Abstract
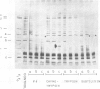
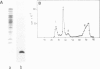
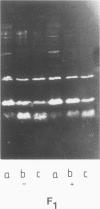
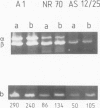
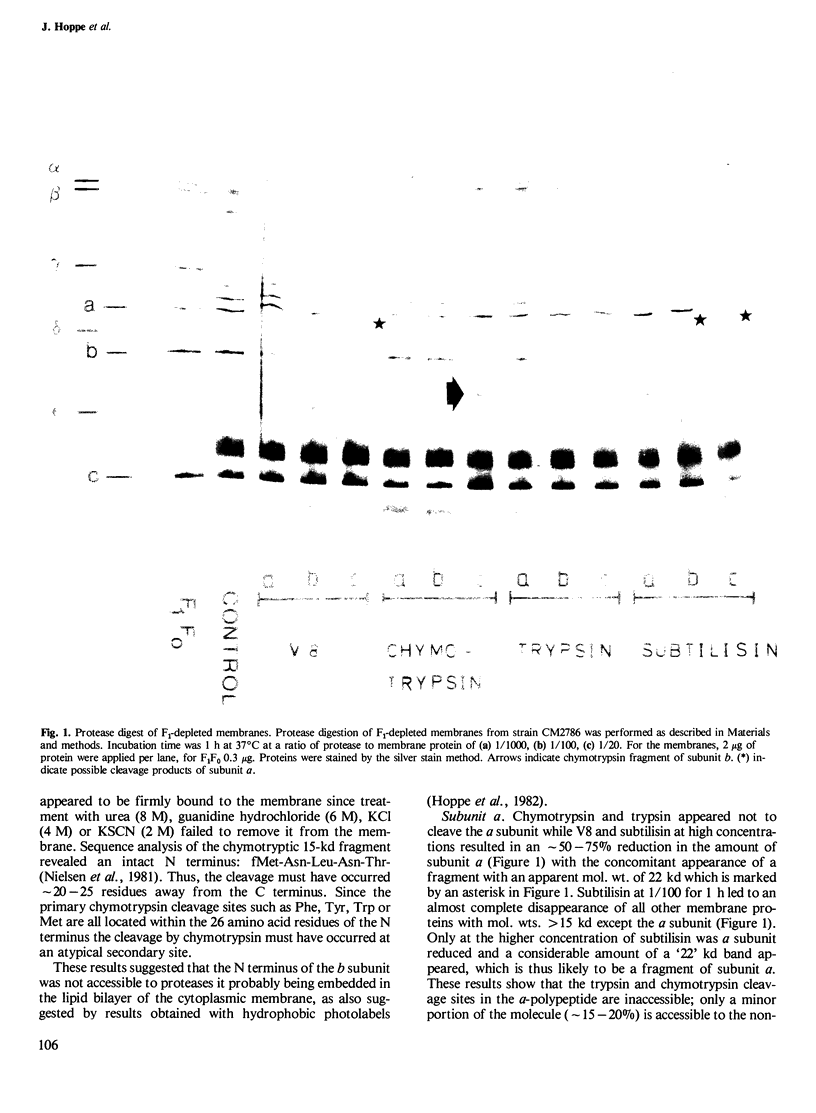
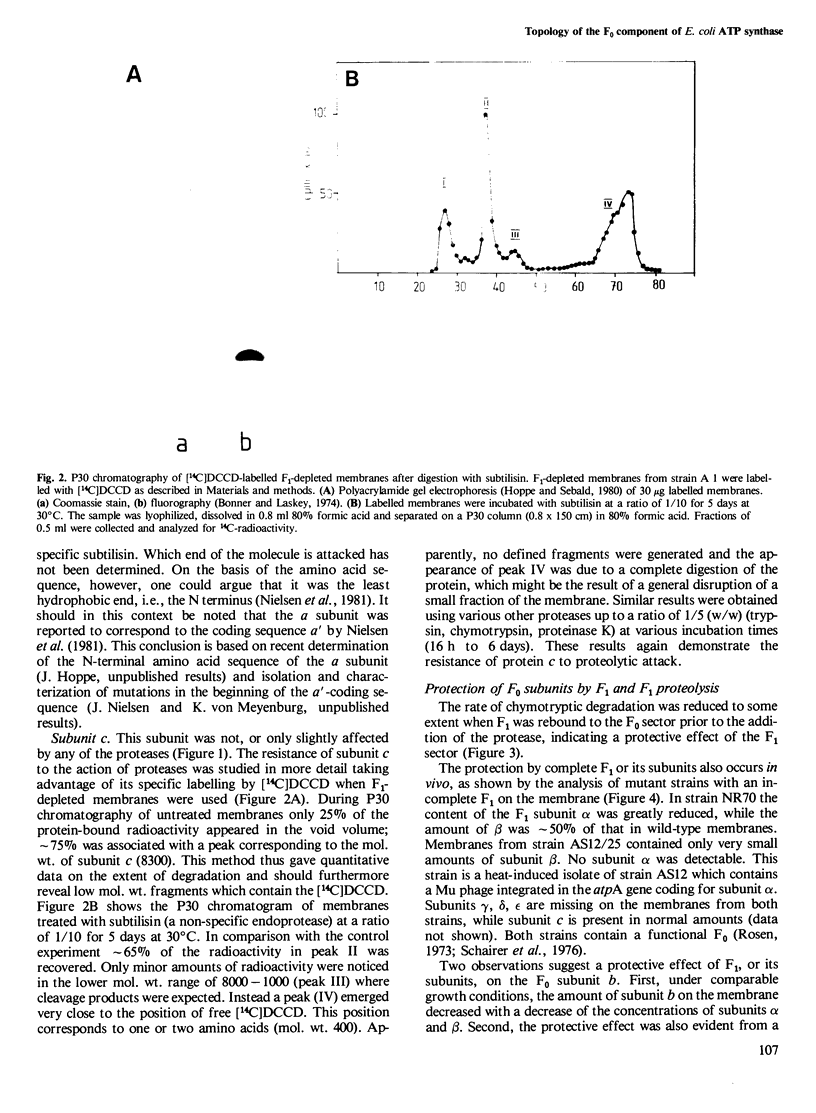
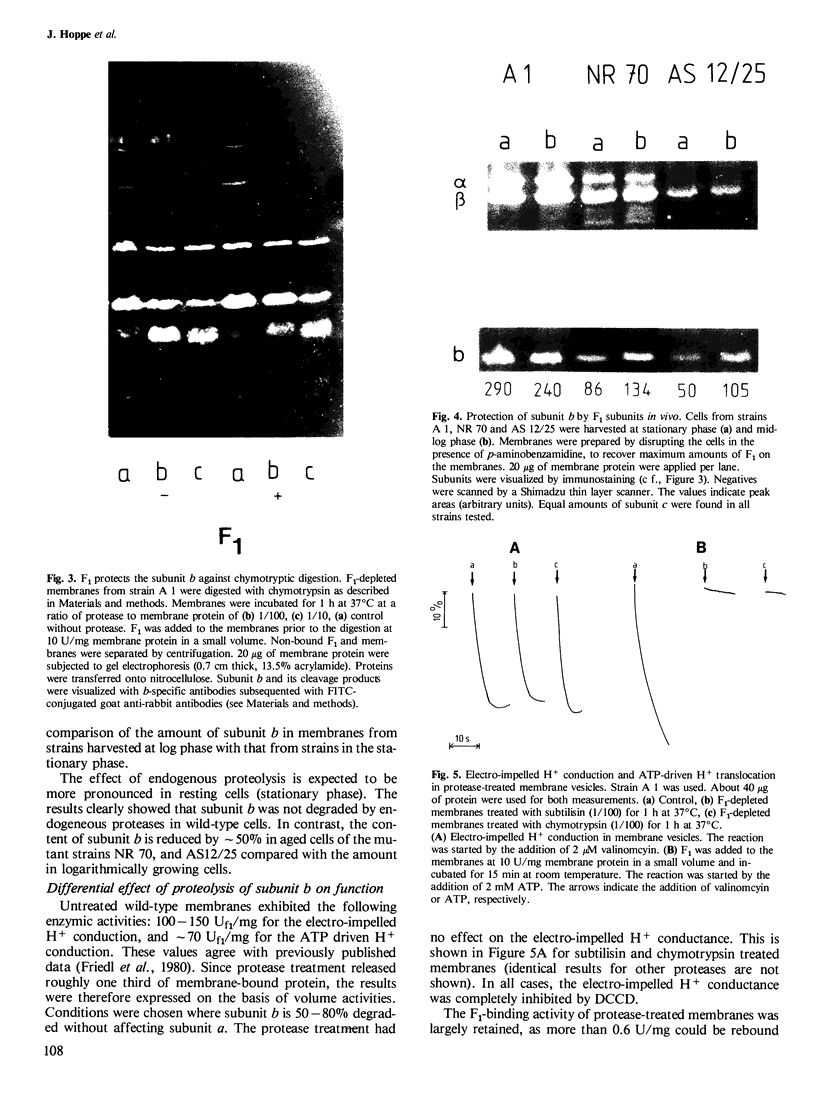
The accessibility of the three F0 subunits a, b and c from the Escherichia coli K12 ATP synthase to various proteases was studied in F1-depleted inverted membrane vesicles. Subunit b was very sensitive to all applied proteases. Chymotrypsin produced a defined fragment of mol. wt. 15,000 which remained tightly bound to the membrane. The cleavage site was located at the C-terminal region of subunit b. Larger amounts of proteases were necessary to attack subunit a (mol. wt. 30,000). There was no detectable cleavage of subunit c. It is suggested that the major hydrophilic part of subunit b extends from the membrane into the cytoplasm and is in contact with the F1 sector. The F1 sector was found to afford some protection against proteolysis of the b subunit in vitro and in vivo. Protease digestion had no influence on the electro-impelled H+ conduction via F0 but ATP-dependent H+ translocation could not be reconstituted upon binding of F1. A possible role for subunit b as a linker between catalytic events on the F1 component and the proton pathway across the membrane is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cattell K. J., Lindop C. R., Knight I. G., Beechey R. B. The identification of the site of action of NN'-dicyclohexylcarbodi-imide as a proteolipid in mitochondrial membranes. Biochem J. 1971 Nov;125(1):169–177. doi: 10.1042/bj1250169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. A., Cox G. B., Langman L., Ash G., Becker M., Gibson F. Three genes coding for subunits of the membrane sector (F0) of the Escherichia coli adenosine triphosphatase complex. J Bacteriol. 1981 Jan;145(1):200–210. doi: 10.1128/jb.145.1.200-210.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. L., Fillingame R. H. Stoichiometry of subunits in the H+-ATPase complex of Escherichia coli. J Biol Chem. 1982 Feb 25;257(4):2009–2015. [PubMed] [Google Scholar]
- Friedl P., Bienhaus G., Hoppe J., Schairer H. U. The dicyclohexylcarbodiimide-binding protein c of ATP synthase from Escherichia coli is not sufficient to express an efficient H+ conduction. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6643–6646. doi: 10.1073/pnas.78.11.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P., Friedl C., Schairer H. U. F0 of Escherichia coli ATP-synthase containing mutant and wild-type carbodiimide-binging proteins is impaired in H+ -conduction. FEBS Lett. 1980 Oct 6;119(2):254–256. doi: 10.1016/0014-5793(80)80265-1. [DOI] [PubMed] [Google Scholar]
- Friedl P., Friedl C., Schairer H. U. The ATP synthetase of Escherichia coli K12: purification of the enzyme and reconstitution of energy-transducing activities. Eur J Biochem. 1979 Oct;100(1):175–180. doi: 10.1111/j.1432-1033.1979.tb02046.x. [DOI] [PubMed] [Google Scholar]
- Friedl P., Hoppe J., Gunsalus R. P., Michelsen O., von Meyenburg K., Schairer H. U. Membrane integration and function of the three F0 subunits of the ATP synthase of Escherichia coli K12. EMBO J. 1983;2(1):99–103. doi: 10.1002/j.1460-2075.1983.tb01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P., Schairer H. U. The isolated F0 of Escherichia coli aTP-synthase is reconstitutively active in H+-conduction and ATP-dependent energy-transduction. FEBS Lett. 1981 Jun 15;128(2):261–264. doi: 10.1016/0014-5793(81)80094-4. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. The atp operon: nucleotide sequence of the promoter and the genes for the membrane proteins, and the delta subunit of Escherichia coli ATP-synthase. Nucleic Acids Res. 1981 Aug 25;9(16):3919–3926. doi: 10.1093/nar/9.16.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen F. G., Nielsen J., Riise E., von Meyenburg K. The genes for the eight subunits of the membrane bound ATP synthase of Escherichia coli. Mol Gen Genet. 1981;183(3):463–472. doi: 10.1007/BF00268766. [DOI] [PubMed] [Google Scholar]
- Hoppe J., Schairer H. U., Sebald W. Identification of amino-acid substitutions in the proteolipid subunit of the ATP synthase from dicyclohexylcarbodiimide-resistant mutants of Escherichia coli. Eur J Biochem. 1980 Nov;112(1):17–24. doi: 10.1111/j.1432-1033.1980.tb04981.x. [DOI] [PubMed] [Google Scholar]
- Hoppe J., Sebald W. Amino acid sequence of the proteolipid subunit of the proton-translocating ATPase complex from the thermophilic bacterium PS-3. Eur J Biochem. 1980;107(1):57–65. doi: 10.1111/j.1432-1033.1980.tb04624.x. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Mabuchi K., Kayano T., Noumi T., Sekiya T., Futai M. Nucleotide sequence of the genes for F0 components of the proton-translocating ATPase from Escherichia coli: prediction of the primary structure of F0 subunits. Biochem Biophys Res Commun. 1981 Nov 30;103(2):613–620. doi: 10.1016/0006-291x(81)90495-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lottspeich F. Identification of the phenylthiohydantoin derivatives of amino acids by high pressure liquid chromatography, using a ternary, isocratic solvent system. Hoppe Seylers Z Physiol Chem. 1980 Dec;361(12):1829–1834. doi: 10.1515/bchm2.1980.361.2.1829. [DOI] [PubMed] [Google Scholar]
- Negrin R. S., Foster D. L., Fillingame R. H. Energy-transducing H+-ATPase of Escherichia coli. Reconstitution of proton translocation activity of the intrinsic membrane sector. J Biol Chem. 1980 Jun 25;255(12):5643–5648. [PubMed] [Google Scholar]
- Nielsen J., Hansen F. G., Hoppe J., Friedl P., von Meyenburg K. The nucleotide sequence of the atp genes coding for the F0 subunits a, b, c and the F1 subunit delta of the membrane bound ATP synthase of Escherichia coli. Mol Gen Genet. 1981;184(1):33–39. doi: 10.1007/BF00271191. [DOI] [PubMed] [Google Scholar]
- Pedersen P. L., Hullihen J., Wehrle J. P. Proton adenosine triphosphatase complex of rat liver. The effect of trypsin on the F1 and F0 moieties of the enzyme. J Biol Chem. 1981 Feb 10;256(3):1362–1369. [PubMed] [Google Scholar]
- Rosen B. P. Restoration of active transport in an Mg2+-adenosine triphosphatase-deficient mutant of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1124–1129. doi: 10.1128/jb.116.3.1124-1129.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schairer H. U., Friedl P., Schmid B. I., Vogel G. The use of several energy-coupling reactions in characterizing mutants of Escherichia coli K12 defective in oxidative phosphorylation. Eur J Biochem. 1976 Jul 1;66(2):257–268. doi: 10.1111/j.1432-1033.1976.tb10515.x. [DOI] [PubMed] [Google Scholar]
- Sebald W. Biogenesis of mitochondrial ATPase. Biochim Biophys Acta. 1977 Jun 21;463(1):1–27. doi: 10.1016/0304-4173(77)90002-7. [DOI] [PubMed] [Google Scholar]
- Sebald W., Machleidt W., Wachter E. N,N'-dicyclohexylcarbodiimide binds specifically to a single glutamyl residue of the proteolipid subunit of the mitochondrial adenosinetriphosphatases from Neurospora crassa and Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Feb;77(2):785–789. doi: 10.1073/pnas.77.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone N., Yoshida M., Hirata H., Kagawa Y. Resolution of the membrane moiety of the H+-ATPase complex into two kinds of subunits. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4219–4223. doi: 10.1073/pnas.75.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vogel G., Steinhart R. ATPase of Escherichia coli: purification, dissociation, and reconstitution of the active complex from the isolated subunits. Biochemistry. 1976 Jan 13;15(1):208–216. doi: 10.1021/bi00646a032. [DOI] [PubMed] [Google Scholar]