Abstract
Objective
The oral cavity represents an initial entry way for oral and gut indigenous colonization. Skin-to-skin (STS) care, in which the mother holds the diaper clad naked preterm (PT) infant between her breasts, is associated with improved digestive function, decreased stress, and improved survival. This study evaluated the development of oral microbial colonization repertoires and health characteristics in PT infants with or without STS exposure.
Methods
Saliva from 42 PT infants (<32 weeks of gestation at birth) was collected prospectively at 1 month and/or at discharge. High-throughput 16S rRNA sequencing identified microbial diversity and prevalence of bacterial signatures correlated with clinical STS or non-STS care.
Results
Corrected for gestational age (CGA) at sampling, bacterial taxa demonstrated increased Streptococcus as a signature of oral repertoire maturation. STS was associated with increased Streptococcus (p < 0.024), while non-STS was associated with greater Corynebacterium (p < 0.023) and Pseudomonas (p < 0.019) in infants ≤ 32 weeks CGA. In infants > 32 weeks CGA, Neisseria and Acinetobacter were more prevalent, 50 vs. 16.7% and 40 vs. 0%, respectively. STS care was associated with shorter hospitalization (p < 0.039).
Conclusion
STS care during earlier gestation was associated with a distinct microbial pattern and an accelerated pace of oral microbial repertoire maturity.
Keywords: skin to skin, preterm infant, oral microbiome
The digestive microbiome of preterm (PT) infants is influenced by nutritional and environmental factors of care, such as mode of feeding, type of birth delivery, maternal separation, and the administration of antenatal and current medications including antibiotics.1–3 Recent information implicates environmental factors as influential in the pace but not the patterned sequence of gut microbial acquisition progression in the PT infant.4 As an entry way to the gut, saliva and the oral cavity in the naive PT infant provides a bacterial community resource that may be important for the initiation of digestion and development of intestinal commensal colonization. Feeding tolerance in the PT infant is challenged due to immature intestinal architecture and function that evolves during the infant’s growth and development in the neonatal intensive care unit (NICU).5 Skin-to-skin (STS) care, also known as kangaroo mother care, where the mother holds her naked infant upright between the breasts for extended periods, is advocated as a method of care that improves PT infant survival and lessens morbidity.6–10 Associated benefits of STS include improved early thermal regulation, decreased infant stress, decreased oxygen need, and increased growth velocity while enhancing maternal breast milk establishment.6,9,11–13 Longer-term effects have been suggested that include less pneumonia and diarrheal disease.9,13
Newborn indigenous microbial colonization may be dependent on resources that include maternal and environmental bacterial repertoire exposure.1,14–16 Additionally, for PT infant, microbial exposure may be impacted by incubator and other respiratory equipment care as well as provider exposures in the NICU. Furthermore, PT infants experience NICU environmental stress that includes skin penetrating procedures, maternal separation after birth, and a variety of touch activities perceived as painful. Stress has been identified as a potential factor in gut microbial dysbiosis.17,18 Developed gut microbial dysbiosis has been associated with later increased risk for allergies and celiac disease.19,20
Saliva and the oropharyngeal area play key roles in early digestion, immune mucosal stimulation, and establishment of gut colonizers important for digestion and immune health.18,21–25 The goal of this study was to examine the development of oral microbial populations in a prospective cohort of PT infants and assess the impact of STS on associated oral microbial colonization repertoire patterns and clinical outcomes.
Material and Methods
Patients
PT infants were enrolled from NICUs at New York University Hospital, Bellevue Hospital Center, and Metropolitan Hospital in New York City. Infants were recruited at <1,500 g and ≤ 32 weeks of completed gestation at the time of birth with parent informed written consent. Forty-two infants met inclusion criteria. This population cohort was chosen as STS care has been used in the very PT infant and to address the understanding of microbial development in this population. This study was approved by the Institutional Review Boards at New York University, Bellevue Hospital Centers, and Metropolitan Hospital. Additional institutional review board approval was obtained at Virginia Commonwealth University for continued sample assessment. Infants with known congenital malformations or infants with lethal conditions were excluded from the study.
Skin-to-Skin Care
The technical application of STS care was standardized in each NICU based on competency training at each center through regional care practices. STS exposure consisted of maternal STS where the mother held the naked diaper clad infant between her breasts. The minimum STS care session was 1 hour. There was no maximum number of care sessions and or duration of STS limit. The initiation of STS care was at the discretion of the clinical team.
Samples
Samples were divided into non-STS exposure and STS exposure within the first 21 days of life. Infant oral saliva samples were assessed at 1 month or at discharge and grouped by corrected gestational age (CGA). Infant samples were obtained prior to feeding using a sterile dry soft cotton swab that was rolled along the infant’s oral mucosal surface of the mouth, inner cheeks, and tongue until saturated with saliva. Saliva samples were placed individually in 2.0 mL of phosphate-buffered saline transported on ice to the laboratory where 1 mL of sample was centrifuged at 14,000 rpm refrigerated for 6 minutes and the pellets were stored at −80°C prior to bacterial DNA extraction. Samples were analyzed as described below to identify microbial repertoires. Clinical data that was collected from infant medical records included maternal and infant birth weight, gestational age, race, medical diagnoses, and medical treatments. All infants were treated with intravenous ampicillin and gentamicin initially and received the standard of care within 30 minutes of admission to the NICU.
Analysis
DNA Extraction
Bacterial DNA was isolated from 1 mL of saliva samples using the PowerLyzer PowerSoil DNA Isolation Kit (MO BIO Laboratories, Inc., Carlsbad, California, United States), as described by the manufacturer. DNA was stored at −20°C until analysis.
16S rRNA Sequence Analysis
The V4–V6 region of the 16s rRNA genes was amplified with barcoded primers for direct sequencing by the Roche 454 Titanium platform, yielding an average of 14,217 (range: 5,742–61,487) reads per sample containing a valid PCR primer and barcode. We utilized universal 16S rRNA primers—OM_515F: GTGCCAGCAGCCGCGGTAA and OM_1061R: TCACRRCACGAGCTGWCGAC targeting 745 species from the oral microbiome database.26 The raw reads were subjected to quality control (QC) filtering with low-quality sequences (average Q-score < 20) and sequences less than 200 nucleotides removed from further analysis.27 High-quality (HQ) sequences were then taxonomically assigned using the Ribosomal Database Project (RDP) Naïve Bayesian Classifier version 2.9 with a bootstrap cutoff of 80%.28 The sequences were also subjected to operational taxonomic unit (OTU) clustering using the UPARSE pipeline at 97% similarity, after trimming the sequences to 450 nucleotides in length and removing singletons.29 OTUs were then assigned taxonomic classifications by aligning OTU sequences against the SILVA database using30 USEARCH31, up to the lowest common ancestor (LCA) in a taxonomic tree. This MEGAN-like32 approach assigns species-specific OTUs to taxa near the leaves of the taxonomy tree, whereas widely conserved OTUs are assigned to higher-order taxa closer to the root. We have implemented a modified approach of obtaining top hits from the USEARCH alignment step and assigning the LCA to each OTU.
Statistical Methods
Analysis
The total bacterial diversity and proportions in saliva were compared between STS and non-STS infant samples. To assess the impact of infant development on microbial repertoires, samples were analyzed by CGA of sample. The analysis of covariance was used to control for the effects of varying gestational age on length of hospitalization and growth velocity with respect to exposure to STS. To associate bacterial patterns with individual population content, we performed post hoc mixed-model regression analysis of patterns discovered visually and Spearman rho test of correlation to assess the strength of correlation of microbial patterns with clinical findings including STS, CGA, and gestational age at birth. The relative reverse Simpson index and the Bray-Curtis dissimilarity index were used to assess microbial diversity among the sample specimens. The paired Student t-test was used with nominal data with categorical data assessed using Fisher exact test. Statistically significant difference was set as p < 0.05.
Results
Patient Characteristics
Forty-two PT infants were included in this study. Demographic characteristics within the STS and non-STS group subdivided by CGA sampling are described in Table 1. The population group’s mean birth gestational age was 28.1 ± 1.6 weeks and mean CGA of sampling was 33.2 ± 2.9 weeks with no significant difference among the STS or non-STS groups within the age delineations. Thirty-five infants (83.3%) were delivered by cesarean delivery and seven by vaginal delivery. The mean birth weight was 1,049 ± 207 g. Thirty-six infants received antenatal steroids (85.7%) and 19 (45.2%) received antenatal ampicillin and gentamicin at the time of delivery. Eighteen infants (42.9%) were males and 24 were females of a variety of racial and ethnic backgrounds. All infants were feeding at the age of sampling by tube feeding alone or in combination with oral feeds based on developmental cues with 6 (14.3%) infants receiving formula and 36 (85.7%) infants receiving human milk. No infant was receiving antibiotic treatment 7 days before or during the time of the specimen sampling.
Table 1.
Preterm infant characteristics, N = 42
| All (N = 42) | STS ≤32 wk (N = 10) | Non-STS ≤32 wk (N = 10) | STS >32 wk (N = 12) | Non-STS > 32 wk (N = 10) | |
|---|---|---|---|---|---|
| Gestational age at birth (weeks mean ± SD) | 28.1 ± 1.6 | 27.5 ± 0.9 | 26.5 ± 1.4 | 29.4 ± 0.5 | 28.7 ± 1.4 |
| Birth weight (grams ± SD) | 1,049 ± 207.2 | 1,053 ± 130.7 | 853 ± 39.7a p < 0.002 |
1,226 ± 123.6 | 1,028 ± 242.9a p < 0.036 |
| History of IUGR < 10%, N (%) | 2 (4.8) | 0 (0) | 0 (0) | 1 (8.3) | 1(10) |
| Gestational age at sampling (weeks mean ± SD) | 33.2 ± 2.9 | 31.7 ± 0.7 | 30.7 ± 1.5 | 34.6 ± 1.8 | 35.4 ± 3.7 |
| Cesarean delivery, N (%) | 35 (83.3) | 8 (80) | 7 (70) | 11 (91.6) | 9 (90) |
| Antenatal steroids, N (%) | 36 (85.7) | 7 (70) | 10 (100) | 10 (83) | 9 (90) |
| Antenatal antibiotics, N (%) | 19 (45.2) | 4 (40) | 2 (20) | 7 (58) | 6 (60) |
| Race/Ethnicity, N (%) | |||||
| Caucasian | 16 (38.1) | 5 (50) | 4 (40) | 5 (41.6) | 2 (20) |
| Black | 8 (19.0) | 3 (30) | 2 (20) | 1 (8.4) | 2 (20) |
| Asian | 10 (23.8) | 1 (10) | 1 (10) | 3 (25) | 5 (50) |
| Hispanic | 8 (19.0) | 1 (10) | 3 (30) | 3 (25) | 1 (10) |
| Male gender, N (%) | 18 (42.9) | 5 (50) | 5 (50) | 5 (41.7) | 3 (30) |
| Human milk, N (%) | 36 (85.7) | 9 (90) | 10 (100) | 11 (91.7) | 6 (60) |
Significant values among ≤32 weeks and >32 weeks groups; other comparisons were not significant at p < 0.05.
Microbiota Characteristics of Samples
Forty-two saliva specimens were sequenced. Sequencing generated a median of 10,731 HQ (3,308–28,830) reads per specimen, and 450 nucleotides per read. Four phylums predominated within these saliva samples. Firmicutes (74.48%), Proteobacteria (20.81%), Actinobacteria (2.02%), and Bacteroidetes (0.84%) were the major phylum representing the total group bacterial sequences. Bacilli (72.64%), Gammaproteobacteria (16.48%), Betaproteobacteria (3.94%), Actinobacteria (2.02%), Negativicutes (1.08%), Bacteroidia (0.55%), Fusobacteria (0.38%), and Alphaproteobacteria (0.22%) accounted for the major classes for >90% of the sequences generated in the specimens (Table 2).
Table 2.
Analysis of major phylum and class of all infant samples (N = 42)
| Taxa level | Taxa name | Number of reads | Total (%) |
|---|---|---|---|
| Rootrank | Root | 465232 | 100 |
| Domain | Bacteria | 464714 | 99.889 |
| Phylum | Firmicutes | 346488 | 74.476 |
| Phylum | Proteobacteria | 96821 | 20.811 |
| Phylum | Actinobacteria | 9417 | 2.024 |
| Phylum | Bacteroidetes | 3911 | 0.841 |
| Phylum | Fusobacteria | 1773 | 0.381 |
| Phylum | Spirochaetes | 107 | 0.023 |
| Class | Bacilli | 337929 | 72.637 |
| Class | Gammaproteobacteria | 76672 | 16.48 |
| Class | Betaproteobacteria | 18349 | 3.944 |
| Class | Actinobacteria | 9416 | 2.024 |
| Class | Negativicutes | 5011 | 1.077 |
| Class | Bacteroidia | 2555 | 0.549 |
| Class | Fusobacteria | 1773 | 0.381 |
| Class | Alphaproteobacteria | 1040 | 0.224 |
| Class | Flavobacteriia | 950 | 0.204 |
| Class | Clostridia | 628 | 0.135 |
| Class | Cytophagia | 249 | 0.054 |
| Class | Spirochaetia | 107 | 0.023 |
Note: Phylum and class reads less than 100 are not shown.
Analysis of Operational Taxonomic Unit Abundance
To refine our analysis and account for possible incorrect representation of the microbial community structure due to sequencing and amplification artifacts, the data were globally trimmed to 450 nucleotides. This was followed by clustering at 97% identity into OTUs, with each OTU representing a bacterial community, using the UPARSE pipeline. All the sequences obtained from the 42 study samples were clustered into a total of 239 unique OTUs. OTUs were then assigned taxonomic classifications up to their LCA using the SILVA database, implementing our modified MEGAN-like algorithm (see “Material and Methods”). Table 3 summarizes OTU abundances within samples and their phylogenetic assignments. The results were in concordance with our RDP analysis, which suggests that Streptococcus is the most dominant genus prevalent in infant saliva, irrespective of their gestational age group.
Table 3.
OTU abundance summary for infants
| OTUa | Number of samplesb | Number of readsc | Total reads (%)d | Taxae |
|---|---|---|---|---|
| OTU_1 | 35 | 142090 | 35.48 | Bacteria; Firmicutes; Bacilli; Lactobacillales; Streptococcaceae; Streptococcus |
| OTU_4 | 29 | 34577 | 8.63 | Bacteria; Firmicutes; Bacilli; Bacillales; Staphylococcaceae; Staphylococcus |
| OTU_2 | 18 | 34047 | 8.5 | Bacteria; Firmicutes; Bacilli; Lactobacillales; Streptococcaceae; Streptococcus |
| OTU_97 | 33 | 18115 | 4.52 | Bacteria; Firmicutes; Bacilli; Bacillales; Staphylococcaceae; Staphylococcus |
| OTU_3 | 6 | 17436 | 4.35 | Bacteria; Proteobacteria; Gammaproteobacteria; Pseudomonadales |
| OTU_154 | 32 | 15717 | 3.92 | Bacteria; Firmicutes; Bacilli; Lactobacillales; Streptococcaceae; Streptococcus |
| OTU_5 | 17 | 15688 | 3.92 | Bacteria; Firmicutes; Bacilli; Bacillales; Family XI; Gemella |
| OTU_7 | 19 | 12968 | 3.24 | Bacteria; Proteobacteria; Gammaproteobacteria; Enterobacteriales; Enterobacteriaceae |
| OTU_55 | 11 | 11453 | 2.86 | Bacteria; Firmicutes; Bacilli; Lactobacillales; Streptococcaceae; Streptococcus |
| OTU_6 | 10 | 9177 | 2.29 | Bacteria; Proteobacteria; Betaproteobacteria; Neisseriales; Neisseriaceae; Neisseria |
| OTU_18 | 20 | 8655 | 2.16 | Bacteria; Proteobacteria; Gammaproteobacteria; Enterobacteriales; Enterobacteriaceae; Klebsiella; Klebsiella pneumoniae |
| OTU_182 | 32 | 7435 | 1.86 | Bacteria; Firmicutes; Bacilli; Lactobacillales; Streptococcaceae; Streptococcus |
| OTU_13 | 2 | 6235 | 1.56 | Bacteria; Proteobacteria; Gammaproteobacteria; Pseudomonadales; Pseudomonadaceae; Pseudomonas |
| OTU_10 | 3 | 4846 | 1.21 | Bacteria; Firmicutes; Bacilli; Lactobacillales; Streptococcaceae; Streptococcus; Streptococcus agalactiae |
| OTU_106 | 17 | 4726 | 1.18 | Bacteria; Proteobacteria; Gammaproteobacteria; Enterobacteriales; Enterobacteriaceae; Klebsiella; Klebsiella pneumoniae |
OTU ID.
Total number of samples showing presence of the OTU.
Total number of reads belonging to the OTU.
Percent of total reads.
Taxonomic classification up to the lowest common ancestor.
Genus Level Differences among STS and Non-STS Groups
Within the majority of specimens, the dominate genus was Streptococcus (Fig. 1A). When grouped by gestational development, the prevalence of Streptococcus was especially noted in the samples > 32 weeks of corrected gestation (samples 21–42) (Fig. 1B).
Fig. 1.
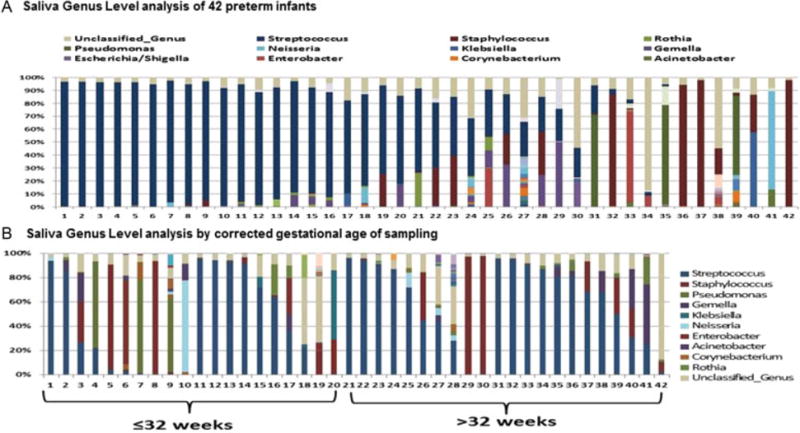
Bacterial taxa at the genus level in oral samples of preterm infants at the corrected age of sampling mean 33.2 ± 2.87 weeks of gestation. (A) Each bar represents the bacterial proportions from an infant’s sample, 1–42. (B) infants 1–20 sample occurred at gestational age was ≤ 32 weeks of gestation while samples 21–42 represents infant samples >32 weeks corrected gestational age. The predominant genera are Streptococcus (dark blue), Staphylococcus (red), Pseudomonas (green), and unclassified genus (tan).
The analyses of differences in bacterial taxa prevalence and abundance at the genus level by CGA groups are summarized in Table 4. In all groups, Streptococcus was present; however, the median level of relative abundance was significantly less in infants ≤32 weeks CGA with non-STS exposure at 4.1% compared with 71.97% in the STS-exposed infants. These non-STS infants, compared with their STS-exposed counterparts, demonstrated greater prevalence and abundance of Pseudomonas median 60.9% compared with 0.03%, Stenotrophomonas median of 3.4% compared with 0.045%, and Neisseria median of 2.7% compared with 0.02% (Table 4). In addition, Corynebacterium was more prevalent in the non-STS (90%) compared with STS (50%) group at an abundance of 0.13% compared with 0.02%, respectively. As development proceeded after birth, samples in infants >32 weeks of CGA tended to demonstrate a predominance of Streptococcus. However, in the non-STS infants compared with the STS group, Neisseria and Acinetobacter genera were more prevalent at 50% compared with 16.67% and at 40% compared with 0%, respectively. Neisseria was also more abundant (median 4.32% compared with 0.03%). At this CGA, several genera were equally prevalent but demonstrated differences in their low abundance levels. These genera included Gemella with a median abundance of 1.01% in non-STS group compared with 5.37% in the STS group; Corynebacterium 1.015% in the non-STS group compared with 0.015% in the STS group; Prevotella, 2.765% in the non-STS group compared with 0.28% in the STS group; and Haemophilus 1.025% in the non-STS group compared with 0.04% in the STS group. It is worth noting that while Streptococcus constituted the major dominant genus in both STS and non-STS infant saliva, STS exposure coincided with a higher presence of Klebsiella in infants corrected to ≤ 32 weeks (60% compared with 0%) and 25% compared with 10% in those >32 weeks of CGA.
Table 4.
Proportions and median abundance of microbial genus among STS and non-STS infants at corrected gestational age of sampling
| Taxa with highest abundance | Corrected gestational age ≤ 32 | Corrected gestational age > 32 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-STS (n = 10) | STS (n = 10) | Difference | Non-STS (n = 10) | STS (n = 12) | Difference | |||||||||||
| Count | Count (%) | Median | Count | Count (%) | Median | Count (%) | Median | Count | Count (%) | Median | Count | Count (%) | Median | Count (%) | Median | |
| Streptococcus | 9 | 90 | 4.12 | 9 | 90 | 71.97 | 0 | 67.85 | 9 | 90 | 71.04 | 12 | 100 | 74.77 | 10 | 3.72 |
| Pseudomonas | 5 | 50 | 60.87 | 2 | 20 | 0.03 | 30 | 60.84 | 1 | 10 | 0.76 | 2 | 17 | 0.20 | 7 | 0.56 |
| Rothia | 1 | 10 | 2.23 | 2 | 20 | 18 | 10 | 15.77 | 6 | 60 | 0.81 | 6 | 50 | 0.62 | 10 | 0.19 |
| Gemella | 3 | 30 | 8.70 | 3 | 30 | 0.18 | 0 | 8.52 | 6 | 60 | 1.01 | 8 | 67 | 5.37 | 7 | 4.34 |
| Corynebacterium | 9 | 90 | 0.13 | 5 | 50 | 0.02 | 40 | 0.11 | 4 | 40 | 1.02 | 4 | 33 | 0.02 | 7 | 1 |
| Enterobacter | 5 | 50 | 0.16 | 4 | 40 | 2.94 | 10 | 2.78 | 2 | 20 | 0.16 | 4 | 33 | 0.05 | 13 | 0.105 |
| Neisseria | 5 | 50 | 2.65 | 2 | 20 | 0.02 | 30 | 2.63 | 5 | 50 | 4.32 | 2 | 17 | 0.03 | 33 | 4.29 |
| Acinetobacter | 5 | 50 | 2.18 | 3 | 30 | 0.02 | 20 | 2.16 | 4 | 40 | 0.06 | 0 | 0 | 0 | 40 | 0.06 |
| Klebsiella | 0 | 0 | 0 | 6 | 60 | 1.63 | 60 | 1.63 | 1 | 10 | 0.12 | 3 | 25 | 0.05 | 15 | 0.07 |
| Staphylococcus | 9 | 90 | 1.33 | 9 | 90 | 1.04 | 0 | 0.29 | 8 | 80 | 0.20 | 9 | 75 | 0.15 | 5 | 0.05 |
| Propionibacterium | 3 | 30 | 0.02 | 1 | 10 | 0.55 | 20 | 0.53 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Enterococcus | 3 | 30 | 0.46 | 5 | 50 | 0.13 | 20 | 0.33 | 3 | 30 | 0.15 | 2 | 17 | 0.92 | 13 | 0.77 |
| Prevotella | 2 | 20 | 0.03 | 0 | 0 | 0 | 20 | 0.03 | 2 | 20 | 2.77 | 2 | 17 | 0.28 | 3 | 2.49 |
| Ureaplasma | 0 | 0 | 0 | 2 | 20 | 0.03 | 20 | 0.03 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cedecea | 3 | 30 | 0.07 | 2 | 20 | 0.21 | 10 | 0.14 | 0 | 0 | 0 | 2 | 17 | 0.11 | 17 | 0.11 |
| Veillonella | 1 | 10 | 0.01 | 0 | 0 | 0 | 10 | 0.01 | 5 | 50 | 0.40 | 7 | 58 | 1.75 | 8 | 1.35 |
| Serratia | 2 | 20 | 0.08 | 2 | 20 | 0.08 | 0 | 0.01 | 0 | 0 | 0 | 3 | 25 | 0.01 | 25 | 0.01 |
| Haemophilus | 2 | 20 | 0.01 | 2 | 20 | 0.01 | 0 | 0 | 4 | 40 | 1.03 | 4 | 33 | 0.04 | 7 | 0.99 |
| Actinomyces | 1 | 10 | 0.08 | 0 | 0 | 0 | 10 | 0.08 | 3 | 30 | 0.49 | 2 | 17 | 0.02 | 13 | 0.47 |
| Stenotrophomonas | 4 | 40 | 3.35 | 2 | 20 | 0.05 | 20 | 3.31 | 2 | 20 | 0.03 | 0 | 0 | 0 | 20 | 0.03 |
| Brevundimonas | 2 | 20 | 0.02 | 0 | 0 | 0 | 20 | 0.02 | 1 | 10 | 0.01 | 0 | 0 | 0 | 10 | 0.01 |
Abbreviation: STS, skin to skin.
Individual Microbial Patterns among STS and Non-STS Groups
Bacterial assessment of individual patterns in the various groups demonstrated that microbial patterns in infants exposed to STS ≤ 32 weeks of gestation were more likely to be dominated by Streptococcus. These infant repertoires were more likely to match individual samples of the older developmental stage of infants who were > 32 weeks of CGA (Fig. 2).
Fig. 2.
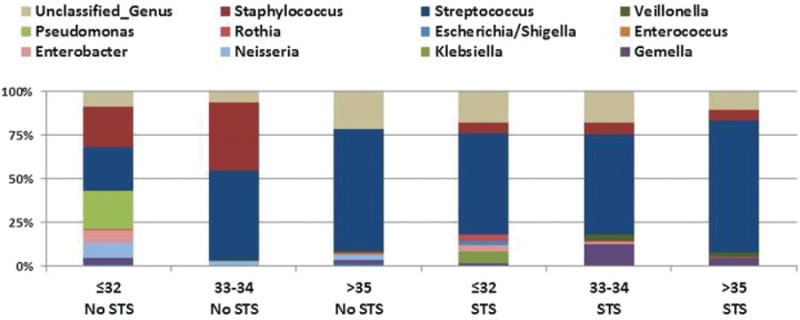
Carriage of microbial taxa at the genus level associated with skin to skin (STS) and non-STS exposure at grouped corrected gestational ages. Proportion of microbes in infant oral microflora in infants exposed to STS care and unexposed non-STS infants at sampling. STS (N = 22) at sampling gestational age ≤32 weeks (N = 10); 33–34 weeks (N = 7), and >35 weeks (N = 5) corrected age. Non-STS (N = 20), at sampling gestational age ≤32 weeks (N = 10); 33–34 weeks (N = 6), and >35 weeks (N = 4) corrected age. The predominant genera are Streptococcus (dark blue), Staphylococcus (red), Pseudomonas (green), and unclassified genus (tan).
STS-exposed individual samples retained a relative paucity of Pseudomonas, Staphylococcus, Neisseria, and Corynebacteria (Fig. 3A, B). Additionally, a greater abundant and dominant Streptococcus pattern was seen as infants aged at CGA of >32 weeks. The effects of formula (F) feeding and vaginal (V) delivery did not impact on this oral development pattern (Fig. 3B).
Fig. 3.
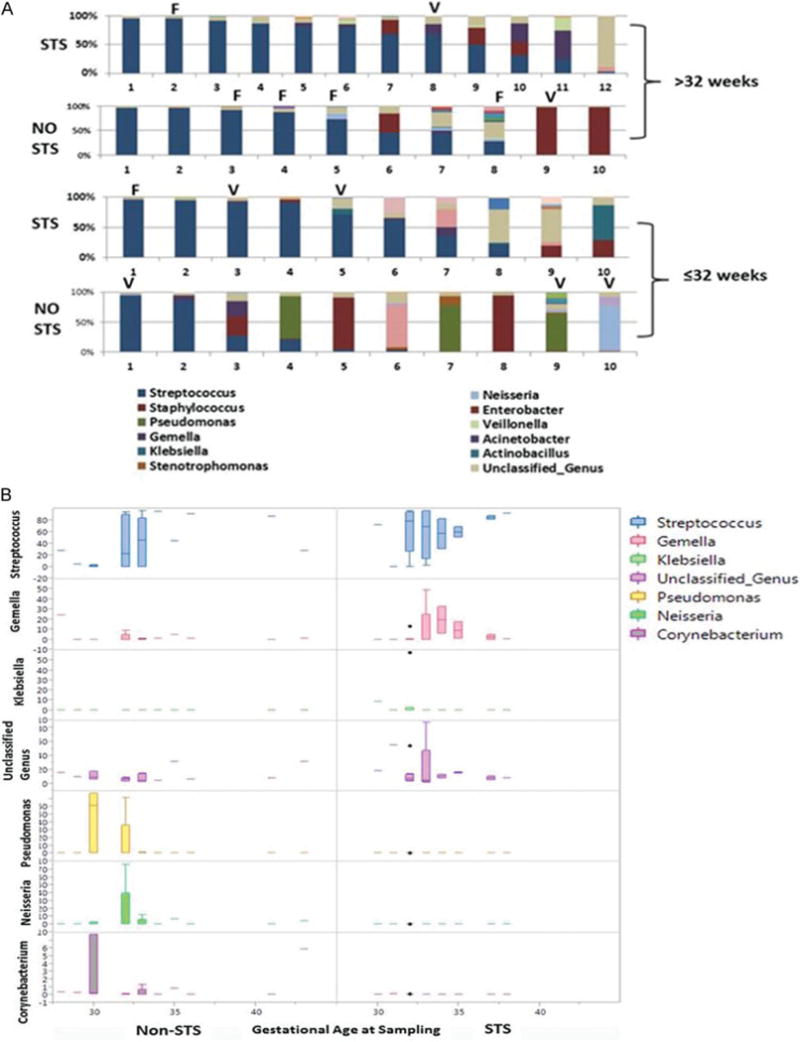
Individual preterm infant microbial genus composition by gestational age and STS exposure. (A) Individual microbial repertoires of infants’ ≤32 weeks of gestational age at sampling and > 32 weeks of gestational age of sampling by exposure to STS or non-STS care. Infants exposed to formula (F) feeding or vaginal (V) delivery are identified. The predominant genus are Streptococcus (dark blue), Staphylococcus (red), Pseudomonas (green), and unclassified genus (tan). (B) Boxplot demonstration of major genera differences in STS-exposed and non-STS-exposed infants at corrected gestational age of sampling.
Sample Diversity Assessment of Sample Groups
Sample alpha-diversity among the groups of STS compared with non-STS or assessed by CGA indicated a relative predominance of 1 or 2 taxa and little difference among the groups (Fig. 4A). Beta-diversity analysis demonstrated increased diversity in the non-STS group (Fig. 4B, inset), which was predominately a result of the non-STS infants ≤ 32 weeks of CGA. The application of STS in infants ≤ 32 weeks of CGA was associated with alignment of beta-diversity similar to infants of older CGAs. Additionally, there was little difference in beta-diversity within the > 32 weeks of CGA groups regardless of exposure to STS (Fig. 4B).
Fig. 4.
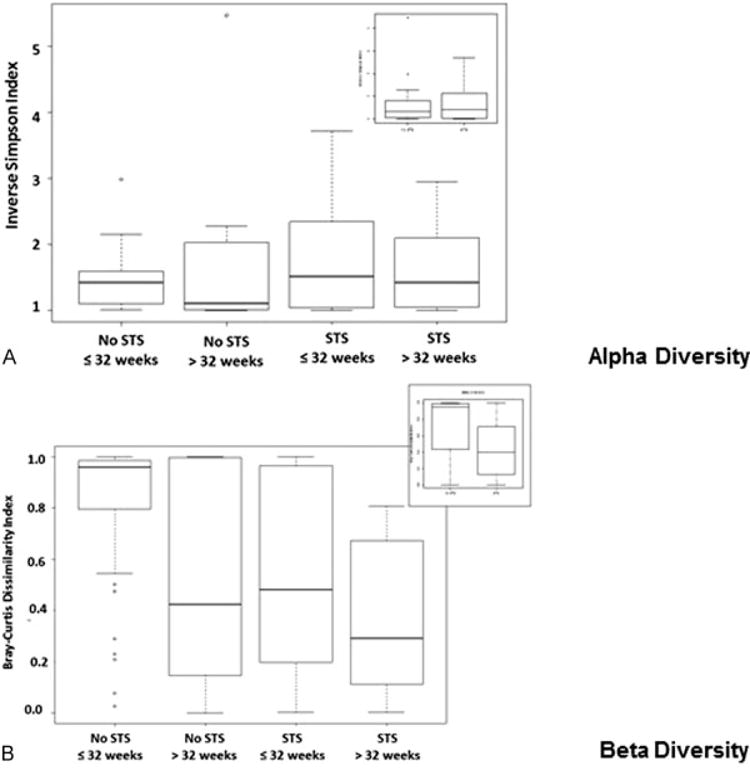
Population diversity within STS and non-STS exposure groups. (A) Alpha-diversity in skin to skin (STS) and non-STS-exposed infants. Alpha-diversity within the two groups by STS or non-STS (inset) or the four groups, as measured using relative inverse Simpson index of genus-level phylotypes, 16S rRNA OTUs. The oral flora demonstrates 1–2 abundant taxa diversity among all groups. (B) Beta-diversity in STS and non-STS-exposed infants. Bray-Curtis dissimilarity index beta-diversity among the two groups (inset) reveals increased microbial diversity within the non-STS as compared with the STS group. Further delineation identified that the non-STS group of ≤32 weeks experienced the greatest difference in microbial diversity within the groups. For each box plot, the middle bar represents the median; the outer horizontal lines of the box represent the 25th and 75th percentiles. The dots indicate the values of individual samples.
Clinical Outcomes of Infants with and without STS
Study infants were comparable in gestational age and severity of illness as identified by SNAPPE II Acuity Scores at birth (Table 5). All infants were intubated with surfactant for respiratory support. Infants were thermo regulated with standard humidified incubators in the NICU with mean day of STS care implemented at 9.1 days of life with a range from day of life 1 to day of life 20 and a mean of 16.3 STS events (Table 5). Evaluation of clinical outcomes demonstrated that STS infants had greater mean growth velocity, with shorter mean length of hospitalization compared with those non-STS infants (Table 5). When corrected for gestational age, length of hospitalization remained significantly decreased in the STS-exposed group, p < 0.0095. No other outcome remained significant. Three infants in the non-STS group were evaluated and treated for necrotizing enterocolitis (NEC) after the sampling period.
Table 5.
Preterm infant clinical outcomes
| ≤ 32 wk STS (N = 10) |
≤ 32 wk non-STS (N = 10) |
> 32 wk STS (N = 12) |
> 32 wk non-STS (N = 10) |
|
|---|---|---|---|---|
| SNAPPE Acuity Score | 15.2 ± 9.21 | 11.1 ± 5.11 | 4.3 ± 6.67 | 7.9 ± 7.29 |
| Antibiotics at birth, N (%) | 9 (90) | 10 (100) | 12 (100) | 10 (100) |
| Respiratory intubation at birth, N (%) | 4 (40) | 10 (100)a p < 0.005 |
7 (58) | 6 (60) |
| Surfactant at birth, N (%) | 4 (40) | 9 (90) | 7 (58) | 6 (60) |
| Age at skin to skin: mean days (range) | 11.1 (1–26) | NA | 10.25 (5–20) | NA |
| Length of hospitalization, number of days ± SD | 61 ± 14.8 | 111.8 ± 43.4a | 46.2 ± 18.5 | 65.6 ± 22.4 p < 0.0095 |
| Necrotizing enterocolitis with surgery, N (%) | 0 (0) | 2 (20) | 0 (0) | 1 (10) |
| Growth velocity, g ± SD | 18.6 ± 4.6 | 15.6 ± 6.8 | 21.6 ± 8.3 | 16.0 ± 3.6 |
After accounting for gestational age and birth weight, length of hospitalization remained significantly different between the STS and non-STS groups.
Discussion
Previous work has demonstrated that STS care for the PT infant is associated with greater intestinal function, superior feeding tolerance, and greater immune stamina including later decreased episodes of pneumonia and diarrhea.8,10,12,16,19 STS also facilitated breastfeeding, thus confounding the possible individual role of STS in improving infant health outcomes. In this study, we demonstrate for the first time in a group of primarily breastfed infants that STS care is associated with altered microbial oral repertoire patterns favoring a pattern in which organisms such as Pseudomonas and Neisseria exhibit a reduced prevalence. Furthermore, STS provided during greater gestational age immaturity was associated with infant acquisition of a mature microbiome repertoire pattern generally identified in the older corrected age group.
It is thought that the infant’s oral cavity, including the oral pharyngeal mucosa, saliva, and their microbiomes, provides a resource for early gut colonizers in the initiation of commensal bacterial colonization important for early immune and gut development. It is not known how changes in oral microbiome development align with later microbial colonization in the pulmonary or gastrointestinal system or the impact of these trajectory changes on later health. Recent data suggest that there is a patterned development of microbiome maturation that can be influenced but not altered by a variety of environmental exposures.4 Our results support a patterned development of oral microbial development and further support a role of early maternal touch as an influence in the pace of oral microbial progression. Benefits attributed to STS have been perceived decreased stress with decreased cortisol levels, increased sleep time, and decreased apnea and need for oxygen therapy.33–37 Previous studies have shown that PT children who receive STS experience fewer medical complications, fewer acute infections, shorter neonatal hospital stays, and better long-term outcomes.9–11,38 Other studies confirm that the vast array of indigenous bacteria that reside on skin and mucosal surfaces are influenced by environmental factors such as maternal–infant interaction at birth.2,3,15,39 It is known that stress-induced physiologic modifications have been associated with changes in bacterial adherence and microbial repertoire patterns.18 Thus, we speculate that STS-associated stress reduction, during earlier gestations, facilitated the acceleration of a mature oral microbial repertoire while infants with non-STS exposure experienced a delay in progression of oral microbial repertoire development. Our data identify that neither delivery mode nor type of feeding altered the expected maturational trajectory of oral microbial repertoire development which is in keeping with recent findings.4 Additionally, our study shows that with further gestational age development, eventually the infant’s oral microbiome in the STS or non-STS counterparts reached similarity.
Our results demonstrate that several common microbial taxa, that is, Pseudomonas, Neisseria, Proteobacteria, and Staphylococcus, were less prevalent or abundant in the saliva of infants ≤ 32 weeks of corrected gestation when exposed to STS care. These organisms have been associated with intestinal dysfunction, including NEC, in the PT infant.40,41 While the incidence of NEC in our total population was small, none of the infants in the STS group developed NEC, whereas three infants in the non-STS group developed NEC after sampling.
The use and efficacy of STS has been well established, and it is applied widely in developed and underdeveloped countries to enhance infant health.10,42–44 Little is known about the impact of other forms of infant touch or stress reduction on the microbial developmental trajectory of the infant. After stabilization in the NICU, STS and other forms of parental touch such as swaddled holding of the infant are routinely provided. These care practices coincide with the infant’s gestational and microbial developmental progression in the NICU. We speculate that the observed accelerated microbial repertoire progression noted in the STS-exposed younger PT infants in this study was a result of the infant’s physiologic changes associated with STS stress reduction. The limitations of our results reflect the varied amount of STS care in the infant population and limited sample size. Additionally, we did not evaluate other forms of NICU stress reduction methods, such as facilitated tuck or touch provided by parents or providers during the hospital stay that may have impacted on microbial colonization development. Furthermore, we did not evaluate physiologic stress levels in the infants with or without STS care application.
Beyond the establishment of microbial repertoire maturation, little is known about the importance of the pace of early microbial colonization patterns or the role of the prevalence of transient lower abundant bacterial populations on later host health in this population. This study supports a conclusion that STS care, a commonly used care practice in the NICU for the PT infant, is associated with an increased pace of oral microbe repertoire maturity development. Furthermore, the STS-exposed oral microbial repertoire exhibited a lower relative prevalence of organisms associated with intestinal dysfunction. Given the current understanding of the importance and long lasting impact of the developing infant’s microbiome, these results add to existing data related to our understanding of infant microbial development. Our results also present a potential role for the observed health impact of STS care and possibly provide an explanation related to the previously recognized enhanced intestinal function observed with STS care globally.
Acknowledgments
This work was supported by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health & Human Development, grant number 1R21HD059047-01A1 (Hendricks-Munoz, PI) and NICHD 8054HD080784, “Multi-’om’ic Analysis of the Vaginal Microbiome During Pregnancy” (Buck, PI). No additional external funding was received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hendricks-Muñoz KD, Perez-Perez G, Xu J, Kim Y, Louie M. Maternal antenatal treatments influence initial oral microbial acquisition in preterm infants. Am J Perinatol. 2013;30(1):47–52. doi: 10.1055/s-0032-1321499. [DOI] [PubMed] [Google Scholar]
- 2.Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr. 2012;96(3):544–551. doi: 10.3945/ajcn.112.037382. [DOI] [PubMed] [Google Scholar]
- 3.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences; June 21, 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.La Rosa PS, Warner BB, Zhou Y, et al. Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci U S A. 2014;111(34):12522–12527. doi: 10.1073/pnas.1409497111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobi SK, Odle J. Nutritional factors influencing intestinal health of the neonate. Adv Nutr. 2012;3(5):687–696. doi: 10.3945/an.112.002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jefferies AL, Canadian Paediatric Society, Fetus and Newborn Committee Kangaroo care for the preterm infant and family. Paediatr Child Health (Oxford) 2012;17(3):141–146. doi: 10.1093/pch/17.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlsson V, Heinemann AB, Sjörs G, Nykvist KH, Agren J. Early skin-to-skin care in extremely preterm infants: thermal balance and care environment. J Pediatr. 2012;161(3):422–426. doi: 10.1016/j.jpeds.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 8.Lawn JE, Davidge R, Paul VK, et al. Born too soon: care for the preterm baby. Reprod Health. 2013;10(Suppl 1):S5. doi: 10.1186/1742-4755-10-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawn JE, Mwansa-Kambafwile J, Barros FC, Horta BL, Cousens S. ‘Kangaroo mother care’ to prevent neonatal deaths due to preterm birth complications. Int J Epidemiol. 2011;42(2):525–528. doi: 10.1093/ije/dyq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charpak N, Ruiz JG, Zupan J, et al. Kangaroo Mother Care: 25 years after. Acta Paediatr. 2005;94(5):514–522. doi: 10.1111/j.1651-2227.2005.tb01930.x. [DOI] [PubMed] [Google Scholar]
- 11.Feldman R, Eidelman AI. Skin-to-skin contact (Kangaroo Care) accelerates autonomic and neurobehavioural maturation in preterm infants. Dev Med Child Neurol. 2003;45(4):274–281. doi: 10.1017/s0012162203000525. [DOI] [PubMed] [Google Scholar]
- 12.Flacking R, Ewald U, Wallin L. Positive effect of kangaroo mother care on long-term breastfeeding in very preterm infants. J Obstet Gynecol Neonatal Nurs. 2011;40(2):190–197. doi: 10.1111/j.1552-6909.2011.01226.x. [DOI] [PubMed] [Google Scholar]
- 13.Nyqvist KH, Anderson GC, Bergman N, et al. Expert Group of the International Network on Kangaroo Mother Care State of the art and recommendations. Kangaroo mother care: application in a high-tech environment. Breastfeed Rev. 2010;18(3):21–28. [PubMed] [Google Scholar]
- 14.Aloisio I, Mazzola G, Corvaglia LT, et al. Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Appl Microbiol Biotechnol. 2014;98(13):6051–6060. doi: 10.1007/s00253-014-5712-9. [DOI] [PubMed] [Google Scholar]
- 15.Fooladi AA, Khani S, Hosseini HM, Mousavi SF, Aghdam EM, Nourani MR. Impact of altered early infant gut microbiota following breastfeeding and delivery mode on allergic diseases. Inflamm Allergy Drug Targets. 2013;12(6):410–418. doi: 10.2174/1871528112666131205113129. [DOI] [PubMed] [Google Scholar]
- 16.Khodayar-Pardo P, Mira-Pascual L, Collado MC, Martínez-Costa C. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J Perinatol. 2014;34(8):599–605. doi: 10.1038/jp.2014.47. [DOI] [PubMed] [Google Scholar]
- 17.Bailey MT, Lubach GR, Coe CL. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J Pediatr Gastroenterol Nutr. 2004;38(4):414–421. doi: 10.1097/00005176-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Bosch JA, Ring C, de Geus EJ, Veerman EC, Amerongen AV. Stress and secretory immunity. Int Rev Neurobiol. 2002;52:213–253. doi: 10.1016/s0074-7742(02)52011-0. [DOI] [PubMed] [Google Scholar]
- 19.Walker WA. Initial intestinal colonization in the human infant and immune homeostasis. Ann Nutr Metab. 2013;63(Suppl 2):8–15. doi: 10.1159/000354907. [DOI] [PubMed] [Google Scholar]
- 20.West CE. Gut microbiota and allergic disease: new findings. Curr Opin Clin Nutr Metab Care. 2014;17(3):261–266. doi: 10.1097/MCO.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 21.Gröschl M, Topf HG, Kratzsch J, Dötsch J, Rascher W, Rauh M. Salivary leptin induces increased expression of growth factors in oral keratinocytes. J Mol Endocrinol. 2005;34(2):353–366. doi: 10.1677/jme.1.01658. [DOI] [PubMed] [Google Scholar]
- 22.Maron JL, Johnson KL, Rocke DM, Cohen MG, Liley AJ, Bianchi DW. Neonatal salivary analysis reveals global developmental gene expression changes in the premature infant. Clin Chem. 2010;56(3):409–416. doi: 10.1373/clinchem.2009.136234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray PA, Prakobphol A, Lee T, Hoover CI, Fisher SJ. Adherence of oral streptococci to salivary glycoproteins. Infect Immun. 1992;60(1):31–38. doi: 10.1128/iai.60.1.31-38.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olsen PS, Poulsen SS, Kirkegaard P, Nexø E. Role of submandibular saliva and epidermal growth factor in gastric cytoprotection. Gastroenterology. 1984;87(1):103–108. [PubMed] [Google Scholar]
- 25.Derrien M, van Passel MW, van de Bovenkamp JH, Schipper RG, de Vos WM, Dekker J. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes. 2010;1(4):254–268. doi: 10.4161/gmic.1.4.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge X, Rodriguez R, Trinh M, Gunsolley J, Xu P. Oral microbiome of deep and shallow dental pockets in chronic periodontitis. PLoS ONE. 2013;8(6):e65520. doi: 10.1371/journal.pone.0065520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fettweis JM, Alves JP, Brooks JP, et al. The vaginal microbiome: disease, genetics and the environment. Nat Preced. 2011;2011 doi: 10.1038/npre.2011.5150.2. [DOI] [Google Scholar]
- 28.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10(10):996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 30.Quast C, Pruesse E, Yilmaz P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(Database issue):D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 32.Huson DH, Auch AF, Qi J, Schuster SC. MEGAN analysis of meta-genomic data. Genome Res. 2007;17(3):377–386. doi: 10.1101/gr.5969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ludington-Hoe SM, Swinth JY. Developmental aspects of kangaroo care. J Obstet Gynecol Neonatal Nurs. 1996;25(8):691–703. doi: 10.1111/j.1552-6909.1996.tb01483.x. [DOI] [PubMed] [Google Scholar]
- 34.Mörelius E, Theodorsson E, Nelson N. Salivary cortisol and mood and pain profiles during skin-to-skin care for an unselected group of mothers and infants in neonatal intensive care. Pediatrics. 2005;116(5):1105–1113. doi: 10.1542/peds.2004-2440. [DOI] [PubMed] [Google Scholar]
- 35.Cong X, Ludington-Hoe SM, Walsh S. Randomized crossover trial of kangaroo care to reduce biobehavioral pain responses in preterm infants: a pilot study. Biol Res Nurs. 2011;13(2):204–216. doi: 10.1177/1099800410385839. [DOI] [PubMed] [Google Scholar]
- 36.Soukka H, Grönroos L, Leppäsalo J, Lehtonen L. The effects of skin-to-skin care on the diaphragmatic electrical activity in preterm infants. Early Hum Dev. 2014;90(9):531–534. doi: 10.1016/j.earlhumdev.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Ardiel EL, Rankin CH. The importance of touch in development. Paediatr Child Health (Oxford) 2010;15(3):153–156. doi: 10.1093/pch/15.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wahlberg V, Affonso DD, Persson BA. Retrospective, Comparative Study Using the Kangaroo Method as a Complement to the Standard Incubator Care. Eur J Public Health. 1992;2(1):34–37. [Google Scholar]
- 39.Brooks B, Firek BA, Miller CS, et al. Microbes in the neonatal intensive care unit resemble those found in the gut of premature infants. Microbiome. 2014;2:1. doi: 10.1186/2049-2618-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart CJ, Marrs EC, Magorrian S, et al. The preterm gut microbiota: changes associated with necrotizing enterocolitis and infection. Acta Paediatr. 2012;101(11):1121–1127. doi: 10.1111/j.1651-2227.2012.02801.x. [DOI] [PubMed] [Google Scholar]
- 42.Conde-Agudelo A, Belizán JM, Diaz-Rossello J. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2011;16(3):CD002771. doi: 10.1002/14651858.CD002771. [DOI] [PubMed] [Google Scholar]
- 43.Conde-Agudelo A, Díaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2014;4(4):CD002771. doi: 10.1002/14651858.CD002771.pub3. [DOI] [PubMed] [Google Scholar]
- 44.Cong X, Cusson RM, Walsh S, Hussain N, Ludington-Hoe SM, Zhang D. Effects of skin-to-skin contact on autonomic pain responses in preterm infants. J Pain. 2012;13(7):636–645. doi: 10.1016/j.jpain.2012.02.008. [DOI] [PubMed] [Google Scholar]


