Abstract
Purpose of Review
In this review, we discuss current thinking in relation to available guidelines for the care of preschool-aged children with recurrent wheezing, while highlighting the gaps in our knowledge and discussing changes that could occur over the next 5 years.
Recent Findings
The Asthma Predictive Index (API) as well as allergen-specific IgE, peripheral eosinophil count, and exhaled nitric oxide are perhaps under-utilized sources of information that can assist in predicting progression to asthma and response to therapies. Inhaled corticosteroids (ICS) and leukotriene receptor antagonists (LTRA) decrease impairment and exacerbation frequency in wheezing children but are not disease modifying. Macrolides may be useful during acute wheezing episodes for preventing progression to more severe symptoms. Monoclonal antibodies targeting IgE and TH2 cytokines have been successful in trials of adults and older children with asthma, but trials in younger children are needed.
Summary
Establishing the phenotype and endotype of young wheezing children can be useful for prognostication of future asthma risk as well as for selection of the most appropriate treatment. Primary asthma prevention strategies are needed during the critical developmental window in early life prior to the onset of irrecoverable loss of lung function.
Keywords: wheezing, asthma, phenotype, endotype, prevention, monoclonal antibodies
Introduction
Asthma is an increasingly prevalent chronic disease among children worldwide and is associated with significant morbidity, mortality, and economic burden. Much research effort has focused on management strategies for established asthma, yet there are significant knowledge gaps in our understanding of young children with recurrent wheezing and progression to asthma. At the 2016 AAAAILife Spectrum of Asthma meeting, workshop groups were asked to discuss a case of an 18-month-old child with recurrent wheezing. This case presented a unique opportunity to assimilate current thinking in relation to available guidelines with significant changes that could occur over the next 5 years.
Case presentation
An 18-month-old male child has experienced four episodes of wheezing, some severe enough to require evaluation in an urgent care clinic and a course of oral corticosteroids. The child has a positive asthma predictive index (API). How would you counsel the parents who want to prevent their child from developing asthma and/or more severe symptoms?
What is this child’s risk of developing asthma?
Approximately 40% of children wheeze during the first year of life, but only one-third of children with recurrent wheezing will have asthma in later childhood (1, 2). In this section, we will discuss techniques to approximate risk of progression to asthma.
The Asthma Predictive Index
Originally developed from the Tucson Children’s Respiratory Study, the Asthma Predictive Index (API) is a well validated tool that uses major and minor criteria to predict which children with early wheezing will go on to have asthma (2–5). The presence of recurrent wheezing in the first 3 years of life plus one major or two minorclinical criteria is considered positive and associated with an OR of 9.8 [5.6–17.2] for asthma at age 6 years (Table 1)(5). Modified versions of the API have been developed and validated and include allergen sensitization as a criterion that increases risk of asthma by school-age (6–8).
Table 1.
Versions of the Asthma Predictive Index
| Criteria | Stringent API5 | Loose API5 | Modified API6, 7 |
|---|---|---|---|
| Wheezing | Early (≤3 years) frequent wheezing* | Early (≤3 years) wheezing | ≥4 episodes/yr during first 3 years of life |
| Major criteria | |||
| Parent with asthma | Yes | Yes | Yes |
| MD-diagnosed Atopic dermatitis | Yes | Yes | Yes |
| Sensitization to ≥1 aeroallergen | Not included | Not included | Yes |
| Minor criteria | |||
| Wheezing unrelated to colds | Yes | Yes | Yes |
| Blood eosinophils ≥4% | Yes | Yes | Yes |
| MD-diagnosed Allergic rhinitis | Yes | Yes | Not included |
| Sensitization to foods (milk, egg, peanut) | Not included | Not included | Yes |
| For a positive API in each version, children must meet the wheezing criterion as well as at least 1 major criterion or at least 2 minor criteria. | |||
Score of ≥3 on scale of 1–5 for wheezing (1 = ”very rarely”, 5 = ”most days”).
The Tucson Children’s Respiratory Study found that young children with persistent wheezing demonstrated reduced maximal expiratory flow at age 6 compared to children who never wheezed. A follow up of this study found that this pulmonary deficit persisted at age 16, suggesting that the insults resulting in reduced lung function occur in the first few years of life (9). The appeal of the API lies in its potential to capture high-risk children during this critical window before development of abnormal lung function so that targeted treatment can be initiated. The API has high specificity and negative predictive value but low sensitivity and positive predictive value, and therefore it cannot be used to definitively rule out future asthma development or predict asthma severity (3–5, 10). Despite its limitations, the API is a well-validated and easily applied screening tool to evaluate asthma risk in young wheezing children.
Allergen-specific IgE
Allergic sensitization appears to be a key player in asthma inception (11, 12), particularly aeroallergen sensitization early in life (13, 14). This relationship appears to be more complex than simply the presence or absence of atopy, with age at onset and type and number of aeroallergens playing an important role in asthma risk (15). Multiple studies have shown a significant relationship between sensitization to multiple allergens in early life and persistent wheezing, reduced lung function, and hospital admissions for asthma (15–19). Additionally, allergen sensitization appears to enhance the risk of rhinovirus-induced wheezing, and the combination of early sensitization and viral infection increases asthma risk (20–22). The National Asthma Education and Prevention Program (NAEPP) suggests that allergy testing be considered in young children with wheezing/asthma as the results can provide information about asthma risk and can impact asthma control (23).
Peripheral Blood Eosinophils
Persistent eosinophilia in early childhood is associated with asthma later in life (24), and blood eosinophils ≥4% is included as a minor criterion in the API (5). The Tucson Children’s Respiratory Study found a linear relationship between blood eosinophils and presence of asthma across the ages of 9 months to 11 years, independent of atopy, with the most chronic asthma found in the group with peripheral eosinophil counts greater than 5% (24). Similarly children under 2 with bronchiolitis and peripheral eosinophil count of >0.45 ×109 cells/L were at increased risk of asthma at ages 7 and 12 years (25, 26). The Individualized Therapy for Asthma in Toddlers (INFANT) study showed that children 12–59 months with blood eosinophils ≥ 300/µL and/or sensitization to at least one aeroallergen showed the most significant improvement in asthma control days and frequency of exacerbations when treated with daily inhaled corticosteroids (ICS) (27), suggesting that these biomarkers can predict steroid-responsiveness (28). Measuring peripheral eosinophilia may represent a minimally invasive surrogate for airway eosinophilia, though the strength of this correlation varies between studies and remains controversial (29, 30). Peripheral eosinophil count may be an under-utilized source of information that could aid in predicting a young wheezing child’s risk of asthma and response to treatment.
Exhaled nitric oxide (FeNO)
Elevated FeNO has been associated with eosinophilic airway inflammation in children, though like peripheral blood eosinophilia, the correlation between FeNO and airway eosinophilia is moderate and varies between studies (30–32). FeNO is increasingly used in both research and clinical care of asthma patients and can be performed by children as young as 4 years (33). FeNO level at 4 years of age is higher in persistent wheezers compared to transient wheezers and those who have never wheezed (34), and higher FeNO at age 4 is associated with greater risk of asthma at age 7 (14, 35). These findings have been replicated in several similar studies (36–39). Moreover, FeNO also appears to predict responsiveness to inhaled corticosteroids (40, 41), with higher FeNO predicting greater improvement in symptoms, lung function, and inflammatory markers in children with asthma treated with ICS compared to montelukast (28, 42). Use of FeNO in clinical practice is increasing and should be considered for establishing the endotype of airway disease and predicting steroid-responsiveness.
What interventions, if any, can impact the risk for and severity of asthma?
Guidelines for management of young wheezing children are lacking. In this section, we will discuss evidence-based treatment options as well as potential primary preventive strategies.
Inhaled Corticosteroids
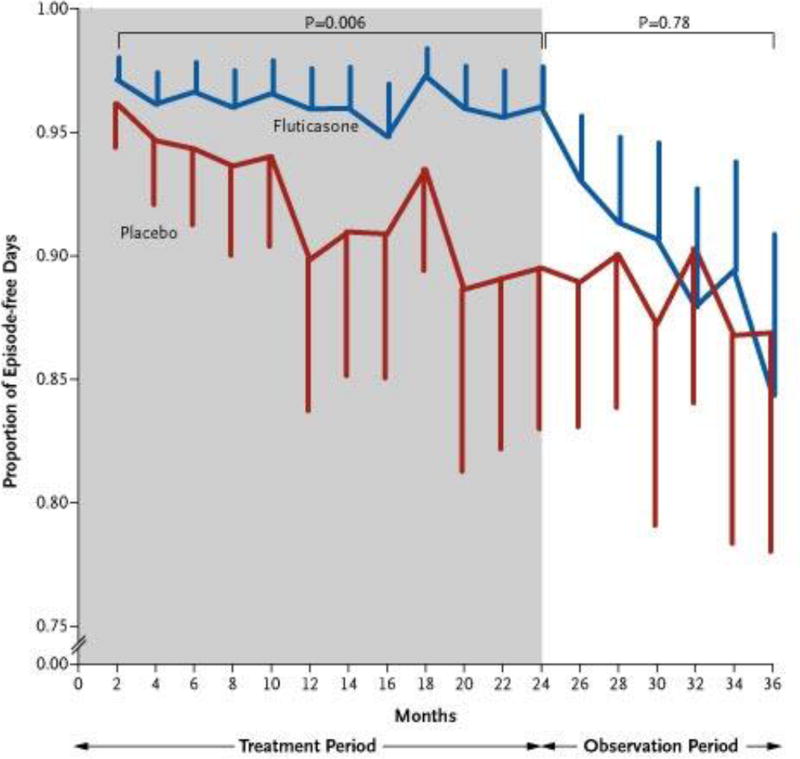
According to the NAEPP, ICS are the preferred long-term controller therapy in children regardless of age to improve symptom control and quality of life (23). Despite this recommendation, there is continuing debate in the field regarding the usefulness of corticosteroids in wheezing preschool-aged children. Some of this debate has arisen from studies showing a lack of efficacy of oral corticosteroids for acute wheezing episodes in this age group (43, 44). However, certain groups of wheezing children may be more responsive to corticosteroids than others, as seen in the INFANT study (27) as well as others showing greater response to ICS in children with higher FeNO and peripheral eosinophilia (28, 42). While effective for reducing impairment and exacerbations, ICS do not appear to impact loss of lung function over time. The Childhood Asthma Management Program (CAMP) study found that daily inhaled budesonide improved asthma control in 5–12 year old children but did not impact the degree of change in lung function over time when compared to placebo (45). The Prevention of Early Asthma in Kids (PEAK) trial found that daily ICS treatment in preschool-aged children with positive modified API was associated with fewer symptoms and exacerbations, but the effect was lost upon discontinuation of ICS, suggesting a lack of disease-modifying effects (6, 7) (Figure 1).
Figure 1. Proportion of Episode-free Days during the Treatment Period (indicated in gray) and the Observation Period (indicated in white).
While fluticasone did increase the proportion of episode-free days during the treatment period compared to placebo, this effect was lost during the observation period after treatment was discontinued. The listed p-values are for comparison between the treatment groups during the treatment and observation periods. The vertical bars represent 95% confidence intervals. (From New England Journal of Medicine, Guilbert, T. et al., Long-Term Inhaled Corticosteroids in Preschool Children at High Risk for Asthma, 354:1985-199. Copyright © (2006) Massachusetts Medical Society. Reprinted with permission. http://www.nejm.org/doi/full/10.1056/NEJMoa051378.)
Most preschool-aged wheezing children experience little or no impairment between exacerbations, which tend to be associated with viral respiratory infections. Many clinicians have begun the practice of using ICS only during acute wheezing episodes or seasonally during fall and winter seasons when viral illnesses are most likely to occur. The Maintenance vs Intermittent Inhaled Steroids in Wheezing Toddlers (MIST) trial compared high dose ICS at the first signs of viral respiratory illness to daily ICS treatment in preschool-aged children with positive API but low degree of impairment (not meeting criteria for Step 2 asthma therapy (23, 46). The authors reported no difference between the groups in frequency of severe exacerbations.
While ICS is not effective for primary prevention of asthma, the evidence suggests some benefit in preschool-aged children with recurrent wheezing episodes. Those children who demonstrate elevated blood eosinophils and/or aeroallergen sensitization may benefit the greatest from ICS treatment. More study is needed to better characterize ICS responders and non-responders to allow for development of additional treatment options.
Leukotriene Receptor Antagonists
Montelukast is approved for children as young as 1 year of age and is recommended as an alternative to ICS when initiating controller therapy for asthma (23). Efficacy studies of montelukast in young children demonstrate benefit particularly in the setting of viral-induced wheezing. The PREVIA study of children 2–5 years of age treated with montelukast daily for 12 months demonstrated reduction in the frequency of viral-induced exacerbations of asthma compared to placebo (47). Another study demonstrated reduced symptoms, need for rescue medications and oral corticosteroids in 2–5 year old children with asthma who received montelukast daily for 12 weeks (48). Children with favorable response to montelukast tend to be of younger age and shorter disease duration with lower FeNO, peripheral eosinophilia, and serum IgE levels according to one study (28). The recently published INFANT trial found that many participants responded best to daily LTRA compared to daily or as-needed ICS but was unable to identify characteristics or biomarkers that predicted this response (27). Montelukast may be an appropriate first line therapy for a select population of preschool-aged wheezing children, but further study is needed to identify those most likely to have a favorable response.
Macrolides
Antibiotics are commonly prescribed during outpatient visits for wheezing, with macrolides being the most common (49). Macrolides are frequently prescribed for cystic fibrosis and COPD due to their anti-inflammatory properties and may have a role in treatment of recurrent wheezing and asthma as well. Bacharier et al conducted a randomized, double-blinded trial of preschool children, half of whom had a positive modified API, using early administration of azithromycin 12 mg/kg for 5 days or placebo at the onset of respiratory illness and found a reduction in episodes of severe lower airway respiratory illness in the azithromycin group compared to placebo (50). Subgroup analyses found no significant effect of API on response to azithromycin. In an unselected Danish birth cohort, COPSAC 2010, a 3-day course of 10 mg/kg azithromycin after the onset of respiratory illness resulted in significant shortening of episodes of asthma-like symptoms compared to placebo in children ages 1–3 (51). In a randomized placebo-controlled study of infants hospitalized with RSV bronchiolitis, azithromycin treatment during the episode prolonged time to the next wheezing episode and resulted in fewer symptomatic days over the subsequent year (52). Macrolides are thought to be more effective in non-eosinophilic airway inflammation, particularly neutrophilic inflammation associated with some types of asthma (53, 54). While not part of the current asthma management guidelines, macrolides could be considered for use during respiratory illness in preschool-aged children with wheezing to prevent progression to more severe symptoms.
Allergen-specific Immunotherapy
Allergen-specific subcutaneous immunotherapy (SCIT) has been shown to effectively reduce asthma symptoms, corticosteroid requirement, and improve quality of life, and may be one of the only available treatment options, apart from avoidance of tobacco smoke, for primary prevention of asthma in children (55–57). The European multicenter Preventive Allergy Treatment (PAT) study randomized children 6–14 years with allergic rhinoconjunctivitis but without asthma who were sensitized to birch and/or grass pollen to SCIT containing birch and/or grass pollen or to an open control group for 3 years. Subjects who received SCIT were less likely to have asthma at 2 years and 7 years after study completion than the control group (58–60). Sublingual immunotherapy (SLIT) trials have shown less consistent benefit in treatment of allergic asthma in older children, and a recent Cochrane review concluded that further research is needed to determine if SLIT is an effective treatment for asthma (61). Clinical trials in preschool aged children are needed to confirm the efficacy and safety of allergen-specific immunotherapy for asthma prevention.
Anti-IgE therapy
Omalizumab is currently approved for adults and children 6 years and older with persistent asthma not well controlled on ICS and with sensitization to perennial allergen(s). In children and adolescents with allergic asthma, omalizumab resulted in decreased ICS dosage (62, 63), frequency of asthma exacerbations (62–65), reduced rescue medication requirement (62), and decreased unscheduled healthcare visits (62) and hospitalizations (65). Recently there has been increasing interest in the use of anti-IgE therapies for primary prevention of asthma, presumably by preventing the cascade of inflammatory events early in life that lead to asthma. Studies in adults have suggested that frequent exacerbations are associated with more rapid decline in lung function over time (66), potentially due to repetitive injury leading to airway remodeling. By preventing exacerbations, omalizumab may have the potential to prevent irrecoverable loss of lung function, but to date this has not been studied. Trials of omalizumab in preschool aged children are likely on the horizon as a potential treatment for primary asthma prevention.
Other Anti-TH2 therapies
Additional biologics aimed at T helper cell type 2 (TH2) cytokines IL-4, 5, and 13 are currently under investigation for treatment of TH2-high asthma. Anti-IL-5 monoclonal antibody (mAb) therapy has been shown to reduce exacerbations in adults with eosinophilic asthma (67, 68), and clinical trials in children are ongoing. Similarly, anti-IL-4Rα therapy appears to reduce the frequency of asthma exacerbations and improves lung function in adults with eosinophilic asthma (69), but no studies have been published in children. Results of anti-IL-13 therapies have shown mixed results in studies of adults with asthma, with some showing a reduction in asthma exacerbations (70) and improved lung function (70, 71), while others showed no improvement in exacerbation rates and or change in asthma questionnaire scores (72). There are currently no active trials of anti-IL-13 mAb in children under 12 years. Use of anti-TH2 biologics in current practice is limited, especially among preschool-aged children due to a lack of evidence demonstrating efficacy and safety in this population. The potential use of biologics for asthma prevention or disease-modification is an exciting area in our field and one that is likely to dramatically change practice in the coming years as further clinical trials in this age group are performed.
Conclusions
While there have been many advances to our understanding of early life wheezing phenotypes, we are still left with many questions regarding how to best treat these young children. As not all children respond the same way to conventional asthma therapies, appropriate phenotyping and endotyping of wheezing children is essential to guide practitioners to the most effective therapies for management of symptoms. The bigger dilemma is the lack of proven treatment options for primary prevention of asthma. Allergen-specific immunotherapy and biologics such as anti-IgE therapy will likely be further explored for their potential for primary prevention of asthma in certain groups of young children with recurrent wheezing.
Key Points.
Insults resulting in reduced lung function occur in the first few years of life, so primary preventive strategies should be targeted towards very young children.
The Asthma Predictive Index (API) and biomarkers such as specific IgE, blood eosinophils, and exhaled nitric oxide (FeNO) are useful tools to estimate asthma risk in young wheezing children.
Daily ICS use in young wheezing children does not modify progression to asthma or prevent decline in lung function but does reduce impairment and exacerbations particularly in children with elevated blood eosinophils and/or aeroallergen sensitization.
Allergen immunotherapy and monoclonal antibodies targeting TH2 inflammation may represent strategies for primary prevention of allergic asthma, but efficacy and safety studies in young children are needed.
Acknowledgments
None.
Financial support and sponsorship: Dr. Szefler reports grants from the Colorado Department of Public Health (#13-FLA-48556 and 17-FHLA-93211), the National Heart, Lung, and Blood Institute Asthma Research Network (1 U10 HL098075-01) and GlaxoSmithKline Inner City Asthma School Program Building Bridges (FLV116794). Dr. Szefler has also served as a consultant for Aerocrine, Astra Zeneca, Boehringer-Ingelheim, Daiichi Sankyo, Genentech, GlaxoSmithKline, Hoffman LaRoche, Merck, Novartis, Roche, Sanofi, and TEVA. Dr. Burbank is supported by 2T32GM086330.
Abbreviations
- API
Asthma predictive index
- NAEPP
National Asthma Education and Prevention Program
- ICS
Inhaled corticosteroid
- LTRA
Leukotriene receptor antagonist
- FeNO
exhaled nitric oxide
- ETS
environmental tobacco smoke
- SCIT
subcutaneous immunotherapy
- TH2 cell
T helper cell type 2
- mAb
monoclonal antibody
Footnotes
Conflicts of Interest: None.
References
Papers of particular interest have been highlighted as:
*of special interest
**of outstanding interest
- 1.Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 2.Taussig LM, Wright AL, Holberg CJ, et al. Tucson Children's Respiratory Study: 1980 to present. J Allergy Clin Immunol. 2003;111:661–675. doi: 10.1067/mai.2003.162. quiz 676. [DOI] [PubMed] [Google Scholar]
- 3.Leonardi NA, Spycher BD, Strippoli MP, et al. Validation of the Asthma Predictive Index and comparison with simpler clinical prediction rules. J Allergy Clin Immunol. 2011;127:1466–1472. e1466. doi: 10.1016/j.jaci.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Martinez CE, Sossa-Briceno MP, Castro-Rodriguez JA. Discriminative properties of two predictive indices for asthma diagnosis in a sample of preschoolers with recurrent wheezing. Pediatr Pulmonol. 2011;46:1175–1181. doi: 10.1002/ppul.21493. [DOI] [PubMed] [Google Scholar]
- 5.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 6.Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 7.Guilbert TW, Morgan WJ, Krawiec M, et al. The Prevention of Early Asthma in Kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Control Clin Trials. 2004;25:286–310. doi: 10.1016/j.cct.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Amin P, Levin L, Epstein T, et al. Optimum predictors of childhood asthma: persistent wheeze or the Asthma Predictive Index? J Allergy Clin Immunol Pract. 2014;2:709–715. doi: 10.1016/j.jaip.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan WJ, Stern DA, Sherrill DL, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172:1253–1258. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savenije OE, Kerkhof M, Koppelman GH, Postma DS. Predicting who will have asthma at school age among preschool children. J Allergy Clin Immunol. 2012;130:325–331. doi: 10.1016/j.jaci.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Kulig M, Bergmann R, Tacke U, et al. Long-lasting sensitization to food during the first two years precedes allergic airway disease. The MAS Study Group, Germany. Pediatr Allergy Immunol. 1998;9:61–67. doi: 10.1111/j.1399-3038.1998.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 12.Zeiger RS, Heller S. The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol. 1995;95:1179–1190. doi: 10.1016/s0091-6749(95)70074-9. [DOI] [PubMed] [Google Scholar]
- 13.Lodge CJ, Lowe AJ, Gurrin LC, et al. House dust mite sensitization in toddlers predicts current wheeze at age 12 years. J Allergy Clin Immunol. 2011;128:782–788. e789. doi: 10.1016/j.jaci.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 14.Caudri D, Wijga AH, Hoekstra MO, et al. Prediction of asthma in symptomatic preschool children using exhaled nitric oxide, Rint and specific IgE. Thorax. 2010;65:801–807. doi: 10.1136/thx.2009.126912. [DOI] [PubMed] [Google Scholar]
- 15.Simpson A, Tan VY, Winn J, et al. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med. 2010;181:1200–1206. doi: 10.1164/rccm.200907-1101OC. [DOI] [PubMed] [Google Scholar]
- 16.Illi S, von Mutius E, Lau S, et al. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 17.Simpson A, Soderstrom L, Ahlstedt S, et al. IgE antibody quantification and the probability of wheeze in preschool children. J Allergy Clin Immunol. 2005;116:744–749. doi: 10.1016/j.jaci.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 18.Lazic N, Roberts G, Custovic A, et al. Multiple atopy phenotypes and their associations with asthma: similar findings from two birth cohorts. Allergy. 2013;68:764–770. doi: 10.1111/all.12134. [DOI] [PubMed] [Google Scholar]
- 19.Sly PD, Boner AL, Bjorksten B, et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372:1100–1106. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson DJ. The role of rhinovirus infections in the development of early childhood asthma. Curr Opin Allergy Clin Immunol. 2010;10:133–138. doi: 10.1097/ACI.0b013e3283352f7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnamoorthy N, Khare A, Oriss TB, et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med. 2012;18:1525–1530. doi: 10.1038/nm.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.3 EPR. Guidelines for the Diagnosis and Management of Asthma. 2007 Available from: www.nhlbi.nih.gov/guidelines/asthma/
- 24.Karakoc F, Remes ST, Martinez FD, Wright AL. The association between persistent eosinophilia and asthma in childhood is independent of atopic status. Clin Exp Allergy. 2002;32:51–56. doi: 10.1046/j.0022-0477.2001.01273.x. [DOI] [PubMed] [Google Scholar]
- 25.Kotaniemi-Syrjanen A, Reijonen TM, Korhonen K, Korppi M. Wheezing requiring hospitalization in early childhood: predictive factors for asthma in a six-year follow-up. Pediatr Allergy Immunol. 2002;13:418–425. doi: 10.1034/j.1399-3038.2002.02091.x. [DOI] [PubMed] [Google Scholar]
- 26.Hyvarinen MK, Kotaniemi-Syrjanen A, Reijonen TM, et al. Eosinophil activity in infants hospitalized for wheezing and risk of persistent childhood asthma. Pediatr Allergy Immunol. 2010;21:96–103. doi: 10.1111/j.1399-3038.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- 27**.Fitzpatrick AM, Jackson DJ, Mauger DT, et al. Individualized Therapy for Persistent Asthma in Young Children. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.09.028. This study demonstrated a differential response to daily ICS among wheezing toddlers with aeroallergen sensitization and elevated blood eosinophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szefler SJ, Phillips BR, Martinez FD, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Schleich FN, Manise M, Sele J, et al. Distribution of sputum cellular phenotype in a large asthma cohort: predicting factors for eosinophilic vs neutrophilic inflammation. BMC Pulm Med. 2013;13:11. doi: 10.1186/1471-2466-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Korevaar DA, Westerhof GA, Wang J, et al. Diagnostic accuracy of minimally invasive markers for detection of airway eosinophilia in asthma: a systematic review and meta-analysis. Lancet Respir Med. 2015;3:290–300. doi: 10.1016/S2213-2600(15)00050-8. In this systematic review, the authors found moderate accuracy of FeNO, blood eosinophils, and total IgE for predicting airway eosinophilia in patients with asthma. [DOI] [PubMed] [Google Scholar]
- 31.Thomas PS, Gibson PG, Wang H, et al. The relationship of exhaled nitric oxide to airway inflammation and responsiveness in children. J Asthma. 2005;42:291–295. doi: 10.1081/jas-200057908. [DOI] [PubMed] [Google Scholar]
- 32.Warke TJ, Fitch PS, Brown V, et al. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax. 2002;57:383–387. doi: 10.1136/thorax.57.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Heijkenskjold-Rentzhog C, Kalm-Stephens P, Nordvall L, et al. New method for single-breath fraction of exhaled nitric oxide measurement with improved feasibility in preschool children with asthma. Pediatr Allergy Immunol. 2015;26:662–667. doi: 10.1111/pai.12447. This study demonstrated the feasibility of an adapted FeNO method using age-adjusted exhalation times in children as young as s4 years of age. [DOI] [PubMed] [Google Scholar]
- 34.van der Valk RJ, Caudri D, Savenije O, et al. Childhood wheezing phenotypes and FeNO in atopic children at age 8. Clin Exp Allergy. 2012;42:1329–1336. doi: 10.1111/j.1365-2222.2012.04010.x. [DOI] [PubMed] [Google Scholar]
- 35.Moeller A, Diefenbacher C, Lehmann A, et al. Exhaled nitric oxide distinguishes between subgroups of preschool children with respiratory symptoms. J Allergy Clin Immunol. 2008;121:705–709. doi: 10.1016/j.jaci.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Oh MA, Shim JY, Jung YH, et al. Fraction of exhaled nitric oxide and wheezing phenotypes in preschool children. Pediatr Pulmonol. 2013;48:563–570. doi: 10.1002/ppul.22705. [DOI] [PubMed] [Google Scholar]
- 37.Collins SA, Pike KC, Inskip HM, et al. Validation of novel wheeze phenotypes using longitudinal airway function and atopic sensitization data in the first 6 years of life: evidence from the Southampton Women's survey. Pediatr Pulmonol. 2013;48:683–692. doi: 10.1002/ppul.22766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singer F, Luchsinger I, Inci D, et al. Exhaled nitric oxide in symptomatic children at preschool age predicts later asthma. Allergy. 2013;68:531–538. doi: 10.1111/all.12127. [DOI] [PubMed] [Google Scholar]
- 39.Shim JY. Association of wheezing phenotypes with fractional exhaled nitric oxide in children. Korean J Pediatr. 2014;57:211–216. doi: 10.3345/kjp.2014.57.5.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Cowan DC, Taylor DR, Peterson LE, et al. Biomarker-based asthma phenotypes of corticosteroid response. J Allergy Clin Immunol. 2015;135:877–883. e871. doi: 10.1016/j.jaci.2014.10.026. This study provides evidence that biomarkers FeNO, sputum eosinophils, and urinary bromotyrosine levels can be useful for predicting steroid-responsiveness in steroid-naïve asthmatics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Debley JS, Stamey DC, Cochrane ES, et al. Exhaled nitric oxide, lung function, and exacerbations in wheezy infants and toddlers. J Allergy Clin Immunol. 2010;125:1228–1234. e1213. doi: 10.1016/j.jaci.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeiger RS, Szefler SJ, Phillips BR, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117:45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360:329–338. doi: 10.1056/NEJMoa0804897. [DOI] [PubMed] [Google Scholar]
- 44.Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent-initiated oral prednisolone for viral wheeze in children aged 1–5 years: randomised controlled trial. Lancet. 2003;362:1433–1438. doi: 10.1016/S0140-6736(03)14685-5. [DOI] [PubMed] [Google Scholar]
- 45.Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 46.Zeiger RS, Mauger D, Bacharier LB, et al. Daily or intermittent budesonide in preschool children with recurrent wheezing. N Engl J Med. 2011;365:1990–2001. doi: 10.1056/NEJMoa1104647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bisgaard H, Zielen S, Garcia-Garcia ML, et al. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med. 2005;171:315–322. doi: 10.1164/rccm.200407-894OC. [DOI] [PubMed] [Google Scholar]
- 48.Knorr B, Franchi LM, Bisgaard H, et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics. 2001;108:E48. doi: 10.1542/peds.108.3.e48. [DOI] [PubMed] [Google Scholar]
- 49.Paul IM, Maselli JH, Hersh AL, et al. Antibiotic prescribing during pediatric ambulatory care visits for asthma. Pediatrics. 2011;127:1014–1021. doi: 10.1542/peds.2011-0218. [DOI] [PubMed] [Google Scholar]
- 50**.Bacharier LB, Guilbert TW, Mauger DT, et al. Early Administration of Azithromycin and Prevention of Severe Lower Respiratory Tract Illnesses in Preschool Children With a History of Such Illnesses: A Randomized Clinical Trial. JAMA. 2015;314:2034–2044. doi: 10.1001/jama.2015.13896. Azithromycin treatment initiated at the onset of respiratory tract illness in preschool-aged children reduced the risk of progression to severe lower respiratory tract illness compared to placebo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51*.Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1–3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19–26. doi: 10.1016/S2213-2600(15)00500-7. Treatment of children aged 1–3 years with azithromycin during asthma-like illness resulted in decreased duration of the illness compared to placebo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Beigelman A, Isaacson-Schmid M, Sajol G, et al. Randomized trial to evaluate azithromycin's effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2015;135:1171–1178. e1171. doi: 10.1016/j.jaci.2014.10.001. The authors found that azithromycin is beneficial in infants with RSV bronchiolitis, prolonging time to the next wheezing episode as well as reducing objective markers of inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friedlander AL, Albert RK. Chronic macrolide therapy in inflammatory airways diseases. Chest. 2010;138:1202–1212. doi: 10.1378/chest.10-0196. [DOI] [PubMed] [Google Scholar]
- 54.Simpson JL, Powell H, Boyle MJ, et al. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med. 2008;177:148–155. doi: 10.1164/rccm.200707-1134OC. [DOI] [PubMed] [Google Scholar]
- 55.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–562. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 56.Bacharier LB, Boner A, Carlsen KH, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63:5–34. doi: 10.1111/j.1398-9995.2007.01586.x. [DOI] [PubMed] [Google Scholar]
- 57.Eifan AO, Shamji MH, Durham SR. Long-term clinical and immunological effects of allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2011;11:586–593. doi: 10.1097/ACI.0b013e32834cb994. [DOI] [PubMed] [Google Scholar]
- 58.Moller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–256. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 59.Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 60.Niggemann B, Jacobsen L, Dreborg S, et al. Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy. 2006;61:855–859. doi: 10.1111/j.1398-9995.2006.01068.x. [DOI] [PubMed] [Google Scholar]
- 61.Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015:CD011293. doi: 10.1002/14651858.CD011293.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milgrom H, Berger W, Nayak A, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab) Pediatrics. 2001;108:E36. doi: 10.1542/peds.108.2.e36. [DOI] [PubMed] [Google Scholar]
- 63.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lanier B, Bridges T, Kulus M, et al. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009;124:1210–1216. doi: 10.1016/j.jaci.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 65*.Rodrigo GJ, Neffen H. Systematic review on the use of omalizumab for the treatment of asthmatic children and adolescents. Pediatr Allergy Immunol. 2015;26:551–556. doi: 10.1111/pai.12405. This systematic review of omalizumab demonstrates reduction in exacerbations without significant adverse events in children and adolescents with asthma. [DOI] [PubMed] [Google Scholar]
- 66.Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J. 2007;30:452–456. doi: 10.1183/09031936.00165106. [DOI] [PubMed] [Google Scholar]
- 67.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184:1125–1132. doi: 10.1164/rccm.201103-0396OC. [DOI] [PubMed] [Google Scholar]
- 69.Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 70*.Hanania NA, Noonan M, Corren J, et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. 2015;70:748–756. doi: 10.1136/thoraxjnl-2014-206719. Lebrikizumab treatment for 24 weeks demonstrated improvement in asthma exacerbations and lung function parameters in adults with asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corren J, Lemanske RF, Hanania NA, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 72.De Boever EH, Ashman C, Cahn AP, et al. Efficacy and safety of an anti-IL-13 mAb in patients with severe asthma: a randomized trial. J Allergy Clin Immunol. 2014;133:989–996. doi: 10.1016/j.jaci.2014.01.002. [DOI] [PubMed] [Google Scholar]