of daily sessions or the cost of treatment (often born by the patient). Hence, these data points were included.
For categorical responses, we defined treatment response as the following: a 50% or greater drop in QIDS-SR or PHQ-9 scores, or a CGI-I score ≤ 2 (“Much improved”), on the last assessment in 6 weeks of treatment. Remission was defined as a QIDS-SR or PHQ-9 score < 5 or CGI-S score as ≤ 2 (‘Borderline mentally ill”), from the last observation in 6 weeks of treatment. For the QIDS-SR and PHQ-9 analysis, patients had to have a baseline score > 5.
For measurement of continuous change, we employed a mixed-model, multiple regression with random coefficients and AR(1) structure, modeling weekly change from baseline with baseline rating as a co-variate. This model was run for the primary and secondary self-report measures. In a moderator analysis of self-report depression measures (QIDS-SR and PHQ-9), we examined the effects of gender, number of prior depressive episodes, failed antidepressant courses, prior history of ECT, ACE scores and stimulation site. Since the number of observations varied between subjects, this mixed approach incorporated all observations by first modeling symptom change at the subject level, and then between subjects.
3. Results
3.1 Subjects entered into analysis
There were 62 evaluable patients from the 81 patients enrolled in the study (Figure 1). The number of evaluable subjects from each site varied from 0 – 21, with a median of 5. As can be seen in Table 1, 88.1% of the sample had a primary diagnosis of MDD. Secondary diagnoses included: anxiety disorder NOS (3.4%), generalized anxiety disorder (5.1%), dysthymia (5.1%), alcohol dependence (3.4%), eating disorder NOS (5.1%), psychosis NOS (1.7%), cognitive disorder NOS (3.4%) and attention deficit disorder NOS (1.7%). Of the patients for whom information was recorded (n=50), all were taking psychotropic medication.
Figure 1.
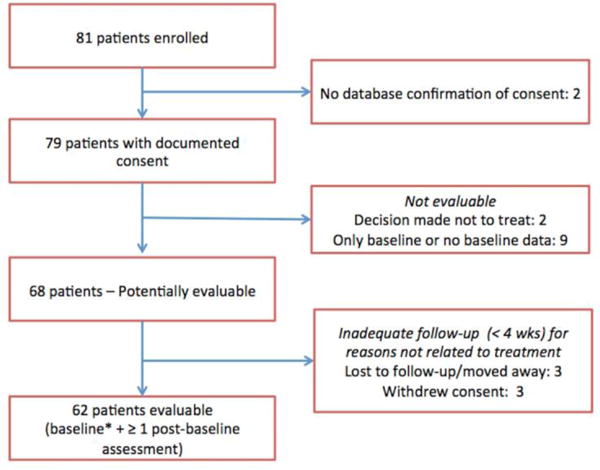
CONSORT diagram depicts enrollment of subjects in the registry.
Table 1.
Demographic characteristics of the sample (n = 62)
| Variable | Value |
|---|---|
| Age, Mean ± SD, (Min – Max) | 48.2 ± 16.8, (20.9 – 78) |
| Gender, % female | 61.3% |
| Race | |
| Asian/Asian American | 3.3% |
| Black/African American | 1.7% |
| White | 95% |
| Education | |
| Advanced/Professional degree | 36.1% |
| Bachelors degree | 37.9% |
| High school grad or GED | 6.6% |
| Some college | 26.2% |
| Some high school | 0% |
| Technical degree/Associates degree | 3.3% |
| Primary diagnosis | |
| Major Depressive Disorder | 88.1 % |
| Bipolar disorder, depressed | 6.8% |
| Bipolar II disorder | 3.4% |
| Mood disorder due to a medical condition | 1.7% |
| Past Psychiatric Hospitalizations | |
| 0 | 46.7% |
| 1 | 4.1% |
| 2 | 37% |
| 3 | 11.1% |
| 4 or more | 11.1% |
| Number of Past Episodes of Depression | |
| 1 | 7% |
| 2 – 4 | 22.8% |
| 5 – 12 | 43.9% |
| 12+ | 26.3% |
| Previous antidepressant therapy |
As Table 2 shows, average baseline depression scores were in the moderate-severe range, although some subjects entered with relatively low QIDS-SR and PHQ-9 scores. Seven patients had QIDS-SR or PHQ-9 scores less than 10.
Table 2.
Baseline symptoms
| Measure | N* | Mean ± SD, (Min – Max) |
|---|---|---|
| CGI-S | 56 | 4.7 ± 0.76, (3–6) |
| ACE | 56 | 2.3 ± 2.1, (0–9) |
| PHQ-9 | 57 | 17.1 ± 5.5, (4 – 27) |
| QIDS -SR | 58 | 15.4 ± 4.7, (3 – 27) |
| GAD_7 | 57 | 11.2 ± 5.6, (0 – 20) |
| WSAS | 56 | 28.4 ± 8.1, (6 – 40) |
Number of assessments available, which is less than the total number of 62 evaluable cases because of missing data
3.2 Delivery of rTMS
Treatment parameters varied across sites. Only 2 sites used an air-cooled figure-8 coil (MagPro, MagVenture, Inc), while the other sites used an iron-core, figure-8 coil (NeuroStar, Neuronetics, Inc). Motor threshold was obtained by visual assessment of movements in the right fingers/thumb for all sites except one, which used electromyography to measure motor evoked potentials. Four sites delivered stimulation 5.0/5.5 cm anterior to motor cortex (George et al., 1997), and 4 sites used the F3 scalp landmark in the 10–20 system (Beam et al., 2009). One site used both methods, in different patients. Stimulation was delivered at 10 Hz to the left prefrontal target, with the following 13 exceptions: 1 Hz to right frontal (3 cases, 1 started out with 10 Hz on the left), 10 Hz on the left and1 Hz on the right (9 cases, of which 8 started with 10 Hz on the left only), 5 Hz on the left (1 case, started with 10 Hz). In general, switches to lower frequencies occurred after the first 10 Hz treatment, whereas switches to combined 1/10 Hz treatment occurred after 7–15 treatments. Stimulation intensity ranged from 80% to 120% of motor threshold. At the initiation of therapy, 41% (n=58) of subjects had stimulation less than 120%, a number that diminished to 19% by treatment #5, but was still 14% at treatment #15 (Table 3). Number of pulses per session for 10 Hz ranged from 3000 – 6000. In the first week of treatment, the mean was 3302 pulses (79% @ 3000, 11% at 4000, 9% @ 5000), which increased to 3951 pulses at week 3 (45% @ 3000, 17% @ 3550–4300, 36% @5000, 2% @ 6000) and 4489 pulses by the 6th week of treatment (18% @ 3000, 14% @ 4000, 68% @ 4700–5000). Number of pulses for 1 Hz treatment ranged from 400 – 1800, with a mean of 1377, and it tended not to increase.
Table 3.
rTMS stimulation intensity increases over treatment sessions
| Treatment # | 1 | 2 | 3 | 4 | 5 | 10 | 15 |
|---|---|---|---|---|---|---|---|
| Mean % of MT* (S.D.) | 109.8 (14.3) | 113.2 (11.5) | 115.4 (9.4) | 116.1 (9.3) | 116.7 (8.4) | 118.1 (5.0) | 118.6 (3.7) |
| % of subjects @ <120% MT | 41% | 36% | 27% | 23% | 19% | 15% | 14% |
| N | 58 | 59 | 52 | 52 | 47 | 48 | 36 |
MT = motor threshold
There was wide variability in the number of treatment sessions delivered. The mean number of sessions was 26.1 (S.D. = 13.7), with a range of 1 – 77 sessions. The 25th percentile was 17 sessions and the 75th percentile was 33 sessions. Three subjects had missing treatment data.
3.3 Categorical response and remission rates
Response and remission rates for the evaluable participants are depicted in Figure 2, for QIDS-SR, PHQ-9 and CGI scales. The highest response and remission rates were elicited by the clinician-rated CGI (50.9% and 17.9%, respectively), whereas the lowest were elicited by the self-rated QIDS-SR (29.4% and 5.9%, respectively), with PHQ-9 in between (39.2% and 15.7%, respectively). The categorical response and remission analysis included 6 subjects with less than 4 weeks of assessments, which may have biased the results (including subjects who went on to improve by 4 weeks, but for whom additional assessments were missing). However, excluding those subjects only changed response/remission rates by a few percentage points. Given the difference in response rates for the self-rated measures compared to the clinician rated CGI, we ran correlations between baseline values for these scales, and found that PHQ-9 and QIDS-SR correlated strongly with each other (r = 0.78, p < 0.0000), whereas correlations of these ratings with the CGI-S were weaker (r = 0.47, p < 0.001; r = 0.38, p < 0.01, respectively).
Figure 2.
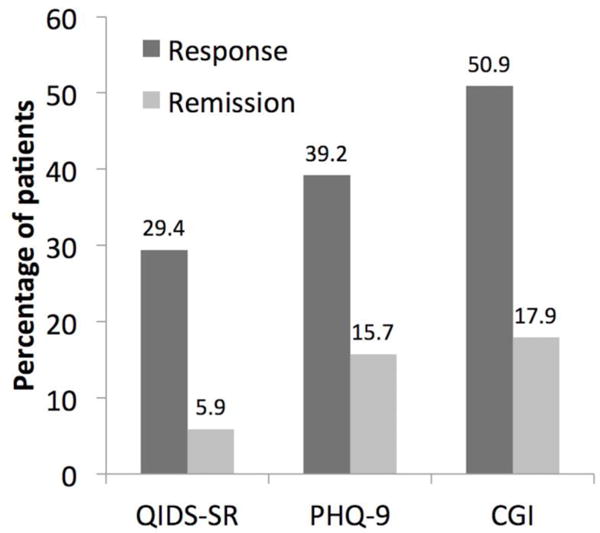
Categorical response rates (numeric % above each bar graph) for primary outcome measures. Fifty-one subjects were evaluated for PHQ-9 and QIDS-SR response/remission, whereas 55 subjects were evaluated for CGI response and 56 for CGI remission. See text for definitions of remission and response.
3.4 Regression analysis of depression outcome measures and moderators
Symptoms scores decreased with treatment – an average weekly drop of 0.7 and 1.09 points on the QIDS-SR and PHQ-9 scales, respectively (Table 3). As expected, subjects with higher baseline scores showed a larger improvement. Moderator analyses showed no significant effects of gender, prior history of ECT or site of treatment (F3 or 5.0/5.5 centimeter rule). The number of prior depressive episodes showed only a trend effect for PHQ-9 scores, driven by an apparent reduced response for patients who had 2–4 prior episodes. There was no effect of prior episodes on QIDS-SR change. Since the categorical variable for failed antidepressant treatments yielded almost 90% of our patients in the category of 2 or more failed treatments, it was not possible to include this variable in the moderator analysis.
We found an effect of ACE scores, such that higher scores were associated with a larger drop – 0.51 on both PHQ-9 and QIDS-SR scales for each point on the ACE. In order to exclude the possibility that gender influenced this relationship (ACE scores were higher in women), we added gender to the regression analysis, which reduced the significance levels somewhat for QIDS-SR (p = 0.012) and PHQ-9 (p = 0.052) change.
3.5 Regression analysis of anxiety and functional outcome measures
Analysis of non-depression, secondary measures showed a similar pattern as the depression scales, with decreasing anxiety, measured by the GAD-7 (0.92 points/week, p<0.0001) and improvement of functioning, as measured by the WSAS (1.3 points/week, p=0.0003). However, for the pain scale, there was only a trend to improvement (p=0.06).
3.6 Safety and tolerability
There were no serious adverse events reported and specifically, no reports of seizures, a rare, but known complication of rTMS therapy (Rossi et al., 2009). The treatments were well tolerated. Although most patients report that initial stimulation is painful, only the following was noted by patient report significant enough to be registered on a standardized assessment form (percentages of sessions): 3.7% for persistent headache, 1.8% for toothache, 1.2% of other pain and 0.2% for eye pain.
4. Discussion
This is the first multi-site, naturalistic study of rTMS treatment for depression in academic centers following FDA clearance of rTMS. Patients treated at centers of the NNDC exhibited significant response and remission rates, demonstrating clinical benefit. Treatment parameters, while generally adhering to the manufacturer-recommended dosing, showed significant variation amongst the sites. Only 5% of the sample met the originally approved FDA-indication for rTMS (since changed) of a reported failure to respond to a single antidepressant treatment. A few findings deserve comment.
4.1 Response to treatment
Response and remission rates were comparable to those reported in the literature, with some differences. As mentioned above, meta-analytic studies have reported a fairly wide range of response and remission rates – 25–50% and 12 – 35%, respectively (Allan et al., 2011; Berlim et al., 2014; Gross et al., 2007; Herrmann and Ebmeier, 2006; Kozel and George, 2002; Lam et al., 2008; Schutter, 2010; Slotema et al., 2010; Xie et al., 2013). More appropriate is the comparison to similar naturalistic studies, where our response/remission rates numbers are comparable, particularly for clinician-reported outcome. Both Connelly and colleagues (Connolly et al., 2012) and Carpenter and colleagues (Carpenter et al., 2012) reported CGI response rates of 50.6% and 58.0%, respectively, nearly identical to our data. Our remission rates were slightly lower, as were the self-report measures of change on the PHQ-9 and QIDS-SR, on which these other studies reported 56.4% and 28.7% response and remission rates on the PHQ-9 (Carpenter et al., 2012), and 38.4% and 24.7% response and remission rates on the QIDS-SR (Connolly et al., 2012). The reason for this difference is unclear, although our analysis, which included subjects with several missing assessment points, may have under-estimated treatment response. At baseline, all three samples exhibited comparable symptom severity and demographics, except for more treatment resistance in our sample, where nearly 90% reported at least 2 failures of antidepressant treatment, compared to 54% in the Carpenter sample, and over 70% had more than 4 prior episodes. The percentage of patients with prior ECT was higher in our sample (31.7%) compared to the Carpenter sample (5.2%). However, we found no effect of prior ECT, and the Connelly study, with a similar proportion of patients receiving ECT (28.2%), also found no effect of prior ECT. Prior treatment resistance has been identified as a predictor of poor response to rTMS (Lisanby et al., 2009), although both the Connelly and Carpenter studies did not replicate this finding.
The differences in the response and remission rates on the self-rated PHQ-9 and QIDS-SR versus the clinician-rated CGI were striking, although perhaps not unexpected. Correlations between CGI and PHQ-9 have been rather modest, with one report of nearly 1800 patients finding correlations from 0.44 to 0.56 (Lowe et al., 2006), similar to what was found in our sample. The most obvious source of error is a bias from clinicians in an open-label study that their patients are improving with the treatment. The different scales may also pick up different aspects of depression, with differential sensitivity to treatment.
4.2 Treatment parameters
As one might expect, treatment parameters in actual practice varied significantly, and this variation provides a useful snapshot of treatment delivery at the time of the study. Practitioners in our study tended to use more stimulation than the manufacturer-recommended dosing of 3000 pulses/session, adjusting this upward with more sessions. While this practice is safe for patients (Hadley et al., 2011), meta-analytic studies have not shown a better response with more than 3000 pulses/session (Berlim et al., 2014). The other notable deviation from the manufacturer-recommending dosing is the selection of less than 120% of MT for stimulation intensity. This intensity is the maximum level allowed for safety reasons (Wassermann, 1998), but many patients have difficulty tolerating this level of stimulation, particularly at the initiation of therapy. Thus, as our data show, clinicians often start with lower intensities, and then ramp up to 120% as patients habituate to the sensation of pain. Even with this strategy, around 15% of the patients in our sample never received 120% of MT.
Several of the patients in the study received 1 Hz (low frequency; LF) stimulation, either exclusively, or in combination with 10 Hz (high frequency; HF). The FDA ‘label’ (users manual) for rTMS specifies HF stimulation (10 Hz) to the left prefrontal cortex, although open-label LF stimulation to the right prefrontal cortex induced remission in 26% of patients who failed to remit with left-sided, HF stimulation in a large, controlled study (McDonald et al., 2011). A recent meta-analysis found similar effects of both LF and HF (Chen et al., 2013) although recent guidelines released by a European group designated LF stimulation a Level B recommendation, in distinction to the Level A recommendation for HF stimulation (Lefaucheur et al., 2014). Patients in our study tended to receive combination HF/LF therapy in the same session, but a recent meta-analysis found no advantage for bilateral, combined treatment (Berlim et al., 2013). Another reason to consider LF stimulation is greater tolerability. For example, one patient in our study started at HF, but then switched to LF stimulation for the remainder of the treatment sessions.
4.3 Moderator analysis
There has been significant interest in moderators or predictors of response to TMS treatment (Fidalgo et al., 2014). We found that more childhood adversity (higher ACE scores) predicted better response. Although early life stress predicts a poor response to pharmacotherapy (Miniati et al., 2010; Tyrka et al., 2013), it has been associated with a better response to cognitive behavioral therapy of depression (Kuyken et al., 2015; Niciu et al., 2015). Prior work has not examined the effect of rTMS on patients with early life stress, and these data, while preliminary, raise the possibility that rTMS may be a better treatment for such individuals. However, with the small numbers of patients in our study, this finding is in need of replication.
4.4 Limitations
Several limitations should be kept in mind. This was an unmonitored registry, with missing assessments, evident in the different numbers of subjects available for analysis for each outcome measure. To minimize the burden of data collection, instruments to measure clinical factors, such as prior history, were coarse-grained, relying mostly on the impressions of the treating clinician. Thus, the moderator analyses for some of these measures did not have high sensitivity. The sample size was relatively small, and type 2 errors are always a possibility. There was considerable variation between sites in treatment parameters, number of treatment sessions and numbers of patients enrolled, introducing some heterogeneity into the analysis. Informal polling of site investigators suggested that small recruitment numbers were due to a scarcity of patients receiving rTMS treatment, rather than low enrollment rates in the registry. The low volumes of rTMS treatment at the time of the registry enrollment reflected the fact that most third party payers did not reimburse for this treatment when patients were enrolling. Lastly, because all of the NNDC sites were located in academic centers, specializing in the treatment of depression, results may not be generalizable to community practice.
4.5 Conclusions
In conclusion, the results demonstrate a significant clinical benefit of rTMS in academic medical centers where patients tend to be slightly more ill than those in the community. These data present a snapshot of current clinical practice, but treatment parameters will likely evolve as additional research provides new guidance about the clinical use of rTMS. Towards that end, the study also highlights the importance of using measurement based care in standard treatment, as well as the value of registries to provide valuable feedback about the effectiveness of therapies used in actual clinical settings.
Table 4.
Primary Outcomes
| Variable | Change in QIDS-SR (primary outcome) | Change in PHQ-9 | ||||
|---|---|---|---|---|---|---|
| beta (SE[beta]) | df | p-value | beta (SE[beta]) | df | p-value | |
| Week | −0.7704 (0.1730) | 1, 50 | <0.0001 | −1.0944 (0.2098) | 1, 48 | <0.0001 |
| Baseline | −0.3293 (0.07670) | 1, 107 | <0.0001 | −0.2040 (0.07981) | 1, 108 | 0.012 |
| Gender – Female* | −0.9643 (0.7753) | 1, 107 | 0.2163 | −1.1525 (0.9752) | 1, 108 | 0.2399 |
| Past depressive episodes* | 3, 100 | 0.4505 | 3, 101 | 0.088 | ||
| 1 | −0.09541 (1.4641) | 0.9482 | 0.3031 (1.7871) | 0.8657 | ||
| 2–4 | 1.5741 (1.0529) | 0.1381 | 3.0741 (1.3178) | 0.0216 | ||
| 5–12 | 0.5900 (0.9576) | 0.5392 | 0.6042 (1.1869) | 0.6118 | ||
| 12+ | Referent | Referent | ||||
| ECT* | −1.0825 (0.7802) | 1, 104 | 0.1683 | 0.5326 (0.9648) | 1, 105 | 0.5821 |
| ACE* | −0.5112 (0.1702) | 1, 106 | 0.0033 | −0.5147 (0.2096) | 1, 107 | 0.0157 |
| Stimulation site* | 0.6130 (0.8327) | 1, 51 | 0.4650 | −0.9038 (1.0322) | 1, 51 | 0.3853 |
With week and baseline as co-variates
Abbreviations: PHQ-9 = Patient Health Questionnaire-9; QIDS-SR = Quick Inventory of Depressive Symptoms, Self-Rating; ECT = electroconvulsive therapy; ACE = Adverse Childhood Experiences
Highlights.
A snapshot of the practice of TMS for major depression in academic centers
Response and remission rates on self-rated scale 29.4% and 5.9%, respectively
Response and remission rates on clinician-rated scale 50.9% and 17.9%, respectively
Early life stress was a predictor of better TMS response
Acknowledgments
Funding Support: This research was supported by the National Network of Depression Centers, the University of Michigan Department of Psychiatry and the Michigan Institute for Clinical and Health Research (UL1TR000433).
Role of the sponsors: The sponsors of this study provided resources and support for the design, analysis, interpretation and reporting of these results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous presentation: This work has been previously presented at the 69th annual meeting of the Society of Biological Psychiatry, May 2013, San Francisco, CA
Potential conflicts of interest: Dr. Taylor has received research support from Neuronetics and St.Jude Medical; Dr. Bahti has received research support from Medtronic, Cyberonics, and NeoSync; Dr. Dubin has no conflicts; Dr. Hawkins has no conflicts; Dr. Lisanby has received research support from Brainsway, NexStim, NeoSync, Magstim and Magventure, and she is listed as an inventor (no royalties) on a patent on TMS technology, owned by Columbia University; Dr. Morales has received research support from Brainsway and he is listed as an inventor (no royalties) on a patent, “Intracranial Electrical Seizure Therapy (ICEST),” owned by McLean Hospital; Dr. Reti has received research support from Neuronetics and Brainsway; Dr. Sampson has received research support from Neuronetics; Dr. Short has no conflicts; Dr. Spino has no conflicts; Dr. Watcharotone has no conflicts; Dr. Wright has an equity interest in Empower Interactive and Mindstreet LLC and he receives book royalties from American Psychiatric Publishing, Inc, Guilford Press, and Simon and Schuster.
References
- Allan CL, Herrmann LL, Ebmeier KP. Transcranial magnetic stimulation in the management of mood disorders. Neuropsychobiology. 2011;64:163–169. doi: 10.1159/000328951. [DOI] [PubMed] [Google Scholar]
- Beam W, Borckardt JJ, Reeves ST, George MS. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul. 2009;2:50–54. doi: 10.1016/j.brs.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlim MT, Van den Eynde F, Daskalakis ZJ. A systematic review and meta-analysis on the efficacy and acceptability of bilateral repetitive transcranial magnetic stimulation (rTMS) for treating major depression. Psychol Med. 2013;43:2245–2254. doi: 10.1017/S0033291712002802. [DOI] [PubMed] [Google Scholar]
- Berlim MT, van den Eynde F, Tovar-Perdomo S, Daskalakis ZJ. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44:225–239. doi: 10.1017/S0033291713000512. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Janicak PG, Aaronson ST, Boyadjis T, Brock DG, Cook IA, Dunner DL, Lanocha K, Solvason HB, Demitrack MA. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29:587–596. doi: 10.1002/da.21969. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou C, Wu B, Wang Y, Li Q, Wei Y, Yang D, Mu J, Zhu D, Zou D, Xie P. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res. 2013;210:1260–1264. doi: 10.1016/j.psychres.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Connolly RK, Helmer A, Cristancho MA, Cristancho P, O’Reardon JP. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. The Journal of clinical psychiatry. 2012;73:e567–573. doi: 10.4088/JCP.11m07413. [DOI] [PubMed] [Google Scholar]
- Fidalgo TM, Morales-Quezada JL, Muzy GS, Chiavetta NM, Mendonca ME, Santana MV, Goncalves OF, Brunoni AR, Fregni F. Biological markers in noninvasive brain stimulation trials in major depressive disorder: a systematic review. J ECT. 2014;30:47–61. doi: 10.1097/YCT.0b013e31828b34d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Wassermann EM, Kimbrell TA, Little JT, Williams WE, Danielson AL, Greenberg BD, Hallett M, Post RM. Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. Am J Psychiatry. 1997;154:1752–1756. doi: 10.1176/ajp.154.12.1752. [DOI] [PubMed] [Google Scholar]
- Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007;22:1596–1602. doi: 10.1007/s11606-007-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M, Nakamura L, Pascual-Leone A, Fregni F. Has repetitive transcranial magnetic stimulation (rTMS) treatment for depression improved? A systematic review and meta-analysis comparing the recent vs. the earlier rTMS studies. Acta psychiatrica Scandinavica. 2007;116:165–173. doi: 10.1111/j.1600-0447.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- Guy W, Bonato R. CGI:Clinical Global Impressions. National Institute of Mental Health; Chevy Chase, MD: 1970. [Google Scholar]
- Hadley D, Anderson BS, Borckardt JJ, Arana A, Li X, Nahas Z, George MS. Safety, tolerability, and effectiveness of high doses of adjunctive daily left prefrontal repetitive transcranial magnetic stimulation for treatment-resistant depression in a clinical setting. J ECT. 2011;27:18–25. doi: 10.1097/YCT.0b013e3181ce1a8c. [DOI] [PubMed] [Google Scholar]
- Herrmann LL, Ebmeier KP. Factors modifying the efficacy of transcranial magnetic stimulation in the treatment of depression: a review. J Clin Psychiatry. 2006;67:1870–1876. doi: 10.4088/jcp.v67n1206. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel FA, George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract. 2002;8:270–275. doi: 10.1097/00131746-200209000-00003. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Hayes R, Barrett B, Byng R, Dalgleish T, Kessler D, Lewis G, Watkins E, Brejcha C, Cardy J, Causley A, Cowderoy S, Evans A, Gradinger F, Kaur S, Lanham P, Morant N, Richards J, Shah P, Sutton H, Vicary R, Weaver A, Wilks J, Williams M, Taylor RS, Byford S. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet. 2015;386:63–73. doi: 10.1016/S0140-6736(14)62222-4. [DOI] [PubMed] [Google Scholar]
- Lam RW, Chan P, Wilkins-Ho M, Yatham LN. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Can J Psychiatry. 2008;53:621–631. doi: 10.1177/070674370805300909. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipovic SR, Hummel FC, Jaaskelainen SK, Kimiskidis VK, Koch G, Langguth B, Nyffeler T, Oliviero A, Padberg F, Poulet E, Rossi S, Rossini PM, Rothwell JC, Schonfeldt-Lecuona C, Siebner HR, Slotema CW, Stagg CJ, Valls-Sole J, Ziemann U, Paulus W, Garcia-Larrea L. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin Neurophysiol. 2014;125:2150–2206. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Husain MM, Rosenquist PB, Maixner D, Gutierrez R, Krystal A, Gilmer W, Marangell LB, Aaronson S, Daskalakis ZJ, Canterbury R, Richelson E, Sackeim HA, George MS. Daily Left Prefrontal Repetitive Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: Clinical Predictors of Outcome in a Multisite, Randomized Controlled Clinical Trial. Neuropsychopharmacology. 2009;34:522–534. doi: 10.1038/npp.2008.118. [DOI] [PubMed] [Google Scholar]
- Lowe B, Schenkel I, Carney-Doebbeling C, Gobel C. Responsiveness of the PHQ-9 to Psychopharmacological Depression Treatment. Psychosomatics. 2006;47:62–67. doi: 10.1176/appi.psy.47.1.62. [DOI] [PubMed] [Google Scholar]
- McDonald WM, Durkalski V, Ball ER, Holtzheimer PE, Pavlicova M, Lisanby SH, Avery D, Anderson BS, Nahas Z, Zarkowski P, Sackeim HA, George MS. Improving the antidepressant efficacy of transcranial magnetic stimulation: maximizing the number of stimulations and treatment location in treatment-resistant depression. Depress Anxiety. 2011;28:973–980. doi: 10.1002/da.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniati M, Rucci P, Benvenuti A, Frank E, Buttenfield J, Giorgi G, Cassano GB. Clinical characteristics and treatment outcome of depression in patients with and without a history of emotional and physical abuse. J Psychiatr Res. 2010;44:302–309. doi: 10.1016/j.jpsychires.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry. 2002;180:461–464. doi: 10.1192/bjp.180.5.461. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Abdallah CG, Fenton LR, Fasula MK, Black A, Anderson GM, Sanacora G. A history of early life parental loss or separation is associated with successful cognitive-behavioral therapy in major depressive disorder. J Affect Disord. 2015;187:241–244. doi: 10.1016/j.jad.2015.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, Demitrack MA, George MS, Sackeim HA. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Schutter DJ. Quantitative review of the efficacy of slow-frequency magnetic brain stimulation in major depressive disorder. Psychological medicine. 2010;40:1789–1795. doi: 10.1017/S003329171000005X. [DOI] [PubMed] [Google Scholar]
- Slotema CW, Blom JD, Hoek HW, Sommer IE. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? a meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010 doi: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Burgers DE, Philip NS, Price LH, Carpenter LL. The neurobiological correlates of childhood adversity and implications for treatment. Acta Psychiatr Scand. 2013;128:434–447. doi: 10.1111/acps.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Xie J, Chen J, Wei Q. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a meta-analysis of stimulus parameter effects. Neurological research. 2013;35:1084–1091. doi: 10.1179/1743132813Y.0000000245. [DOI] [PubMed] [Google Scholar]


