Abstract
Hybridomas producing a monoclonal IgG1 antibody to a human plasminogen-activating enzyme with an apparent mol. wt. of 66,000 (66 K, HPA66) from human melanoma cells were obtained by fusion of NSI-Ag 4/1 mouse myeloma cells with spleen cells from a mouse immunized with a partially purified preparation of the enzyme. Screening for clones of hybridomas producing antibodies to HPA66 was performed with the impure enzyme preparation. A preliminary screening included enzyme-linked immunosorbent assay and SDS-polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting; the final identification was based on inhibition of the enzymatic activity of HPA66 which was complete at high antibody concentrations. No inhibition of three other human and murine plasminogen activators or of plasmin was observed. Employing a one-step affinity procedure with the antibody coupled to Sepharose, HPA66 was purified approximately 200-fold from conditioned medium from the melanoma cells with a yield of 79%. The purified HPA66 was homogeneous as evaluated by SDS-PAGE. Electrophoresis under reducing conditions indicated that it consisted of one polypeptide chain. The binding constant between the antibody and 125I-labelled HPA66 was approximately 2.5 x 10(9) l/mol. The antibody did not bind to a variety of other plasminogen activators, including 52-K and 36-K human enzymes and 48-K and 75-K murine enzymes. Previously, a monoclonal antibody against another enzyme was derived by the sole use of enzyme inhibition for screening. The present study represents a modification of this procedure that can be used when antibody-unrelated inhibitors of the enzyme are present in hybridoma culture fluid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. A., Pepper D. S. Isolation and properties of human vascular plasminogen activator. Thromb Haemost. 1981 Feb 23;45(1):43–50. [PubMed] [Google Scholar]
- Aoki N. Preparation of plasminogen activator from vascular trees of human cadavers. Its comparison with urokinase. J Biochem. 1974 Apr;75(4):731–741. doi: 10.1093/oxfordjournals.jbchem.a130446. [DOI] [PubMed] [Google Scholar]
- Aoki N., Von Kaulla K. N. Dissimilarity of human vascular plasminogen activator and human urokinase. J Lab Clin Med. 1971 Sep;78(3):354–362. [PubMed] [Google Scholar]
- Astedt B. No crossreaction between circulating plasminogen activator and urokinase. Thromb Res. 1979;14(4-5):535–539. doi: 10.1016/0049-3848(79)90109-9. [DOI] [PubMed] [Google Scholar]
- Beers W. H., Strickland S., Reich E. Ovarian plasminogen activator: relationship to ovulation and hormonal regulation. Cell. 1975 Nov;6(3):387–394. doi: 10.1016/0092-8674(75)90188-9. [DOI] [PubMed] [Google Scholar]
- Binder B. R., Spragg J., Austen K. F. Purification and characterization of human vascular plasminogen activator derived from blood vessel perfusates. J Biol Chem. 1979 Mar 25;254(6):1998–2003. [PubMed] [Google Scholar]
- Bonsall R. W., Hunt S. Characteristics of interactions between surfactants and the human erythrocyte membrane. Biochim Biophys Acta. 1971 Oct 12;249(1):266–280. doi: 10.1016/0005-2736(71)90104-0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Christman J. K., Silverstein S. C., Acs G. Immunological analysis of plasminogen activators from normal and transformed hamster cells. Evidence that the plasminogen activators produced by SV40 virus-transformed hamster embryo cells and normal hamster lung cells are antigenically identical. J Exp Med. 1975 Aug 1;142(2):419–434. doi: 10.1084/jem.142.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole E. R., Bachmann F. W. Purification and properties of a plasminogen activator from pig heart. J Biol Chem. 1977 Jun 10;252(11):3729–3737. [PubMed] [Google Scholar]
- Danø K., Dabelsteen E., Nielsen L. S., Kaltoft K., Wilson E. L., Zeuthen J. Plasminogen activating enzyme in cultured glioblastoma cells. An immunofluorescence study with monoclonal antibody. J Histochem Cytochem. 1982 Nov;30(11):1165–1170. doi: 10.1177/30.11.6183313. [DOI] [PubMed] [Google Scholar]
- Danø K., Moller V., Ossowski L., Nielsen L. S. Purification and characterization of a plasminogen activator from mouse cells transformed by an oncogenic virus. Biochim Biophys Acta. 1980 Jun 13;613(2):542–555. doi: 10.1016/0005-2744(80)90110-2. [DOI] [PubMed] [Google Scholar]
- Danø K., Nielsen L. S., Møller V., Engelhart M. Inhibition of a plasminogen activator from oncogenic virus-transformed mouse cells by rabbit antibodies against the enzyme. Biochim Biophys Acta. 1980 Jun 5;630(1):146–151. doi: 10.1016/0304-4165(80)90146-4. [DOI] [PubMed] [Google Scholar]
- Danø K., Reich E. Serine enzymes released by cultured neoplastic cells. J Exp Med. 1978 Mar 1;147(3):745–757. doi: 10.1084/jem.147.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Rotman B. Functional diversity of antibodies elicited by bacterial beta-D-galactosidase. Monoclonal activating, inactivating, protecting, and null antibodies to normal enzyme. J Biol Chem. 1980 Jun 10;255(11):5286–5290. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
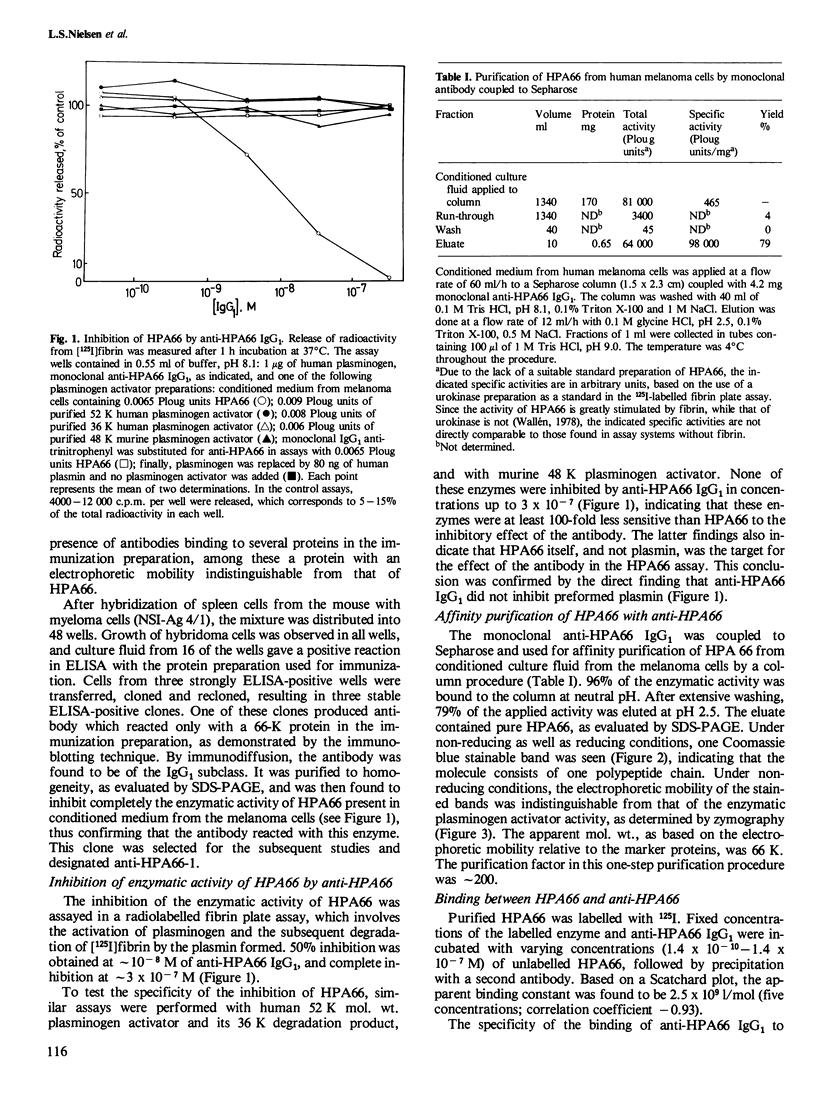
- Kaltoft K., Nielsen L. S., Zeuthen J., Danø K. Monoclonal antibody that specifically inhibits a human Mr 52,000 plasminogen-activating enzyme. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3720–3723. doi: 10.1073/pnas.79.12.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsuo O., Rijken D. C., Collen D. Thrombolysis by human tissue plasminogen activator and urokinase in rabbits with experimental pulmonary embolus. Nature. 1981 Jun 18;291(5816):590–591. doi: 10.1038/291590a0. [DOI] [PubMed] [Google Scholar]
- Mattsson C., Nyberg-Arrhenius V., Wallén P. Dissolution of thrombi by tissue plasminogen activator, urokinase and streptokinase in an artificial circulating system. Thromb Res. 1981 Mar 15;21(6):535–545. doi: 10.1016/0049-3848(81)90254-1. [DOI] [PubMed] [Google Scholar]
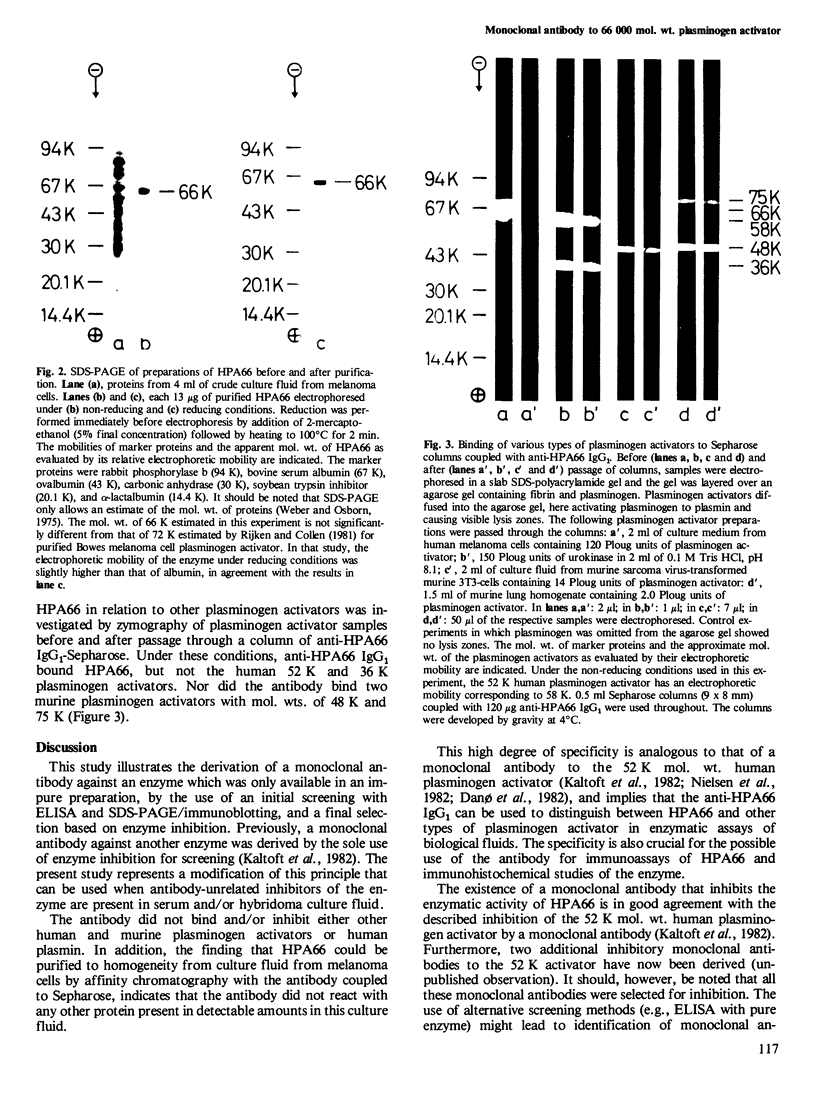
- Nielsen L. S., Hansen J. G., Skriver L., Wilson E. L., Kaltoft K., Zeuthen J., Danø K. Purification of zymogen to plasminogen activator from human glioblastoma cells by affinity chromatography with monoclonal antibody. Biochemistry. 1982 Dec 7;21(25):6410–6415. doi: 10.1021/bi00268a014. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Biegel D., Reich E. Mammary plasminogen activator: correlation with involution, hormonal modulation and comparison between normal and neoplastic tissue. Cell. 1979 Apr;16(4):929–940. doi: 10.1016/0092-8674(79)90108-9. [DOI] [PubMed] [Google Scholar]
- Porath J., Carlsson J., Olsson I., Belfrage G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 1975 Dec 18;258(5536):598–599. doi: 10.1038/258598a0. [DOI] [PubMed] [Google Scholar]
- Radcliffe R., Heinze T. Isolation of plasminogen activator from human plasma by chromatography on lysine-sepharose. Arch Biochem Biophys. 1978 Jul;189(1):185–194. doi: 10.1016/0003-9861(78)90131-5. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Loeb J. N., Moore G., Reich E. Properties of plasminogen activators formed by neoplastic human cell cultures. J Exp Med. 1974 May 1;139(5):1317–1328. doi: 10.1084/jem.139.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijken D. C., Collen D. Purification and characterization of the plasminogen activator secreted by human melanoma cells in culture. J Biol Chem. 1981 Jul 10;256(13):7035–7041. [PubMed] [Google Scholar]
- Rijken D. C., Wijngaards G., Welbergen J. Relationship between tissue plasminogen activator and the activators in blood and vascular wall. Thromb Res. 1980 Jun 15;18(6):815–830. doi: 10.1016/0049-3848(80)90204-2. [DOI] [PubMed] [Google Scholar]
- Rijken D. C., Wijngaards G., Zaal-de Jong M., Welbergen J. Purification and partial characterization of plasminogen activator from human uterine tissue. Biochim Biophys Acta. 1979 Sep 29;580(1):140–153. doi: 10.1016/0005-2795(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Roblin R., Young P. L. Dexamethasone regulation of plasminogen activator in embryonic and tumor-derived human cells. Cancer Res. 1980 Aug;40(8 Pt 1):2706–2713. [PubMed] [Google Scholar]
- Skriver L., Nielsen L. S., Stephens R., Danø K. Plasminogen activator released as inactive proenzyme from murine cells transformed by sarcoma virus. Eur J Biochem. 1982 May 17;124(2):409–414. doi: 10.1111/j.1432-1033.1982.tb06608.x. [DOI] [PubMed] [Google Scholar]
- Strickland S., Reich E., Sherman M. I. Plasminogen activator in early embryogenesis: enzyme production by trophoblast and parietal endoderm. Cell. 1976 Oct;9(2):231–240. doi: 10.1016/0092-8674(76)90114-8. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J., Dano K., Kellerman G. M., Reich E. Fibrinolysis associated with oncogenic transformation. Partial purification and characterization of the cell factor, a plasminogen activator. J Biol Chem. 1974 Jul 10;249(13):4295–4305. [PubMed] [Google Scholar]
- Vetterlein D., Young P. L., Bell T. E., Roblin R. Immunological characterization of multiple weight forms of human cell plasminogen activators. J Biol Chem. 1979 Feb 10;254(3):575–578. [PubMed] [Google Scholar]
- Wilson E. L., Becker M. L., Hoal E. G., Dowdle E. B. Molecular species of plasminogen activators secreted by normal and neoplastic human cells. Cancer Res. 1980 Mar;40(3):933–938. [PubMed] [Google Scholar]