ABSTRACT
Wolbachia bacteria are widespread, maternally transmitted endosymbionts of insects. Maintenance of sufficient Wolbachia titer in maternal germline cells is required for transmission efficacy. The mechanisms that regulate Wolbachia titer are not well understood; however, dietary sucrose was reported to elevate oocyte Wolbachia titer in Drosophila melanogaster whereas dietary yeast decreased oocyte titer. To further investigate how oocyte Wolbachia titer is controlled, this study analyzed the response of wMel Wolbachia to diets enriched in an array of natural sugars and other sweet tastants. Confocal imaging of D. melanogaster oocytes showed that food enriched in dietary galactose, lactose, maltose and trehalose elevated Wolbachia titer. However, oocyte Wolbachia titers were unaffected by exposure to the sweet tastants lactulose, erythritol, xylitol, aspartame and saccharin as compared to the control. Oocyte size was generally non-responsive to the nutrient-altered diets. Ovary size, however, was consistently smaller in response to all sugar- and sweetener-enriched diets. Furthermore, most dietary sugars administered in tandem with dietary yeast conferred complete rescue of oocyte titer suppression by yeast. All diets dually enriched in yeast and sugar also rescued yeast-associated ovary volume changes. This indicates oocyte colonization by Wolbachia to be a nutritionally sensitive process regulated by multiple mechanistic inputs.
KEY WORDS: Symbiosis, Wolbachia, Drosophila, Transmission, Oocyte, Titer
Summary: The density of Wolbachia infection in Drosophila oocytes is significantly increased by dietary sugars, but not sugar alcohols or artificial sweeteners.
INTRODUCTION
Metazoan organisms are increasingly recognized as communities of prokaryotic and eukaryotic cells. Symbiotic interactions within the collective unit of an organism range from mutualistic to parasitic (Dale and Moran, 2006). Endosymbiotic Wolbachia bacteria are unique in that they occupy a wide range of the symbiotic spectrum. Wolbachia are Alphaproteobacteria that reside within the cells of mites, crustaceans, filarial nematodes (Werren et al., 2008) and approximately 52% of all insect species based on a handful of typing loci (Weinert et al., 2015). At least 470 distinct Wolbachia strains have been reported to date (Baldo et al., 2006). Of those, some are reported to provide essential cofactors to the host (Ghedin et al., 2007; Hosokawa et al., 2010; Nikoh et al., 2014), promote host reproduction (Dedeine et al., 2001; Landmann et al., 2011; Starr and Cline, 2002) and protect the host from lethal RNA viruses (Chrostek et al., 2013; Hedges et al., 2008; Martinez et al., 2014; Teixeira et al., 2008). Conversely, the wMelPop Wolbachia variant lyses brain cells and shortens insect lifespan (Min and Benzer, 1997). This positions Wolbachia as a uniquely informative system for elucidating the cellular mechanisms of symbiosis.
A consensus requirement for Wolbachia success across diverse hosts is robust vertical transmission. Though Wolbachia occupy the germline stem cells (GSC) of male and female hosts, removal of the bacteria during spermatogenesis creates a ‘dead end’ with respect to transmission (Bressac and Rousset, 1993; Serbus et al., 2008). Thus, persistence of Wolbachia in maternal germline cells is of critical importance for transmission to progeny. In the Drosophila melanogaster model system that naturally carries wMel Wolbachia (O'Neill et al., 1992; Riegler et al., 2005), the GSC are infected with these bacteria. This ensures that differentiating daughter cells (cystoblasts) inherit Wolbachia during mitosis (Ferree et al., 2005; King, 1970; Serbus et al., 2008). While the cystoblast undergoes mitosis to generate an interconnected cyst of 16 germline cells, Wolbachia exiting the nearby somatic cell niche also invade the germline cyst (Toomey et al., 2013). After the cyst is coated with a blanket of somatic follicle cells, creating a unit referred to as an egg chamber (King, 1970), additional horizontal invasion events may also occur (Casper-Lindley et al., 2011). Wolbachia also replicate to populate the germline cells of the egg chamber, including the oocyte cell that ultimately takes over to form a completed egg (King, 1970; Serbus et al., 2011). Similar germline loading mechanisms are expected to apply to other Wolbachia-Drosophila combinations, with differential contributions to germline colonization by GSC loading and horizontal invasion in each case (Toomey et al., 2013).
Maternal transmission relies upon sufficient Wolbachia titer within the germline cells. One strategy of Wolbachia transmission in embryogenesis is the use of mass action to promote inclusion of bacteria in embryonic germline cells (Veneti et al., 2004). A complementary strategy to facilitate bacterial transmission is through strategic subcellular localization (Breeuwer and Werren, 1990; Hadfield and Axton, 1999; Rasgon and Scott, 2003; Stouthamer et al., 1993; Veneti et al., 2004; Zchori-Fein et al., 1998). In D. melanogaster, the host microtubule motor proteins Dynein and Kinesin-1 act sequentially to elevate Wolbachia concentration at the oocyte posterior cortex (Ferree et al., 2005; Serbus and Sullivan, 2007). This is followed by association of Wolbachia with a cortical mixture of components referred to as pole plasm (Ashburner, 1989; Riechmann and Ephrussi, 2001; Serbus and Sullivan, 2007). This positions the bacteria for envelopment by embryonic germline cells specified by the pole plasm (Ashburner, 1989; Hadfield and Axton, 1999; Serbus and Sullivan, 2007). Maternal Wolbachia transmission rates documented in D. melanogaster are near 97% in the field (Hoffmann et al., 1998) and 100% in the lab (Turelli and Hoffmann, 1995), indicating this maternal transmission strategy is effective.
The molecular mechanisms that regulate Wolbachia titer are not well understood. Body-wide Wolbachia titer has been reported to vary up to 180,000-fold in lab-reared offspring of mosquitoes collected from nature (Ahantarig et al., 2008), and 20,000-fold between wild-caught Drosophila innubila individuals (Unckless et al., 2009). This titer variation may be due in part to sensitivity to host temperature (Bordenstein and Bordenstein, 2011; Mouton et al., 2006, 2007; Wiwatanaratanabutr and Kittayapong, 2009, 2006), host crowding (Hoffmann et al., 1998; Wiwatanaratanabutr and Kittayapong, 2009), host genetic background (Boyle et al., 1993; Poinsot et al., 1998; Veneti et al., 2004; Serbus et al., 2011) and host age (Tortosa et al., 2010; Unckless et al., 2009).
A set of studies has particularly highlighted the impact of diet on Wolbachia titers in vivo, implicating roles for dietary cholesterol (Caragata et al., 2013) and other macronutrients (Ponton et al., 2015). It was recently shown that dietary yeast, known to trigger insulin signaling in Drosophila (Géminard, 2009 #1357; Teleman, 2010 #1333), suppresses Wolbachia titer in developing oocytes (Serbus et al., 2015 #1785). By contrast, dietary sucrose, which is expected to induce insulin resistance in Drosophila (Broughton et al., 2010; Morris et al., 2012; Musselman et al., 2011; Norseen et al., 2012; Pasco and Léopold, 2012; Yang et al., 2005), led to elevated oocyte titers (Serbus, 2015 #1785). Understanding how diet affects oocyte Wolbachia titer is expected to inform the mechanisms supporting Wolbachia colonization of host cells and ultimately, Wolbachia transmission. To address the mechanisms underlying Wolbachia titer control in oogenesis, an array of structurally diverse dietary sugars and sweet tastants was selected, and their impact on colonization investigated as described below.
RESULTS
Dietary sugars elevate oocyte Wolbachia titer in D. melanogaster
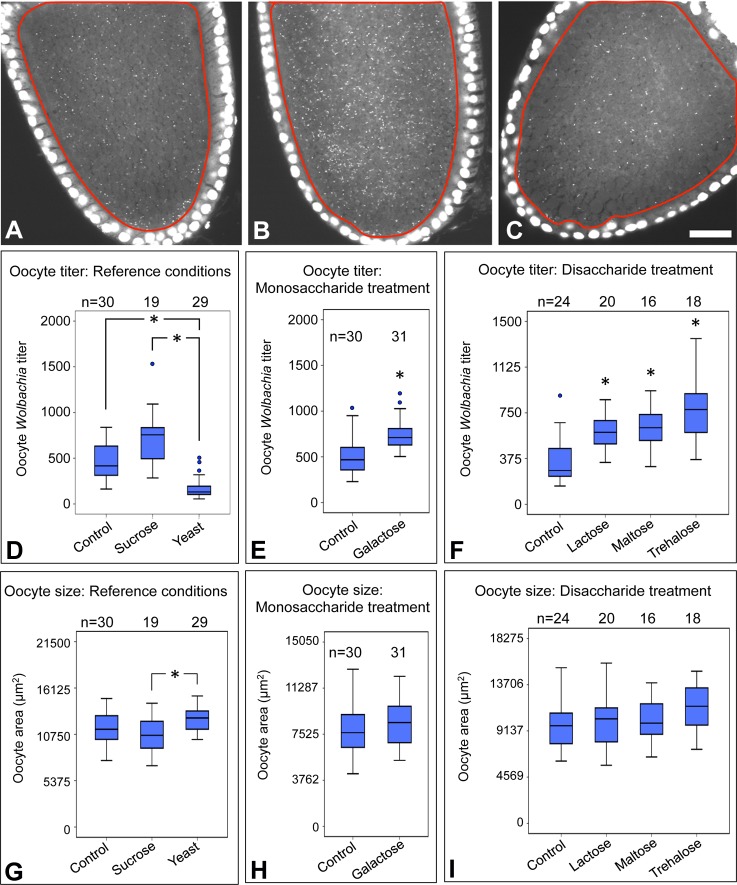
Prior results indicated that yeast-enriched food reduces Wolbachia titers in oogenesis, whereas sucrose-enriched food elevates oocyte Wolbachia titer (Serbus et al., 2015). To confirm this, two-day-old flies were exposed to yeast- and sucrose-enriched food for 3 days (Fig. 1E, Table 1; Table S1). The ovarian tissues were dissected, fixed, stained with propidium iodide and imaged by confocal microscopy. Each punctate nucleoid that is labeled by the DNA stain is interpreted as representing a single bacterium. The resulting images suggested overall more Wolbachia puncta in the sucrose condition, and fewer Wolbachia puncta in the yeast condition (Fig. 2A-C). For finer resolution of oocyte Wolbachia titer, Wolbachia were quantified from representative oocyte focal planes, and analyzed by Kruskal–Wallis ANOVA. According to these criteria, oocyte Wolbachia titer in the yeast-enriched condition was significantly lower than the control [χ2(2)=27.3, P<0.001] (Fig. 2D). Though higher oocyte Wolbachia titers were detected in the sucrose-enriched condition, the values did not differ significantly from the control [χ2(2)=15.6, P=0.056]. Significant oocyte titer differences were detected between the yeast and sucrose conditions, however, with sucrose exhibiting a 577% higher median titer value than yeast [χ2(2)=42.9, P<0.001] (Fig. 2D). Overall, this outcome corroborates opposing effects of dietary sucrose and yeast on oocyte Wolbachia titer.
Fig. 1.
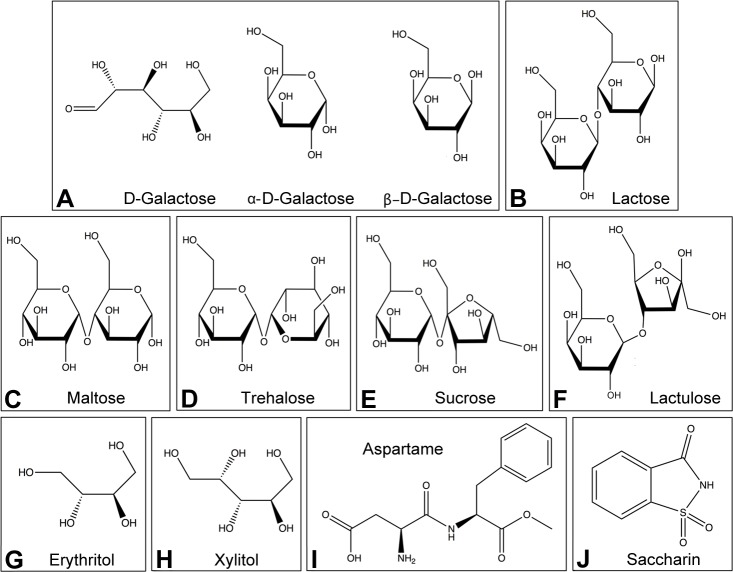
Structures of the sugars, sugar alcohols and artificial sweeteners used. Natural saccharides included: (A) D-galactose, shown in open ring, alpha-pyranose and beta-pyranose conformations; (B) lactose; (C) maltose; (D) trehalose; (E) sucrose. Synthetic disaccharide: (F) lactulose. Sugar alcohols: (G) erythritol; (H) xylitol. Artificial sweeteners: (I) aspartame; (J) saccharin.
Table 1.
Structural and chemical properties of sugars and sweet tastants used in this study
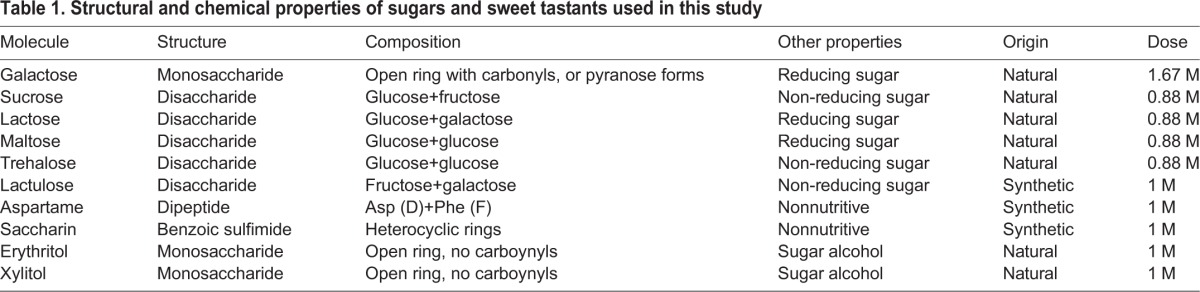
Fig. 2.
Effects of dietary sugars on oocyte Wolbachia titer and oocyte size. (A-C) Stage 10 D. melanogaster oocytes imaged by confocal microscopy are outlined in red. DNA staining indicates the Drosophila DNA as large circles and Wolbachia nucleoids as small puncta. Treatment conditions: (A) control food; (B) sucrose-enriched food; (C) yeast-enriched food. Scale bar: 25 μm. (D-I) Graphs indicate the average number of Wolbachia nucleoids displayed by single oocyte focal planes. Oocyte Wolbachia titer was scored in response to foods enriched with the following nutrients: (D) sucrose and yeast, (E) the monosaccharide galactose, and (F) the disaccharides lactose, maltose and trehalose. Oocyte size was also assessed from the same set of confocal images, to determine the profile of oocyte area for the following nutrient-enriched diets: (G) sucrose and yeast, (H) the monosaccharide galactose and (I) the disaccharides lactose, maltose and trehalose. To collect these data, three biological replicates were performed, with 20 flies dissected per condition per replicate. The sample size (n) for all experimental conditions is included in the figure. Median values are displayed as the middle line within each boxplot, and the boxed areas represent the interquartile range. The box whiskers indicate minimal and maximum values of the dataset, except for the outliers which are shown as solid blue circles. Significance is indicated by asterisks, as according to Kruskal–Wallis ANOVA. Significance values by panel are: (D,E) *P<0.001; (F) control vs lactose: *P=0.002, control vs maltose:*P<0.001, control vs trehalose: *P<0.001; (G) oocyte size: sucrose vs yeast: *P=0.004.
A surprising finding from prior work was that food enriched in the monosaccharide constituents of sucrose, namely glucose and fructose, did not recapitulate high oocyte titer responses analogous to sucrose (Serbus et al., 2015). This raised questions as to whether any monosaccharide is capable of affecting oocyte Wolbachia titer. To test this, two-day-old flies were collected and exposed to galactose-enriched food for 3 days (Fig. 1A, Table 1; Table S1). Wolbachia quantification indicated that galactose-fed flies carried significantly more Wolbachia than control oocytes [χ2(1)=18.2, P<0.001] (Fig. 2E). This indicates galactose to be the first dietary monosaccharide capable of elevating oocyte Wolbachia titer.
To test the extent to which other dietary disaccharides affect oocyte Wolbachia titer, flies were exposed to lactose-, maltose- and trehalose-enriched foods (Fig. 1B-D, Table 1; Table S1). These treatments elevated oocyte Wolbachia titer, with magnitude increasing from lactose to maltose to trehalose (Fig. 2F). Oocyte Wolbachia titers in disaccharide enriched conditions were also identified as significantly different from the control [lactose χ2(3)=25.0, P=0.002; maltose χ2(3)=28.8, P<0.001; trehalose χ2(3)=39.0, P<0.001] (Fig. 2F). This indicates that exposure to a range of disaccharide-enriched diets increases oocyte Wolbachia titer.
Oocyte size is generally non-responsive to sugar-enriched foods
To consider the basis for oocyte titer changes, oocyte size was tested. Specifically, the two-dimensional area of every oocyte image used for titer quantification above was measured. As all sample compression and oocyte focal plane selection were standardized for each experiment, the resulting area values are a proxy estimate for oocyte size. According to this analysis, no significant differences in oocyte area were identified between control and sucrose-enriched conditions [χ2(2)=12.2, P=0.085], nor control and yeast-enriched conditions [χ2(2)=6.6, P=0.811] (Fig. 2G). Significance was detected when comparing oocyte area values between sucrose- and yeast-enriched conditions [χ2(2)=18.7, P=0.004]. The median area of sucrose-treated oocytes was 84% of the yeast condition (Fig. 2G), in contrast to the 577% disparity between median oocyte Wolbachia titers in these conditions (Fig. 2D). Oocyte area did not differ significantly between control and galactose-fed oocytes [χ2(1)=1.27, P=0.26] (Fig. 2H), nor between control, lactose-, maltose-, and trehalose-fed oocytes [χ2(3)=6.72, P=0.083] (Fig. 2I). Thus, oocyte area did not parallel the significantly higher oocyte Wolbachia titer responses to natural saccharides (Fig. 2E,F). This suggests that oocyte size changes are not responsible for sugar-induced increases in oocyte Wolbachia titer.
Ovary size is consistently smaller in response to sugar-enriched diets
D. melanogaster ovary size is responsive to nutritional conditions. Through apparent impacts on systemic insulin signaling, sucrose-rich diets have been shown to reduce ovary size, whereas yeast-rich diets increase it (Fig. 3A) (Geminard et al., 2009; LaFever and Drummond-Barbosa, 2005; Morris et al., 2012). Direct measurement of ovary volume in response to these diets confirms that the size changes are substantial (Fig. 3B). Ovary volumes were significantly different between control and sucrose-fed oocytes [χ2(2)=29.1, P<0.001], and control and yeast-fed oocytes [χ2(2)=30.2, P<0.001] as well as sucrose- and yeast-fed oocytes [χ2(2)=59.3, P<0.001] (Fig. 3B). As dietary sucrose and yeast exert opposite impacts on ovary volume and Wolbachia titer, these data open the possibility that oocyte titer reflects ovary size.
Fig. 3.
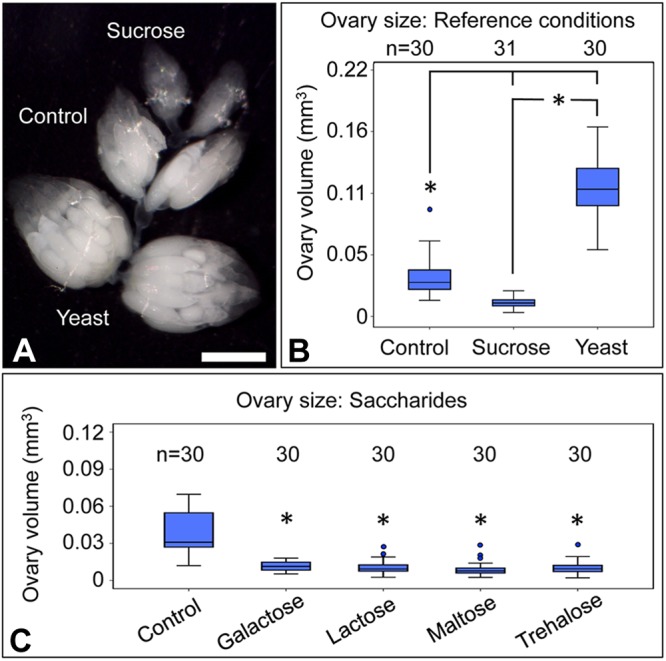
Ovary size response to sugar-enriched diets. (A) Image shows examples of ovaries dissected from flies exposed to control, sucrose, and yeast. Scale bar: 0.5 mm. The graphs show quantification of ovary volume after exposure to food enriched in (B) sucrose and yeast and (C) galactose, lactose, maltose, and trehalose. Ovary pairs were dissected in the context of three biological replicates, with 5 flies dissected per condition per replicate. The size of each ovary was measured independently. The sample size (n) for all experimental conditions is included in the figure. Median values are displayed as the middle line within each boxplot, and the boxed areas represent the interquartile range. The box whiskers indicate minimal and maximum values of the dataset, except for the outliers which are shown as solid blue circles. Significance is indicated by asterisks, as according to Kruskal–Wallis ANOVA; *P<0.001.
To further assess the relationship between ovary size and oocyte Wolbachia titer, ovary volume was assessed across sugar-enriched dietary conditions. This analysis indicated consistently small ovary volumes for galactose-, lactose-, maltose- and trehalose-fed oocytes [χ2(4)≥57.0, P<0.001 for all] (Fig. 3C). As these conditions significantly elevated oocyte Wolbachia titers (Fig. 2E,F), this outcome is consistent with oocyte Wolbachia titer as an inverse correlate of ovary size. Furthermore, median ovary volumes ranged from 25% of control in the maltose condition, to 36% of the control in the galactose condition (Fig. 3C). This is analogous to size reductions seen in sucrose-fed ovaries (39% of the control) (Fig. 3B). Taken together, the data suggest that sugar-enriched diets generally lead to ovary size reduction.
Sweet tastants affect ovary size, but not oocyte Wolbachia titer or oocyte size
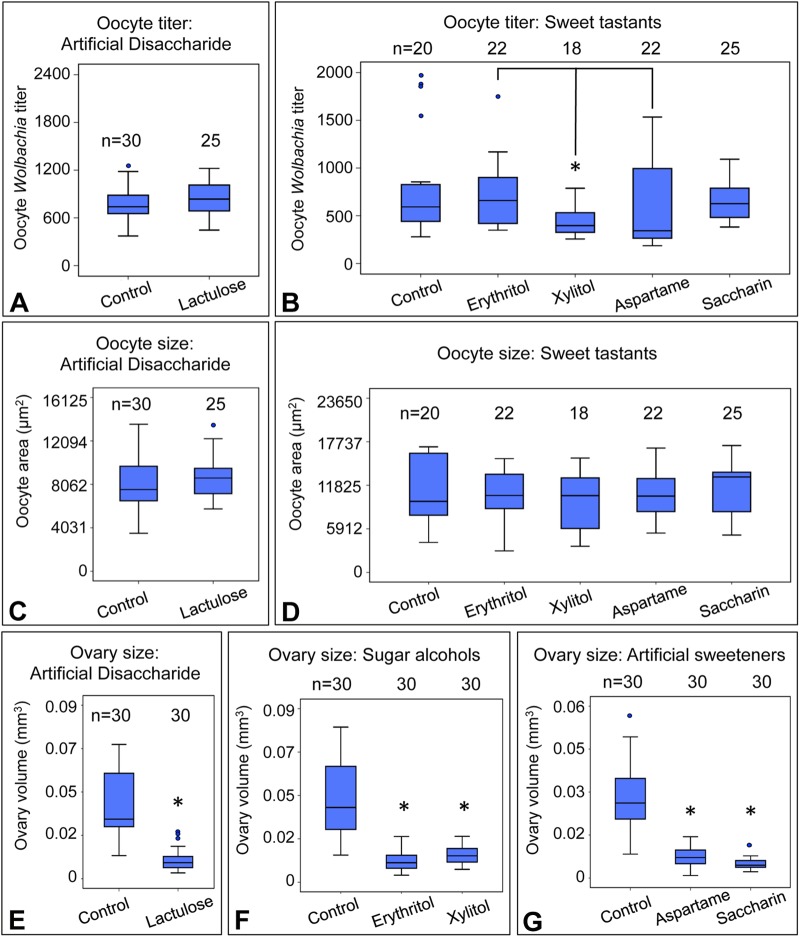
To test whether oocyte titer is selectively responsive to natural dietary sugars, an array of other sweet tastants was tested (Table 1; Table S1). Unlike natural sugars, the synthetic disaccharide lactulose (Fig. 1F) is reportedly indigestible by eukaryotes (Schmidl and Labuza, 2000). Flies exposed to lactulose-enriched diets did not exhibit any significant oocyte titer difference from the control [χ2(1)=1.57, P=0.211] (Fig. 4A). The impact of the sugar alcohols, erythritol and xylitol, as well as the artificial sweeteners aspartame and saccharin, were also tested (Fig. 1G-J). Oocyte Wolbachia titer was significantly different between xylitol and erythritol conditions [χ2(4)=28.9, P=0.034] as well as xylitol and saccharin conditions [χ2(4)=27.6, P=0.040] (Fig. 4B). However, no significant differences were evident when comparing the sweet tastant treatments against the control [χ2(4)≤26.5, P≥0.087 for all] (Fig. 4B). These data suggest that properties outside of taste recognition are responsible for sugar-driven increases in oocyte Wolbachia titer.
Fig. 4.
Analysis of conditions enriched in artificial- and non-saccharide sweet tastants. Oocyte Wolbachia titer is shown from flies exposed to diets enriched in (A) lactulose and (B) the sweet tastants erythritol, xylitol, aspartame and saccharin. Oocyte size is shown for dietary conditions enriched in: (C) lactulose and (D) erythritol, xylitol, aspartame and saccharin. For these experiments, three biological replicates were performed, with 20 flies dissected per condition per replicate. Stage 10 oocytes were selected at random for imaging by confocal microscopy, then analyzed to define oocyte Wolbachia titer and oocyte area. Ovary size was also measured in response to dietary conditions enriched in: (E) lactulose, (F) erythritol and xylitol, and (G) aspartame and saccharin. To perform this work, ovary pairs were dissected from three biological replicates, with 5 flies used per condition per replicate. Sizing of each ovary was measured independently. The sample size (n) for all experimental conditions is included in the figure. Median values are displayed as the middle line within each boxplot, and the boxed areas represent the interquartile range. The box whiskers indicate minimal and maximum values of the dataset, except for outliers, shown as solid blue circles. Significance is indicated by asterisks, as according to Kruskal–Wallis ANOVA. Significance values by panel are: (B) oocyte titer: xylitol vs erythritol: *P=0.034; xylitol vs saccharin: *P=0.040; (E-G) *P<0.001.
To consider the basis for the oocyte titers observed in sweet tastant conditions, oocyte and ovary sizing were also examined. No significant changes in oocyte area were detected in response to lactulose [χ2(1)=3.15, P=0.076], (Fig. 4C) nor artificial sweeteners and sugar alcohols [χ2(4)=1.75, P<0.782] (Fig. 4D). However, consistently small ovary size was detected in response to all sweet tastant treatments, with median ovary volumes ranging from 17% of the control for saccharin [χ2(2)=50.7, P<0.001] to 36% of the control for xylitol [χ2(2)=35.8, P<0.001] (Fig. 4E-G). Thus, ovary volume reduction associated with sweet tastants parallels that induced by sugar-enriched diets. However, as sugar-enriched diets elevate oocyte Wolbachia titer and sweet tastants do not, this indicates that oocyte Wolbachia titer is not specified exclusively by ovary size.
Desiccation-associated host diet lowers oocyte Wolbachia titer
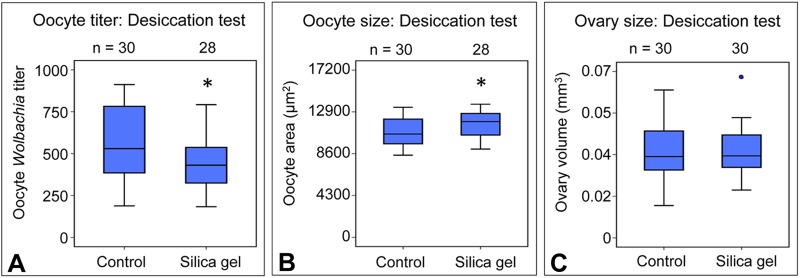
To further investigate the basis for sugar-associated oocyte titer increases, candidate hypotheses were pursued. During food preparation, the sugar solutions were distinctively thick in consistency compared to other treatments. The apparently hygroscopic properties of the sugars opened the possibility that they may act as a desiccant after ingestion. To test the impact of desiccation on oocyte Wolbachia titer, flies were exposed to standard fly food containing dehydrating silica gel in a 2:1 volumetric ratio. After 3 days of exposure, samples were examined. Analysis of oocyte Wolbachia titer indicated that fewer Wolbachia were carried by the silica gel condition, with the median oocyte titer value at 81% of the control [χ2(1)=4.95, P=0.026] (Fig. 5A). Oocyte size assessment indicated that oocyte area was significantly larger in the silica gel condition, with the median area value at 112% of the control [χ2(1)=4.09, P=0.043] (Fig. 5B). No differences in ovary size were observed between control and silica gel conditions [χ2(1)=0.056, P=0.813] (Fig. 5C). These data suggest that desiccation impacts on Wolbachia and oogenesis are entirely distinct from that of the dietary sugars. Thus, desiccation is not responsible for the titer-increasing effects of dietary saccharides.
Fig. 5.
Assessing response to desiccated food diet. Graphical representations of (A) oocyte Wolbachia titer, (B) oocyte size, and (C) ovary size response to desiccated food conditions. For oocyte Wolbachia titer and oocyte size analysis, three biological replicates of the experiment were performed, 20 flies were dissected per condition per replicate. For ovary size analysis, ovary pairs were dissected from 15 flies total, 5 flies per replicate. Each ovary was measured independently. The sample size (n) for all experimental conditions is included in the figure. Median values are displayed as the middle line within each boxplot, and the boxed areas represent the interquartile range. The box whiskers indicate minimal and maximum values of the dataset, except for outliers, shown as solid blue circles. Significance is indicated by asterisks, as according to Kruskal–Wallis ANOVA. Significance values by panel are: (A) oocyte titer: control vs silica gel: P=0.026; (B) oocyte size: control vs silica gel: P=0.043.
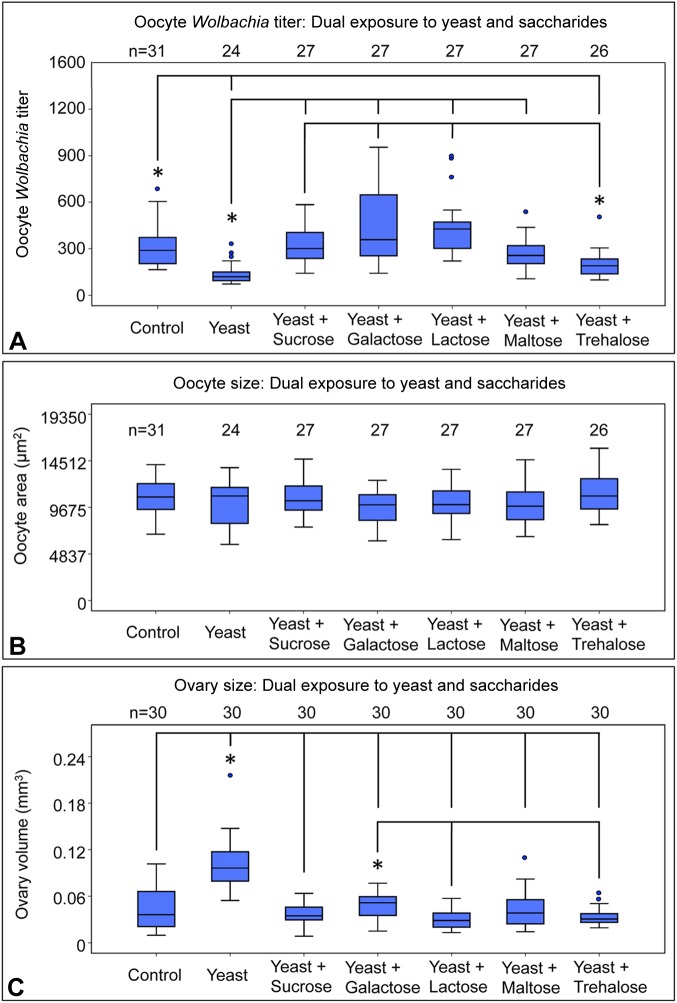
Dietary sugars differentially rescue dietary yeast impact on oocyte Wolbachia titer
Another possibility is that titer-elevating sugars generally affect oocyte Wolbachia titer through impact on core nutritional signaling processes. It was previously demonstrated that dietary yeast and sucrose exert opposite effects on oocyte Wolbachia titer in an insulin-dependent manner (Serbus et al., 2015). If dietary sugars are generally antagonistic to insulin signaling, one possibility is that they will rescue the impact of dietary yeast on oocyte Wolbachia titer. To test this, flies were exposed to diets dually enriched in yeast and dietary saccharides. An array of responses was evident (Fig. 6A). No dual feedings of yeast and sugar elevated oocyte titer significantly above control levels. Relative to the control, oocytes exposed to diets enriched in either yeast or yeast+trehalose showed significant depletion of Wolbachia [χ2(6)=75.5, P<0.001 and χ2(6)=47.9, P<0.021, respectively]. Yeast-fed oocytes showed the overall lowest titer levels, differing significantly from all dual yeast-sugar feeding conditions [χ2(6)≥59, P≤0.002 for all] except yeast+trehalose [χ2(6)=27.5, P=1.000]. The yeast+trehalose condition displayed significantly lower oocyte Wolbachia titer as compared to dual feedings of yeast+sucrose, galactose, or lactose [χ2(6)≥53.6, P≤0.008 for all] (Fig. 6A). Thus, the yeast+trehalose oocyte titer profile paralleled many of the outcomes associated with exposure to dietary yeast alone. These data overall indicate that dietary sucrose, galactose, lactose and maltose rescue oocyte titer suppression by dietary yeast, whereas dietary trehalose does not.
Fig. 6.
Assessing impact of diets dually enriched in yeast and natural saccharides. The sugars co-administered with yeast included: sucrose, galactose, lactose, maltose and trehalose. Graphs represent changes in quantification of (A) oocyte Wolbachia titer, (B) oocyte area, and (C) ovary volume in response to dual feeding conditions. For oocyte Wolbachia titer and oocyte size analyses, three biological replicates of the experiment were performed, 20 flies were dissected per condition per replicate. Stage 10 oocytes were selected randomly for confocal microscopy imaging, then followed up for quantification. For assessment of ovary size, ovary pairs were dissected from 15 flies total per condition, 5 flies per replicate. Each ovary was measured independently. The sample size (n) for all experimental conditions is included in the figure. Median values are displayed as the middle line within each boxplot, and the boxed areas represent the interquartile range. The box whiskers indicate minimal and maximum values of the dataset, except for outliers, shown as solid blue circles. Significance is indicated by asterisks, as according to Kruskal–Wallis ANOVA. Significance values by panel are: (A) Oocyte titer: *P<0.001 with the exception of: control vs yeast + trehalose: P=0.021; yeast vs yeast + maltose: P=0.002; and yeast + trehalose vs yeast + sucrose: P=0.008. (C) Ovary size: *P<0.001 with the exception of: yeast + galactose vs yeast + lactose: P=0.005; and yeast + galactose vs yeast + trehalose: P=0.035.
Dietary sugars consistently rescue yeast-driven ovary enlargement
To investigate the basis for differential oocyte Wolbachia titer responses to yeast+sugar diets, sizing controls were also performed. No significant differences in oocyte area were observed between any of the feeding conditions used [χ2(6)=9.03, P=0.172] (Fig. 6B). By contrast, ovary size was responsive to nutrient-altered diets. Ovary volumes in all yeast+sugar feeding conditions were significantly lower than in the yeast-fed condition [χ2(6)≥67.5, P<0.001 for all cases] (Fig. 6C). Furthermore, no significant ovary volume differences were observed between the control and any of the yeast+sugar dual feeding conditions [χ2(6)≤36.8, P≥0.397 for all]. This indicates that dietary sugars consistently rescued yeast-driven ovary enlargement. Some variation was detected in the extent of ovary size modification by dual yeast+sugar diets. Ovary volumes in the yeast+galactose condition were distinguished as significantly greater than the yeast+lactose condition [χ2(6)=57.6, P<0.005] and the yeast+trehalose condition [χ2(6)=49.3, P<0.035] (Fig. 6C). As these trends do not parallel oocyte titer outcomes in a consistent manner, the implication is that dietary sugars affect ovary development and Wolbachia colonization dynamics through mechanisms that are at least partially independent.
DISCUSSION
The impact of diverse dietary sugars on insulin signaling has not been fully defined in D. melanogaster. From the perspective of Wolbachia endosymbiosis, this study suggests that dietary sugars induce different classes of mechanistic responses. This work showed that D. melanogaster diets enriched in galactose, lactose, maltose and trehalose significantly elevated oocyte Wolbachia titer. As oocyte size was also unaffected by most dietary sugar treatments, all observed titer increases are interpreted to represent true elevation of bacterial quantity per oocyte and not a concentration artifact of cell size. No titer-related trends were evident in terms of reported caloric value (Table S1), gustatory preferences (Stafford et al., 2012), nor the magnitude of neural responses to single tastants (Freeman et al., 2014). These outcomes are most readily reconciled with the structural content of the sugars, as maltose and trehalose are both glucose disaccharides, and lactose contains galactose as one of its major constituents. The absence of titer-elevating effects by lactulose is consistent with a possible requirement for glucose as a constituent of titer-influencing disaccharides.
Though these sugars were selected for analysis due to their structural features, D. melanogaster may reasonably encounter some of these sugars in nature. Maltose is reportedly the major starch breakdown product released from chloroplasts at night (Niittayla et al., 2004; Wiese et al., 2004) and is commonly associated with starchy plant products such as grains (Halford et al., 2011). Natural exposure to trehalose is less likely, as it is carried at low levels in higher plants, serving as a signaling cue (Grennan, 2007; Lunn et al., 2014). Galactose is a core component of raffinose-containing oligosaccharides that are widespread in higher plants (Sengupta et al., 2015) and carried within dozens of fruits and vegetables (Gross and Acosta, 1991). Though lactose is not expected to appear in a natural D. melanogaster diet, one highly speculative possibility is that lactose digestion by microbes occupying the food vial or gut microbiome releases galactose, ultimately inducing titer responses. Future analyses of sugar uptake by D. melanogaster are needed to further inform the relevance of ingested doses. At this time, only two (Meyer et al., 2011; Wang and Wang, 1993) of the 26 predicted sugar transporters in D. melanogaster (Adams et al., 2000; Marygold et al., 2016) have been characterized. However, 17 of the predicted transporter proteins are expressed by females and detected in digestive tissues, and are thus potentially relevant for consideration (Table S2).
Another finding of this study was that ovary size responses to dietary cues did not consistently correspond to oocyte titer changes. As ovary size and oocyte Wolbachia titer are oppositely affected by sugar- and yeast-enriched diets, the simplest interpretation is that spatial re-allocation of Wolbachia within the ovary is responsible for increased oocyte titers. This hypothesis invokes horizontal Wolbachia invasion between cells of the ovary as influential in oocyte colonization by Wolbachia. Invasion has been reported to contribute to Wolbachia colonization of the distal tip of the Drosophila ovary (Toomey et al., 2013), early stages of Drosophila oogenesis (Casper-Lindley et al., 2011) and ovarian cells of mosquitoes and nematodes (Hughes et al., 2012; Landmann et al., 2012). Sugar alcohol and artificial sweetener treatments deviated from this invasion paradigm, as their reductions of ovary size were not paralleled by oocyte titer increases. Thus, ovary size may modulate oocyte colonization by Wolbachia titer in some cases, but the data argue against ovary size as a binary predictor of oocyte titer. One possibility is that changes in bacterial loading and replication in the germline offset changes in the extent of horizontal invasion. Reduced oocyte titers seen in xylitol-fed oocytes suggest that antibiotic properties of this sugar alcohol (Katsuyama et al., 2005; Renko et al., 2008) may exaggerate such tendencies. Another possibility is that dietary saccharides and sweet tastants each alter ovary size in a different manner. Ovary size reflects the number of productive ovarioles as well as the rates of egg chamber production, egg development and egg laying by each female (King, 1970). At this time, it cannot be ruled out that the physical basis for small ovary size may differ between saccharide and sweet tastant conditions.
This study further sought to address the mechanistic basis for saccharide impacts on oocyte Wolbachia titer. One possibility was that the concentrated sugar additives may increase oocyte Wolbachia titer as an indirect consequence of ovarian responses to desiccation. However, dietary desiccation tests showed reduction of oocyte Wolbachia titer rather than an increase, suggesting that sugar-based titer responses are unrelated to hydration. Another formal possibility is that Wolbachia responsiveness to dietary sugars is due to uptake of these sugars and/or their derivatives after ingestion. Though Wolbachia are predicted to encode a single hexose phosphate transporter, WD_0619, homologous to GlpT/PgpT/UhpT of Escherichia coli (Fann and Maloney, 1998; Kadner et al., 1992), there is no information to suggest the Wolbachia homolog of this transporter is sufficiently permissive to take up the diverse dietary sugars analyzed in this study.
The impact of dietary sugars on oocyte Wolbachia titer is currently best explained through nutritional impacts on the host. Dietary yeast is expected to activate multiple nutritional signaling branches, including insulin signaling, that converge upon activation of the mTORC1 kinase complex (Geminard et al., 2009; Teleman, 2010). Prior work showed that chemical inhibition of mTORC1 increased oocyte Wolbachia titer analogous to dietary sucrose, while loss of mTORC1 suppression lowered oocyte titers (Serbus et al., 2015). These findings and others implicated insulin as a suppressor of oocyte Wolbachia titer and inherently suggested that yeast-associated phenotypes should be ameliorated by dietary sugars. This study showed that dietary sugars did suppress yeast-associated ovary enlargement across the board, consistent with such a prediction. However, oocyte Wolbachia titers showed a range of responses, with trehalose exerting no impact on yeast-driven titer depletion, whereas sucrose, galactose, lactose and maltose restored oocyte titer to control levels. The disparity is surprising, as trehalose-enriched diets elicited the largest recorded oocyte titer increase to date. Examination of ovary size further indicated that galactose rescue of yeast-driven ovary enlargement was significantly less effective than lactose and trehalose. These findings suggest that saccharide treatments, all singly capable of elevating oocyte Wolbachia titer, may exert distinct functional impacts on oocyte Wolbachia titer. Integrated quantitative analyses will play an important role going forward in elucidating the mechanisms of oocyte colonization by Wolbachia.
MATERIALS AND METHODS
Fly food preparation
The standard food used in this study is based upon a recipe by the Bloomington Drosophila Stock Center (http://flystocks.bio.indiana.edu/Fly_Work/media-recipes/bloomfood.htm). Our fly food was prepared in large batches that consisted of 20 liters water, 337 g yeast, 190 g soy flour, 1325 g yellow corn meal, 96 g agar, 1.5 liters Karo light corn syrup and 94 ml propionic acid. This standard food was used as a base for all nutrient-altered foods that were prepared in this study (Table S3). The sugar-enriched foods were prepared by first making a stock sugar solution of 20 g sugar in 10 ml ddH2O, solubilized with rounds of 15 s in the microwave and then stirring, repeated until the sugar dissolved. 1.5 ml amounts of these sugar solutions were immediately mixed with 3.5 ml of melted standard food. As aspartame, erythritol, saccharin, and xylitol were not uniformly soluble, the sweetener-enriched foods were generated through direct addition of powder equivalents directly into 5 ml of melted standard food to a final concentration of 1 M (Table S3). Yeast-enriched food was prepared by mixing 1.5 ml of heat-killed yeast paste into 3.5 ml melted standard food. Dually enriched food was prepared through addition of 1.5 ml sugar solution and 1.5 ml heat-killed yeast to 2 ml standard food. Desiccated food was prepared by addition of 2.5 g silica gel (roughly 2.5 ml volume) to vials containing 5 ml standard food (Table S3). To ensure homogeneous suspensions of nutrient-altered diet preparations, all food vials were immediately transferred to an ice bucket to be cooled with additional stirring every 10 min until the food completely solidified. Kimwipe strips were inserted into the food to wick away excess moisture.
All feeding experiments were done using flies of the genotype w; Sp/Cyo; Sb/Tm6B, reared on standard food and in a controlled, 25°C environment. This stock carries the wMel Wolbachia strain as confirmed previously (Christensen et al., 2016). 0−24-hour-old adult flies were selected at random and transferred into new bottles of standard food and aged for 2 days. Then flies were transferred to vials of nutrient-altered food and incubated for 3 days. Controls were run in parallel with all treatment conditions in all experiments.
Tissue staining, imaging, and analysis
Ovarian tissues were dissected in PBS and fixed in 2% formaldehyde for 20 min as previously described (Serbus et al., 2015). The ovaries were rinsed with PBS-Triton 0.1% (PBS-T), incubated overnight in 10 mg ml−1 RNAse, and rinsed extensively with PBS-T the next day. Then tissues were infused with 70% glycerol that contained 0.015 mg ml−1 propidium iodide, and mounted on a slide. All replicates were imaged by laser scanning confocal microscopy on either Leica SP2 or an Olympus FV1200 confocal microscope at 63× magnification with 1.5× zoom. The Z-height of oocytes on each slide was standardized against the control slide for each replicate. Z-series images were acquired from randomly selected stage 10 egg chambers at 1.5 μm intervals. Uniform intensity settings were applied to all egg chambers imaged in each replicate.
To quantify oocyte Wolbachia titer, stacks of confocal images were examined to identify the deepest possible focal plane where Wolbachia are clearly visible across all samples of the replicate (Serbus et al., 2015). Images were manually processed in Photoshop to remove extraneous signal outside the oocyte, and remaining oocyte puncta were quantified using the Analyze Particles feature in Image J version 2.0.0-rc-43/1.51d (NIH). Thus, these data quantify the Wolbachia titer carried within one focal plane of each oocyte. This has been verified as a representative measure for comparing oocyte Wolbachia titer between different conditions (Serbus et al., 2011). Three or more experimental replicates were performed for all treatment conditions examined. Significance of differences between conditions was determined by ANOVA analysis of the raw data.
For measurement of oocyte area, the same representative oocyte focal planes used for Wolbachia titer assessment were re-analyzed. Oocytes were manually outlined in Microsoft PowerPoint, and the resulting two-dimensional shapes flood-filled in with color. Screen shots of these ovary fill diagrams were then imported into Fiji (Image J version 2.0.0-rc-43/1.51d, NIH) for conversion into 8-bit, thresholded black and white images. The area of the ovary fill diagrams was determined in terms of pixels2 by the Analyze Particles function in Fiji. A scale bar was also used to calculate a pixel2 to micron2 ratio (9.3025:1) that was applied to all oocyte area data, for presentation and discussion purposes only. Statistical differences were determined through analysis of the primary data in terms of pixel2 units.
For measurement of ovary volume, tissues were dissected from adult flies and imaged using an AmScope MD500 5.0 megapixel digital Camera mounted upon a Jenco ST-F803 dissection microscope set at 1× magnification. The pixel length and width of each ovary was assessed with the ‘Measure’ tool in Fiji. These values used to approximate ovary volume using the standard ellipsoid formula for volume; V=4 3πabc−1, where a=½ the length and b and c=½ the width. Three biological replicates were assessed for all treatment conditions. The area of a reference object was measured to determine the pixel to mm ratio (148.62:1) appropriate for describing the volumetric data. This conversion was applied in the context of presentation and discussion purposes only. All statistical analyses of ovary volumes were based upon primary data in terms of the pixel3 units.
Statistical analysis
All data in this study were analyzed with the IBM SPSS statistics program, v.23. The descriptive statistics function was used to analyze the distribution of the data. According to the residuals as well as metrics such as skewedness and kurtosis, the current data did not fit a normal distribution. However, the data met the assumptions of the two-tailed Kruskal–Wallis ANOVA, and thus this test was systematically applied for data analysis. Variation within each experimental condition is indicated by boxplot format used to graphically display the data. Post hoc data presented here were generated by SPSS as standard outputs of the analysis, including the adjusted P-values reported throughout the manuscript. Though subtle differences in oocyte titer, oocyte area and ovary volume may not be detected by this analysis framework, the data empirically demonstrated that the sample ‘n’ for these experiments was sufficient to identify clear cases of significance.
Acknowledgements
We thank A. J. M. Zehadee Momtaz, Jessica Pintado Silva, Steen Christensen, Pamela White, Christopher Chin, Yongjun Huang, M. Alejandro Barbieri and Erasmo Perera for their assistance and helpful discussions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: L.R.S.; Methodology: M.C., L.R.S.; Validation: L.R.S.; Formal analysis: M.C., M.O., L.R.S.; Investigation: M.C., M.O., L.R.S.; Resources: L.R.S.; Data curation: M.C., M.O., L.R.S.; Writing - original draft: M.C., L.R.S.; Writing - review & editing: M.C., M.O., L.R.S.; Visualization: M.C., M.O., L.R.S.; Supervision: L.R.S.; Project administration: L.R.S.; Funding acquisition: M.O, L.R.S.
Funding
Support for this work was provided by startup funds from Florida International University and FIU MBRS Rise Program (grant no. 5R25GM061347-16).
Supplementary information
Supplementary information available online at http://bio.biologists.org/lookup/doi/10.1242/bio.023895.supplemental
References
- Adams M. D., Celniker S. E., Holt R. A., Evans C. A., Gocayne J. D., Amanatides P. G., Scherer S. E., Li P. W., Hoskins R. A., Galle R. F. et al. (2000). The genome sequence of Drosophila melanogaster. Science 287, 2185-2195. 10.1126/science.287.5461.2185 [DOI] [PubMed] [Google Scholar]
- Ahantarig A., Trinachartvanit W. and Kittayapong P. (2008). Relative Wolbachia density of field-collected Aedes albopictus mosquitoes in Thailand. J. Vector Ecol. 33, 173-177. 10.3376/1081-1710(2008)33[173:RWDOFA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ashburner M. (1989). Developmental biology. In Drosophila, a Laboratory Handbook, pp. 139-204. Cold Spring Harbor: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Baldo L., Dunning Hotopp J. C., Jolley K. A., Bordenstein S. R., Biber S. A., Choudhury R. R., Hayashi C., Maiden M. C. J., Tettelin H. and Werren J. H. (2006). Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 72, 7098-7110. 10.1128/AEM.00731-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomington Drosophila Stock Center. (2014). Standard medium in use at Indiana University, Bloomington Drosophila Stock Center. http://flystocks.bio.indiana.edu/Fly_Work/media-recipes/bloomfood.htm [Google Scholar]
- Bordenstein S. R. and Bordenstein S. R. (2011). Temperature affects the tripartite interactions between bacteriophage WO, Wolbachia, and cytoplasmic incompatibility. PLoS ONE 6, e29106 10.1371/journal.pone.0029106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle L., O'Neill S. L., Robertson H. M. and Karr T. L. (1993). Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260, 1796-1799. 10.1126/science.8511587 [DOI] [PubMed] [Google Scholar]
- Breeuwer J. A. J. and Werren J. H. (1990). Microorganisms associated with chromosome destruction and reproductive isolation between two insect species. Nature 346, 558-560. 10.1038/346558a0 [DOI] [PubMed] [Google Scholar]
- Bressac C. and Rousset F. (1993). The reproductive incompatibility system in Drosophila simulans: DAPI-staining analysis of the Wolbachia symbionts in sperm cysts. J. Invertebr. Pathol. 61, 226-230. 10.1006/jipa.1993.1044 [DOI] [PubMed] [Google Scholar]
- Broughton S. J., Slack C., Alic N., Metaxakis A., Bass T. M., Driege Y. and Partridge L. (2010). DILP-producing median neurosecretory cells in the Drosophila brain mediate the response of lifespan to nutrition. Aging Cell 9, 336-346. 10.1111/j.1474-9726.2010.00558.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caragata E. P., Rancès E., Hedges L. M., Gofton A. W., Johnson K. N., O'Neill S. L. and McGraw E. A. (2013). Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 9, e1003459 10.1371/journal.ppat.1003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper-Lindley C., Kimura S., Saxton D. S., Essaw Y., Simpson I., Tan V. and Sullivan W. (2011). Rapid fluorescence-based screening for wolbachia endosymbionts in Drosophila germ line and somatic tissues. Appl. Environ. Microbiol. 77, 4788-4794. 10.1128/AEM.00215-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S., Pérez Dulzaides R., Hedrick V. E., Momtaz A. J. M. Z., Nakayasu E. S., Paul L. N. and Serbus L. R. (2016). Wolbachia endosymbionts modify Drosophila ovary protein levels in a context-dependent manner. Appl. Environ. Microbiol. 82, 5354-5363. 10.1128/AEM.01255-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrostek E., Marialva M. S. P., Esteves S. S., Weinert L. A., Martinez J., Jiggins F. M. and Teixeira L. (2013). Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genet. 9, e1003896 10.1371/journal.pgen.1003896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale C. and Moran N. A. (2006). Molecular interactions between bacterial symbionts and their hosts. Cell 126, 453-465. 10.1016/j.cell.2006.07.014 [DOI] [PubMed] [Google Scholar]
- Dedeine F., Vavre F., Fleury F., Loppin B., Hochberg M. E. and Bouletreau M. (2001). Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc. Natl. Acad. Sci. USA 98, 6247-6252. 10.1073/pnas.101304298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann M.-C. and Maloney P. C. (1998). Functional symmetry of UhpT, the sugar phosphate transporter of Escherichia coli. J. Biol. Chem. 273, 33735-33740. 10.1074/jbc.273.50.33735 [DOI] [PubMed] [Google Scholar]
- Ferree P. M., Frydman H. M., Li J. M., Cao J., Wieschaus E. and Sullivan W. (2005). Wolbachia utilizes host microtubules and Dynein for anterior localization in the Drosophila oocyte. PLoS Pathog. 1, e14 10.1371/journal.ppat.0010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman E. G., Wisotsky Z. and Dahanukar A. (2014). Detection of sweet tastants by a conserved group of insect gustatory receptors. Proc. Natl. Acad. Sci. USA 111, 1598-1603. 10.1073/pnas.1311724111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Géminard C., Rulifson E. J. and Léopold P. (2009). Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 10, 199-207. 10.1016/j.cmet.2009.08.002 [DOI] [PubMed] [Google Scholar]
- Ghedin E., Wang S., Spiro D., Caler E., Zhao Q., Crabtree J., Allen J. E., Delcher A. L., Guiliano D. B., Miranda-Saavedra D. et al. (2007). Draft genome of the filarial nematode parasite Brugia malayi. Science 317, 1756-1760. 10.1126/science.1145406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grennan A. K. (2007). The role of trehalose biosynthesis in plants. Plant Physiol. 144, 3-5. 10.1104/pp.104.900223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross K. C. and Acosta P. B. (1991). Fruits and vegetables are a source of galactose: implications in planning the diets of patients with galactosaemia. J. Inherit. Metab. Dis. 14, 253-258. 10.1007/BF01800599 [DOI] [PubMed] [Google Scholar]
- Hadfield S. J. and Axton J. M. (1999). Germ cells colonized by endosymbiotic bacteria. Nature 402, 482 10.1038/45002 [DOI] [PubMed] [Google Scholar]
- Halford N. G., Curtis T. Y., Muttucumaru N., Postles J. and Mottram D. S. (2011). Sugars in crop plants. Ann. Appl. Biol. 158, 1-25. 10.1111/j.1744-7348.2010.00443.x [DOI] [Google Scholar]
- Hedges L. M., Brownlie J. C., O'Neill S. L. and Johnson K. N. (2008). Wolbachia and virus protection in insects. Science 322, 702 10.1126/science.1162418 [DOI] [PubMed] [Google Scholar]
- Hoffmann A. A., Hercus M. and Dagher H. (1998). Population dynamics of the Wolbachia infection causing cytoplasmic incompatibility in Drosophila melanogaster. Genetics 148, 221-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa T., Koga R., Kikuchi Y., Meng X.-Y. and Fukatsu T. (2010). Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl. Acad. Sci. USA 107, 769-774. 10.1073/pnas.0911476107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes G. L., Pike A. D., Xue P. and Rasgon J. L. (2012). Invasion of Wolbachia into Anopheles and other insect germlines in an ex vivo organ culture system. PLoS ONE 7, e36277 10.1371/journal.pone.0036277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., Murphy G. P. and Stephens C. M. (1992). Two mechanisms for growth inhibition by elevated transport of sugar phosphates in Escherichia coli. J. Gen. Microbiol. 138, 2007-2014. 10.1099/00221287-138-10-2007 [DOI] [PubMed] [Google Scholar]
- Katsuyama M., Ichikawa H., Ogawa S. and Ikezawa Z. (2005). A novel method to control the balance of skin microflora. Part 1. Attack on biofilm of Staphylococcus aureus without antibiotics. J. Dermatol. Sci. 38, 197-205. 10.1016/j.jdermsci.2005.01.006 [DOI] [PubMed] [Google Scholar]
- King R. C. (1970). Ovarian Development in Drosophila melanogaster. New York: Academic Press. [Google Scholar]
- LaFever L. and Drummond-Barbosa D. (2005). Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science 309, 1071-1073. 10.1126/science.1111410 [DOI] [PubMed] [Google Scholar]
- Landmann F., Voronin D., Sullivan W. and Taylor M. J. (2011). Anti-filarial activity of antibiotic therapy is due to extensive apoptosis after Wolbachia depletion from filarial nematodes. PLoS Pathog. 7, e1002351 10.1371/journal.ppat.1002351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann F., Bain O., Martin C., Uni S., Taylor M. J. and Sullivan W. (2012). Both asymmetric mitotic segregation and cell-to-cell invasion are required for stable germline transmission of Wolbachia in filarial nematodes. Biol. Open 1, 536-547. 10.1242/bio.2012737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn J. E., Delorge I., Figueroa C. M., Van Dijck P. and Stitt M. (2014). Trehalose metabolism in plants. Plant J. 79, 544-567. 10.1111/tpj.12509 [DOI] [PubMed] [Google Scholar]
- Martinez J., Longdon B., Bauer S., Chan Y.-S., Miller W. J., Bourtzis K., Teixeira L. and Jiggins F. M. (2014). Symbionts commonly provide broad spectrum resistance to viruses in insects: a comparative analysis of Wolbachia strains. PLoS Pathog. 10, e1004369 10.1371/journal.ppat.1004369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marygold S. J., Crosby M. A., Goodman J. L. and The Flybase Consortium (2016). Using FlyBase, a database of Drosophila genes and genomes. Methods Mol. Biol. 1478, 1-31. 10.1007/978-1-4939-6371-3_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H., Vitavska O. and Wieczorek H. (2011). Identification of an animal sucrose transporter. J. Cell Sci. 124, 1984-1991. 10.1242/jcs.082024 [DOI] [PubMed] [Google Scholar]
- Min K.-T. and Benzer S. (1997). Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. USA 94, 10792-10796. 10.1073/pnas.94.20.10792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. N. S., Coogan C., Chamseddin K., Fernandez-Kim S. O., Kolli S., Keller J. N. and Bauer J. H. (2012). Development of diet-induced insulin resistance in adult Drosophila melanogaster. Biochim. Biophys. Acta 1822, 1230-1237. 10.1016/j.bbadis.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton L., Henri H., Bouletreau M. and Vavre F. (2006). Effect of temperature on Wolbachia density and impact on cytoplasmic incompatibility. Parasitology 132, 49-56. 10.1017/S0031182005008723 [DOI] [PubMed] [Google Scholar]
- Mouton L., Henri H., Charif D., Bouletreau M. and Vavre F. (2007). Interaction between host genotype and environmental conditions affects bacterial density in Wolbachia symbiosis. Biol. Lett. 3, 210-213. 10.1098/rsbl.2006.0590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman L. P., Fink J. L., Narzinski K., Ramachandran P. V., Hathiramani S. S., Cagan R. L. and Baranski T. J. (2011). A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis. Model. Mech. 4, 842-849. 10.1242/dmm.007948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoh N., Hosokawa T., Moriyama M., Oshima K., Hattori M. and Fukatsu T. (2014). Evolutionary origin of insect–Wolbachia nutritional mutualism. Proc. Natl Acad. Sci. USA 111, 10257-10262. 10.1073/pnas.1409284111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niittayla T., Messerli G., Trevisan M., Chen J., Smith A. M. and Zeeman S. C. (2004). A previously unknown maltose transporter essential for starch degradation in leaves. Science 303, 87-89. 10.1126/science.1091811 [DOI] [PubMed] [Google Scholar]
- Norseen J., Hosooka T., Hammarstedt A., Yore M. M., Kant S., Aryal P., Kiernan U. A., Phillips D. A., Maruyama H., Kraus B. J. et al. (2012). Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol. Cell. Biol. 32, 2010-2019. 10.1128/MCB.06193-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill S. L., Giordano R., Colbert A. M., Karr T. L. and Robertson H. M. (1992). 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl. Acad. Sci. USA 89, 2699-2702. 10.1073/pnas.89.7.2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco M. Y. and Léopold P. (2012). High sugar-induced insulin resistance in Drosophila relies on the lipocalin Neural Lazarillo. PLoS ONE 7, e36583 10.1371/journal.pone.0036583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinsot D., Bourtzis K., Markakis G., Savakis C. and Mercot H. (1998). Wolbachia transfer from Drosophila melanogaster into D. simulans: Host effect and cytoplasmic incompatibility relationships. Genetics 150, 227-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton F., Wilson K., Holmes A., Raubenheimer D., Robinson K. L. and Simpson S. J. (2015). Macronutrients mediate the functional relationship between Drosophila and Wolbachia. Proc. Biol. Sci. 282, 20142029 10.1098/rspb.2014.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon J. L. and Scott T. W. (2003). Wolbachia and cytoplasmic incompatibility in the California Culex pipiens mosquito species complex: parameter estimates and infection dynamics in natural populations. Genetics 165, 2029-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renko M., Valkonen P., Tapiainen T., Kontiokari T., Mattila P., Knuuttila M., Svanberg M., Leinonen M., Karttunen R. and Uhari M. (2008). Xylitol-supplemented nutrition enhances bacterial killing and prolongs survival of rats in experimental pneumococcal sepsis. BMC Microbiol. 8, 45 10.1186/1471-2180-8-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann V. and Ephrussi A. (2001). Axis formation during Drosophila oogenesis. Curr. Opin. Genet. Dev. 11, 374-383. 10.1016/S0959-437X(00)00207-0 [DOI] [PubMed] [Google Scholar]
- Riegler M., Sidhu M., Miller W. J. and O'Neill S. L. (2005). Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr. Biol. 15, 1428-1433. 10.1016/j.cub.2005.06.069 [DOI] [PubMed] [Google Scholar]
- Schmidl M. K. and Labuza T. P. (2000). Essentials of Functional Foods. Gaithersburg, Maryland: Aspen Publishers Inc. [Google Scholar]
- Sengupta S., Mukherjee S., Basak P. and Majumder A. L. (2015). Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Front. Plant Sci 6, 656 10.3389/fpls.2015.00656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbus L. R. and Sullivan W. (2007). A cellular basis for Wolbachia recruitment to the host germline. PLoS Pathog. 3, e190 10.1371/journal.ppat.0030190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbus L. R., Casper-Lindley C., Landmann F. and Sullivan W. (2008). The genetics and cell biology of Wolbachia-host interactions. Annu. Rev. Genet. 42, 683-707. 10.1146/annurev.genet.41.110306.130354 [DOI] [PubMed] [Google Scholar]
- Serbus L. R., Ferreccio A., Zhukova M., McMorris C. L., Kiseleva E. and Sullivan W. (2011). A feedback loop between Wolbachia and the Drosophila gurken mRNP complex influences Wolbachia titer. J. Cell Sci. 124, 4299-4308. 10.1242/jcs.092510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbus L. R., White P. M., Silva J. P., Rabe A., Teixeira L., Albertson R. and Sullivan W. (2015). The impact of host diet on Wolbachia titer in Drosophila. PLoS Pathog. 11, e1004777 10.1371/journal.ppat.1004777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford J. W., Lynd K. M., Jung A. Y. and Gordon M. D. (2012). Integration of taste and calorie sensing in Drosophila. J. Neurosci. 32, 14767-14774. 10.1523/JNEUROSCI.1887-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr D. J. and Cline T. W. (2002). A host–parasite interaction rescues Drosophila oogenesis defects. Nature 418, 76-79. 10.1038/nature00843 [DOI] [PubMed] [Google Scholar]
- Stouthamer R., Breeuwert J. A. J., Luck R. F. and Werren J. H. (1993). Molecular identification of microorganisms associated with parthenogenesis. Nature 361, 66-68. 10.1038/361066a0 [DOI] [PubMed] [Google Scholar]
- Teixeira L., Ferreira A. and Ashburner M. (2008). The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6, e2 10.1371/journal.pbio.1000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleman A. A. (2010). Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem. J. 425, 13-26. 10.1042/BJ20091181 [DOI] [PubMed] [Google Scholar]
- Toomey M. E., Panaram K., Fast E. M., Beatty C. and Frydman H. M. (2013). Evolutionarily conserved Wolbachia-encoded factors control pattern of stem-cell niche tropism in Drosophila ovaries and favor infection. Proc. Natl. Acad. Sci. USA 110, 10788-10793. 10.1073/pnas.1301524110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortosa P., Charlat S., Labbé P., Dehecq J.-S., Barré H. and Weill M. (2010). Wolbachia age-sex-specific density in Aedes albopictus: a host evolutionary response to cytoplasmic incompatibility? PLoS ONE 5, e9700 10.1371/journal.pone.0009700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M. and Hoffmann A. A. (1995). Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics 140, 1319-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unckless R. L., Boelio L. M., Herren J. K. and Jaenike J. (2009). Wolbachia as populations within individual insects: causes and consequences of density variation in natural populations. Proc. Biol. Sci. 276, 2805-2811. 10.1098/rspb.2009.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneti Z., Clark M. E., Karr T. L., Savakis C. and Bourtzis K. (2004). Heads or tails: host-parasite interactions in the Drosophila-Wolbachia system. Appl. Environ. Microbiol. 70, 5366-5372. 10.1128/AEM.70.9.5366-5372.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. and Wang C. (1993). Characterization of glucose transport system in Drosophila Kc cells. FEBS Lett. 317, 241-244. 10.1016/0014-5793(93)81284-7 [DOI] [PubMed] [Google Scholar]
- Weinert L. A., Araujo-Jnr E. V., Ahmed M. Z. and Welch J. J. (2015). The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. Biol. Sci. 282, 20150249 10.1098/rspb.2015.0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren J. H., Baldo L. and Clark M. E. (2008). Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6, 741-751. 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- Wiese S. E., Weber A. P. M. and Sharkey T. D. (2004). Maltose is the major form of carbon exported from the chloroplast at night. Planta 218, 474-482. 10.1007/s00425-003-1128-y [DOI] [PubMed] [Google Scholar]
- Wiwatanaratanabutr S. and Kittayapong P. (2006). Effects of temephos and temperature on Wolbachia load and life history traits of Aedes albopictus. Med. Vet. Entomol. 20, 300-307. 10.1111/j.1365-2915.2006.00640.x [DOI] [PubMed] [Google Scholar]
- Wiwatanaratanabutr I. and Kittayapong P. (2009). Effects of crowding and temperature on Wolbachia infection density among life cycle stages of Aedes albopictus. J. Invertebr. Pathol. 102, 220-224. 10.1016/j.jip.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Yang Q., Graham T. E., Mody N., Preitner F., Peroni O. D., Zabolotny J. M., Kotani K., Quadro L. and Kahn B. B. (2005). Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436, 356-362. 10.1038/nature03711 [DOI] [PubMed] [Google Scholar]
- Zchori-Fein E., Roush R. T. and Rosen D. (1998). Distribution of parthenogenesis-inducing symbionts in ovaries and eggs of Aphytis (Hymenoptera: Aphelinidae). Curr. Microbiol. 36, 1-8. 10.1007/s002849900270 [DOI] [PubMed] [Google Scholar]