Abstract
Global wind patterns affect flight strategies in many birds, including pelagic seabirds, many of which use wind-powered soaring to reduce energy costs during at-sea foraging trips and migration. Such long-distance movement patterns are underpinned by local interactions between wind conditions and flight behaviour, but these fine-scale relationships are far less well understood. Here we show that remotely sensed ocean wind speed and direction are highly significant predictors of soaring behaviour in a migratory pelagic seabird, the Manx shearwater (Puffinus puffinus). We used high-frequency GPS tracking data (10 Hz) and statistical behaviour state classification to identify two energetic modes in at-sea flight, corresponding to flap-like and soar-like flight. We show that soaring is significantly more likely to occur in tailwinds and crosswinds above a wind speed threshold of around 8 m s−1, suggesting that these conditions enable birds to reduce metabolic costs by preferentially soaring over flapping. Our results suggest a behavioural mechanism by which wind conditions may shape foraging and migration ecology in pelagic seabirds, and thus indicate that shifts in wind patterns driven by climate change could impact this and other species. They also emphasize the emerging potential of high-frequency GPS biologgers to provide detailed quantitative insights into fine-scale flight behaviour in free-living animals.
Keywords: movement ecology, seabirds, tracking, remote sensing, wind, soaring
1. Introduction
The effects of global-scale environmental variables such as temperature and precipitation on animal ecology are well known, but similar relationships with wind have been much less extensively studied. Wind conditions affect phenology, migration routes, ecological interactions and foraging success in many volant animals including birds, bats and insects (e.g. [1–4]). Recent GPS tracking studies have shown that global winds affect long-distance patterns of foraging and migration behaviour in various wide-ranging bird species [5–8]; however much less is known about the effect of more localized wind conditions. Understanding such fine-scale interactions between flight behaviour and the environment is key to understanding how individual behavioural responses to wind scale up to shape movement patterns at large spatial scales and over evolutionary time, such as the evolution of stable migration routes [8,9]. In a conservation context, such knowledge is also important to predict how shifts in atmospheric conditions under climate change [10–12] may impact many migratory birds.
Pelagic seabirds are top marine predators that regularly travel hundreds of kilometres during foraging and migration [13], making them particularly reliant on ocean wind patterns [14–17]. During these journeys many albatrosses and shearwaters (Procellariiformes) engage in specialized modes of wind-powered soaring behaviour, thought to be metabolically less costly than flapping flight [18–20]. Data from GPS and accelerometer tags are now providing insights into soaring in free-living albatrosses and other birds [21–23]; however much remains unknown about the fine-scale relationship between local winds and soaring behaviour. In this study we use very high-frequency GPS tracking (10 Hz) to show that wind speed and direction, measured via satellite remote sensing, are highly significant predictors of soaring behaviour in a migratory pelagic seabird, the Manx shearwater (Puffinus puffinus). Manx shearwaters are small (approx. 400 g), burrow nesting, pelagic seabirds. They are Amber listed in the UK [24] where most (approx. 80%) of the global Manx shearwater population nests. They forage from breeding colonies around the UK coastline each summer before migrating to overwinter off southern Argentina, making an annual round trip of over 20 000 km [9,25–27].
We tracked breeding adults during at-sea foraging trips using custom GPS loggers that record bursts of three-dimensional location fixes at 10 Hz, and distinguished flight behaviour from each burst's mechanical energy characteristics. A bird's total mechanical energy at any time consists of the two components kinetic (related to speed) and gravitational potential energy (related to altitude). During flight, total energy can increase either through flapping, when stored chemical energy is converted to power in the wing muscles, or through input from an external energy source, e.g. wind [28]. Relative changes to the kinetic and potential energy components are determined both by the magnitude of energy input and the bird's current mode of movement. Different flight behaviours therefore show markedly different patterns of mechanical energy change over time, which can be calculated from high-frequency three-dimensional GPS positional data (e.g. [22]). During soaring, tracked albatrosses show large cyclical variations in both potential (derived from altitude) and kinetic energy (derived from ground speed) as they ascend and descend through the shear wind gradient above the sea surface [22]. Although Manx shearwaters are ‘flap-gliders’, mixing intermittent wingbeat pulses with gliding and soaring [13], we hypothesized that wind-powered soaring in this species would show similar variations in energy and ground speed.
We therefore aimed to assess the prevalence of wind-powered soaring in Manx shearwaters and how this may vary under different environmental conditions. If, as might be expected, wind conditions play a role in how frequently soaring can occur, and soaring represents an energetically favourable mode of flight, then this has implications for the cost of movement during travel and foraging. This can have knock-on effects upon how much effort is expended during reproduction, which has been demonstrated to impact breeding success in subsequent years [29]. Furthermore, quantifying the impacts of environmental conditions on the energetics of movement has potential implications for understanding the timing and success of migration and stopover [9]. This study also represents a proof of concept, demonstrating the potential of high-frequency GPS to analyse predictive relationships between movement and environmental conditions, with implications for understanding distribution, space-use and conservation of seabird species.
2. Methods
2.1. GPS tracking procedure
We tracked breeding adult birds during the chick-rearing season, between 12 and 25 August 2012 at the study colony on Lundy Island, Devon, UK (51.1781°N, 4.6673°W). We deployed our own custom GPS loggers (mataki.org [30]) on eight birds. Devices were positioned on the back above the bird's centre of gravity and attached to feathers with marine tape, ensuring that if loggers could not be retrieved they would loosen and fall off within 2–3 weeks (see details in [25,31]). Study individuals weighed between 415 and 470 g, and complete mass of devices including tape was less than 17 g, under 3.6% of body mass. To maximize the proportion of foraging trips recorded, devices were programmed to record 10 Hz bursts of GPS fixes for 60 s, at 30 min intervals. Each fix recorded latitude, longitude and altitude, so each discrete sequence of 10 Hz fixes (hereafter ‘burst’) forms a detailed track of the bird's movement through its environment. All loggers were retrieved from recaptured birds and data were downloaded for analysis. One bird remained in its burrow for the study duration, so at-sea GPS tracks were obtained from seven birds (table 1). Seven complete foraging trips were recorded from these birds, with durations of 17.1–53.5 h (mean 44.9 ± 23.8 h), and four incomplete foraging trips during which the device battery expired before the bird returned to the colony.
Table 1.
Summary tracking statistics for all seven birds, including proportion of recorded bursts classified as soar-like, flap-like, sitting and colony-associated, and average wind speed (mean ± s.d.) encountered during the tracking period.
| ID | number of bursts | body mass before tracking (g) | tracking time (h) | total distance (km) | flap-like % |
soar-like % |
sitting % |
colony % |
wind speed (mean ± s.d.) (m s−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 114 | 445 | 79 | 625.1 | 21.9 | 26.3 | 35.1 | 16.7 | 11.05 ± 1.6 |
| 2 | 115 | 430 | 85.8 | 483.7 | 11.3 | 9.6 | 72.2 | 6.9 | 6.54 ± 3.59 |
| 3 | 56 | 440 | 46.8 | 282.6 | 17.8 | 25.0 | 51.8 | 5.3 | 10.97 ± 2.52 |
| 4 | 33 | 470 | 25.9 | 200.8 | 21.2 | 9.1 | 48.5 | 21.2 | 1.9 ± 0.25 |
| 5 | 44 | 465 | 28.5 | 123.1 | 22.7 | 4.5 | 43.2 | 29.5 | 7.09 ± 0.15 |
| 6 | 101 | 450 | 94.9 | 700.8 | 26.7 | 2.9 | 58.4 | 11.9 | 4.82 ± 3.0 |
| 7 | 96 | 440 | 76.9 | 462.8 | 8.3 | 31.3 | 37.5 | 22.9 | 10.58 ± 1.76 |
2.2. Track processing and movement analysis
All analyses were carried out in R v. 3.1.2 [32]. Complete GPS tracks were filtered to exclude fixes with erroneous timestamps and those derived using fewer than four satellites, the minimum required for a precise three-dimensional location and time fix [25]. Each bird's track was split into its constituent bursts and the median latitude and longitude of each burst were assigned as its location. Since this study concerns at-sea activity, colony-associated bursts (within 1500 m radius around Lundy, n = 84) were excluded, as were information-poor bursts of fewer than 20 points (n = 35), leaving a total of n = 475 at-sea bursts. Within each burst we calculated distance and ground speed (velocity with respect to Earth's surface) between successive fixes. Fixes with speeds exceeding 40 m s−1 were excluded as likely GPS errors [25]. To reduce the effect of any small GPS positional errors or missed fixes, we smoothed ground speed and altitude along each burst by applying a 15-point (1.5 s) rolling mean.
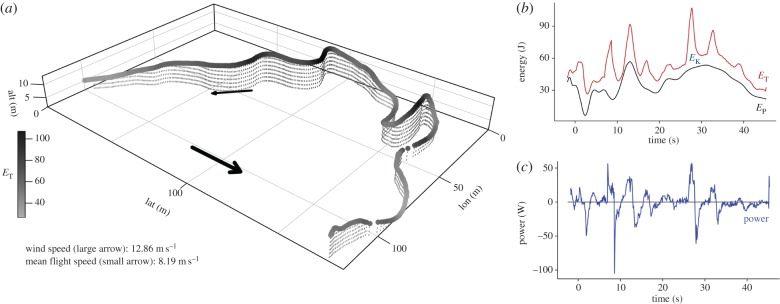
Following [23], from each fix's ground speed and altitude we calculated mechanical energy components kinetic (EK), gravitational potential (EP) and total energy (ET = EK + EP), and also mechanical power (P), which measures the rate of ET change across each between-fix time interval. These describe a bird's in-flight mechanical energy relative to the Earth's surface (as inertial frame of reference), and their relative changes across a 60 s tracked burst describe flight dynamics in detail [22]. Although not directly related to metabolic energy expenditure, power values in excess of 0 indicate a net increase in mechanical energy over time, which could either be due to metabolic energy input (from wing muscles) or from the wind [28]. There is an upper limit to the power a bird can generate by flapping; therefore high power values and very large variation in EK and power are strongly suggestive of wind energy input [22]. Further detail on track processing is provided in electronic supplementary material (S1).
Tracking data are inherently statistically non-independent, with an animal's movement at any time being influenced by its recent activities, internal state and environment [33,34]. However, between-burst time intervals were sufficiently large (minimum 31.08 min) to allow each to be treated as functionally independent. We therefore compared bursts by calculating the following summary parameters for each burst: (i) beeline distance (straight-line distance between burst start and endpoints); (ii) mean ground speed (‘mean speed’); (iii) standard deviation of kinetic energy (‘EK variance’); (iv) standard deviation of power (‘power variance’); and (v) straightness index (beeline distance divided by total path length), a measure of path tortuosity ranging from completely straight (SI = 1) to randomly oriented (SI = 0) [35].
Clusters in the distribution of summary parameters corresponding to putative flight modes were identified by fitting multivariate Gaussian mixture models (GMMs) by expectation-maximization (EM), using mclust v.4.4 [36,37]. GMMs estimate the probability of each observation belonging to each cluster, and as such are a useful framework for identifying energetic modes from 60 s bursts of tracked flight, which often contain mixtures of flap-powered and wind-powered flight rather than single discrete behaviours. All variables were transformed to have mean 0 and standard deviation 1 before model fitting.
2.3. Modelling behavioural responses to environment
Metop/ASCAT remotely sensed wind data (24-h averaged at 0.25° resolution) were obtained from CERSAT (http://www.ifremer.fr/cersat). For each burst location this provided both total wind speed and separate zonal and meridional components, from which we calculated wind direction. Each burst's flight direction relative to wind (‘flight direction’) was calculated as the difference between burst beeline bearing and wind direction, and categorized as ‘tailwind’ (a difference of 0° to 50°), ‘crosswind’ (50° to 130°) or ‘headwind’ (130° to 180°), following [13]. We also obtained remotely sensed data for sea surface chlorophyll a concentration (CHL), net primary productivity (NPP) and sea surface temperature (SST), to test possible relationships between flight mode and ocean productivity as proxy for prey abundance (see electronic supplementary material, S2). CHL and SST from Aqua and Terra MODIS were obtained from NASA OceanColor (4 km, 8640 × 4320, 8-day composite, http://oceancolor.gsfc.nasa.gov/cms/). Aqua and Terra values were averaged where both were available, and missing data values were removed. Modelled NPP was obtained from Oregon State University Ocean Productivity (2160 × 4320, 8-day composite, http://www.science.oregonstate.edu/ocean.productivity/). We modelled relationships between flight mode, wind and ocean productivity using logistic mixed-effects regression (lme4 v.1.1-7 [38]).
3. Results
3.1. Flight mode classification
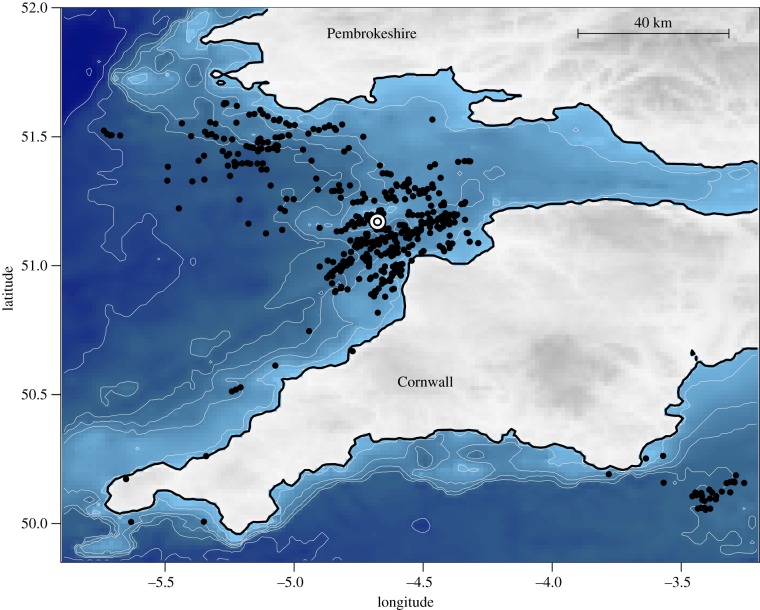
Foraging was mostly concentrated locally around Lundy and northwest towards Wales (figure 1; mean distance from colony 33.0 ± 35.4 km). Track processing yielded a final dataset of at-sea 10 Hz bursts (n = 475) (figure 2; for more examples see electronic supplementary material, S4). Although most recorded for a full 60 s, some bursts were shorter due to device error (burst length mean 43.9 s, median 59.9 s). We were only interested in bursts recorded during flight, so following [25], we first classified bursts as either in-flight (n = 193) or sitting on sea surface (n = 282) by fitting a two-component GMM to the bimodal distribution of mean speeds (BIC = −625.7, log-lik = −297.44; electronic supplementary material, figure S5). Flight bursts showed high mean speed (10.89 ± 3.31 m s−1) while sitting bursts showed low mean speed and variance (1.33 ± 0.61 m s−1). Sitting bursts were excluded from subsequent analysis.
Figure 1.
The distribution of Manx shearwaters foraging around Lundy Island (white circle), tracked between 12 and 25 August 2012 (n = 7). Black points denote 60 s bursts of tracked movement (n = 475) and are shown relative to the underlying bathymetry, accessed from National Oceanic and Atmospheric Administration (NOAA) via marmap [39].
Figure 2.
A high-frequency GPS flight burst. The bird's three-dimensional path through space (a) is shown with track shaded by total mechanical energy (ET), with arrows showing wind direction (large) and flight direction (small). Separate graphs show mechanical energy components ET, EP and EK (b) and power (c) against time. This burst contains both low-power flapping and spikes in power suggesting wind energy input.
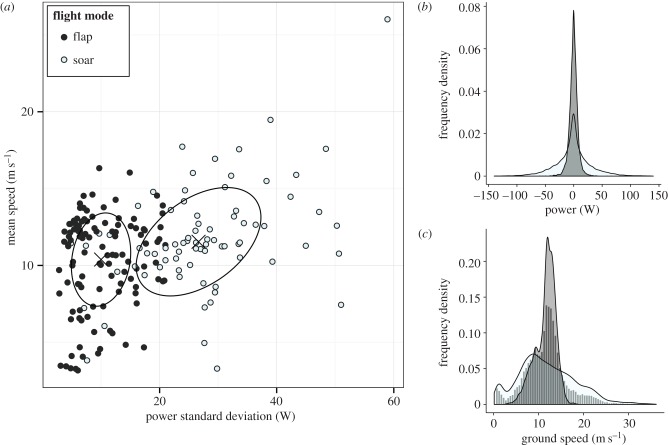
For all flight bursts (n = 193) we identified clusters in the distribution of mean speed, power variance and EK variance by iteratively fitting trivariate GMMs with an increasing number of clusters. Although Bayesian information criterion (BIC) was maximized with a 3-component model, by far the greatest BIC increase was observed between 1 and 2 component models, identifying a clear knee-point [40]. We therefore selected a mixture of 2 ellipsoidal Gaussian components as most parsimonious (BIC = −1270.09, log-lik = −585.05, d.f. = 19). The first component's high speed and low energetic variance was consistent with powered flapping flight, while the second component showed high speed and high energetic variance, consistent with wind-powered soaring (figure 3a). Each flight burst was classified to either flap-like (n = 115) or soar-like (n = 78) by maximum probability. Bursts classified with under 95% probability (low-certainty bursts, n = 74) were of intermediate energetic variance and visual inspection suggested that most contained mixtures of flight modes, although the GMM classified the majority as flap-like (n = 55). However, the resolution of the available environmental covariates meant that it would not be possible to resolve finer-scale relationships between the environment and within-burst variations in flight mode. We therefore decided to classify bursts in their entirety to either flap-like or soar-like for subsequent analyses.
Figure 3.
Energetic characteristics of flap-like and soar-like flight. (a) Relationship between burst summary parameters power variance and mean speed, with ellipses showing modelled Gaussian components (mean + s.d.). Density curves show within-burst distributions of fine-scale power (b) and ground speed (c) for flap-like (grey) and soar-like flight (white), produced by combining 10 Hz points from all bursts classified to either mode with over 95% probability. The distribution of ground speeds across all flight bursts (both flap-like and soar-like) was trimodal (histogram in c). (Online version in colour.)
Summary energetic parameter values for flap-like and soar-like bursts classified with over 95% probability (high-certainty bursts) are reported in table 2. High-certainty flap-like and soar-like bursts contained markedly different distributions of fine-scale in-flight power and ground speed (figure 3b,c). Energetic dynamics within soar-like bursts generally consisted of large oscillations in power, often due to rapid EK gains. There were large differences in the amount of time that different individuals spent engaging in different behaviours (table 1). There were overlaps in foraging areas between birds, but no obvious visible spatial trends in the at-sea distribution of flap-like and soar-like flight (electronic supplementary material, figure S3).
Table 2.
Summary movement and energetic characteristics of bursts classified to flap-like and soar-like with over 95% probability (n = 119). Values reported are mean ± s.d. Asterisks denote a significant difference between flap-like and soar-like bursts (p < 0.0001), tested using either two-sided t-test (*) or Wilcoxon sum-ranks (**).
| flight mode | number of bursts | mean ground speed (m s−1) | power variance (W) (*) | EK variance (J) (*) | beeline distance (m) | straightness index (**) |
|---|---|---|---|---|---|---|
| flap-like | 60 | 11.46 ± 2.23 | 7.22 ± 2.63 | 4.98 ± 2.74 | 422.3 ± 268.3 | 0.88 ± 0.12 |
| soar-like | 59 | 11.97 ± 3.84 | 30.62 ± 10.5 | 27.29 ± 10.68 | 418.0 ± 299.5 | 0.72 ± 0.25 |
3.2. Environmental predictors of soaring
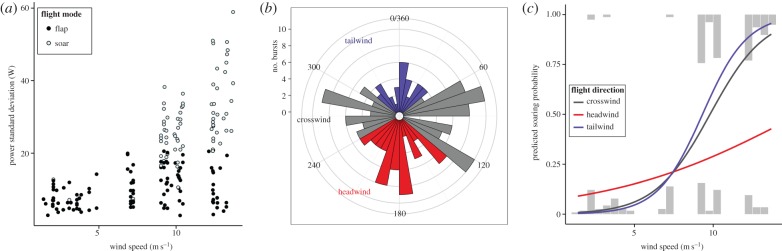
Wind speed data were accessed for 189 bursts (data for four bursts were missing from the METOP/ASCAT dataset, possibly because of cloud cover). Tracked birds encountered wind speeds between 1.41 and 13.69 m s−1, with each bird experiencing a range of wind speeds during tracking (table 1; electronic supplementary material, figure S10). Wind speed had a clear strong effect on power variance, with soar-like bursts with high power variance almost exclusively observed in winds above 8 m s−1 (figure 4a). Mean ground speeds were mostly concentrated between 11 and 15 m s−1 in low winds, becoming more variable at higher wind speeds (electronic supplementary material, figure S9). Birds were more often recorded flying in crosswind (n = 99) than headwind (n = 57) or tailwind (n = 33) (figure 4b).
Figure 4.
The relationship between flight and wind conditions, for all flight bursts for which wind data were available (n = 189). (a) Relationship between wind speed and burst power variance, with flap-like bursts shown in black and soar-like bursts in white. (b) Histogram of the per-burst difference in degrees between the bird's flight direction (beeline bearing) and wind direction, where a value of 0° denotes no difference (i.e. flight direction is the same as wind direction). For modelling, flight direction was categorized as headwind (shown in red), crosswind (grey) or tailwind (blue). (c) Wind speed is a highly significant predictor of soar-like flight (logistic mixed-effects regression controlling for individual and day), with soaring less likely to occur in strong headwind than tailwind or crosswind. Grey histograms show density of wind speeds for all bursts classified as either flap-like (bottom) or soar-like (top).
We modelled the relationship between wind speed, flight direction and flight mode using logistic mixed-effects regression, including an interaction between wind speed and flight direction and including individual and day as random effects (n = 189, AIC = 204.1, model outputs are reported in electronic supplementary material). Model deviance was significantly reduced with wind speed and flight direction included as predictors, compared to an intercept-only null model (ΔAIC = 19.5; χ2 = 29.5, null–residual deviance 217.57–188.11, d.f. = 5, p < 0.0001). The model showed a highly significant effect of wind speed on flight mode, with likelihood of soaring increasing at higher wind speeds (figure 4c). There was also a significant interaction between wind speed and flight direction, with soaring occurring less frequently in strong headwinds than in tailwinds or crosswinds (for separate plots for each flight direction, see electronic supplementary material). The strength and significance of both these relationships increased and model fit improved when low-certainty bursts were excluded (n = 119, AIC = 93.9, residual deviance = 77.9). The second model additionally showed a significant effect of flight direction on flight mode, with reduced soaring in headwind compared to crosswind and tailwind. We found no significant relationships between flight mode and oceanic productivity (electronic supplementary material, S2).
4. Discussion
Ocean wind patterns are important drivers of seabird ecology and evolution [13,41], and recent research integrating information from multiple biologger types has revealed that winds exert a major influence on timing and distribution of foraging and migration in many species [1,8,14,15]. The relationship we demonstrate between flight behaviour and local wind conditions illuminates some of the behavioural mechanisms that underpin these large-scale patterns. Crosswinds and tailwinds above a wind speed threshold of around 8 m s−1 are highly significant predictors of soar-like behaviour in foraging Manx shearwaters. While we emphasize that these results come from a population sample of seven individuals, they support the inference that suitable wind conditions enable birds to engage in soar-like flight, which is likely to reduce overall energy costs during journeys. Statistical behaviour state classification is increasingly used to analyse animal tracking data [26,33,42]. However, to our knowledge this is the first time such an approach has been used to both identify distinct modes of flight behaviour and demonstrate their predictive relationship to environmental conditions.
4.1. Tracking and modelling of flight behaviour
The effect of tags on study animals is a key consideration in tracking research. Previous tests with devices of equal weight reported minimal impacts on movement and reproductive success in Manx shearwaters [42]; however we tracked movement at much finer temporal resolution than any previous study, and it is impossible to rule out the effects that a device weighing up to 4% of body mass could have on behaviour (e.g. [43]). Nonetheless, we observed the same responses to wind speed across several individuals that encountered both low and high winds during tracking. We suggest that although tag weight may impact flight to some degree, this is unlikely to significantly alter overall behavioural trends.
Using mean ground speed, kinetic energy (EK) and power as variables in the GMM offered several advantages for distinguishing wind-powered from flap-powered flight behaviour. Although the relative three-dimensional positional accuracy of successive GPS fixes is very high, absolute GPS accuracy is more reliable horizontally (used to calculate ground speed and EK; absolute error of ±2.5 m) than vertically (used to calculate potential energy EP). Visually inspecting all flight bursts showed no abrupt changes in altitude that were obviously artefacts; however we opted to exclude absolute EP values (which are derived from absolute altitude) from the GMM in order to minimize any potential effects of GPS error. Mechanical power measures the rate of energy change across each between-fix time interval t (P = (ΔEK + ΔEP)/t), so by including power (derived from change in altitude) as an input variable we ensured that the GMM still incorporated relative changes in EP, an important aspect of soaring flight. For additional model validation we also independently hand-classified bursts as soar-like or flap-like based on visual inspection of their shape, and the results closely resembled model outputs (electronic supplementary material, figure S7), improving our confidence that the clusters identified by the GMM correspond to these behaviours.
The GMM clearly distinguished bursts that contained mostly flap-like or soar-like movement, due to their markedly different mechanical characteristics. However, it appeared slightly biased towards classifying low-certainty bursts (those classified with under 95% probability) as flap-like (n = 55) rather than soar-like (n = 17), despite visual inspection suggesting that most were mixed-mode. These intermediate energy bursts mostly occurred in wind speeds above the soaring threshold (electronic supplementary material, figure S8) suggesting that our models may slightly underestimate use of soaring in wind speeds above 8 m s−1. This emphasizes that although a behavioural state framework is a useful abstraction for modelling relationships between flight mode and environment, Manx shearwater flight is complex and responsive to local heterogeneity in wind and wave conditions. 60 s tracked flight bursts exist on a continuum of mixed behaviours, ranging from mostly flap-like to mostly soar-like (e.g. figure 2). This variability reflects the smaller wingspan and flap-gliding flight of this species compared to that of large soaring specialists such as albatrosses, which travel long distances without flapping their wings. Soar-like bursts occasionally showed regular EP and EK oscillations resembling those observed in albatrosses, albeit with shorter soar cycle lengths (5 s compared to 15 s) [22]; however such stereotyped movement was relatively uncommon (see electronic supplementary material, S4). The resolution of the available environmental covariates meant that it was not feasible to model the effect of environment on within-burst variability in flight behaviour. However, in future, either accessing wind data at a higher spatio-temporal resolution (e.g. collected using on-animal tags) or recording much longer high-frequency GPS bursts (e.g. 5–10 min or above) could potentially facilitate analysis of the effect of wind on flight behaviour at an even finer scale.
4.2. Flap-like and soar-like flight characteristics
Birds engaging in powered flapping flight are predicted to minimize net energy expenditure by travelling close to maximum range velocity (Vmr), the speed at which maximum distance is covered per unit of fuel [28]. Previous studies tracked Manx shearwaters at mean ground speeds of 10–11 m s−1, slower than their estimated Vmr of 14 m s−1, suggesting some use of wind while travelling [25,27]. Our results confirm this, and show that soar-like flight enables shearwaters to travel at equivalent mean ground speeds as flapping (table 2). Within flap-like bursts the highest density of ground speeds occurred between 12 and 14 m s−1 (figure 3c), with birds apparently maximizing efficiency by travelling close to Vmr. The relationship between ground speed and airspeed varies with flight direction relative to wind; we hypothesize that birds maintain airspeeds close to Vmr throughout flapping, and that much of the observed within-burst variability in ground speed is due to birds flying with or against the wind, as indeed is the broader distribution of mean ground speeds observed in high winds (electronic supplementary material, figure S9).
In contrast, during soar-like flight regular kinetic energy boosts from the wind generate power levels far exceeding those available through flapping alone (figure 3b), with maximum available power appearing to increase as a function of wind speed (figure 4a). Accelerating and slowing repeatedly as they change flight path and body orientation relative to wind, soaring birds cover a far broader range of ground speeds (figure 3c) along significantly more tortuous flight paths (table 2). Soar-like flight in Manx shearwaters involves more flapping activity than in albatrosses [13], whose metabolic costs while soaring are extremely low [18]. Nonetheless, we find that soaring shearwaters cover equivalent distances as in flap-like flight (table 2) while spending much more time flying at closer to their estimated minimum power velocity (Vmp) of 7.5 m s−1 (figure 3c), which strongly suggests that energy expenditure is lower during soar-like flight. The second smaller peak between 0 and 2.5 m s−1 emphasizes the distinction between ground speed and airspeed; it corresponds to phases during soaring when birds ascend into oncoming wind, sharply decreasing in ground speed but simultaneously increasing in airspeed [22]. Birds were also more likely to soar in suitably strong tailwinds and crosswinds than headwinds, although the relative coarseness of our wind data (24-h averaged vectors) means that these categorized directions may be inexact. This nonetheless makes intuitive sense, since soaring against strong headwind is both time-inefficient and metabolically costly [18,20].
More broadly, these insights emphasize the emerging potential of high-frequency GPS biologgers, either solo or paired with other sensor types [20,44], as tools for studying fine-scale movement behaviour in wild animals. Tri-axial accelerometers are typically used to quantify metabolic energy expenditure in tracked animals (e.g. [45,46]). However, since these measure body acceleration rather than an individual's position in space they can present challenges for studying soaring and gliding in birds, in which body posture often remains relatively fixed and much muscle work is isometric [44]. In future, combining high-resolution GPS with co-deployed accelerometer tags would enable more precise estimation of the relative metabolic costs of different flight modes in this and other bird species, providing even more detailed insights into dynamic relationships between flight behaviour and the local environment.
4.3. Ecological relevance and future directions
Global wind patterns affect migration strategies in many birds [6,7,14] and the foraging ecology of pelagic seabirds [1,8]. Breeding and migration success may depend on minimizing energy costs during these trips [1,28]. Our results support the inference that soaring in tailwinds and crosswinds above an 8 m s−1 threshold enables Manx shearwaters to reduce flight energy expenditure, and therefore suggest a local-scale behavioural mechanism by which the wind conditions experienced by birds during flight modulate the net cost of at-sea journeys. Wind conditions are therefore likely to affect route choice, for example sufficiently high speed crosswinds and tailwinds may provide low-cost soaring corridors to foraging areas. This may contribute to the costs of foraging during reproduction, and may be an important factor to consider in future analysis of carry-over effects (e.g. [29]). Such a mechanism may also underpin some of the considerable variety in foraging routes observed during several years' tracking of Manx shearwaters around the UK [27], as well as the flexible route choice strategies of other seabirds in response to wind [15,16]. Our data provide some support for this, in that the tracked birds travelling furthest northwest towards Wales were those that encountered the strongest winds and soared the most (table 1).
Migratory birds are predicted to evolve migration strategies that minimize energetic costs [28]. Following favourable conditions for soaring may be one behavioural mechanism by which long-term trends in oceanic wind patterns, including the persistence of stable atmospheric features, affect the evolution of migration routes and timing in the Manx shearwater and other seabirds [8]. By identifying the wind conditions that favour soar-like flight, our results therefore present opportunities for a more predictive approach to understanding seabird life histories. We suggest that a future research direction, applying our model outputs, would be to combine global-scale wind data with the multiple years of geolocator and GPS migration tracks now collected for this species [26,27], in order to further assess how local behavioural responses to wind influence its global spatial distribution and migratory routes. Such an approach may also have conservation management implications for this and other seabirds. For example, although Manx shearwaters are generally considered low risk for collision with offshore wind turbines due to their relatively low altitude flight [47], applying similar methods to assess the predictive relationship between wind conditions, flight behaviour and route choice in other, more vulnerable species may assist in predicting regions of present and future collision risk.
Our results also suggest that climate change-driven wind pattern shifts [10] have the potential to affect the costs of long-distance journeys in this species. Recent wind changes in the Southern Ocean have affected foraging routes and life-history traits in wandering albatrosses [1], suggesting fitness impacts but also some behavioural plasticity in response to changing atmospheric conditions. However, as much smaller birds reliant on favourable winds for both foraging and migration [5], Manx shearwaters may be highly sensitive to such changes. If future global wind pattern shifts result in either increased energy expenditure during flight or extended travel times while at sea, this could have long-term population impacts on survival and reproductive success [8]. Similar impacts may also be expected in other pelagic seabird species, whose populations are already in global decline due to human impacts on the marine environment [48].
5. Conclusion
Data from on-board biologgers are fast improving our understanding of free-living animal movement. Using high-frequency GPS, here we have shown for the first time that wind speed, measured via satellite remote sensing, is an accurate predictor of soar-like flight in a wide-ranging pelagic seabird. Tailwinds and crosswinds above an 8 m s−1 wind speed threshold predict significantly increased likelihood of soaring flight. Both wind speed and direction are therefore likely to modulate flight costs during at-sea trips, suggesting a mechanism by which oceanic wind conditions could affect population-level foraging and migration strategies in this and other species. Our results highlight that high-frequency GPS should be considered within an emerging toolbox of tracking technologies that enable detailed quantitative study of the interactions between animal movement and the environment.
Supplementary Material
Acknowledgements
Thanks to Igor Boczarow for assistance during data collection in the field, and to Chris Carbone and Rachel Lane. We also thank our two reviewers for their helpful comments on the first version of the manuscript.
Ethics
Research took place under review by the British Trust for Ornithology Unconventional Methods Panel (permit C/5311) and the University of Oxford's Local Ethical Review Process.
Data accessibility
Data and code are archived on figshare (https://figshare.com/s/bd14e3a32e3ad340e323).
Authors' contributions
R.G. and R.F. conceived the paper, R.F. and A.S. collected the data, and R.G. and R.F. analysed the data. All authors contributed to writing the manuscript.
Competing interests
The authors have no competing interests.
Funding
This work was funded by the Lundy Field Society, Microsoft Research Cambridge, the Department of Zoology of Oxford University and the RSPB. A.L.F. was also funded by scholarships from the Biotechnology and Biological Sciences Research Council grant ATGAAB9, Microsoft Research Cambridge, the British Council Entente Cordiale Scheme, the Mary Griffiths Foundation, and an award from the British Federation for Women Graduates.
References
- 1.Weimerskirch H, Louzao M, Grissac S, Delord K. 2012. Changes in wind pattern alter albatross distribution and life-history traits. Science 335, 211–214. ( 10.1126/science.1210270) [DOI] [PubMed] [Google Scholar]
- 2.Krauel JJ, Westbrook JK, McCracken GF. 2015. Weather-driven dynamics in a dual-migrant system: moths and bats. J. Anim. Ecol. 84, 604–614. ( 10.1111/1365-2656.12327) [DOI] [PubMed] [Google Scholar]
- 3.Chapman JW, Reynolds DR, Wilson K. 2015. Long-range seasonal migration in insects: mechanisms, evolutionary drivers and ecological consequences. Ecol. Lett. 18, 287–302. ( 10.1111/ele.12407) [DOI] [PubMed] [Google Scholar]
- 4.Kranstauber B, Weinzierl R, Wikelski M, Safi K. 2015. Global aerial flyways allow efficient travelling. Ecol. Lett. 18, 1338–1345. ( 10.1111/ele.12528) [DOI] [PubMed] [Google Scholar]
- 5.González-Solís J, Felicísimo A, Fox JW, Afanasyev V, Kolbeinsson Y, Muñoz J. 2009. Influence of sea surface winds on shearwater migration detours. Mar. Ecol. Prog. Ser. 391, 221–230. ( 10.3354/meps08128) [DOI] [Google Scholar]
- 6.Klaassen RHG, Hake M, Strandberg R, Alerstam T. 2011. Geographical and temporal flexibility in the response to crosswinds by migrating raptors. Proc. R. Soc. B 278, 1339–1346. ( 10.1098/rspb.2010.2106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidal-Mateo J, Mellone U, Lopez-Lopez P, De La Puente J, Garcia-Ripolles C, Bermejo A, Urios V. 2016. Wind effects on the migration routes of trans-Saharan soaring raptors: geographical, seasonal, and interspecific variation. Curr. Zool. 62, 89–97. ( 10.1093/cz/zow008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weimerskirch H, Bishop C, Jeanniard-du-Dot T, Prudor A, Sachs G. 2016. Frigate birds track atmospheric conditions over months-long transoceanic flights. Science 353, 74–78. ( 10.1126/science.aaf4374) [DOI] [PubMed] [Google Scholar]
- 9.Guilford T, Meade J, Willis J, Phillips RA, Boyle D, Roberts S, Collett M, Freeman R, Perrins CM. 2009. Migration and stopover in a small pelagic seabird, the Manx shearwater Puffinus puffinus: insights from machine learning. Proc. R. Soc. B 276, 1215–1223. ( 10.1098/rspb.2008.1577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young IR, Zeiger S, Babanin AV. 2011. Global trends in wind speed and wave height. Science 332, 451–455. ( 10.1126/science.1197219) [DOI] [PubMed] [Google Scholar]
- 11.McVicar TR, et al. 2012. Global review and synthesis of trends in observed terrestrial near-surface wind speeds: implications for evaporation. J. Hydrol. 416-417, 182–205. ( 10.1016/j.jhydrol.2011.10.024) [DOI] [Google Scholar]
- 12.IPCC Core Writing Team, Pachauri RK, Meyer L. 2014. Climate change 2014: synthesis report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland: IPCC. [Google Scholar]
- 13.Spear LB, Ainley DG. 1997. Flight behaviour of seabirds in relation to wind speed and direction. Ibis 139, 234–251. ( 10.1111/j.1474-919X.1997.tb04620.x) [DOI] [Google Scholar]
- 14.Felicísimo ÁM, Muñoz J, González-Solis J. 2008. Ocean surface winds drive dynamics of transoceanic aerial movements. PLoS ONE 3, e2928 ( 10.1371/journal.pone.0002928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paiva VH, Guilford T, Meade J, Geraldes P, Ramos JA, Garthe S. 2010. Flight dynamics of Cory's shearwater foraging in a coastal environment. Zoology 113, 47–56. ( 10.1016/j.zool.2009.05.003) [DOI] [PubMed] [Google Scholar]
- 16.Tarroux A, et al. 2016. Flexible flight response to challenging wind conditions in a commuting Antarctic seabird: do you catch the drift? Anim. Behav. 113, 99–112. ( 10.1016/j.anbehav.2015.12.021) [DOI] [Google Scholar]
- 17.Kogure Y, Sato K, Watanuki Y, Wanless S, Daunt F. 2016. European shags optimize their flight behavior according to wind conditions. J. Exp. Biol. 219, 311–318. ( 10.1242/jeb.131441) [DOI] [PubMed] [Google Scholar]
- 18.Weimerskirch H, Guionnet T, Martin J, Shaffer SA, Costa DP. 2000. Fast and fuel efficient? Optimal use of wind by flying albatrosses. Proc. R. Soc. Lond. B 267, 1869–1874. ( 10.1098/rspb.2000.1223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pennycuick CJ. 2002. Gust soaring as a basis for the flight of petrels and albatrosses (Procellariiformes). Avian Sci. 2, 1–12. [Google Scholar]
- 20.Spivey RJ, Stansfield S, Bishop CM. 2014. Analysing the intermittent flapping flight of a Manx shearwater, Puffinus puffinus, and its sporadic use of a wave-meandering wing-sailing flight strategy. Prog. Oceanogr. 125, 62–73. ( 10.1016/j.pocean.2014.04.005) [DOI] [Google Scholar]
- 21.Halsey LG, Portugal SJ, Smith JA, Murn CP, Wilson RP. 2009. Recording raptor behavior on the wing via accelerometry. J. Field Ornithol. 80, 171–177. ( 10.1111/j.1557-9263.2009.00219.x) [DOI] [Google Scholar]
- 22.Sachs G, Traugott J, Nesterova AP, Bonadonna F. 2013. Experimental verification of dynamic soaring in albatrosses. J. Exp. Biol. 216, 4222–4232. ( 10.1242/jeb.085209) [DOI] [PubMed] [Google Scholar]
- 23.Sachs G, Traugott J, Nesterova AP, Dell'Omo G, Kümmeth F, Heidrich W, Vyssotski AL, Bonadonna F. 2012. Flying at no mechanical energy cost: disclosing the secret of wandering albatrosses. PLoS ONE 7, e41449 ( 10.1371/journal.pone.0041449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eaton MA, Brown AF, Hearn R, Noble DG, Musgrove AJ, Lock L, Stroud D, Gregory RD. 2015. Birds of conservation concern 4: the population status of birds in the United Kingdom, Channel Islands and Isle of Man. Br. Birds 108, 708–746. [Google Scholar]
- 25.Guilford TC, Meade J, Freeman R, Biro D, Evans T, Bonadonna F, Boyle D, Roberts S, Perrins CM. 2008. GPS tracking of the foraging movements of Manx shearwaters Puffinus puffinus breeding on Skomer Island, Wales. Ibis 150, 462–473. ( 10.1111/j.1474-919X.2008.00805.x) [DOI] [Google Scholar]
- 26.Freeman R, Dean B, Kirk H, Leonard K, Phillips RA, Perrins CM, Guilford T. 2013. Predictive ethoinformatics reveals the complex migratory behaviour of a pelagic seabird, the Manx shearwater. J. R. Soc. Interface 10, 20130279 ( 10.1098/rsif.2013.0279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dean B, Kirk H, Fayet A, Shoji A, Freeman R, Leonard K, Perrins CM, Guilford T. 2015. Simultaneous multi-colony tracking of a pelagic seabird reveals cross-colony utilization of a shared foraging area. Mar. Ecol. Prog. Ser. 538, 239–248. ( 10.3354/meps11443) [DOI] [Google Scholar]
- 28.Pennycuick CJ. 1969. The mechanics of bird migration. Ibis 111, 525–556. ( 10.1111/j.1474-919X.1969.tb02566.x) [DOI] [Google Scholar]
- 29.Fayet AL, Freeman R, Shoji A, Kirk HL, Padget O, Perrins CM, Guilford T, Verhulst S. 2016. Carry-over effects on the annual cycle of a migratory seabird: an experimental study. J. Anim. Ecol. 85, 1516–1527. ( 10.1111/1365-2656.12580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeman R, Naumowicz T. MATAKI Tracking Devices. UCL CoMPLEX, Zoological Society of London, Microsoft Research Cambridge. http://mataki.org/.
- 31.Freeman R, Shoji A, Fayet A, Dean B, Kirk H, Perrins C, Guilford T. 2012. Tracking the migration and foraging dynamics of Lundy's Manx shearwaters. Lundy F. Soc. Annu. Rep. 2012, 101–106. [Google Scholar]
- 32.Core Team R. 2014. R: a language & environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 33.Calenge C, Dray S, Royer-Carenzi M. 2009. The concept of animals’ trajectories from a data analysis perspective. Ecol. Inform. 4, 34–41. ( 10.1016/j.ecoinf.2008.10.002) [DOI] [Google Scholar]
- 34.Dray S, Royer-Carenzi M, Calenge C. 2010. The exploratory analysis of autocorrelation in animal-movement studies. Ecol. Res. 25, 673–681. ( 10.1007/s11284-010-0701-7) [DOI] [Google Scholar]
- 35.Benhamou S. 2004. How to reliably estimate the tortuosity of an animal's path: straightness, sinuosity, or fractal dimension? J. Theor. Biol. 229, 209–220. ( 10.1016/j.jtbi.2004.03.016) [DOI] [PubMed] [Google Scholar]
- 36.Fraley C, Raftery A. 2002. Model-based clustering, discriminant analysis and density estimation. J. Am. Stat. Assoc. 97, 611–631. ( 10.1198/016214502760047131) [DOI] [Google Scholar]
- 37.Fraley C, Raftery A, Murphy T, Scrucca L. 2012. mclust Version 4 for R: Normal Mixture Modeling for Model-Based Clustering, Classification, and Density Estimation. Technical Report No. 597, Department of Statistics, University of Washington.
- 38.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7.
- 39.Pante E, Simon-Bouhet B. 2013. marmap: a package for importing, plotting and analyzing bathymetric and topographic data in R. PLoS ONE 8, e73051 ( 10.1371/journal.pone.0073051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Q, Xu M, Fränti P. 2008. Knee point detection on Bayesian information criterion. In Proc. 20th IEEE Int. Conf. on Tools with Artificial Intelligence, Dayton, OH, USA, 3–5 November 2008, pp. 431–438. ( 10.1109/ICTAI.2008.154) [DOI] [Google Scholar]
- 41.Davies RG, Irlich UM, Chown SL, Gaston KJ. 2010. Ambient, productive and wind energy, and ocean extent predict global species richness of procellariiform seabirds. Glob. Ecol. Biogeogr. 19, 98–110. ( 10.1111/j.1466-8238.2009.00498.x) [DOI] [Google Scholar]
- 42.Dean B, Freeman R, Kirk H, Leonard K, Phillips RA, Perrins CM, Guilford T. 2012. Behavioural mapping of a pelagic seabird: combining multiple sensors and a hidden Markov model reveals the distribution of at-sea behaviour. J. R. Soc. Interface 10, 20120570 ( 10.1098/rsif.2012.0570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vandenabeele SP, Grundy E, Friswell MI, Grogan A, Votier SC, Wilson RP. 2014. Excess baggage for birds: inappropriate placement of tags on gannets changes flight patterns. PLoS ONE 9, e92657 ( 10.1371/journal.pone.0092657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gleiss AC, Wilson RP, Shepard ELC. 2011. Making overall dynamic body acceleration work: on the theory of acceleration as a proxy for energy expenditure. Methods Ecol. Evol. 2, 23–33. ( 10.1111/j.2041-210X.2010.00057.x) [DOI] [Google Scholar]
- 45.Wilson RP, White CR, Quintana F, Halsey LG, Liebsch N, Martin GR, Butler PJ. 2006. Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: the case of the cormorant. J. Anim. Ecol. 75, 1081–1090. ( 10.1111/j.1365-2656.2006.01127.x) [DOI] [PubMed] [Google Scholar]
- 46.Wilson RP, Quintana F, Hobson VJ. 2012. Construction of energy landscapes can clarify the movement and distribution of foraging animals. Proc. R. Soc. B 279, 975–980. ( 10.1098/rspb.2011.1544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langston RHW. 2010. Offshore wind farms and birds: Round 3 zones, extensions to Round 1 & Round 2 sites & Scottish Territorial Waters. RSPB research report no. 39. Sandy, UK: RSPB. https://www.rspb.org.uk/Images/langston_2010_tcm9-203501.pdf.
- 48.Paleczny M, Hammill E, Karpouzi V, Pauly D. 2015. Population trend of the world's monitored seabirds, 1950–2010. PLoS ONE 10, e0129342 ( 10.1371/journal.pone.0129342) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code are archived on figshare (https://figshare.com/s/bd14e3a32e3ad340e323).