Abstract
Thousands of fungal species use surface energy to power the launch of their ballistospores. The surface energy is released when a spherical Buller's drop at the spore's hilar appendix merges with a flattened drop on the adaxial side of the spore. The launching mechanism is primarily understood in terms of energetic models, and crucial features such as launching directionality are unexplained. Integrating experiments and simulations, we advance a mechanistic model based on the capillary–inertial coalescence between the Buller's drop and the adaxial drop, a pair that is asymmetric in size, shape and relative position. The asymmetric coalescence is surprisingly effective and robust, producing a launching momentum governed by the Buller's drop and a launching direction along the adaxial plane of the spore. These key functions of momentum generation and directional control are elucidated by numerical simulations, demonstrated on spore-mimicking particles, and corroborated by published ballistospore kinematics. Our work places the morphological and kinematic diversity of ballistospores into a general mechanical framework, and points to a generic catapulting mechanism of colloidal particles with implications for both biology and engineering.
Keywords: Basidiomycota, spore dispersal, Buller's drop, adaxial drop, surface tension catapult, coalescence-induced jumping
1. Introduction
Spore morphologies and behaviours are essential descriptors of fungal species, and the mechanisms of spore discharge define two of the major phyla of fungi, Ascomycota and Basidiomycota [1–3]. Ascospores are launched by the turgor pressure built up within a liquid-filled tubular sac, termed the ascus, which shapes the direction of spore launch [1,4,5]. Basidiospores are launched by the surface tension (or surface energy) associated with liquid droplets accumulated on the spores [1,6,7]. However, the physical mechanism determining the launching directionality of basidiospores, referred to as ballistospores below [7], remains unknown.
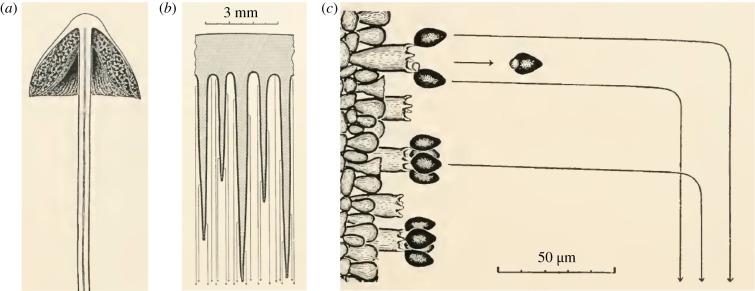
Basidiomycete ballistospore launching is frequently observed in gilled mushrooms (figure 1a) [1]. Spores develop on the tightly spaced gills and have a radius of gyration typically below 5 μm. A micrometric spore is first projected horizontally from a vertically oriented gill, and then driven by gravity to sediment in the interlamellar space (figure 1b), until the spore escapes the mushroom cap and is subsequently dispersed by air flows. Despite a large initial launching velocity (on the order of 1 m s−1), the tiny spore can only travel a short distance (on the order of 100 μm) because of the viscous drag exerted by the surrounding air. Using mathematical models (vol. 1, ch. 17 [1]), Buller demonstrated that the launching trajectory can be divided into two phases (figure 1c). In the first phase, the spore follows the initial launching direction, but only for a short period (typically below 1 ms) because of the excessive air drag. In the second phase, the spore falls vertically at a small sedimentation velocity (typically below 1 mm s−1), again due to air drag. Since the first phase is brief, gravity can only displace the spore vertically by an insignificant distance (less than a spore diameter). This insignificant gravitational effect in the first phase enabled Buller to accurately record, without high-speed imaging tools, the entire launching trajectory consisting of both phases (figure 1c). Within vertically oriented gills, the launching trajectories reported by Buller are predominantly in the horizontal direction. In figure 1c as well as figs 49 and 107 of volume 3 [1], initial spore movement is always orthogonal to the spore-bearing structure, and the maximum deviation from the horizontal direction is within ±15°.
Figure 1.
Ballistospore launching in a gilled mushroom. (a) Vertical section of a Panaeolus campanulatus sporocarp. (b) Magnified view depicting the vertically orientated gills (shaded area) on which spores are formed. The trajectories of the launched spores are indicated by arrowed light grey lines. (c) Further magnified view showing the trajectories of the spores discharged from the sterigmata on a single vertical gill. At the base of the launched spore is a liquid droplet resulting from the drop coalescence that powers the explosive launch. Images adapted from figs 84, 85 and 97 of volume 2 by Buller [1]. (Online version in colour.)
Why are the ballistospores launched orthogonally to the spore-bearing structure (the hymenium)? As Buller noted, these spores are sticky. If a spore attaches to any surrounding surface before reaching dispersive air flows, it will not disperse [1]. A likely explanation for directionality, albeit difficult to test as a hypothesis [8], stems from the advantage provided to a spore that clears the hymenium. An orthogonal arrangement maximizes horizontal travel distance in the first phase, creating a safety margin against small air currents and the random Brownian motion that might inadvertently carry the spores back to the initial supporting structure or to surrounding lamellar tissues. Of course, for a spore to successfully disperse, it cannot be launched so far that the spore sticks to the opposing gill. The same argument for directionality applies to species with either gilled or pored mushrooms. (Note that some ballistospores are launched directly into the open air, e.g. in yeasts and rusts, with typically larger spores. In those cases, an orthogonal launching direction maximizes the probability of escaping the viscous boundary layer formed around the hymenium [5,9].)
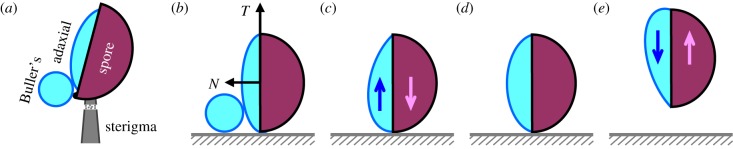
How do physical processes shape the orthogonal direction taken by a launching spore, the directionality consistently reported since 1909 [1]? Our paper attempts to address this unanswered question by advancing the mechanistic understanding of ballistospory beyond the energetic model. Note that the orthogonal direction is typically aligned with the spore's long axis, which in turn is strongly correlated with the longitudinal axis of the sterigma that initially supports the spore (figures 1c and 2a).
Figure 2.
Ballistospore launching upon drop coalescence. (a) Prior to the launching of a ballistospore, a spherical Buller's drop forms at the hilar appendix which is at the base of the spore, and a lens-shaped drop forms on the adaxial side. The spore is supported by a sterigma with an abscission zone indicated by the dotted area. (b–e) Conceptual model for ballistospore launching, where a spore-shaped particle is launched from a supporting substrate by the capillary–inertial process. The blue (dark) arrow denotes the force exerted on the merged drop by the particle, and the purple (light) arrow denotes the counter-force exerted on the particle. At the point of launching in (d), the net acceleration of the merged drop is zero and the compressive force between the particle and the substrate vanishes. Note that the sterigma is rotated 90° relative to figure 1.
1.1. Energetic model
Ballistospore discharge is powered by the surface energy released upon drop coalescence [1,7,10]. Although initial research has focused on the spherical drop (Buller's drop) at the hilar appendix of the spore [1,7], recent research using better imaging techniques suggests the typical involvement of a second drop [6,11–13], the lens-shaped adaxial drop that spreads longitudinally on the spore surface above the Buller's drop (figure 2a). A typical mature spore is shaped like a prolate ellipsoid, distorted asymmetrically with a flatter adaxial face and a more curved abaxial face along its long axis [2]. The long radius (semi-major axis) of a spore ranges approximately from 1.5 to 15 μm [2,9], while the radius of the full-grown Buller's drop ranges approximately from 0.3 to 10 μm [9]. The Buller's and adaxial drops are formed by the condensation of ambient water vapour, a process facilitated by hygroscopic substances secreted from the spore, e.g. mannitol, which is also known as manna sugar [14,15]. When the Buller's and adaxial drops grow large enough to touch each other, drop coalescence leads to spore launching.
The coalescence-induced launching process is primarily understood in terms of a lumped energetic model that links the kinetic energy of the merged drop to the surface energy released during drop coalescence [6,12,13,16]. Using Buller's drop radius (rB) as the reference length scale, the released surface energy scales as σr2B where σ is the liquid–air surface tension, and the kinetic energy scales as ρLr3Bu2ci, where ρL is the liquid density and uci is a characteristic velocity. Equating these two energies, the capillary–inertial velocity is obtained as
| 1.1 |
The associated capillary–inertial time is
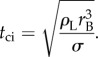 |
1.2 |
For an aqueous Buller's drop with rB ∼ 1 μm, uci ∼ 10 m s−1 and tci ∼ 0.1 μs. Such a high-speed motion is difficult to resolve, especially given the microscopic length scale. As a result, mechanical analogies have been proposed to explain the launching mechanism, ranging from a two-part solid model launched with a stretched elastic band [10] to a ‘hinged’ human body jumping with its internal biochemical energy [12]. The essence of these analogies is a two-stage launching process [6,12]. In the first stage, the drop coalescence converts surface energy to kinetic energy, leading to liquid motion that scales with the capillary–inertial velocity. In the second stage, the momentum of the merged drop is partially transferred to the spore, resulting in the launch of the drop–spore complex that conserves the total momentum. The energetic model along with the two-stage momentum transfer is useful in understanding the self-propelled launching process [6,10,12], especially in explaining the launching speed of the ballistospore [12].
However, the energetic model has limitations. First, an energetic argument cannot explain the launching direction, which is crucial given that the spore must be launched away from supporting structures prior to dispersal in airflows [16–18]. Since the energetic model is insensitive to detailed configurations, prior work largely ignores the role played by the adaxial drop. (The adaxial plane is important in guiding the launching direction, as shown in this paper.) Second, the energetic model typically calculates the launching speed by assuming a perfect capillary–inertial energy conversion, an assumption that does not hold for a related phenomenon of coalescence-induced jumping drops. When pure liquid drops jump upon coalescence, the released surface energy is converted into translational kinetic energy with wide-ranging efficiencies, from 4% to 40% [19–21]. Third, the two-stage launching process outlined above is essentially a rigid-body model and cannot represent the true launching mechanism induced by liquid drop coalescence. In fact, a similar two-stage process has been used to explain the jumping of a nematode worm, a form of locomotion that is also powered by surface energy but hardly resembles the launching of a fungal spore [22,23].
To move beyond the energetic model, we will construct a model system (figure 2) that is conducive to experimental demonstration and numerical simulation. Our work reveals the different roles played by the Buller's drop and the adaxial drop, a pair that is asymmetric in size, shape and relative position: The spherical Buller's drop of smaller radius governs the launching momentum, while the flattened adaxial drop on the spore's adaxial plane governs the launching direction. We then validate these physical insights and discuss the biological implications for ballistospore launching.
2. Model system
We first construct a conceptual model of ballistospore launching. The model simplifies the mechanical role of the sterigma, but otherwise retains the essence of the Buller–adaxial drop coalescence. The conceptual model is experimentally implemented and numerically simulated. The results suggest the association of the point of launching with the instant of peak tangential momentum.
2.1. Conceptual model
Right before spore launch, the sterigma initially supporting the spore develops an abscission zone [24–26], facilitating sterigmal rupture to release the spore at the instant of launching [6,12] (figure 2a). The sterigmal walls dissolve around the abscission zone [26], forfeiting the main structural components that resist tensile loading. However, the sterigma still anchors the spore against bending and twisting, as indicated by the schematics in Miller & Miller [26]. The role of the sterigma in anchoring the spore is supported by published videos showing an apparently still spore prior to its launching [11,12]. Taken together, these observations suggest the sterigma's mechanical role as follows: the sterigma with an abscission zone can anchor the spore and withstand compressive force, but not any significant tensile force.
Given that the sterigma anchors the spore with a weakened tensile strength, how does the Buller–adaxial drop coalescence launch the spore with the precise directionality observed in figure 1? Since the launching direction is strongly correlated with the sterigma's axial direction, which in turn correlates with the tangential direction of the spore's adaxial plane [1,2], the coalescence process should impart a launching momentum to the spore that is predominantly in the tangential direction (essentially along the spore's long axis). The mechanism for imparting the tangential launching momentum is revealed by our model system (figure 2).
In figure 2b–e, the sterigma is replaced with a substrate, and a spore-like particle is launched from the substrate by a two-stage process. In the first stage, a round Buller's drop coalesces with a flattened adaxial drop at its bottom edge. The Buller's drop with a higher Laplace pressure drains towards the adaxial drop, and the merged drop first accelerates upwards as a result (figure 2c). The acceleration of the merged drop eventually turns into deceleration owing to the capillarity of the pinned drop. A zero acceleration corresponds to vanishing compressive force on the substrate and signifies the point of launching (figure 2d), marking the beginning of the second stage. Without any tensile force between the particle and the substrate, the particle is pulled upwards by the decelerating drop, and the drop–particle complex is launched into the air (figure 2e), along the tangential direction of the adaxial plane. (The tangential–normal TN coordinate system is attached to the spore, as in figure 2b.) The launching momentum is ultimately attributed to the impulse provided by the substrate, integrated over the first stage of drop acceleration represented by figure 2c. Although the adaxial drop is depicted as spanning the entire adaxial plane in figure 2b, partial coverage is also possible and the adaxial surface may be somewhat curved [2,6]. The same principle as depicted in figure 2 applies as long as the contact line is pinned at the near edge where Buller–adaxial drop coalescence initiates. The near-edge pinning is a reasonable assumption given the apparent wettability contrast leading to preferential growth on the adaxial (not abaxial) side of the spore.
Building upon existing two-stage models [6,12], our conceptual model accounts for the fluid dynamical process of the asymmetric drop coalescence. The essence of the asymmetric coalescence is captured: the globular Buller's drop has a smaller spherical radius and is placed off-centre to the flattened adaxial drop on the spore surface. Note that the schematics in figure 2b–d imply a fixed (non-rotating) spore, which preserves the sterigmal anchoring. The fixed spore is adopted in the numerical simulations below. For the experimental demonstration in figure 3, we mimic the sterigmal anchoring by placing the model spore at an orientation that minimizes prelaunching rotation.
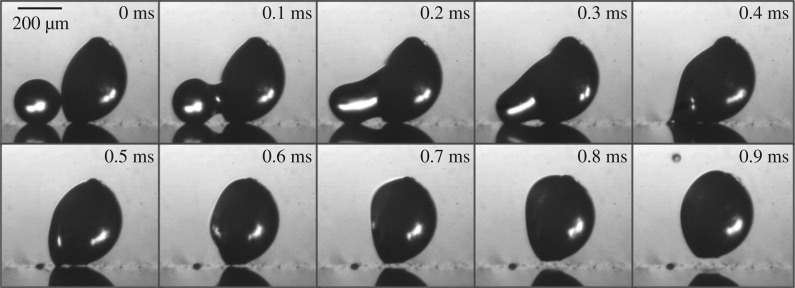
Figure 3.
Experimental launching process of a spore-shaped particle. The polystyrene spherical cap is launched from a flat superhydrophobic substrate, following the coalescence of two inkjet-printed drops: the Buller's drop with a radius rB = 104 μm and the adaxial drop with a base radius rA = 200 μm. The two-stage launching process is demarcated by the point of launching at approximately 0.5 ms. After launching, the particle-drop complex continues to move along the spore's long axis; see also electronic supplementary material, movie S1 for later times.
2.2. Experimental demonstration
Our conceptual model is demonstrated in figure 3. The spore-shaped polystyrene particle is rendered hydrophilic with plasma oxidization, and placed on a superhydrophobic substrate [27] with the long axis pointing upwards. The adaxial drop is first deposited by cumulative inkjet printing on the flat face of the spore, and then the Buller's drop is accumulated on the superhydrophobic surface until coalescence occurs. Further experimental details can be found in electronic supplementary material, section S2. Compared to vapour condensation employed by real ballistospores [14], the inkjet accumulation alleviates the difficulty in capturing live launching processes involving two disparate time scales, the long period for drop accumulation and the short duration for drop coalescence.
The coalescence-induced launching process in figure 3 can be divided into two stages. In the first stage, the Buller's drop is absorbed into the adaxial drop and accelerated upwards. The accelerating drop exerts a compressive force on the particle against the substrate. The upwards acceleration of the drop turns zero (and then negative) at 0.5 ± 0.1 ms, which is the point of launching because no tensile force can be supported between the particle and the substrate. In the second stage that commences at the point of launching, the launched particle continues to move along the flat face (the long axis) of the spherical cap (see electronic supplementary material, movie S1).
A few aspects of the experimental implementation in figure 3 are worth noting. (i) The model particle is much larger than live spores and the characteristic capillary–inertial time in equation (1.2) is much longer, enabling temporal resolution of the launching process. For real ballistospores that are much smaller, high-speed imaging of microsecond resolution is not enough to capture the dynamic coalescence process [11,12]. Apart from the larger scale, the geometrical configuration resembles that in some real ballistospores (e.g. fig. 3D4 in Webster et al. [6]). (ii) The cap-shaped particle is oriented with its long axis almost perpendicularly to the substrate, minimizing the rotation of the spore prior to launching (see electronic supplementary material, movie S1). Such an initial orientation is critical to the experiment, and the minimal rotation is consistent with the anchoring of a real ballistospore by the sterigma. (iii) A water–ethanol mixture is chosen as the working fluid to facilitate the experiments. The Buller's drop made of 70%vol water is still approximately spherical (superhydrophobic) on the substrate, and there is just enough adhesion to the substrate to prevent any bouncy motion during accumulative printing. In comparison, real Buller's and adaxial drops are aqueous solutions of sugars such as mannitol and hexoses [14,15]. These aspects of the drop–particle system are chosen to facilitate experimentation and are inconsequential to the capillary–inertial coalescence process leading to launching. The Ohnesorge number in figure 3 is approximately 0.04, which is in the capillary–inertial regime as discussed below.
2.3. Numerical simulation
The two-stage launching process inspires numerical simulations that focus on the first stage. The spore is immobile during the first stage, at the end of which the launching momentum is already determined. Therefore, the coalescence is simulated on the flat face of a fixed spore in this paper. Note that the fixed spore preserves the aforementioned sterigmal anchoring in all our simulations.
The interfacial flow is governed by the Navier–Stokes equations described in electronic supplementary material, section S3. The momentum equation for the merged liquid drop reads
| 2.1 |
where V is the velocity vector, t is the time, P is the pressure and μL is the liquid viscosity. As shown below, the asymmetric Buller–adaxial drop coalescence is governed by the Buller's drop radius, rB, which is the smaller one of the drop pair. The properties of the Buller's drop are used to non-dimensionalize the momentum equation, e.g. length scales with rB, stress scales with the capillary pressure σ/rB, and the coalescence dynamics is governed by the capillary–inertial velocity uci and time tci. The resulting non-dimensional momentum equation is
| 2.2 |
where the dimensionless variables are represented with an asterisk, and the Ohnesorge number is defined as
| 2.3 |
When Oh ≪ 1, the system is in the capillary–inertial regime [20], in which the viscous term plays a secondary role and the actual Ohnesorge number has little bearing on the dynamics, as shown below. The nomenclature along with procedures for non-dimensionalization can be found in electronic supplementary material, section S1.
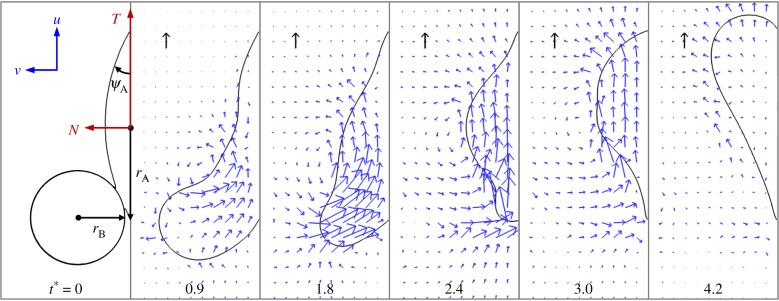
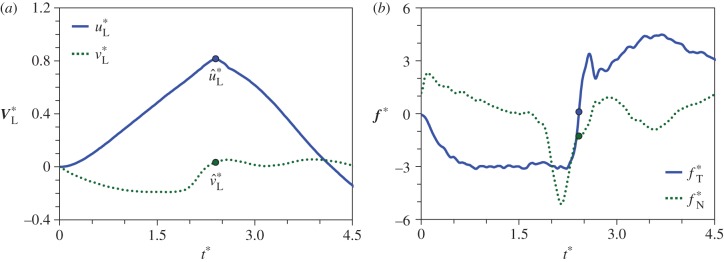
The coalescence process is simulated using the phase-field method [20,28,29] detailed in electronic supplementary material, section S3. The adaxial drop is attached to a flat substrate with a pinned contact line. The pinned area is effectively the wetted adaxial surface of the spore. The Buller's drop is situated tangent to the adaxial drop, with an off-centred configuration resembling the drop position in real ballistospores. The coalescence is initiated by the overlapping of diffuse interfaces in the phase-field implementation. In the three-dimensional (3D) simulations in figure 4 (see electronic supplementary material, movie S2), the relative position and size of the two coalescing drops approximate the experiment in figure 3. The corresponding velocity evolution is shown in figure 5a. Although the Buller's drop has an appreciable normal momentum at the beginning (t* = 0.9), it is quickly redirected by capillarity to the tangential direction, parallel to the impermeable substrate (t* = 1.8). When the tangential momentum reaches its peak value (t* = 2.4), the normal component at that instant is an order of magnitude smaller than its tangential counterpart. Beyond this point, the merged drop swings upwards (t* = 3.0), until the net tangential momentum reaches a nearly null value with a highly deformed drop (t* = 4.2). Despite the fact that the spore is fixed in place in the simulations, the numerical results in figure 4 capture the essence of the coalescence process in figure 3, evident in the shape oscillation of the merged drop with respect to the spore.
Figure 4.
Numerical simulation of the coalescence process on a fixed substrate with Oh = 0.0126. The contact line of the adaxial drop is pinned, and the pinned area is effectively the wetted spore surface. The cap-shaped adaxial drop has a base radius rA (not the spherical radius) given by rA/rB = 2. The cap angle of the initial adaxial drop is ψA = 30°. The unit velocity vectors (black arrows) represent uci and the dimensionless time is indicated by t* = t/tci. See also electronic supplementary material, movie S2 for the entire process.
Figure 5.
Velocity and force evolution resulting from the coalescence process depicted in figure 4. (a) The average velocity of the merged drop, V*L = VL/uci; and (b) the force exerted on the particle by the merged drop, f* = f/(σrB). The tangential and normal components are defined in the TN coordinate system in figure 4. At 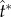 (indicated by dots), the point when the tangential momentum peaks and the tangential force vanishes, the tangential velocity
(indicated by dots), the point when the tangential momentum peaks and the tangential force vanishes, the tangential velocity 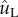 is an order of magnitude larger than the normal velocity
is an order of magnitude larger than the normal velocity 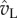 . (Online version in colour.)
. (Online version in colour.)
2.4. Launching condition
In our model, the point of launching coincides with the vanishment of tangential force on the particle (figure 2d). The force exerted on the particle by the merged drop is
| 2.4 |
where ℘ is the liquid momentum and VL is the instantaneous velocity averaged over the entire liquid mass mL. For the process in figure 4, when the tangential velocity uL reaches the maximum at 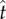 (figure 5a), the corresponding tangential force fT vanishes (figure 5b). At
(figure 5a), the corresponding tangential force fT vanishes (figure 5b). At 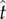 , the tangential liquid velocity (
, the tangential liquid velocity (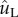 ) dominates the normal velocity (
) dominates the normal velocity (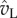 ). If the particle is mobile, it will be launched beyond
). If the particle is mobile, it will be launched beyond 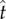 , the point of peak tangential momentum.
, the point of peak tangential momentum.
The point of launching, 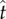 , plays a central role in our model. Experimentally, the launching in figure 3 occurs at
, plays a central role in our model. Experimentally, the launching in figure 3 occurs at 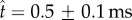 or
or 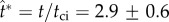 , where tci = 0.17 ms is calculated using equation (1.2) based on rB = 104 μm and the liquid properties in electronic supplementary material, section S2. Numerically, the point of peak tangential momentum occurs at
, where tci = 0.17 ms is calculated using equation (1.2) based on rB = 104 μm and the liquid properties in electronic supplementary material, section S2. Numerically, the point of peak tangential momentum occurs at 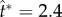 in figure 5a. These results agree very well, especially considering the delay in launching caused by the finite adhesion between the coalescing water–ethanol drop and the superhydrophobic substrate. In both experiments and simulations, the merged drop approaches the shape of a spherical cap at the point of launching. Such a round shape is a signature of the pseudoequilibrium configuration [20] in the oscillation process with a zero net acceleration.
in figure 5a. These results agree very well, especially considering the delay in launching caused by the finite adhesion between the coalescing water–ethanol drop and the superhydrophobic substrate. In both experiments and simulations, the merged drop approaches the shape of a spherical cap at the point of launching. Such a round shape is a signature of the pseudoequilibrium configuration [20] in the oscillation process with a zero net acceleration.
Our model adopts the vanishment of tangential force (fT = 0) as the criterion for launching, and neglects the role of normal force fN. During the coalescence process leading to launching, the normal force is small compared to the tangential force except for two periods in figure 5b. (i) In the beginning period of the coalescence (t* = 0 to 0.4) when the normal momentum dominates. The presence of a significant normal force may explain the slight rotation of the spore at the beginning of the coalescence, most clearly seen in electronic supplementary material, movie S1. (ii) In the short period before the point of launching (t* = 2.0 to 2.4) when the normal momentum quickly vanishes. By then, the tangential momentum is already close to the peak value and is the dominating component (figure 5a). Given the short durations of both periods with a significant fN, the influence of the normal force on the launching process should be secondary.
2.5. Launching velocity
Figure 4 suggests that the Buller's drop plays an active role in the capillary–inertial coalescence process, and the adaxial drop acts more or less as a passive liquid reservoir. By the time the tangential momentum reaches its peak value in figure 4, the Buller's drop with a mass 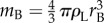 has been drained into the adaxial drop at a velocity comparable to the capillary–inertial velocity uci, and the merged drop's motion is predominantly in the tangential direction because of capillary and solid confinements. Accordingly, the magnitude of liquid momentum is governed by the Buller's drop, while the direction approximately follows the adaxial plane. At the point of launching (
has been drained into the adaxial drop at a velocity comparable to the capillary–inertial velocity uci, and the merged drop's motion is predominantly in the tangential direction because of capillary and solid confinements. Accordingly, the magnitude of liquid momentum is governed by the Buller's drop, while the direction approximately follows the adaxial plane. At the point of launching (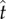 ), the liquid momentum follows:
), the liquid momentum follows:
| 2.5 |
and
| 2.6 |
where the hatted variables are evaluated at the point of peak tangential momentum (as in figure 5a), mL = mA + mB is the combined mass of the adaxial drop (mA) and the Buller's drop (mB), and 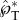 and
and 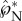 are, respectively, the tangential and normal momentum non-dimensionalized by mBuci.
are, respectively, the tangential and normal momentum non-dimensionalized by mBuci.
At the point of launching, the spore–liquid complex acquires the momentum of the merged drop and conserves it afterwards, giving rise to 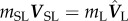 , where mSL = mS + mL is the combined spore–liquid mass and VSL is the centre-of-mass launching velocity. Accordingly,
, where mSL = mS + mL is the combined spore–liquid mass and VSL is the centre-of-mass launching velocity. Accordingly,
| 2.7 |
where 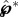 is the non-dimensional liquid momentum at the point of launching, and m* = mB/mSL is the ratio of the ‘active’ Buller's drop mass over the combined spore–liquid mass. Note that the ‘passive’ adaxial drop mass only appears in the denominator of this mass ratio through mSL = mS + mA + mB. With
is the non-dimensional liquid momentum at the point of launching, and m* = mB/mSL is the ratio of the ‘active’ Buller's drop mass over the combined spore–liquid mass. Note that the ‘passive’ adaxial drop mass only appears in the denominator of this mass ratio through mSL = mS + mA + mB. With 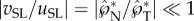 , the launching velocity is dominated by the tangential component, uSL, which can be reduced to
, the launching velocity is dominated by the tangential component, uSL, which can be reduced to
| 2.8 |
where the non-dimensional launching velocity (u*SL = uSL/uci) is proportional to both the effectiveness of the coalescence-induced momentum generation (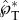 ) and the mass fraction of the active Buller's drop (m*).
) and the mass fraction of the active Buller's drop (m*).
The capillary–inertial velocity (uci) scaling has long been recognized [6,9,10,12,13,16], along with the momentum conservation at the transition of the two-stage launching process [6,12]. Using these ideas, Noblin et al. [12] have calculated the magnitude of the launching velocity with the assumption of perfect capillary–inertial energy conversion. The energy conversion cannot be perfect for any real system because of the inevitable viscous dissipation [20]. Using the simulation in figure 4 as an example, the surface energy released by coalescence is ΔE = 0.77(4πσr2B), assuming that the merged drop eventually takes the shape of a spherical cap of the same base radius as rA. If the energetic calculation in the spirit of Noblin et al. is adopted, the useful part of the translational kinetic energy would be given by 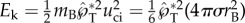 , assuming that all the kinetic energy is carried by the Buller's drop as in [12] (in reality, the adaxial drop is also partially mobilized). For such an energetic model to yield the same launching momentum as the numerically simulated value,
, assuming that all the kinetic energy is carried by the Buller's drop as in [12] (in reality, the adaxial drop is also partially mobilized). For such an energetic model to yield the same launching momentum as the numerically simulated value, 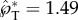 (figure 8), an energy conversion efficiency of Ek/ΔE = 48% would need to be assumed. In addition to this quantitative limitation, the scalar energetic model is more fundamentally limited by its inability to predict the launching direction.
(figure 8), an energy conversion efficiency of Ek/ΔE = 48% would need to be assumed. In addition to this quantitative limitation, the scalar energetic model is more fundamentally limited by its inability to predict the launching direction.
Figure 8.
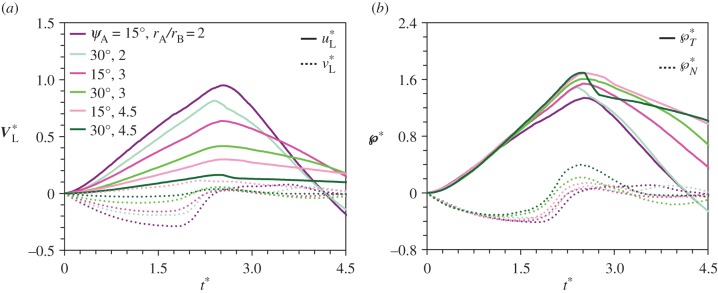
The momentum scaling of the merged drop. (a) The liquid velocity non-dimensionalized as V*L = VL/uci. The scatter suggests that the mass-averaged velocity does not follow a naive capillary–inertial scaling. (b) The liquid momentum rescaled as ℘* = mLVL/(mBuci) = (mL/mB)V*L. The collapse suggests that the momentum of the merged drop scales with mBuci. The parametric variations are the same as those in figure 7.
Given the limitations of the energetic model, we shall adopt the more appropriate capillary–inertial momentum scaling in equations (2.5) and (2.6). Our mechanistic model (along with numerical simulations) can accurately predict both the magnitude and direction of the launching velocity with equations (2.7) and (2.8). Indeed, our analysis of the launching velocity is confirmed by the trajectory of the launched particle in figure 3, which is approximately along the long axis of the initial particle, despite an appreciable self-rotation after launching (see electronic supplementary material, movie S1). Such a trajectory is consistent with the predominantly tangential liquid momentum at the point of launching (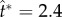 in figures 4 and 5a), which is retained by the launched particle–drop complex. Our mechanistic model is further tested below with numerical parametric variations and available experimental evidence, with a focus on the launching velocity prediction in equations (2.7) and (2.8) and the associated liquid momentum scaling in equations (2.5) and (2.6).
in figures 4 and 5a), which is retained by the launched particle–drop complex. Our mechanistic model is further tested below with numerical parametric variations and available experimental evidence, with a focus on the launching velocity prediction in equations (2.7) and (2.8) and the associated liquid momentum scaling in equations (2.5) and (2.6).
3. Liquid momentum scaling
The asymmetric coalescence of the Buller–adaxial drop pair is crucial to the liquid momentum scaling. For real ballistospores, the approximate range of the Buller's drop radius can be extracted from existing reports, but the parameters related to the adaxial drop are scarcely reported. Using numerical simulations, we now establish that equations (2.5) and (2.6) are applicable in the low-Ohnesorge-number regime, which is mainly governed by the radius of the aqueous Buller's drop. The capillary–inertial momentum scaling in this regime is insensitive to other parameters, as long as the drop pair is asymmetric in size, shape and relative position.
3.1. Capillary–inertial regime
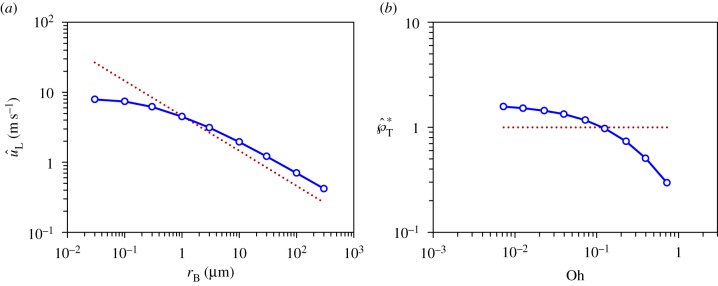
The Buller–adaxial drop coalescence results in the capillary–inertial momentum scaling in equations (2.5) and (2.6). These equations apply as long as the coalescence is in the capillary–inertial regime, signified by a small Ohnesorge number [20]. In figure 6, the Buller's drop radius varies from 0.03 to 300 μm in the 3D simulations, assuming negligible gravitational effects. The average velocity of the merged drop follows the capillary–inertial scaling 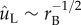 at rB ≥ 0.3 μm. Correspondingly, the non-dimensional tangential momentum
at rB ≥ 0.3 μm. Correspondingly, the non-dimensional tangential momentum 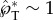 at Oh ≤ 0.230, assuming properties of pure water at room temperature. At smaller drop radii, viscous effects become more prominent and the liquid motion no longer follows the capillary–inertial momentum scaling, although launching may still be possible at a reduced speed. The viscous ‘cutoff’ is set at rB = 0.3 μm with
at Oh ≤ 0.230, assuming properties of pure water at room temperature. At smaller drop radii, viscous effects become more prominent and the liquid motion no longer follows the capillary–inertial momentum scaling, although launching may still be possible at a reduced speed. The viscous ‘cutoff’ is set at rB = 0.3 μm with 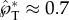 , which is approximately half of the value at the small-Ohnesorge-number limit.
, which is approximately half of the value at the small-Ohnesorge-number limit.
Figure 6.
The simulated velocity of the merged drop follows the capillary–inertial scaling (dotted line) in the small-Ohnesorge-number limit. (a) The maximum liquid velocity in the tangential direction 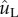 as a function of the Buller's drop radius rB; (b) The non-dimensional peak tangential momentum,
as a function of the Buller's drop radius rB; (b) The non-dimensional peak tangential momentum, 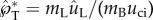 , as a function of the Ohnesorge number,
, as a function of the Ohnesorge number, 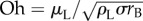 . The conditions are the same as those in figure 4 except for the Ohnesorge number which varies with the Buller's drop radius. With pure water as the working fluid, the viscous ‘cutoff’ occurs around rB = 0.3 μm (Oh = 0.230). At smaller radii,
. The conditions are the same as those in figure 4 except for the Ohnesorge number which varies with the Buller's drop radius. With pure water as the working fluid, the viscous ‘cutoff’ occurs around rB = 0.3 μm (Oh = 0.230). At smaller radii, 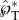 is significantly less than unity. (Online version in colour.)
is significantly less than unity. (Online version in colour.)
When Oh ≲ 0.2, the drop coalescence is in the capillary–inertial regime according to figure 6, and the specific Ohnesorge number is of little relevance to the scaling of the ensuing liquid momentum. For real ballistospores, the reported range of the Buller's drop radius (rB) is between 0.3 and 10 μm [9], and the corresponding Ohnesorge number (Oh) is between 0.2 and 0.04 assuming the properties of pure water. Therefore, ballistospore launching is expected to follow the capillary–inertial momentum scaling.
3.2. Asymmetric drop arrangement
Based on published images [6,9,11–13], the drop coalescence is between two asymmetrically arranged drops, a spherical Buller's drop and a flattened adaxial drop that are off-centered to each other. As long as the asymmetric coalescence is in the capillary–inertial regime, the momentum scaling is insensitive to parameters associated with the adaxial drop, including the adaxial cap angle ψA, the radius ratio rA/rB, and the positioning angle β between the Buller's and adaxial drops. The asymmetric arrangement leads to the capillary–inertial momentum scaling with a dominant tangential component.
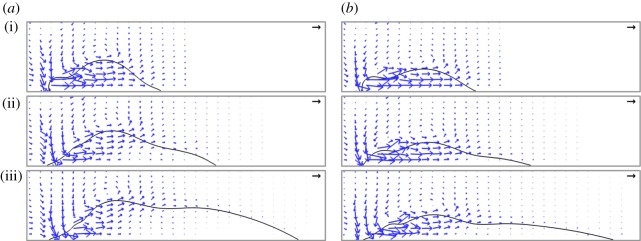
The shape and size of the adaxial drop are varied in figures 7 and 8 with different combinations of adaxial cap angle ψA and base radius rA. Note that the conditions for the top case in figure 7a are identical to those in figure 4. For all cases in figure 7, the peak tangential momentum occurs around 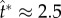 , which is essentially the time required to drain the Buller's drop mass over a distance equal to its diameter (t ≈ 2rB/uci = 2tci). This observation is consistent with the active role of the Buller's drop. Further, the fluid flow at
, which is essentially the time required to drain the Buller's drop mass over a distance equal to its diameter (t ≈ 2rB/uci = 2tci). This observation is consistent with the active role of the Buller's drop. Further, the fluid flow at 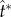 is predominantly in the tangential direction. The passive role of the adaxial drop is apparent in the strong similarity in the velocity fields among different adaxial radii (rA), especially at the near edge where the coalescence initiates. In figure 8a, the average liquid velocity VL does not simply scale with uci. However, the liquid momentum (℘ = mLVL) does scale with mBuci in figure 8b, where the tangential and normal momentum under various conditions each collapse around a single curve. (The divergence after the peak tangential momentum is inconsequential since it is after the point of launching.) For all cases in figure 8b,
is predominantly in the tangential direction. The passive role of the adaxial drop is apparent in the strong similarity in the velocity fields among different adaxial radii (rA), especially at the near edge where the coalescence initiates. In figure 8a, the average liquid velocity VL does not simply scale with uci. However, the liquid momentum (℘ = mLVL) does scale with mBuci in figure 8b, where the tangential and normal momentum under various conditions each collapse around a single curve. (The divergence after the peak tangential momentum is inconsequential since it is after the point of launching.) For all cases in figure 8b, 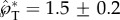 while
while 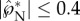 .
.
Figure 7.
The velocity fields at the point of peak tangential momentum (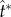 ): (a) ψA = 30°; (b) ψA = 15°. At each cap angle ψA, the radius ratio rA/rB = 2, 3, 4.5 from (i) to (iii), and the corresponding
): (a) ψA = 30°; (b) ψA = 15°. At each cap angle ψA, the radius ratio rA/rB = 2, 3, 4.5 from (i) to (iii), and the corresponding 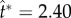 , 2.50, 2.48 in (a) and
, 2.50, 2.48 in (a) and 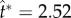 , 2.52, 2.56 in (b). The conditions are otherwise identical to those in figure 4, although the orientation is rotated 90°. The unit vectors (black arrows) represent uci. See also electronic supplementary material, movies S2–S7. (Online version in colour.)
, 2.52, 2.56 in (b). The conditions are otherwise identical to those in figure 4, although the orientation is rotated 90°. The unit vectors (black arrows) represent uci. See also electronic supplementary material, movies S2–S7. (Online version in colour.)
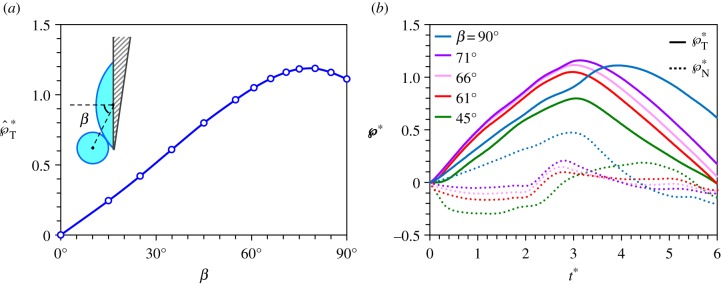
The role of the relative drop position is explored in figure 9 with two-dimensional (2D) simulations (detailed in electronic supplementary material, section S3), which are more versatile than their 3D counterparts in accommodating complex boundary conditions. The positioning angle β varies from 0°, where the symmetric coalescence leads to no net tangential momentum, to 90°, where the Buller's drop centre is on the adaxial plane and the asymmetric coalescence results in a large tangential momentum. As long as the Buller's drop is sufficiently off-centred (β≥ 45° when rA/rB = 2 and ψA = 30°), the normal momentum is redirected and turned into the tangential momentum, and the asymmetric coalescence ends up imparting a capillary–inertial velocity to the Buller's drop. The resulting peak tangential momentum is a weak function of the relative position with 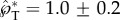 (figure 9a), and this launching momentum is predominantly tangential with
(figure 9a), and this launching momentum is predominantly tangential with 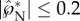 (figure 9b).
(figure 9b).
Figure 9.
Variation of the positioning angle β defined in the inset, using 2D simulations with Oh = 0.0126, rA/rB = 2 and ψA = 30°. (a) The peak tangential momentum 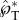 as a function of β, indicating that
as a function of β, indicating that 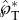 is approximately unity for β≥45°. The slight reduction of the launching momentum when β is close to 90° is due to the placement of the Buller's drop past the adaxial plane, leading to a normal momentum initially pointing away from the substrate. (b) Evolution of the tangential and normal momentum for β≥45°, indicating the dominance of the tangential component. This is the only figure adopting 2D simulations in order to accommodate large β angles. The 2D results are slightly different in magnitude from their 3D counterparts. See also electronic supplementary material, movie S8 with β = 61°.
is approximately unity for β≥45°. The slight reduction of the launching momentum when β is close to 90° is due to the placement of the Buller's drop past the adaxial plane, leading to a normal momentum initially pointing away from the substrate. (b) Evolution of the tangential and normal momentum for β≥45°, indicating the dominance of the tangential component. This is the only figure adopting 2D simulations in order to accommodate large β angles. The 2D results are slightly different in magnitude from their 3D counterparts. See also electronic supplementary material, movie S8 with β = 61°.
4. Ballistospore launching velocity
At the point of launching, the liquid momentum is transferred to the spore–liquid complex, giving rise to the launching velocity predicted by equations (2.7) and (2.8). We next validate our model with existing measurements on real ballistospores, and discuss the biological implications of the asymmetric drop coalescence.
4.1. Launching speed and direction
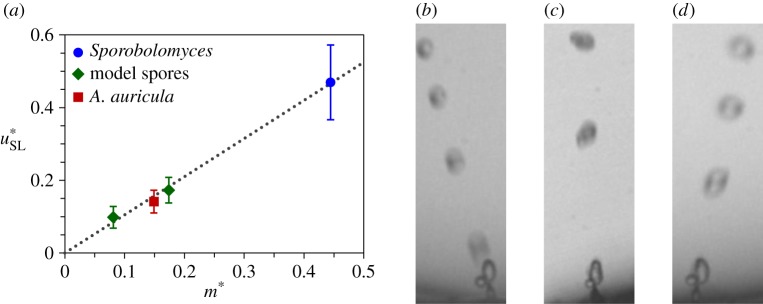
Our insights into the launching speed and direction are verified in figure 10 with experimental data on both live ballistospores and model particles. In terms of the launching speed, there are two data points in Noblin et al. [12] for which both the velocity and the masses can be accurately extracted. These data on Auricularia auricula and Sporobolomyces spores are plotted in figure 10a. The other two measurements in figure 10a are the tangential launching velocities of our model spores. All four data points fall closely onto the prediction of equation (2.8) with 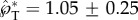 . The agreement is remarkable considering that both live and model spores are plotted, and the Buller's drop radius varies by more than an order of magnitude between these two categories. The slight discrepancy in the numerical and experimental
. The agreement is remarkable considering that both live and model spores are plotted, and the Buller's drop radius varies by more than an order of magnitude between these two categories. The slight discrepancy in the numerical and experimental 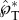 may be due to the approximation of the adaxial drop as a perfect spherical cap, implying the unlikely shape of a circular adaxial plane on the spore. Interestingly, the 2D simulations in figure 9 give rise to a peak tangential momentum
may be due to the approximation of the adaxial drop as a perfect spherical cap, implying the unlikely shape of a circular adaxial plane on the spore. Interestingly, the 2D simulations in figure 9 give rise to a peak tangential momentum 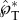 that is closer to the experimental value.
that is closer to the experimental value.
Figure 10.
The speed and trajectory of launched ballistospores. (a) The launching velocity of live ballistospores is calculated from the velocities and Buller's drop radii reported in tables 1 and 2 of Noblin et al. [12], and that of spore-shaped particles is extracted from the launching trajectories in electronic supplementary material, movies S1 and S9 using procedures described in electronic supplementary material, section S2 and Liu et al. [30]. The linear fit according to equation (2.8) is 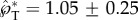 , where the uncertainty indicates 95% CI of the fitting coefficient. The calculation of u*SL is based on the properties of pure water (fungal spores) or water–ethanol mixture (model spores), both at 20°C. (b–d) The launching trajectories of fungal spores, adapted from supplemental videos 14, 4 and 13 of Pringle et al. [11]. Each trajectory is rotated so that the long axis of the spore is approximately vertical. The trajectories in (b) and (d) represent the maximum deviation from the vertical (tangential) direction found in all videos in Pringle et al. [11], with deviations of 20° (b) and −15° (d). (Online version in colour.)
, where the uncertainty indicates 95% CI of the fitting coefficient. The calculation of u*SL is based on the properties of pure water (fungal spores) or water–ethanol mixture (model spores), both at 20°C. (b–d) The launching trajectories of fungal spores, adapted from supplemental videos 14, 4 and 13 of Pringle et al. [11]. Each trajectory is rotated so that the long axis of the spore is approximately vertical. The trajectories in (b) and (d) represent the maximum deviation from the vertical (tangential) direction found in all videos in Pringle et al. [11], with deviations of 20° (b) and −15° (d). (Online version in colour.)
In terms of the launching trajectory, figure 10b–d are adapted from Pringle et al. [11], confirming that the launching of A. auricula spores is approximately along the long axis. Based on all the supplemental videos in Pringle et al. [11], the launching trajectory is within 20° of the tangential direction of the spore. The observed deviation can be partially attributed to the small normal component of the launching momentum. Numerical simulations in figure 8b suggest that 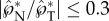 , which corresponds to a deviation within 17°. In our experiments, the model spores are also launched approximately along the tangential direction, as long as the spore is carefully oriented to minimize its prelaunching rotation (electronic supplementary material, movies S1 and S9). Additional corroboration for the predominantly tangential launching direction can be found in the trajectories of Itersonilia perplexans spores in fig. 11 of Webster et al. [6], and in the trajectories of A. auricula spores in fig. 3 of Noblin et al. [12].
, which corresponds to a deviation within 17°. In our experiments, the model spores are also launched approximately along the tangential direction, as long as the spore is carefully oriented to minimize its prelaunching rotation (electronic supplementary material, movies S1 and S9). Additional corroboration for the predominantly tangential launching direction can be found in the trajectories of Itersonilia perplexans spores in fig. 11 of Webster et al. [6], and in the trajectories of A. auricula spores in fig. 3 of Noblin et al. [12].
The numerical work in this paper mainly applies to elongated ballistospores, for which the adaxial surface can be approximated as a plane to first order. Although ballistospores typically adopt a somewhat elongated shape, rounded spores are occasionally encountered [1,2]. The tangential launching along the long axis appears to work well for the relatively elongated spores of I. perplexans and A. auricula, but less well for the more rounded spores of Sporobolomyces yeasts for which the long axis is ill-defined (e.g. fig. 4 of Noblin et al. [12]). Interestingly, the launching speed of the Sporobolomyces spore still follows the momentum scaling in equation (2.8).
4.2. Role of asymmetric coalescence
In the capillary–inertial regime, the asymmetric drop coalescence offers an intriguing division of roles in the generation of launching momentum: the Buller's drop in the form of a liquid globule ensures effective release of its surface energy to impart a substantial momentum, while the adaxial plane of the solid spore (covered with a flattened drop) orients the launching direction.
In terms of the effectiveness of momentum generation, the asymmetric coalescence not only releases a large fraction of the surface energy stored on the Buller's drop, but also converts a large portion of the released surface energy into translational kinetic energy. The effective conversion is evident in the dominance of translational over oscillatory motion at the point of launching (figure 4). Such a dominance gives rise to 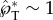 , which is impressive considering that the capillary–inertial velocity is about the best attainable in self-propelled motion powered by surface energy. The effectiveness will be significantly reduced if the drop coalescence is symmetric instead, as shown in a related capillary–inertial colloidal catapult where the merged drop has a predominantly oscillatory motion [31].
, which is impressive considering that the capillary–inertial velocity is about the best attainable in self-propelled motion powered by surface energy. The effectiveness will be significantly reduced if the drop coalescence is symmetric instead, as shown in a related capillary–inertial colloidal catapult where the merged drop has a predominantly oscillatory motion [31].
In terms of the robustness of directional control, the predominantly tangential launching direction (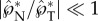 ) is insensitive to parametric variations, as long as the asymmetry in size, shape and position is preserved (e.g. with rA/rB ≳ 2, ψA ≲ 30° and β ≳ 45°). Such insensitivity is important given the natural variations in the drop/spore shape and the associated wettability across thousands of ballistospore species. Although the Buller's drop is well documented, the adaxial drop is insufficiently studied. With the model system represented by figure 3, we have experimentally observed the spore-shaped particle to launch with different adaxial cap angles ψA (see electronic supplementary material, movies S1 and S9). The observed insensitivity corroborates the conclusion that the adaxial drop plays a passive role in the asymmetric coalescence, but the adaxial plane guides the launching direction. Note that the asymmetry plays a critical role in the launching directionality, evident in figure 4 with the initially normal momentum turning into the eventually tangential one. Without the asymmetry, e.g. when the coalescence is between symmetric drops [31], it will not be possible to create a launching momentum that is tangential to the solid surface supporting the drops.
) is insensitive to parametric variations, as long as the asymmetry in size, shape and position is preserved (e.g. with rA/rB ≳ 2, ψA ≲ 30° and β ≳ 45°). Such insensitivity is important given the natural variations in the drop/spore shape and the associated wettability across thousands of ballistospore species. Although the Buller's drop is well documented, the adaxial drop is insufficiently studied. With the model system represented by figure 3, we have experimentally observed the spore-shaped particle to launch with different adaxial cap angles ψA (see electronic supplementary material, movies S1 and S9). The observed insensitivity corroborates the conclusion that the adaxial drop plays a passive role in the asymmetric coalescence, but the adaxial plane guides the launching direction. Note that the asymmetry plays a critical role in the launching directionality, evident in figure 4 with the initially normal momentum turning into the eventually tangential one. Without the asymmetry, e.g. when the coalescence is between symmetric drops [31], it will not be possible to create a launching momentum that is tangential to the solid surface supporting the drops.
Our model simplifies the role of the sterigma and translates it into an anchoring support that cannot withstand any tensile force, thereby ignoring the finite rupture force between the mature spore and the sterigma. By implicitly accounting for the sterigmal anchoring with a fixed spore in our simulations, we have focused on the role of the asymmetric drop coalescence in producing tangential launching momentum. Further work is needed to differentiate the roles of the two solid components with strongly correlated directions—the spore with an adaxial plane and the sterigma with a longitudinal axis.
5. Concluding remarks
Integrating simulations and experiments, we have elucidated the capillary–inertial mechanism of ballistospore discharge arising from the asymmetric coalescence of a round Buller's drop and a lens-shaped adaxial drop. During coalescence, the Buller's drop drains towards the adaxial drop and is redirected by capillary and solid confinements, such that the merged liquid acquires a predominantly tangential momentum along the longitudinal axis of an elongated spore. The peak tangential momentum of the merged liquid is approximately given by the Buller's drop mass multiplied by the capillary–inertial velocity based on the Buller's drop radius. After the tangential momentum peaks, the decelerating liquid exerts a tensile force to pull the spore away from the sterigma. The launched spore–liquid complex retains the peak liquid momentum and follows a trajectory along the adaxial plane of the initial spore.
The ballistospore launching mechanism is analogous to a cannon. The rapid capillary–inertial coalescence process quickly releases the surface energy accumulated by the much slower condensation, providing the high power required to launch small projectiles [32]. The high quality of the ballistospore ‘cannon’ is reflected in the large tangential momentum (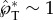 ) as well as the accurate directional control (
) as well as the accurate directional control (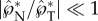 ). In equation (2.8), the speed of the ballistospore ‘projectile’ (u*SL) is largely determined by the relative quantity of ‘ammunition’ (m*), which is proportional to the mass of the active Buller's drop. Consistent with the need to maximize m*, the spherical Buller's drop is much more prominent compared to the flattened adaxial drop, so much so that A. H. R. Buller focused exclusively on this globular drop, which was eventually named after him.
). In equation (2.8), the speed of the ballistospore ‘projectile’ (u*SL) is largely determined by the relative quantity of ‘ammunition’ (m*), which is proportional to the mass of the active Buller's drop. Consistent with the need to maximize m*, the spherical Buller's drop is much more prominent compared to the flattened adaxial drop, so much so that A. H. R. Buller focused exclusively on this globular drop, which was eventually named after him.
The asymmetric Buller–adaxial drop arrangement ensures that a ballistospore achieves effective momentum generation and robust directional control by a division of roles: the Buller's drop in the form of a liquid globule is used to maximize the release and conversion of surface energy, while the adaxial plane of the solid spore (covered with a flattened drop) is responsible for robustly orientating the launch. By distinguishing the two key functions of ballistospore launching, momentum generation and directional control, our mechanistic model offers a general mechanical framework to study the morphological and kinematic diversity of ballistospores. Beyond fungal biology, our work on the capillary–inertial catapulting mechanism has broader implications for both biology and engineering, such as ballistic launching systems for small projectiles [32–34] and self-cleaning systems for colloidal contaminants [31,35,36].
Supplementary Material
Acknowledgments
The authors are grateful to the late Prof. Steven Vogel for inspiring discussions. C.H.C. acknowledges an anonymous reviewer of [19] for pointing him to the ballistospore literature.
Data accessibility
Electronic supplementary material is available online.
Author's contributions
C.-H.C. conceived the project. F.L., R.L.C., J.J.F. and C.-H.C. designed the research. F.L. conducted simulations and R.L.C. performed experiments. All authors analysed data and wrote the paper. F.L. and R.L.C contributed equally to this work.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the National Science Foundation (CBET-1236373 and DMR-1121107) and the National Institute of Health (T32-GM008555). S.N.P was supported by the National Science Foundation (IOS-1439850). A.P. was supported by the Human Frontier Science Program. J.J.F. was supported by the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Buller AHR. 1909–1950 Researches on fungi, vol. 1–7 London, UK: Longmans, Green, and Co. [Google Scholar]
- 2.Webster J, Weber R. 2007. Introduction to fungi, 3rd edn Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Blackwell M. 2011. The fungi: 1, 2, 3 … 5.1 million species? Am. J. Bot. 98, 426–438. ( 10.3732/ajb.1000298) [DOI] [PubMed] [Google Scholar]
- 4.Trail F. 2007. Fungal cannons: explosive spore discharge in the ascomycota. FEMS Microbiol. Lett. 276, 12–18. ( 10.1111/j.1574-6968.2007.00900.x) [DOI] [PubMed] [Google Scholar]
- 5.Pringle A, Brenner MP, Fritz JA, Roper M, Seminara A. 2017. Reaching the wind: Boundary layer escape as a constraint on ascomycete spore dispersal. In The fungal community: its organization and role in the ecosystem (eds Dighton J, White JF), 4th edn Boca Raton, FL: CRC Press. [Google Scholar]
- 6.Webster J, Davey RA, Duller GA, Ingold CT. 1984. Ballistospore discharge in Itersonilia perplexans. Trans. Br. Mycol. Soc. 82, 13–29. ( 10.1016/S0007-1536(84)80207-7) [DOI] [Google Scholar]
- 7.Money NP. 1998. More g's than the space shuttle: ballistospore discharge. Mycologia 90, 547–558. ( 10.2307/3761212) [DOI] [Google Scholar]
- 8.Gould SJ, Lewontin RC. 1979. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc. R. Soc. Lond. B 205, 581–598. ( 10.1098/rspb.1979.0086) [DOI] [PubMed] [Google Scholar]
- 9.Fischer MW, Stolze-Rybczynski JL, Cui Y, Money NP. 2010. How far and how fast can mushroom spores fly? Physical limits on ballistospore size and discharge distance in the Basidiomycota. Fungal Biol. 114, 669–675. ( 10.1016/j.funbio.2010.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webster J, Proctor MCF, Davey RA, Duller GA. 1988. Measurement of the electrical charge on some basidiospores and an assessment of two possible mechanisms of ballistospore propulsion. Trans. Br. Mycol. Soc. 91, 193–203. ( 10.1016/S0007-1536(88)80205-5) [DOI] [Google Scholar]
- 11.Pringle A, Patek SN, Fischer M, Stolze J, Money NP. 2005. The captured launch of a ballistospore. Mycologia 97, 866–871. ( 10.1080/15572536.2006.11832777) [DOI] [PubMed] [Google Scholar]
- 12.Noblin X, Yang S, Dumais J. 2009. Surface tension propulsion of fungal spores. J. Exp. Biol. 212, 2835–2843. ( 10.1242/jeb.029975) [DOI] [PubMed] [Google Scholar]
- 13.Stolze-Rybczynski JL, Cui YL, Stevens MHH, Davis DJ, Fischer MWF, Money NP. 2009. Adaptation of the spore discharge mechanism in the Basidiomycota. PLoS ONE 4, e4163 ( 10.1371/journal.pone.0004163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webster J, Davey RA, Ingold CT. 1984. Origin of the liquid in Buller drop. Trans. Br. Mycol. Soc. 83, 524–527. ( 10.1016/S0007-1536(84)80055-8) [DOI] [Google Scholar]
- 15.Webster J, Davey RA, Smirnoff N, Fricke W, Hinde P, Tomos D, Turner JCR. 1995. Mannitol and hexoses are components of Buller's drop. Mycol. Res. 99, 833–838. ( 10.1016/S0953-7562(09)80737-5) [DOI] [Google Scholar]
- 16.Ingold CT. 1971. Fungal spores: their liberation and dispersal. Oxford, UK: Clarendon Press. [Google Scholar]
- 17.Aylor DE. 1990. The role of intermittent wind in the dispersal of fungal pathogens. Annu. Rev. Phytopathol. 28, 73–92. ( 10.1146/annurev.py.28.090190.000445) [DOI] [Google Scholar]
- 18.Dressaire E, Yamada L, Song B, Roper M. 2016. Mushrooms use convectively created airflows to disperse their spores. Proc. Natl Acad. Sci. USA 113, 2833–2838. ( 10.1073/pnas.1509612113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boreyko JB, Chen C-H. 2009. Self-propelled dropwise condensate on superhydrophobic surfaces. Phys. Rev. Lett. 103, 184501 ( 10.1103/PhysRevLett.103.184501) [DOI] [PubMed] [Google Scholar]
- 20.Liu F, Ghigliotti G, Feng JJ, Chen C-H. 2014. Numerical simulations of self-propelled jumping upon drop coalescence on non-wetting surfaces. J. Fluid Mech. 752, 39–65. ( 10.1017/jfm.2014.320) [DOI] [Google Scholar]
- 21.Zhang K, Liu F, Williams AJ, Qu X, Feng JJ, Chen C-H. 2015. Self-propelled droplet removal from hydrophobic fiber-based coalescers. Phys. Rev. Lett. 115, 074502 ( 10.1103/PhysRevLett.115.074502) [DOI] [PubMed] [Google Scholar]
- 22.Reed E, Wallace H. 1965. Leaping locomotion by an insect-parasitic nematode. Nature 206, 210–211. ( 10.1038/206210a0) [DOI] [Google Scholar]
- 23.Campbell JF, Kaya HK. 2000. Mechanism, kinematic performance, and fitness consequences of jumping behavior in entomopathogenic nematodes (steinernema spp.). Can. J. Zool. 77, 1947–1955. ( 10.1139/z99-178) [DOI] [Google Scholar]
- 24.McLaughlin DJ, Beckett A, Yoon KS. 1985. Ultrastructure and evolution of ballistosporic basidiospores. Bot. J. Linnean Soc. 91, 253–271. ( 10.1111/j.1095-8339.1985.tb01149.x) [DOI] [Google Scholar]
- 25.Goates BJ, Hoffmann JA. 1986. Formation and discharge of secondary sporidia of the bunt fungus, Tilletia foetida. Mycologia 78, 371–379. ( 10.2307/3793040) [DOI] [Google Scholar]
- 26.Miller SL, Miller OK. 1988. Spore release in hypogeous, gasteroid and agaricoid russulales. Trans. Br. Mycol. Soc. 90, 513–526. ( 10.1016/S0007-1536(88)80001-9) [DOI] [Google Scholar]
- 27.Miljkovic N, Enright R, Nam Y, Lopez K, Dou N, Sack J, Wang EN. 2013. Jumping-droplet-enhanced condensation on scalable superhydrophobic nanostructured surfaces. Nano Lett. 13, 179–187. ( 10.1021/nl303835d) [DOI] [PubMed] [Google Scholar]
- 28.Yue P, Feng JJ, Liu C, Shen J. 2004. A diffuse-interface method for simulating two-phase flows of complex fluids. J. Fluid Mech. 515, 293–317. ( 10.1017/S0022112004000370) [DOI] [Google Scholar]
- 29.Yue P, Feng JJ. 2011. Can diffuse-interface models quantitatively describe moving contact lines? Eur. Phys. J. Spec. Top. 197, 37–46. ( 10.1140/epjst/e2011-01434-y) [DOI] [Google Scholar]
- 30.Liu F, Ghigliotti G, Feng JJ, Chen CH. 2014. Self-propelled jumping upon drop coalescence on leidenfrost surfaces. J. Fluid Mech. 752, 22–38. ( 10.1017/jfm.2014.319) [DOI] [Google Scholar]
- 31.Chavez RL, Liu F, Feng JJ, Chen CH. 2016. Capillary-inertial colloidal catapults upon drop coalescence. Appl. Phys. Lett. 109, 011601 ( 10.1063/1.4955085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogel S. 2009. Glimpses of creatures in their physical worlds. Princeton, NJ: Princeton Univ Press. [Google Scholar]
- 33.Koh J-S. 2015. Jumping on water: surface tension–dominated jumping of water striders and robotic insects. Science 349, 517–521. ( 10.1126/science.aab1637) [DOI] [PubMed] [Google Scholar]
- 34.Sakes A, van der Wiel M, Henselmans PW, van Leeuwen JL, Dodou D, Breedveld P. 2016. Shooting mechanisms in nature: a systematic review. PLoS ONE 11, e0158277 ( 10.1371/journal.pone.0158277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wisdom KM, Watson JA, Qu X, Liu F, Watson GS, Chen C-H. 2013. Self-cleaning of superhydrophobic surfaces by self-propelled jumping condensate. Proc. Natl Acad. Sci. USA 110, 7992–7997. ( 10.1073/pnas.1210770110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson GS, Schwarzkopf L, Cribb BW, Myhra S, Gellender M, Watson JA. 2015. Removal mechanisms of dew via self-propulsion off the gecko skin. J. R. Soc. Interface 12, 20141396 ( 10.1098/rsif.2014.1396) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Electronic supplementary material is available online.