Abstract
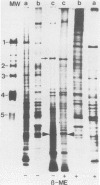
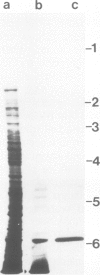
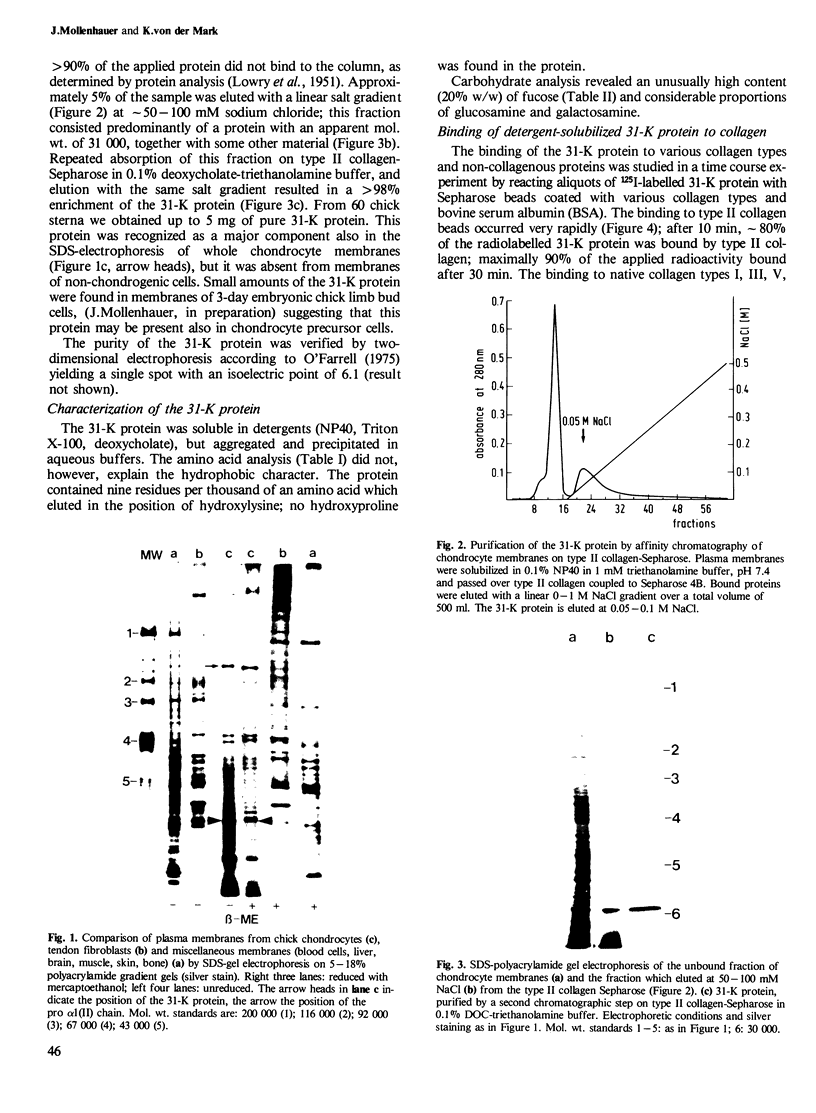
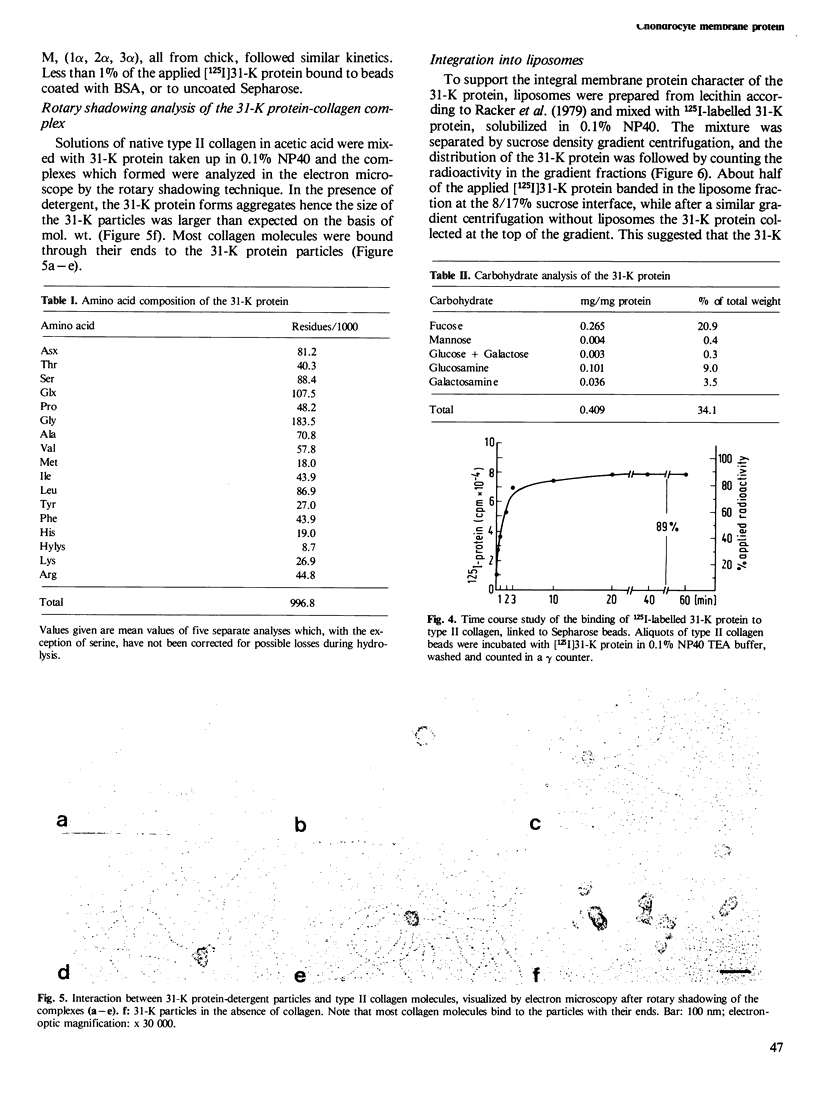
A collagen-binding glycoprotein was isolated from purified chick chondrocyte surface membranes by affinity chromatography on type II collagen-Sepharose. The purified glycoprotein has an apparent mol. wt. of 31,000 and binds to native chick collagen types I, II, III, V and M. Although it contains 30% carbohydrates, the majority of which is fucose, it is hydrophobic and soluble only in detergents. The integral membrane protein character of the 31-K protein became apparent from its ability to insert into lecithin vesicles. Liposome-inserted 31-K protein binds 125I-labelled type II collagen in the presence of 0.5 M NaCl, while detergent-solubilized 31-K protein is dissociated from type II collagen by 0.05-0.1 M NaCl. Electron microscopic studies employing the rotary shadowing technique indicate that 31-K protein particles bind to the ends of collagen molecules. We propose that this glycoprotein serves as anchorage site for extracellular collagen to the chondrocyte membrane and thus may be involved in cell-matrix interactions in cartilage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cates G. A., Holland P. C. Biosynthesis of plasma-membrane proteins during myogenesis of skeletal muscle in vitro. Biochem J. 1978 Sep 15;174(3):873–881. doi: 10.1042/bj1740873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T. M., Beachey E. H., Kang A. H. Binding of collagen alpha1 chains to human platelets. J Clin Invest. 1977 Mar;59(3):405–411. doi: 10.1172/JCI108653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T. M., Kang A. H. Isolation and purification of collagen alpha 1(I) receptor from human platelet membrane. J Biol Chem. 1982 Jul 10;257(13):7581–7586. [PubMed] [Google Scholar]
- Chiang T. M., Postlethwaite A. E., Beachey E. H., Seyer J. M., Kang A. H. Binding of chemotactic collagen-derived peptides to fibroblasts. The relationship to fibroblast chemotaxis. J Clin Invest. 1978 Nov;62(5):916–922. doi: 10.1172/JCI109219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehm P., Prockop D. J. Biosynthesis of cartilage procollagen. Eur J Biochem. 1973 May;35(1):159–166. doi: 10.1111/j.1432-1033.1973.tb02821.x. [DOI] [PubMed] [Google Scholar]
- Dessau W., Sasse J., Timpl R., Jilek F., von der Mark K. Synthesis and extracellular deposition of fibronectin in chondrocyte cultures. Response to the removal of extracellular cartilage matrix. J Cell Biol. 1978 Nov;79(2 Pt 1):342–355. doi: 10.1083/jcb.79.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessau W., Vertel B. M., von der Mark H., von der Mark K. Extracellular matrix formation by chondrocytes in monolayer culture. J Cell Biol. 1981 Jul;90(1):78–83. doi: 10.1083/jcb.90.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay S., Müller P. K., Lemmen C., Remberger K., Matzen K., Kühn K. Immunohistological study on collagen in cartilage-bone metamorphosis and degenerative osteoarthrosis. Klin Wochenschr. 1976 Oct 15;54(20):969–976. doi: 10.1007/BF01468947. [DOI] [PubMed] [Google Scholar]
- Goldberg B. Binding of soluble type I collagen molecules to the fibroblast plasma membrane. Cell. 1979 Feb;16(2):265–275. doi: 10.1016/0092-8674(79)90004-7. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Minter D. Attachment and spreading of baby hamster kidney cells to collagen substrata: effects of cold-insoluble globulin. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4408–4412. doi: 10.1073/pnas.75.9.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979 Jan 1;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper P. A., Juliano R. L. Fibronectin-independent adhesion of fibroblasts to the extracellular matrix: mediation by a high molecular weight membrane glycoprotein. J Cell Biol. 1981 Dec;91(3 Pt 1):647–653. doi: 10.1083/jcb.91.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H., Dessau W., Fessler L. I., von der Mark K. Synthesis of types I, III and AB2 collagen by chick tendon fibroblasts in vitro. Eur J Biochem. 1980 Mar;105(1):63–74. doi: 10.1111/j.1432-1033.1980.tb04474.x. [DOI] [PubMed] [Google Scholar]
- Hewitt A. T., Kleinman H. K., Pennypacker J. P., Martin G. R. Identification of an adhesion factor for chondrocytes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):385–388. doi: 10.1073/pnas.77.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt A. T., Varner H. H., Silver M. H., Dessau W., Wilkes C. M., Martin G. R. The isolation and partial characterization of chondronectin, an attachment factor for chondrocytes. J Biol Chem. 1982 Mar 10;257(5):2330–2334. [PubMed] [Google Scholar]
- Hughes R. C., Butters T. D., Aplin J. D. Cell surface molecules involved in fibronectin-mediated adhesion. A study using specific antisera. Eur J Cell Biol. 1981 Dec;26(1):198–207. [PubMed] [Google Scholar]
- Jilek F., Hörmann H. Fibronectin (cold-insoluble globulin), VI. Influence of heparin and hyaluronic acid on the binding of native collagen. Hoppe Seylers Z Physiol Chem. 1979 Apr;360(4):597–603. doi: 10.1515/bchm2.1979.360.1.597. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler J. K., Nudelman E. D., Hakomori S. A collagen-binding protein on the surface of ejaculated rabbit spermatozoa. J Cell Biol. 1980 Aug;86(2):529–536. doi: 10.1083/jcb.86.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosher R. A., Church R. L. Stimulation of in vitro somite chondrogenesis by procollagen and collagen. Nature. 1975 Nov 27;258(5533):327–330. doi: 10.1038/258327a0. [DOI] [PubMed] [Google Scholar]
- Kühn K., Wiedemann H., Timpl R., Risteli J., Dieringer H., Voss T., Glanville R. W. Macromolecular structure of basement membrane collagens. FEBS Lett. 1981 Mar 9;125(1):123–128. doi: 10.1016/0014-5793(81)81012-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lash J. W., Vasan N. S. Somite chondrogenesis in vitro. Stimulation by exogenous extracellular matrix components. Dev Biol. 1978 Sep;66(1):151–171. doi: 10.1016/0012-1606(78)90281-6. [DOI] [PubMed] [Google Scholar]
- Lehto V. P., Vartio T., Virtanen I. Enrichment of a 140 KD surface glycoprotein in adherent, detergent-resistant cytoskeletons of cultured human fibroblasts. Biochem Biophys Res Commun. 1980 Aug 14;95(3):909–916. doi: 10.1016/0006-291x(80)91559-4. [DOI] [PubMed] [Google Scholar]
- Linsenmayer T. F., Gibney E., Toole B. P., Gross J. Cellular adhesion to collagen. Exp Cell Res. 1978 Oct 15;116(2):470–474. doi: 10.1016/0014-4827(78)90473-1. [DOI] [PubMed] [Google Scholar]
- Niedermeier W., Tomana M. Gas chromatographic analysis of hexosamines in glycoproteins. Anal Biochem. 1974 Feb;57(2):363–368. doi: 10.1016/0003-2697(74)90090-6. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Racker E., Violand B., O'Neal S., Alfonzo M., Telford J. Reconstitution, a way of biochemical research; some new approaches to membrane-bound enzymes. Arch Biochem Biophys. 1979 Dec;198(2):470–477. doi: 10.1016/0003-9861(79)90521-6. [DOI] [PubMed] [Google Scholar]
- Rubin K., Hök M., Obrink B., Timpl R. Substrate adhesion of rat hepatocytes: mechanism of attachment to collagen substrates. Cell. 1981 May;24(2):463–470. doi: 10.1016/0092-8674(81)90337-8. [DOI] [PubMed] [Google Scholar]
- Schrager J., Oates M. D. The carbohydrate components of hydrolysates of gastric secretion and extracts from mucous glands of the gastric body mucosa and antrum. Biochem J. 1968 Jan;106(2):523–529. doi: 10.1042/bj1060523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Mosher D. F. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim Biophys Acta. 1978 Sep 18;516(1):1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]
- von der Mark H., von der Mark K., Gay S. Study of differential collagen synthesis during development of the chick embryo by immunofluorescence. I. Preparation of collagen type I and type II specific antibodies and their application to early stages of the chick embryo. Dev Biol. 1976 Feb;48(2):237–249. doi: 10.1016/0012-1606(76)90088-9. [DOI] [PubMed] [Google Scholar]
- von der Mark K., Gauss V., von der Mark H., Müller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977 Jun 9;267(5611):531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]