Abstract
How extinct, non-avian theropod dinosaurs locomoted is a subject of considerable interest, as is the manner in which it evolved on the line leading to birds. Fossil footprints provide the most direct evidence for answering these questions. In this study, step width—the mediolateral (transverse) distance between successive footfalls—was investigated with respect to speed (stride length) in non-avian theropod trackways of Late Triassic age. Comparable kinematic data were also collected for humans and 11 species of ground-dwelling birds. Permutation tests of the slope on a plot of step width against stride length showed that step width decreased continuously with increasing speed in the extinct theropods (p < 0.001), as well as the five tallest bird species studied (p < 0.01). Humans, by contrast, showed an abrupt decrease in step width at the walk–run transition. In the modern bipeds, these patterns reflect the use of either a discontinuous locomotor repertoire, characterized by distinct gaits (humans), or a continuous locomotor repertoire, where walking smoothly transitions into running (birds). The non-avian theropods are consequently inferred to have had a continuous locomotor repertoire, possibly including grounded running. Thus, features that characterize avian terrestrial locomotion had begun to evolve early in theropod history.
Keywords: step width, locomotion, theropods, birds, fossil footprints
1. Introduction
How extinct dinosaurs stood and moved has always been a question of great interest for palaeontologists. Locomotion in non-avian theropods is a controversial topic, owing to interest surrounding their carnivorous lifestyle and often gigantic size. Additionally, the recognition that Theropoda includes birds [1–5] provides a further rationale for understanding locomotion in extinct theropods: charting the evolution of locomotion on the line to birds [6–8].
Terrestrial locomotion in modern birds is distinct from that of humans, the only other extant obligate, striding biped [9,10]. It is characterized by a crouched, digitigrade, parasagittal posture, with a subhorizontally oriented femur during much of the stride [11–27]. A further curious aspect is that birds employ ‘grounded running’ at certain speeds [14,16,21,24,28–32]. Here, the whole-body centre-of-mass (COM) exhibits little exchange of kinetic energy (KE) and potential energy (PE), characteristic of running, despite there being double-support phases in the stride (i.e. duty factors are greater than 0.5; characteristic of walking). Therefore, birds run without an aerial phase; only at greater speeds do duty factors decrease below 0.5, resulting in an aerial run. Birds hence show more ‘continuous’ gaits compared with humans. Reflecting this, many kinematic parameters that have been previously measured in birds often show a continuous change with increasing speed, whereas in humans, they typically change discontinuously at the walk–run transition [14,16,17,21,26,29–31,33–37].
The distinct locomotor repertoire of modern birds raises the question as to whether their non-avian ancestors exhibited a similar repertoire, and if not, when did this distinct suite of behaviours first evolve? In answering this question, the most direct evidence of locomotion in extinct, non-avian theropods is fossil footprints and trackways, because they record the actual placement and motions of the feet during locomotion [38–40]. All known non-avian theropod footprints and trackways show that they were digitigrade, obligatorily striding, parasagittal bipeds that did not drag their tails along the ground, much like modern birds [39,40].
Fossil theropod trackways have also facilitated estimates of trackmaker speeds, using stride length as a proxy [39,41–44], but how locomotor kinematics changed with speed remains less investigated. The patterns exhibited by birds and humans lead to the prediction that whether a given non-avian theropod trackmaker exhibited continuous or discontinuous locomotor behaviour, this should be detectable in measurements of its tracks. One such measurement is step width, the mediolateral (transverse) distance between successive footfalls, which can be measured for both fossil trackways and modern animals. Step width is linked to the maintenance of stability and energetic efficiency during locomotion in humans [45–50]. Indeed, stability may be paramount for large bipeds, such as giant non-avian theropods, because the consequences of falling could be dire [51]. Consequently, analyses of step width have the potential to offer insight into non-avian theropod locomotor biomechanics.
There are only three known non-avian theropod trackway sites where the trackmakers made an appreciable change in speed. These sites provide the opportunity to examine how locomotion changed with speed in non-avian theropods, enabling comparison to locomotion in birds and humans. One site, in England, suggests that step width decreased with increasing speed [52,53], but it is unclear whether this occurred gradually or abruptly. A second site in South Korea [54] is also too small for thorough analysis. The third site, the Culpeper Crushed Stone Quarry trackways, from the Late Triassic (approx. 211 Ma) of Virginia, USA, comprises 20 individual trackways [55–57], some of which are quite extensive. Based on measured stride lengths, it is clear that in some trackways, the trackmaker progressed from a slow walk to a fast run [57], so a large part of their speed spectrum was captured in these trackways.
This study investigated the pattern of step width change versus speed in non-avian theropods, using the dataset of the Culpeper Quarry trackways. The results obtained from analysis of the trackways were compared with three-dimensional kinematic measurements collected for humans and a range of extant, ground-dwelling birds. It is hypothesized that birds show a more continuous change in step width with increasing speed, whereas humans show a discontinuous change. Comparison of the patterns observed in the trackways to those observed in modern bipeds will hence provide insight into non-avian theropod locomotion. Additionally, the importance of mediolateral limb movements in bipedal animals (living and extinct) can be better clarified.
2. Material and methods
A summary of the methodology is given below, with a detailed outline provided in the electronic supplementary material.
2.1. Theropod trackways
2.1.1. Data collection
Morphological similarity in all of the footprints examined suggests that a single genus, if not species, was recorded in the trackways [56,57]. Moreover, the size of the footprints indicates that the trackmakers were similar in size [57]. Hence, by focusing only on the Culpeper Quarry trackways, this study effectively controlled for the potentially confounding factors of differing species, body sizes or shapes, patterns of leg movements and substrate conditions. The only factor likely responsible for any systematic change of step width with speed is therefore speed itself.
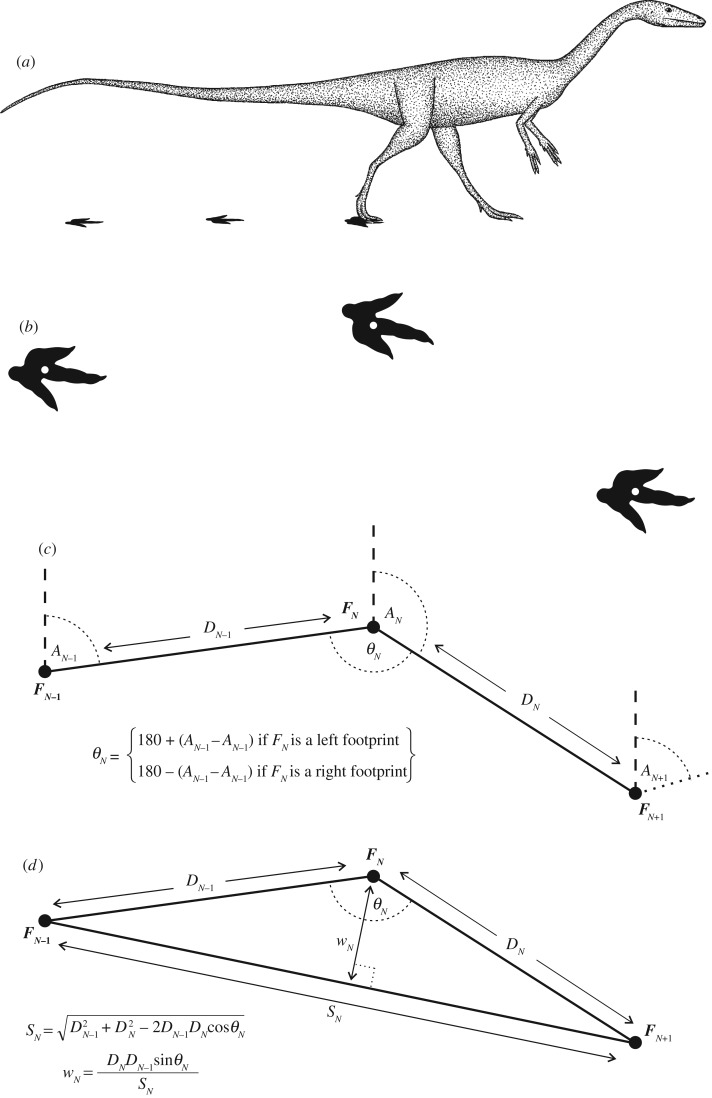
Two measurements were made along each trackway (figure 1a–c): pace length (distance from one footprint to the next) and pace bearing (compass bearing from one footprint to the next). The reference point for each footprint was at the posterior end of the digit III impression (figure 1b). None of the trackways studied showed any indication of pathology, such as limping [58].
Figure 1.
The quantitative analysis of trackways to determine step width and stride length. (a) Life reconstruction of the trackmaker, likely to be some form of basal neotheropod. (b) The footprints in plan view; white dots mark the common reference point from which measurements were made. (c) Two principal measurements were made: pace length D, and bearing from one footprint to the next A. This allowed the calculation of pace angulation θ. (d) Step width w and stride length S were then calculated trigonometrically from pace lengths and angulations.
2.1.2. Data analysis
From measured pace bearings and pace lengths, the pace angulation at each footprint (θN) was determined (figure 1c). The stride length (SN) and step width (wN) at each footprint was then calculated trigonometrically (figure 1d). Step width was defined as the perpendicular distance from a footprint to the line of its corresponding stride length. If the feet crossed over the body midline, pace angulation becomes reflex (θN > 180°) and step width becomes negative. Calculated stride lengths and step widths were then normalized to the estimated hip height of the trackmakers, to minimize potential size effects on comparisons; hip height was estimated via eqn (1) of Thulborn [43]. Normalization of S and w to hip height (producing S* and w*, respectively) facilitated fair comparison across the trackways. Additionally, relative stride length is a common proxy for speed of locomotion when speed itself cannot be measured, because animals tend to take longer strides at faster speeds [16,21,29–31,41,42,59].
2.2. Humans
2.2.1. Data collection
Three healthy, recreationally active adults (two male and one female) were studied (height 179.3 ± 3.2 cm, mass 79.7 ± 16.8 kg, means ± s.d.). A sample size of three was deemed sufficient, because this study aimed to elucidate major patterns, for broad comparative purposes [16,41,59–61]. Reflective markers were placed on the end of the left and right hallux, and their three-dimensional trajectories during locomotion were recorded at 200 Hz using a 10-camera VICON MX T40-S motion capture system (Vicon Oxford Metrics, Oxford, UK). The subjects walked and ran barefoot on a split-belt instrumented treadmill (Bertec Limited, Columbus, OH, USA) integrated with the motion capture system.
Each subject undertook a number of steady-state walking and running trials at increments of 0.25 m s−1, ranging from slow walking through fast running speeds. Subjects were also tested twice at their (predetermined) walk–run transition speed, in one trial using walking, in the other using running. To elucidate if there was any difference in how step width changes with increasing speed between accelerating and steady-state locomotion, two subjects also undertook accelerating trials. Using the programmable treadmill, each subject undertook a slow and fast acceleration of 1.0 m s−2 and 2.5 m s−2, respectively, up to a peak tread speed of 3.25 m s−1.
2.2.2. Data analysis
Step width was measured from the three-dimensional trajectory data for the hallux markers, and normalized to the hip height of the subjects; this was estimated using standard anthropometry [62] based on the subject's total standing height.
2.3. Birds
2.3.1. Animals studied
Eleven species of ground-dwelling bird were studied (table 1). Given logistical limitations and the study's objectives, preference was given to maximizing the diversity of species investigated, rather than achieving more replicates for fewer species. All birds investigated in this study were considered to be adults based on skeletal maturity, with the possible exception of the domestic turkeys, which had sizeable chondroepiphyses (revealed in postmortem dissection) but were still of adult size. Following the conclusion of experiments, birds were euthanized and immediately weighed. Data collected in this study were combined with data collected previously for ostriches [18] and emus [63].
Table 1.
The species of bird studied, along with sample sizes and mean (±s.d.) masses and hip heights.
| species |
n | mass (kg) | hip height (mm) | data collected | |
|---|---|---|---|---|---|
| scientific name | common name | ||||
| Coturnix chinensis | Chinese painted quail | 3♂, 2♀ | 0.047 ± 0.002 | 58.6 ± 4.0 | this study |
| Colinus virginianus | northern bobwhite quail | 3♂, 2♀ | 0.170 ± 0.014 | 77.8 ± 10.4 | this study |
| Coturnix japonica | Japanese quail | 1♂, 3♀ | 0.301 ± 0.077 | 106.25 ± 7.5 | this study |
| Porphyrio porphyrio | purple swamphen | 3♀ | 0.623 ± 0.058 | 239.0 ± 14.1 | this study |
| Numida meleagris | helmeted guineafowl | 2♂, 1♀ | 1.257 ± 0.114 | 201.7 ± 15.5 | this study |
| Alectura lathami | Australian brush turkey | 2♀ | 1.490 ± 0.057 | 267.0 ± 15.6 | this study |
| Threskiornis moluccus | Australian white ibis | 2♂ | 1.54 ± 0.057 | 282.5 ± 30.4 | this study |
| Gallus gallus | domestic chicken (white leghorn breed) | 1♂, 2♀ | 1.710 ± 0.521 | 254.3 ± 47.8 | this study |
| Meleagris gallopavo | domestic turkey (various mixed breeds) | 2♂, 3♀ | 3.228 ± 0.90 | 365.2 ± 47.4 | this study |
| Dromaius novaehollandiae | emu | 6♀ | 38.58 ± 2.69 | 903.3 ± 35.0 | [63] |
| Struthio camelus | ostrich | 3♂ | 74.87 ± 4.44 | 1129.3 ± 10.3 | [18] |
2.3.2. Data collection
Two experimental set-ups were used in this study, one small indoor racetrack for the quail species, and a larger outdoor one for the remaining species (electronic supplementary material, figure S1). Both racetracks were walled around their entire perimeter, but the middle of one side was replaced with clear acrylic or fine wire mesh, through which filming took place. Birds were filmed at 50–250 frames s−1 with two high-speed light video cameras (IL3-100 and HiSpec1, Fastec Imaging, San Diego, CA, USA), synchronized using a manual trigger pulse. For both racetracks, a calibration volume for each day's trials was established using an 11-coefficient direct linear transform [64].
Prior to data collection, feathers from the back and wings were clipped, to allow the placement of small (2–5 mm) markers that were unobstructed from the cameras' views during locomotion. Up to three markers were placed on the midline of the back, as part of another study. A single marker was placed over the trochanteric crest of each hip, which in all species was easily palpable. Non-toxic, white paint was used to mark the base of the claw of digit III of both feet.
Birds moved down the racetrack at a self-selected speed, although sometimes additional motivation was used, such as making loud noises. The speeds used varied from slow walking speeds through fast running speeds. Birds were also filmed during quiet standing, allowing the capture and measurement of the height of the hip marker (taken as standing hip height).
2.3.3. Data analysis: this study and ostrich data
Toe markers were digitized and their three-dimensional coordinates calculated using DLTdv5 [64], a program written for Matlab v. 8.0 (MathWorks, Natick, MA, USA). Markers were digitized when the feet were planted on the ground and fully stationary. Additionally, the hip marker in standing trials was digitized and its coordinates were calculated, either using DLTdv5 or using a reference object of known dimensions within the cameras' fields of view. The coordinates of the toe markers were then used to determine stride lengths and step widths trigonometrically (electronic supplementary material, figure S2). Stride lengths and step widths were normalized to the standing hip height of the birds as for the theropod footprints. The calculations followed the same convention as used for the theropod footprints; if the feet crossed over the midline, step width was negative. A similar process was used to extract and analyse the three-dimensional kinematic data collected for the ostriches [18].
2.3.4. Data analysis: emu data
In the emus [63], a marker was placed only on the right digit III, in addition to two markers on the back midline. While true step width and stride length could not be calculated, they could be estimated, using the trajectory of the back markers as a proxy for the body midline axis (electronic supplementary material, figure S3). Step width and stride length were then normalized to standing hip height.
2.4. Statistics
Two statistical analyses were performed. The first tested whether w* varied significantly with S* for a given species, using major axis (MA) regression [65]. As assumptions of normality or homoscedasticity were frequently not met by the data, as determined in PAST v. 3.09 [66], a permutation test of the slope was performed [67], with significance levels conservatively set to p = 0.01. The second statistical analysis examined whether a given dataset showed continuous or discontinuous change in w* with respect to S*. Curve fitting was performed in R v. 3.2.2 (R Foundation, Vienna, Austria), using two continuous curves (linear, power) and one discontinuous curve (logistic). The Akaike information criterion was then used to determine which curve provided the best fit.
3. Results
3.1. Theropod trackways
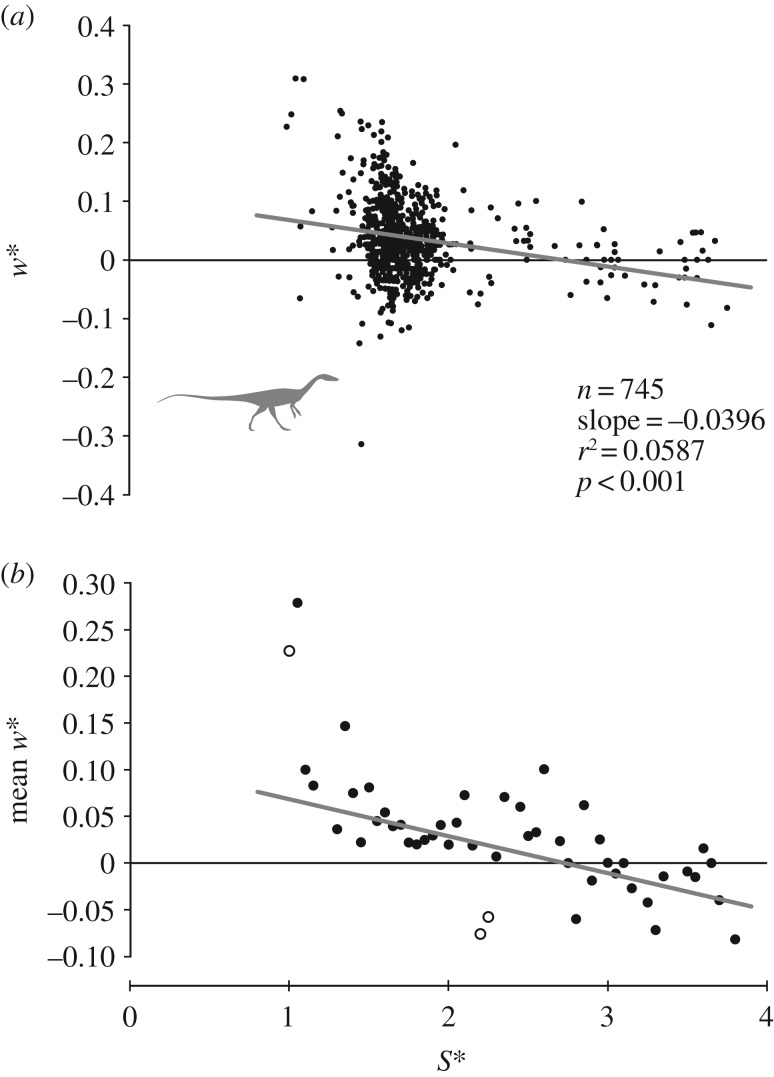
Step width decreased with increasing speed (figure 2a), although the correlation is not strong (r2 = 0.0587, p < 0.001). After binning w* into narrow intervals of S* and taking the mean for each interval, this pattern is more clearly illustrated (figure 2b): as the theropods moved faster, they placed their feet closer to the midline. At slow speeds, step width was approximately 5–10% of hip height; at fast speeds (S* > 3.0), the feet typically crossed the midline, as indicated by negative values for w*. Importantly, the decrease in w* versus S* was best modelled by a power function (electronic supplementary material, table S1), indicating no discontinuity.
Figure 2.
Comparison of how step width changes with stride length in the non-avian theropods. (a) The raw data, with MA regression plotted. (b) Mean values of w* for each bin of S*, with MA regression derived from raw data again plotted. In (b), hollow circles are single data points; they are not means of multiple points for a given bin. Hence, their apparent outlier nature can be accounted for as simply the only points that fell into those particular bins.
3.2. Humans
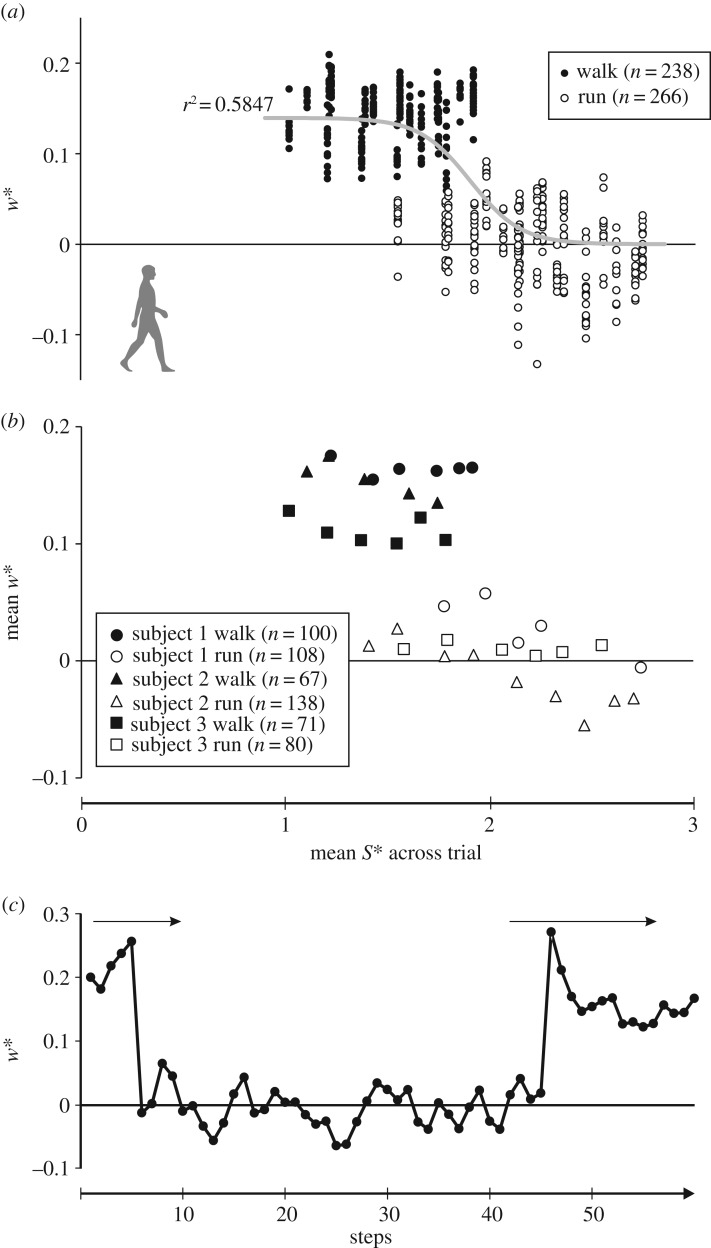
Step width decreased with increasing stride length (p < 0.001), particularly at the transition from walking to running, with a distinct difference in w* between the gaits (figure 3a,b). This was well modelled by a logistic function (electronic supplementary material, table S1; figure 3a), indicating a pronounced discontinuity. Abrupt, discontinuous change in w* was also observed in accelerating–decelerating trials (figure 3c); at the transition from walking to running (or vice versa), w* changed suddenly over one step. This indicates no difference in how w* varies with S*, whether through acceleration within a trial, or across steady-state trials at different speeds. This supports the validity of comparing steady-state locomotion in humans and birds with largely non-steady-state locomotion in the Culpeper theropod trackways.
Figure 3.
Comparison of how step width changes with stride length in humans. (a) The raw data, with logistic curve plotted (cf. electronic supplementary material, table S1); data for running at walk–run transition speeds were excluded to be more reflective of naturally preferred gaits. S* is the mean across the trial for each speed tested. (b) Mean values of w* plotted against mean S* across each trial; data for running at walk–run transition speeds were included, to emphasize how w* remains distinctly different between the two gaits, even at the transition. (c) Plot of w* through 60 consecutive steps for subject 2, across an acceleration of 1.0 m s−2, followed by a slow deceleration. Acceleration and deceleration phases are signified by arrows; subject begins moving at step 1. Note that the change from walking-like to running-like values for w* takes place over a single step during both acceleration and deceleration.
3.3. Birds
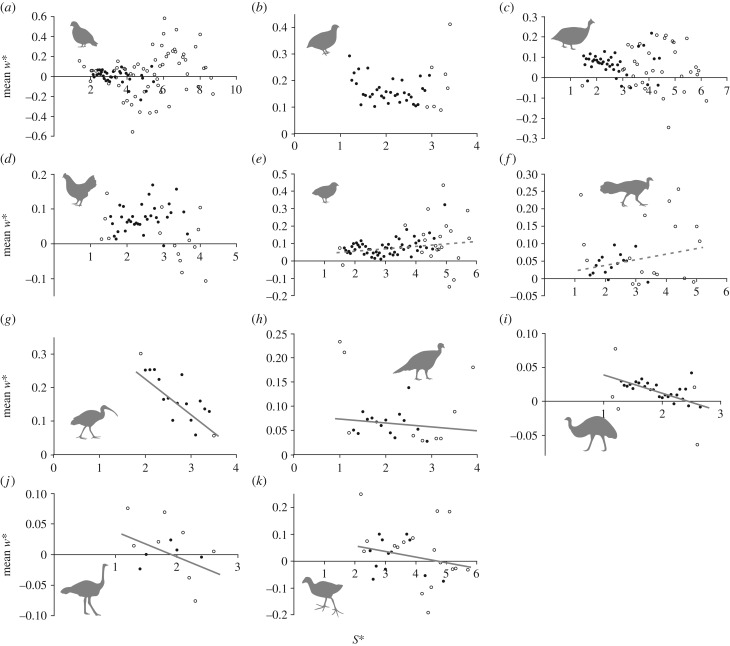
Four species studied (Colinus virginianus, Coturnix japonica, Numida meleagris and Gallus gallus) did not show a statistically significant pattern of w* versus S*; values for w* largely varied between 0 and 0.2 (figure 4a–d). Two species (Coturnix chinensis and Alectura lathami) showed a significant pattern, but the slope of the MA regression was positive (figure 4e,f; w* increased slightly with S*). The remaining five species (Threskiornis moluccus, Meleagris gallopavo, Porphyrio porphyrio, Dromaius novaehollandiae and Struthio camelus) all showed a significant pattern with negative MA regression slopes, i.e. w* decreased with increasing S* (figure 4g–k). In each of these five species, the data were best modelled by a linear or power function (electronic supplementary material, table S1), indicating no discontinuity.
Figure 4.
Comparison of how step width changes with stride length in birds. For visualization, the raw data were binned to intervals of S*, and the mean w* determined for each bin. An MA regression derived from the raw data is also plotted for those species in which p < 0.01. (a) Colinus virginianus (n = 221, n.s.). (b) Coturnix japonica (n = 419, n.s.). (c) Numida meleagris (n = 442, n.s.). (d) Gallus gallus (n = 233, n.s.). (e) Coturnix chinensis (n = 840, slope = 0.0138, r2 = 0.007). (f) Alectura lathami (n = 154, slope = 0.0159, r2 = 0.024). (g) Threskiornis moluccus (n = 124, slope = −0.1067, r2 = 0.162). (h) Meleagris gallopavo (n = 185, slope = −0.0081, r2 = 0.003). (i) Dromaius novaehollandiae (n = 1140, slope = −0.0268, r2 = 0.027). (j) Struthio camelus (n = 34, slope = −0.0411, r2 = 0.107). (k) Porphyrio porphyrio (n = 78, slope = −0.0209, r2 = 0.019). Hollow circles are single or double data points; they are not means of many points for a given bin. Hence, in some instances, their apparent outlier nature may be accounted for as simply very few points that fell into those particular bins. Note differences in horizontal and vertical scales.
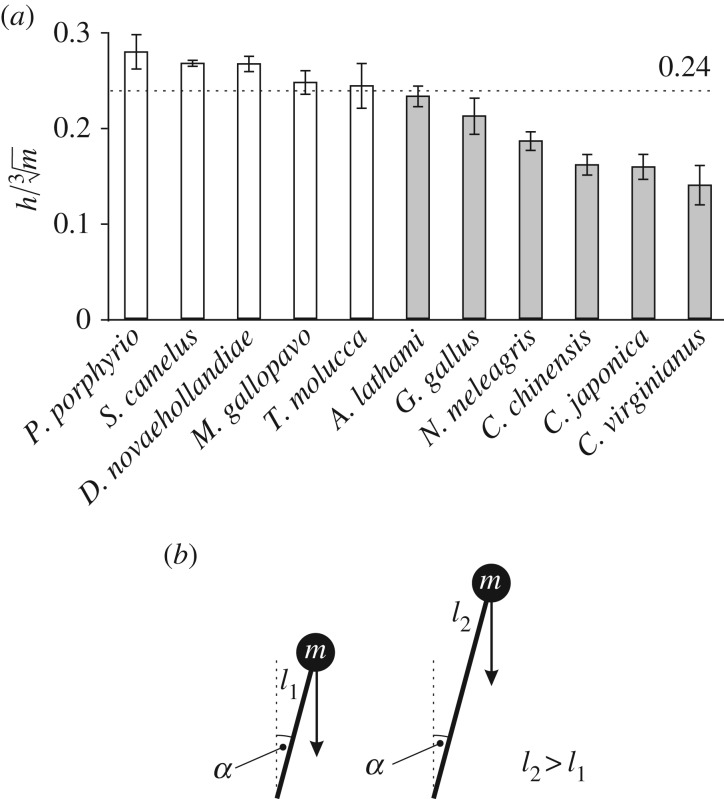
Following these mixed results, further analysis revealed that a measure of bipedal stability could discriminate between species that showed a decrease in w* with increasing S* and those that did not (figure 5a). This measure, 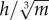 , expresses hip height (h) relative to body mass (m), assuming isometry. Birds that are relatively tall for their mass have a higher
, expresses hip height (h) relative to body mass (m), assuming isometry. Birds that are relatively tall for their mass have a higher 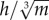 , and are inherently more unstable, because stability decreases as the height of the COM above the ground increases [68] (figure 5b). All species for which the mean value of
, and are inherently more unstable, because stability decreases as the height of the COM above the ground increases [68] (figure 5b). All species for which the mean value of 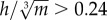 demonstrated a pattern of decreasing w* with increasing S*. This ‘threshold’ may be specific to birds, however, because for the humans studied,
demonstrated a pattern of decreasing w* with increasing S*. This ‘threshold’ may be specific to birds, however, because for the humans studied, 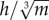 was 0.222 ± 0.012, yet w* still decreased with increasing S*.
was 0.222 ± 0.012, yet w* still decreased with increasing S*.
Figure 5.
Bipedal stability in birds. (a) Comparison of mean (±s.d.) value of 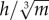 for each species studied. Species with a mean value of
for each species studied. Species with a mean value of 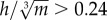 (white bars) ubiquitously demonstrated a pattern of decreasing w* with increasing S*. (b) Bipedal stability is influenced by the length of the legs (or equivalently, hip height) relative to body mass. For a point mass m on a massless leg of length l1 or l2 (where l2 > l1), the mass with leg length l2 exerts a greater moment (by virtue of weight) about the base of support than the mass with leg length l1, for the same leg orientation α. Therefore, it is easier for the mass to be shifted away from a stable state and tipped over (i.e. relatively longer legs decrease stability).
(white bars) ubiquitously demonstrated a pattern of decreasing w* with increasing S*. (b) Bipedal stability is influenced by the length of the legs (or equivalently, hip height) relative to body mass. For a point mass m on a massless leg of length l1 or l2 (where l2 > l1), the mass with leg length l2 exerts a greater moment (by virtue of weight) about the base of support than the mass with leg length l1, for the same leg orientation α. Therefore, it is easier for the mass to be shifted away from a stable state and tipped over (i.e. relatively longer legs decrease stability).
4. Discussion
This study investigated how step width varies with stride length in one extinct and two extant groups of obligate biped. The non-avian theropods showed a pattern of continuous decrease in w* with increasing S*, a pattern that was also observed in about half of the bird species. These species were those that are relatively tall (long-legged) for their mass; in the other bird species, no strong patterns were apparent. In contrast with the non-avian theropods and birds, humans showed a marked and abrupt decrease in w* at the walk–run transition.
4.1. Step width in bipedal locomotion
For all three kinds of biped investigated, it was shown in general that if step width changed with speed, it decreased with increasing speed: at quicker speeds, the feet were placed closer to the body midline, and in fast running cross-over could occur. The observed pattern of step width versus speed in the Triassic-age Culpeper trackways parallels the observation of Day et al. [52,53], of a large theropod trackway of Middle Jurassic age. Step width has therefore been shown to decrease with increasing speed in two non-avian theropod trackways, more than 40 Ma apart in age. Given that step width also decreased with increasing speed in several of the bird species investigated here, it is phylogenetically conceivable that such a pattern was present in many, if not most, extinct non-avian theropods.
The findings for humans also accord with those of previous studies that have measured step width, for both treadmill and overground locomotion [46,48,50,69]; step width was approximately 10–20% of hip height in walking and was close to zero in running. However, this study is the first to show how step width changes with speed in humans, across the walk–run transition. Almost no previous data exist for how step width changes with speed in modern bird species. One exception is a recent study of walking in broiler chickens [70], which showed that step width decreased with increasing speed in a continuous fashion. That no such pattern was observed for chickens in this study is possibly due to different breeds studied.
4.2. Step width and bipedal stability
Step width is closely tied to mediolateral stability during bipedal locomotion [45–50], yet why step width decreases with speed remains undetermined. It is suggested here that this phenomenon may represent, at least partly, a trade-off between mediolateral stability and energy economy.
At slow speeds, the body has limited linear momentum directed forwards, so it is relatively easy for lateral forces to displace the trajectory of the COM laterally, leading to postural instability. To counteract this, the feet assume a sizeable non-zero lateral placement relative to the midline. However, this incurs an energetic cost, as energy is expended in mediolateral movement of the COM (successively towards each footfall) instead of forward movement of the COM [69]. This cost, derived from the KE of lateral movement, would be comparatively greater in larger bipeds, as KE is proportional to the square of velocity, and hence linear dimensions (e.g. hip height). With increasing speed, dynamic stability increases as linear momentum in the forward direction increases, decreasing the effect of lateral forces on the trajectory of the COM. Consequently, the feet are not required to have such large lateral placements, so step width can be reduced. By reducing step width, and therefore lateral oscillations of the COM [69,71], less energy is expended in mediolateral movement of the COM and more is expended in forward movement of the COM, improving economy.
The above scenario of a stability–economy trade-off may explain why five of the 11 bird species studied showed a pattern of decreasing w* with increasing S*, yet the others did not. Given the expectation that the energetic cost of unnecessarily large lateral displacements is disproportionally greater in taller bipeds, and given that taller bipeds are less stable (figure 5), this makes it more likely that a stability–economy trade-off will exist in relatively taller birds. In turn, step width would be more tightly modulated with respect to speed. An example illustrating this is the comparison between swamphen and guineafowl. The swamphen were only about half the mass of the guineafowl, and yet they were almost 20% taller at the hips; the swamphen showed a significant decrease in step width with increasing speed, while the guineafowl did not.
An additional reason for why step width decreases with speed may be simple anatomical constraints. As speed increases, stride length also increases, providing more room between each step for the legs to be placed closer towards the midline, something that may not be as easily achieved at lower speeds (and stride lengths) without considerable yawing or rolling of the body.
4.3. The gait patterns of non-avian theropods
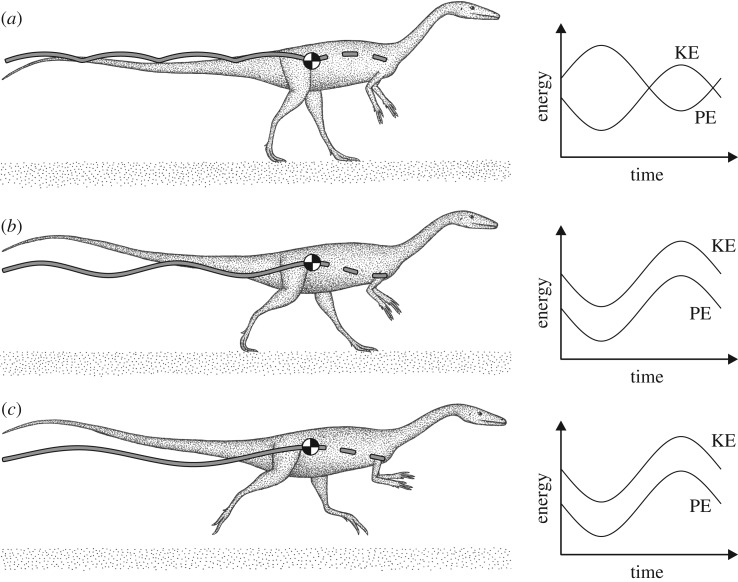
In the modern bipeds studied, step width displayed the same speed-related pattern as many other previously investigated kinematic parameters: a continuous change with speed reflects a continuous locomotor repertoire, while an abrupt change with speed reflects a discontinuous locomotor repertoire, with discrete and distinct gaits defined by those abrupt changes [72]. In the non-avian theropod trackways, step width decreased with increasing speed in a continuous fashion. In the light of the patterns exhibited by (long-legged) modern obligate bipeds, this suggests that the theropod trackmakers may not have had discrete gaits, instead having a continuous locomotor repertoire, as in modern birds. That is, grounded running was possibly a component of their locomotor repertoire (figure 6). This realization should not be as surprising as it may first appear, for there are a wide variety of extant animals that habitually employ a continuous locomotor repertoire (to one degree or another), in addition to birds. These animals use a variety of limb postures, and include opossums [73,74], other marsupials [74], frogs [75], elephants [76], primates [77,78], sheep [79] and even arthropods [80–82]. That grounded running was possibly present in an early, basal theropod suggests that it may have been present in the majority of extinct theropods, both non-avian and avian. Indeed, grounded running may have even been primitive for theropods.
Figure 6.
The Culpeper theropod trackmakers are inferred to have had a continuous gait pattern, probably including grounded running in their locomotor repertoire. (a) At slow speeds of locomotion, the theropods would have walked in a fashion comparable to slow walking in both modern birds and humans. The COM (black and white disc) would have been lowest during double-support phases and highest at single-support phases, and KE and PE of the COM would have oscillated more or less out of phase. (b) At faster speeds, the theropods would have employed grounded running, still having periods of double-support, yet where the COM is highest at double-support and lowest at single-support. KE and PE would oscillate more or less in phase. (c) At faster speeds still, the theropods would have become airborne (i.e. aerial running) as the vertical oscillations in the COM increase. KE and PE continue to oscillate in phase.
The possibility of grounded running in extinct, non-avian theropods has implications for understanding their palaeobiology. Grounded running could confer several advantages to a bipedal predator, despite potentially higher energy expenditure [83]. Periods of double limb support make grounded running more stable than aerial running at the same speed, including over uneven terrain [16,84]. Double limb support also reduces the peak ground reaction forces experienced compared with aerial running [32,77,83], which would subsequently reduce bone and muscle stresses. Therefore, the predator can move faster while still maintaining stability and lowered musculoskeletal stresses. This would be particularly advantageous for the large to giant species (e.g. Allosaurus, Tyrannosaurus and Giganotosaurus), whose athletic capabilities have previously been questioned [85–88]. Grounded running may also improve visual acuity by increasing head stability, particularly in the vertical direction [30,83], which would be beneficial when pursuing prey. Given these advantages to a predatory biped, it is tempting to speculate that these advantages may have facilitated the evolution of grounded running in theropods in the first instance.
4.4. Limitations of the study
This study has some potential limitations arising from logistical constraints on data collection. Firstly, the study compared steady-state locomotion data for humans and birds with largely non-steady-state locomotion data from theropod trackways, although the accelerating trials for humans suggest this had little effect. Secondly, locomotion in the non-avian theropods and birds was overground, whereas locomotion in the human subjects was treadmill-based. While some kinematic differences have previously been found between treadmill and overground locomotion [89], more recent, detailed studies tend to suggest that the kinematics of the two are quantitatively and qualitatively similar [90–93]. Thirdly, human kinematics were recorded with barefoot subjects, possibly influencing their gait because they may have been accustomed to wearing shoes. Although wearing shoes may alter some kinematics compared with barefoot locomotion [94,95], it is unknown if it affects many variables in the coronal plane, including step width. Moreover, any influence of wearing shoes may also vary with shoe design; hence, for consistency, the subjects walked and ran barefoot in the experiments. Despite these potential limitations, they are considered unlikely to alter the main findings of this study, which is concerned with major patterns of similarity and difference across species.
5. Conclusion
By integrating ichnological and comparative biomechanical datasets, this study has revealed new insight into the biomechanics of terrestrial locomotion of theropods, and bipeds in general. Across the groups studied, step width tended to decrease with increasing speed, but the manner in which step width changed with speed was decidedly different between humans and theropods. Humans exhibited an abrupt decrease at the walk–run transition, whereas in both non-avian and avian theropods, step width decreased gradually with speed, with considerable variability among avian taxa. These differences reflect a discontinuous locomotor repertoire in humans, and a continuous locomotor repertoire in theropods.
The non-avian theropods that made the trackways investigated seemed to have used a more continuous locomotor behaviour, much like modern birds, possibly including grounded running as part of their repertoire. The age and likely basal phylogenetic position of the trackmakers would therefore suggest that the distinct locomotor behaviour of modern birds had begun to appear very early in theropod evolution. The results of this study also indicate that future analyses of locomotion in non-avian theropods cannot simply pigeonhole it into discrete ‘walking’ or ‘running’ gaits. Moreover, given that mediolateral limb movements were apparently important in terrestrial locomotion in these animals, future analyses also need to be three-dimensional in order to fully capture the range of limb postures used.
Supplementary Material
Acknowledgements
The staff of the Geosciences Program of the Queensland Museum are thanked for the provision of workspace and access to literature. Much appreciation is extended to the human subjects for their time and cooperation during experiments; likewise, a great debt of gratitude is owed to the volunteers who assisted with the bird experiments. Technical support provided by Vicon Oxford Metrics is also gratefully acknowledged, as are the constructive comments of three anonymous reviewers.
Ethics
All protocols used were approved by institutional research ethics committees (approvals AHS/01/14/AEC, SBS/102/14/ARC). Collection of the three wild bird species was approved by the Queensland Department of Environment and Heritage Protection (permit no. WISP14699514). Written informed consent was obtained from the human subjects prior to the study.
Data accessibility
All data and scripts used are held in the Geosciences Collection of the Queensland Museum, and will be made available upon request to the Collections Manager.
Authors' contributions
P.J.B., C.J.C., S.A.H., R.S.B. and D.G.L. conceived the study design. P.J.B., C.J.C., R.E.W., D.F.G., L.P.L., J.R.H., J.R. and D.G.L. contributed or collected data. All authors contributed to data analysis, interpretation and drafting of the manuscript. All authors approved the final draft.
Competing interests
We declare we have no competing interests.
Funding
Supported by an Australian Government Research Training Program Scholarship (to P.J.B.), an ISB Matching Dissertation Grant (to P.J.B.), an ARC DECRA Fellowship (DE120101503, to C.J.C.) and an ARC Future Fellowship (FT150100492, to R.S.W.).
References
- 1.Ostrom JH. 1976. Archaeopteryx and the origin of birds. Biol. J. Linn. Soc. 8, 91–182. ( 10.1111/j.1095-8312.1976.tb00244.x) [DOI] [Google Scholar]
- 2.Gauthier JA. 1986. Saurischian monophyly and the origin of birds. Mem. Calif. Acad. Sci. 8, 1–55. [Google Scholar]
- 3.Sereno PC. 1999. The evolution of dinosaurs. Science 284, 2137–2147. ( 10.1126/science.284.5423.2137) [DOI] [PubMed] [Google Scholar]
- 4.Chiappe LM, Witmer LM. 2002. Mesozoic birds: above the heads of the dinosaurs. Berkeley, CA: University of California Press. [Google Scholar]
- 5.Weishampel DB, Dodson P, Osmólska H. 2004. The dinosauria, 2nd edn Berkeley, CA: University of California Press. [Google Scholar]
- 6.Gatesy SM. 2002. Locomotor evolution on the line to modern birds. In Mesozoic birds: above the heads of the dinosaurs (eds Chiappe LM, Witmer LM), pp. 432–447. Berkeley, CA: University of California Press. [Google Scholar]
- 7.Hutchinson JR, Allen V. 2009. The evolutionary continuum of limb function from early theropods to birds. Naturwissenschaften 96, 423–448. ( 10.1007/s00114-008-0488-3) [DOI] [PubMed] [Google Scholar]
- 8.Heers AM, Dial KP. 2012. From extant to extinct: locomotor ontogeny and the evolution of avian flight. Trends Ecol. Evol. 27, 296–305. ( 10.1016/j.tree.2011.12.003) [DOI] [PubMed] [Google Scholar]
- 9.Alexander RM. 2004. Bipedal animals, and their differences from humans. J. Anat. 204, 321–330. ( 10.1111/j.0021-8782.2004.00289.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller R, Birn-Jeffery AV, Blum Y. 2016. Human and avian running on uneven ground: a model-based comparison. J. R. Soc. Interface 13, 20160529 ( 10.1098/rsif.2016.0529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cracraft J. 1971. The functional morphology of the hind limb of the domestic pigeon, Columba livia. Bull. Am. Mus. Nat. Hist. 144, 171–268. [Google Scholar]
- 12.Gatesy SM. 1990. Caudofemoral musculature and the evolution of theropod locomotion. Paleobiology 16, 170–186. ( 10.1017/S0094837300009866) [DOI] [Google Scholar]
- 13.Gatesy SM. 1991. Hind limb scaling in birds and other theropods: implications for terrestrial locomotion. J. Morphol. 209, 83–96. ( 10.1002/jmor.1052090107) [DOI] [PubMed] [Google Scholar]
- 14.Gatesy SM. 1999. Guineafowl hindlimb function I: cineradiographic analysis and speed effects. J. Morphol. 240, 115–125. ( 10.1002/(SICI)1097-4687(199905)240:2%3C115::AID-JMOR3%3E3.0.CO;2-Y) [DOI] [PubMed] [Google Scholar]
- 15.Gatesy SM. 1999. Guineafowl hindlimb function II: electromyographic analysis and motor pattern evolution. J. Morphol. 240, 127–142. ( 10.1002/(SICI)1097-4687(199905)240:2%3C127::AID-JMOR4%3E3.0.CO;2-Q) [DOI] [PubMed] [Google Scholar]
- 16.Gatesy SM, Biewener AA. 1991. Bipedal locomotion: effects of speed, size and limb posture in birds and humans. J. Zool. 224, 127–147. ( 10.1111/j.1469-7998.1991.tb04794.x) [DOI] [Google Scholar]
- 17.Abourachid A, Renous S. 2000. Bipedal locomotion in ratites (Paleognatiform): examples of cursorial birds. Ibis 142, 538–549. ( 10.1111/j.1474-919X.2000.tb04455.x) [DOI] [Google Scholar]
- 18.Rubenson J, Lloyd DG, Besier TF, Heliams DB, Fournier PA. 2007. Running in ostriches (Stuthio camelus): three-dimensional joint axes alignment and joint kinematics. J. Exp. Biol. 210, 2548–2562. ( 10.1242/jeb.02792) [DOI] [PubMed] [Google Scholar]
- 19.Smith NC, Jespers KJ, Wilson AM. 2010. Ontogenetic scaling of locomotor kinetics and kinematics of the ostrich (Struthio camelus). J. Exp. Biol. 213, 1347–1355. ( 10.1242/jeb.020271) [DOI] [PubMed] [Google Scholar]
- 20.Abourachid A, Hackert R, Herbin M, Libourel PA, Lambert F, Gioanni H, Provini P, Blazevic P, Hugel V. 2011. Bird terrestrial locomotion as revealed by 3-D kinematics. Zoology 114, 360–368. ( 10.1016/j.zool.2011.07.002) [DOI] [PubMed] [Google Scholar]
- 21.Nyakatura JA, Andrada E, Grimm N, Weise H, Fischer MS. 2012. Kinematics and center of mass mechanics during terrestrial locomotion in northern lapwings (Vanellus vanellus, Charadriiformes). J. Exp. Zool. 317A, 580–594. ( 10.1002/jez.1750) [DOI] [PubMed] [Google Scholar]
- 22.Stoessel A, Fischer MS. 2012. Comparative intralimb coordination in avian bipedal locomotion. J. Exp. Biol. 215, 4055–4069. ( 10.1242/jeb.070458) [DOI] [PubMed] [Google Scholar]
- 23.Allen V, Bates KT, Li Z, Hutchinson JR. 2013. Linking the evolution of body shape and locomotor biomechanics in bird-line archosaurs. Nature 497, 104–107. ( 10.1038/nature12059) [DOI] [PubMed] [Google Scholar]
- 24.Andrada E, Nyakatura JA, Bergmann F, Blickhan R. 2013. Adjustments of global and local hindlimb properties during terrestrial locomotion of the common quail (Coturnix coturnix). J. Exp. Biol. 216, 3906–3916. ( 10.1242/jeb.085399) [DOI] [PubMed] [Google Scholar]
- 25.Grossi B, Iriarte-Díaz J, Larach O, Canals M, Vásquez RA. 2014. Walking like dinosaurs: chickens with artificial tails provide clues about non-avian theropod locomotion. PLoS ONE 9, e88458 ( 10.1371/journal.pone.0088458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kilbourne BM, Andrada E, Fischer MS, Nyakatura JA. 2016. Morphology and motion: hindlimb proportions and swing phase kinematics in terrestrially locomoting charadriiform birds. J. Exp. Biol. 219, 1405–1416. ( 10.1242/jeb.124081) [DOI] [PubMed] [Google Scholar]
- 27.Rode C, Sutedja Y, Kilbourne BM, Blickhan R, Andrada E. 2016. Minimizing the cost of locomotion with inclined trunk predicts crouched leg kinematics of small birds at realistic levels of elastic recoil. J. Exp. Biol. 219, 485–490. ( 10.1242/jeb.127910) [DOI] [PubMed] [Google Scholar]
- 28.Clark J, Alexander RM. 1975. Mechanics of running by quail (Coturnix). J. Zool. 176, 87–113. ( 10.1111/j.1469-7998.1975.tb03189.x) [DOI] [Google Scholar]
- 29.Rubenson J, Heliams DB, Lloyd DG, Fournier PA. 2004. Gait selection in the ostrich: mechanical and metabolic characteristics of walking and running with and without an aerial phase. Proc. R. Soc. Lond. B 271, 1091–1099. ( 10.1098/rspb.2004.2702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hancock JA, Stevens NJ, Biknevicius AR. 2007. Whole-body mechanics and kinematics of terrestrial locomotion in the elegant-crested tinamou Eudromia elegans. Ibis 149, 605–614. ( 10.1111/j.1474-919X.2007.00688.x) [DOI] [Google Scholar]
- 31.Nudds RL, Folkow LP, Lees JJ, Tickle PG, Stokkan K-A, Codd JR. 2011. Evidence for energy savings from aerial running in the Svalbard rock ptarmigan (Lagopus muta hyperborea). Proc. R. Soc. B 278, 2654–2661. ( 10.1098/rspb.2010.2742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrada E, Rode C, Blickhan R. 2013. Grounded running in quails: simulations indicate benefits of observed fixed aperture angle between legs before touch-down. J. Theor. Biol. 335, 97–107. ( 10.1016/j.jtbi.2013.06.031) [DOI] [PubMed] [Google Scholar]
- 33.Hreljac A. 1995. Determinants of the gait transition speed during human locomotion: kinematic factors. J. Biomech. 28, 669–677. ( 10.1016/0021-9290(94)00120-S) [DOI] [PubMed] [Google Scholar]
- 34.Muir GD, Gosline JM, Steeves JD. 1996. Ontogeny of bipedal locomotion: walking ad running in the chick. J. Physiol. 493, 589–601. ( 10.1113/jphysiol.1996.sp021406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. 2006. Motor patterns in human walking and running. J. Neurophysiol. 95, 3426–3437. ( 10.1152/jn.00081.2006) [DOI] [PubMed] [Google Scholar]
- 36.Jones EA. 2010. Characterisation of limb development and locomotion in the brown kiwi (Apteryx mantelli). Palmerston North, New Zealand: Massey University. [Google Scholar]
- 37.Rose KA, Bates KT, Nudds RL, Codd JR. 2016. Ontogeny of sex differences in the energetics and kinematics of terrestrial locomotion in leghorn chickens (Gallus gallus domesticus). Sci. Rep. 6, 24292 ( 10.1038/srep24292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gillette DD, Lockley MG. 1989. Dinosaur tracks and traces. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 39.Thulborn T. 1990. Dinosaur tracks. London, UK: Chapman and Hall. [Google Scholar]
- 40.Lockley MG. 1991. Tracking dinosaurs. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 41.Alexander RM. 1976. Estimates of speeds of dinosaurs. Nature 261, 129–130. ( 10.1038/261129a0) [DOI] [Google Scholar]
- 42.Thulborn RA. 1982. Speeds and gaits of dinosaurs. Palaeogeogr. Palaeoclimatol. Palaeoecol. 38, 227–256. ( 10.1016/0031-0182(82)90005-0) [DOI] [Google Scholar]
- 43.Thulborn RA. 1984. Preferred gaits of bipedal dinosaurs. Alcheringa 8, 243–252. ( 10.1080/03115518408618947) [DOI] [Google Scholar]
- 44.Thulborn RA, Wade M. 1984. Dinosaur trackways in the Winton Formation (mid-Cretaceous) of Queensland. Mem. Queensl. Mus. 21, 413–517. [Google Scholar]
- 45.Bauby CE, Kuo AD. 2000. Active control of lateral balance in human walking. J. Biomech. 33, 1433–1440. ( 10.1016/S0021-9290(00)00101-9) [DOI] [PubMed] [Google Scholar]
- 46.Donelan JM, Kram R, Kuo AD. 2001. Mechanical and metabolic determinants of the preferred step width in human walking. Proc. R. Soc. Lond. B 268, 1985–1992. ( 10.1098/rspb.2001.1761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donelan JM, Shipman DW, Kram R, Kuo AD. 2004. Mechanical and metabolic requirements for active lateral stabilization in human walking. J. Biomech. 37, 827–835. ( 10.1016/j.jbiomech.2003.06.002) [DOI] [PubMed] [Google Scholar]
- 48.Arellano CJ, Kram R. 2011. The effects of step width and arm swing on energetic cost and lateral balance during running. J. Biomech. 44, 1291–1295. ( 10.1016/j.jbiomech.2011.01.002) [DOI] [PubMed] [Google Scholar]
- 49.Arellano CJ, Kram R. 2011. The energetic cost of maintaining lateral balance during human running. J. Appl. Physiol. 112, 427–434. ( 10.1152/japplphysiol.00554.2011) [DOI] [PubMed] [Google Scholar]
- 50.Collins SH, Kuo AD. 2013. Two independent contributions to step variability during over-ground human walking. PLoS ONE 7, e73597 ( 10.1371/journal.pone.0073597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farlow JO, Smith MB, Robinson JM. 1995. Body mass, bone ‘strength indicator’, and cursorial potential of Tyrannosaurus rex. J. Vertebr. Paleontol. 15, 713–725. ( 10.1080/02724634.1995.10011257) [DOI] [Google Scholar]
- 52.Day JJ, Norman DB, Upchurch P, Powell HP. 2002. Dinosaur locomotion from a new trackway. Nature 415, 494–495. ( 10.1038/415494a) [DOI] [PubMed] [Google Scholar]
- 53.Day JJ, Norman DB, Gale AS, Upchurch P, Powell HP. 2004. A Middle Jurassic dinosaur trackway site from Oxfordshire, UK. Palaeontology 47, 319–348. ( 10.1111/j.0031-0239.2004.00366.x) [DOI] [Google Scholar]
- 54.Kim BS, Huh M. 2010. Analysis of the acceleration phase of a theropod dinosaur based on a Cretaceous trackway from Korea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 293, 1–8. ( 10.1016/j.palaeo.2010.04.020) [DOI] [Google Scholar]
- 55.Weems RE. 1987. A late Triassic footprint fauna from the Culpeper Basin northern Virginia (U.S.A.). Trans. Am. Phil. Soc. 77, 1–79. ( 10.2307/1006417) [DOI] [Google Scholar]
- 56.Weems RE. 1992. A re-evaluation of the taxonomy of the Newark Supergroup saurischian dinosaur tracks, using extensive statistical data from a recently exposed tracksite near Culpeper, Virginia. Virginia Div. Mineral Resources Pub. 119, 113–127. [Google Scholar]
- 57.Weems RE. 2006. Locomotor speeds and patterns of running behaviour in non-maniraptoriform theropod dinosaurs. New Mexico Mus. Nat. Hist. Sci. Bull. 37, 379–389. [Google Scholar]
- 58.Lockley MG, Hunt AP, Moratella J, Matsukawa M. 1994. Limping dinosaurs? Trackway evidence for abnormal gaits. Ichnos 3, 193–202. ( 10.1080/10420949409386388) [DOI] [Google Scholar]
- 59.Alexander RM, Jayes AS. 1983. A dynamic similarity hypothesis for the gaits of quadrupedal mammals. J. Zool. 201, 135–152. ( 10.1111/j.1469-7998.1983.tb04266.x) [DOI] [Google Scholar]
- 60.Alexander RM, Jayes AS. 1978. Vertical movements in walking and running. J. Zool. 185, 27–40. ( 10.1111/j.1469-7998.1978.tb03311.x) [DOI] [Google Scholar]
- 61.Rubenson J, Lloyd DG, Heliams DB, Besier TF, Fournier PA. 2011. Adaptations for economical bipedal running: the effect of limb structure on three-dimensional joint mechanics. J. R. Soc. Interface 8, 740–755. ( 10.1098/rsif.2010.0466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winter DA. 2009. Biomechanics and motor control of human movement, 4th edn Hoboken, NJ: John Wiley & Sons, Inc. [Google Scholar]
- 63.Lamas LP. 2015. Musculoskeletal biomechanics during growth on emu (Dromaius; Aves): an integrative experimental and modelling analysis. PhD thesis, Royal Veterinary College, University of London UK. [Google Scholar]
- 64.Hedrick TL. 2008. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir. Biomim. 3, 034001 ( 10.1088/1748-3182/3/3/034001) [DOI] [PubMed] [Google Scholar]
- 65.Warton DI, Wright IJ, Falster DS, Westoby M. 2006. Bivariate line-fitting methods for allometry. Biol. Rev. 81, 259–291. ( 10.1017/S1464793106007007) [DOI] [PubMed] [Google Scholar]
- 66.Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4, 4. [Google Scholar]
- 67.Legendre P, Legendre L. 2012. Numerical ecology, 3rd English edn Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 68.Hildebrand M. 1985. Walking and running. In Functional vertebrate morphology (eds Hildebrnad M, Bramble DM, Liem KF, Wake DB), pp. 38–57. Cambridge, MA: Harvard University Press. [Google Scholar]
- 69.Orendurff MS, Segal AD, Klute GK, Berge JS, Rohr ES, Kadel NJ. 2004. The effect of walking speed on center of mass displacement. J. Rehabil. Res. Dev. 41, 829–834. ( 10.1682/JRRD.2003.10.0150) [DOI] [PubMed] [Google Scholar]
- 70.Paxton H, Daley MA, Corr SA, Hutchinson JR. 2013. The gait dynamics of the modern broiler chicken: a cautionary tale of selective breeding. J. Exp. Biol. 216, 3237–3248. ( 10.1242/jeb.080309) [DOI] [PubMed] [Google Scholar]
- 71.Ortega JD, Farley CT. 2005. Minimizing centre of mass vertical movement increases metabolic cost in walking. J. Appl. Physiol. 99, 2099–2107. ( 10.1152/japplphysiol.00103.2005) [DOI] [PubMed] [Google Scholar]
- 72.Alexander RM. 1989. Optimization and gaits in the locomotion of vertebrates. Physiol. Rev. 69, 1199–1227. [DOI] [PubMed] [Google Scholar]
- 73.Parchman AJ, Reilly SM, Biknevicius AR. 2003. Whole-body mechanics and gaits in the gray short-tailed opossum Monodelphis domestica: integrating patterns of locomotion in a semi-erect mammal. J. Exp. Biol. 206, 1379–1388. ( 10.1242/jeb.00267) [DOI] [PubMed] [Google Scholar]
- 74.Biknevicius AR, Reilly SM, McElroy EJ, Bennett MB. 2013. Symmetrical gaits and center of mass mechanics in small-bodied, primitive mammals. Zoology 116, 67–74. ( 10.1016/j.zool.2012.05.005) [DOI] [PubMed] [Google Scholar]
- 75.Ahn AN, Furrow E, Biewener AA. 2004. Walking and running in the red-legged running frog, Kassina maculata. J. Exp. Biol. 207, 399–410. ( 10.1242/jeb.00761) [DOI] [PubMed] [Google Scholar]
- 76.Hutchinson JR, Famini D, Lair R, Kram R. 2003. Are fast-moving elephants really running? Nature 422, 493–494. ( 10.1038/422493a) [DOI] [PubMed] [Google Scholar]
- 77.Schmitt D. 1999. Compliant walking in primates. J. Zool. 248, 149–160. ( 10.1111/j.1469-7998.1999.tb01191.x) [DOI] [Google Scholar]
- 78.Demes B, O'Neill MC. 2013. Ground reaction forces and centre of mass mechanics of bipedal capuchin monkeys: implications for the evolution of human bipedalism. Am. J. Phys. Anthropol. 150, 76–86. ( 10.1002/ajpa.22176) [DOI] [PubMed] [Google Scholar]
- 79.Jayes AS, Alexander RM. 1978. Mechanics of locomotion of dogs (Canis familiaris) and sheep (Ovis aries). J. Zool. 185, 289–308. ( 10.1111/j.1469-7998.1978.tb03334.x) [DOI] [PubMed] [Google Scholar]
- 80.Blickhan R, Full RJ. 1987. Locomotion energetics of the ghost crab. II. Mechanics of the centre of mass during walking and running. J. Exp. Biol. 130, 155–174. [Google Scholar]
- 81.Full RJ, Tu MS. 1990. Mechanics of six-legged runners. J. Exp. Biol. 148, 129–146. [DOI] [PubMed] [Google Scholar]
- 82.Reinhardt L, Blickhan R. 2014. Level locomotion in wood ants: evidence for grounded running. J. Exp. Biol. 217, 2358–2370. ( 10.1242/jeb.098426) [DOI] [PubMed] [Google Scholar]
- 83.McMahon TA, Valiant G, Frederick EC. 1987. Groucho running. J. Appl. Physiol. 62, 2326–2337. [DOI] [PubMed] [Google Scholar]
- 84.Andrada E, Rode C, Sutedja Y, Nyakatura JA, Blickhan R. 2014. Trunk orientation causes asymmetries in leg function in small bird terrestrial locomotion. Proc. R. Soc. B 281, 20141405 ( 10.1098/rspb.2014.1405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blanco RE, Mazzetta GV. 2001. A new approach to evaluate the cursorial ability of the giant theropod Giganotosaurus carolinii. Acta Palaeontol. Pol. 46, 193–202. [Google Scholar]
- 86.Hutchinson JR, Garcia M. 2002. Tyrannosaurus was not a fast runner. Nature 415, 1018–1021. ( 10.1038/4151018a) [DOI] [PubMed] [Google Scholar]
- 87.Hutchinson JR. 2004. Biomechanical modeling and sensitivity analysis of bipedal running ability. II. Extinct taxa. J. Morphol. 262, 441–461. ( 10.1002/jmor.10240) [DOI] [PubMed] [Google Scholar]
- 88.Sellers WI, Manning PL. 2007. Estimating dinosaur maximum running speeds using evolutionary robotics. Proc. R. Soc. B 274, 2711–2716. ( 10.1098/rspb.2007.0846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nigg BM, De Boer RW, Fisher V. 1995. A kinemaric comparison of overground and treadmill running. Med. Sci. Sports Exerc. 27, 98–105. ( 10.1249/00005768-199501000-00018) [DOI] [PubMed] [Google Scholar]
- 90.Pereira JE, et al. 2006. A comparison analysis of hindlimb kinematics during overground and treadmill locomotion in rats. Behav. Brain Res. 172, 212–218. ( 10.1016/j.bbr.2006.04.027) [DOI] [PubMed] [Google Scholar]
- 91.Riley PO, Paolini G, Croce UD, Paylo KW, Kerrigan DC. 2007. A kinematic and kinetic comparison of overground and treadmill walking in healthy subjects. Gait Posture 26, 17–24. ( 10.1016/j.gaitpost.2006.07.003) [DOI] [PubMed] [Google Scholar]
- 92.Riley PO, Dicharry J, Franz J, Croce UD, Wilder RP, Kerrigan DC. 2008. A kinematics and kinetic comparison of overground and treadmill running. Med. Sci. Sports Exerc. 40, 1093–1100. ( 10.1249/MSS.0b013e3181677530) [DOI] [PubMed] [Google Scholar]
- 93.Lee SJ, Hidler J. 2008. Biomechanics of overground vs. treadmill walking in healthy individuals. J. Appl. Physiol. 104, 747–755. ( 10.1152/japplphysiol.01380.2006) [DOI] [PubMed] [Google Scholar]
- 94.Squadrone R, Gallozzi C. 2009. Biomechanical and physiological comparison of barefoot and two shod conditions in experienced barefoot runners. J. Sports Med. Phys. Fitness 49, 6–13. [PubMed] [Google Scholar]
- 95.Franklin S, Grey MJ, Heneghan N, Bowen L, Li F-X. 2015. Barefoot vs common footwear: a systematic review of the kinematic, kinetic and muscle activity differences during walking. Gait Posture 42, 230–239. ( 10.1016/j.gaitpost.2015.05.019) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and scripts used are held in the Geosciences Collection of the Queensland Museum, and will be made available upon request to the Collections Manager.