Abstract
Crosstalk between mesenchymal and epithelial cells influences organogenesis in multiple tissues, such as lung, pancreas, liver, and the nervous system. Lung mesenchyme comprises multiple cell types, however, and precise identification of the mesenchymal cell type(s) that drives early events in lung development remains unknown. Endothelial cells have been shown to be required for some aspects of lung epithelial patterning, lung stem cell differentiation, and regeneration after injury. Furthermore, endothelial cells are involved in early liver and pancreas development. From these observations we hypothesized that endothelial cells might also be required for early specification of the respiratory field and subsequent lung bud initiation. We first blocked VEGF signaling in E8.5 cultured foreguts with small molecule VEGFR inhibitors and found that lung specification and bud formation were unaltered. However, when we examined E9.5 mouse embryos carrying a mutation in the VEGFR Flk-1, which do not develop endothelial cells, we found that respiratory progenitor specification was impeded. Because the E9.5 embryos were substantially smaller than control littermates, suggesting the possibility of developmental delay, we isolated and cultured foreguts from mutant and control embryos on E8.5, when no size differences were apparent. We found that both specification of the respiratory field and lung bud formation occurred in mutant and control explants. These observations were unaffected by the presence or absence of serum. We also observed that hepatic specification and initiation occurred in the absence of endothelial cells, and that expansion of the liver epithelium in culture did not differ between mutant and control explants. Consistent with previously published results, we also found that pancreatic buds were not maintained in cultured foreguts when endothelial cells were absent. Our observations support the conclusion that endothelial cells are not required for early specification of lung progenitors and bud initiation, and that the diminished lung specification seen in E9.5 Flk−/− embryos is likely due to developmental delay resulting from the insufficient delivery of oxygen, nutrients, and other factors in the absence of a vasculature.
Keywords: Lung development, Lung specification, Endothelial cells
1. Introduction
Early lung organogenesis comprises the processes of progenitor specification and bud initiation. Specification of respiratory progenitors, which is the commitment and demarcation of endodermal cells fated to become lung or trachea, is evident on embryonic day (E) 8.5– 9.0 in the mouse. The respiratory progenitors are identified by expression of the transcription factor Nkx2-1, which is the earliest known marker of lung specification, along the ventral foregut endoderm. Following specification, lung morphogenesis ensues with the formation of two primary buds that evaginate into the surrounding splanchnic mesoderm at E9.5, followed by elaboration of the conducting airways through branching morphogenesis (Havrilak and Shannon, 2015a; Herriges and Morrisey, 2014; Metzger et al., 2008).
Events in early lung development are driven by reciprocal tissue interactions between the mesenchyme and the epithelium (Havrilak and Shannon, 2015a; Hines and Sun, 2014; McCulley et al., 2015; Shannon et al., 1998). These interactions are mediated by diffusible paracrine factors, and intensive research over the past two decades has identified many of the conserved signaling pathways governing early lung specification and morphogenesis. The retinoic acid (RA), WNT/β-catenin, fibroblast growth factor (FGF), hedgehog (HH), and bone morphogenetic protein (BMP) signaling pathways form gene regulatory networks that interact to orchestrate lung development (Herriges and Morrisey, 2014; Hines and Sun, 2014; Rankin and Zorn, 2014). For example, RA produced by splanchnic mesenchyme cells stimulates SHH expression, which in turn supports both mesenchymal survival (Weaver et al., 2003) and its expression of Wnt2/2b (Rankin et al., 2016).
Although much has been learned about the gene regulatory networks controlling lung specification and development, precise identification of the cell types within the surrounding mesenchyme that produce critical signaling factors for the epithelium in early lung development remains unknown. Of the myriad of different cell types found in the developing lung mesenchyme (Kumar et al., 2014), some available evidence suggests a potential role for endothelial cells. Several studies have shown that endothelial cells contribute to some processes in the developing lung, such as patterning and alveoligenesis (Lazarus et al., 2011; van Tuyl et al., 2005; Zhao et al., 2005). A requirement for endothelial cells for early lung branching is not absolute, however, since we have recently demonstrated that embryonic (E12.5) lung endoderm is fully capable of branching in vitro in the absence of endothelial cells (Havrilak and Shannon, 2015b). In the adult lung, endothelial cells act to influence adult lung stem cell differentiation (Lee et al., 2014), as well as in promoting alveolar regeneration following unilateral pneumonectomy (Ding et al., 2011).
Observations from other endoderm-derived tissues, notably the pancreas and liver, have suggested that endothelial cells are required for organ initiation, specifically during bud formation. Utilizing recombination techniques, Lammert et al. showed that endoderm recombined with dorsal aorta initiated expression of the pancreas markers Pdx1 and insulin (Lammert et al., 2001). They also found that Xenopus embryos lacking a dorsal aorta showed a significant decrease in pancreatic gene expression. Gain of function experiments using the Pdx1 promoter to drive VEGF expression demonstrated that increased vascularization led to hypertrophy of pancreatic islets, ectopic expression of insulin expressing cells in the stomach near the areas of increased vascularization, and ectopic pancreatic buds in the anterior duodenum (Lammert et al., 2001). Studies examining liver initiation in mouse embryos null for the VEGF receptor Flk-1, which lack mature endothelial cells, showed that although early liver genes such as Alb, Ttr and Hex were expressed in these embryos, liver epithelial cells did not migrate into the adjacent septum transversum, either in vivo or in vitro (Matsumoto et al., 2001).
The known role of endothelial cells in some aspects of lung development, combined with observations in the pancreas and liver, raised the possibility that endothelial cells might also play a critical role in respiratory field specification and lung bud initiation. In the present study we have examined the role of endothelial cells during lung specification and bud initiation. Using pharmacological inhibitors of VEGF signaling, as well as Flk-1 mutant mouse embryos lacking endothelial cells, we show here that a lack of endothelial cells does not interfere with specification of respiratory progenitors or subsequent lung bud initiation.
2. Results
2.1. Endothelial cells are associated with early respiratory progenitors
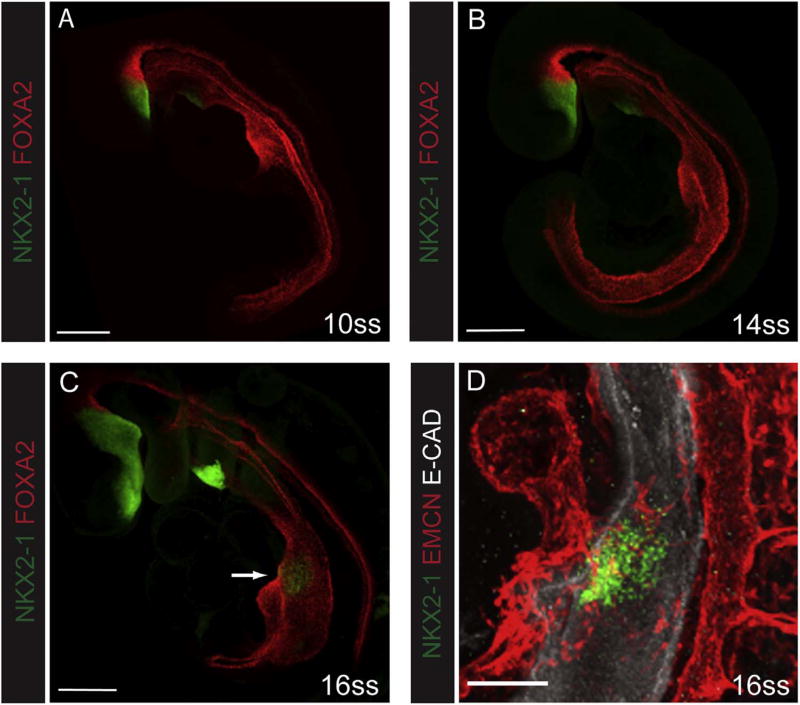
Previous studies from our lab have shown that pulmonary vascular development begins in the lateral plate mesoderm as soon as the lung bud emerges from the ventral foregut endoderm (Gebb and Shannon, 2000). Being closely apposed to the lung bud at early stages of development, lung vascular development is temporally and spatially correlated with that of the developing lung bud. The relationship of endothelial cells to the field of specified respiratory progenitors prior to lung bud formation has not been previously described. This required that we first precisely determine when lung specification occurs, using the early lung marker NKX2-1 as a benchmark. Whole mount immunostaining of E8.5 embryos examined at the 10 and 14 somite stage (ss) (Fig. 1A and B) showed clearly detectable NKX2-1 positive cells in both the forebrain and thyroid regions, but no positive cells in the presumptive respiratory field, which lies in the ventral foregut endoderm dorsal to the developing heart. At 16ss (Fig. 1C), however, NKX2-1 positive cells were clearly apparent in the respiratory field. Notably, these progenitors were in proximity to endothelial cells, as detected by the endothelial cell specific maker endomucin (EMCN, Fig. 1D) (Brachtendorf et al., 2001). The expression data for NKX2-1 in the presumptive lung region were confirmed using qPCR for Nkx2-1 mRNA (data not shown). As has been previously described (Gebb and Shannon, 2000; Schachtner et al., 2000), the proximate association of endothelial cells with the developing lung endoderm was even more apparent after nascent lung buds had emerged.
Fig. 1.
Endothelial cells are associated with respiratory progenitors. Early (E8–8.5) embryos were stained by whole mount immunofluorescence for NKX2-1 (green) to visualize the emergence of the respiratory progenitors. At 10ss (A) or 14ss (B), NKX2-1 is only detected in the forebrain and thyroid region. At 16ss NKX2-1 positive cells are evident in the presumptive lung field (C, arrow). FOXA2 (red) stains the gut tube endoderm on the ventral side of the embryo, and the notochord and floor plate dorsally (A–C). Higher magnification of a 16ss embryo (D) stained for EMCN (red) demonstrates that endothelial cells are in proximity to respiratory progenitors emerging from the ventral side of the foregut; E-CAD staining (white) marks the foregut endoderm in this panel. Scale bars: A–C =200 µm, D =100 µm.
2.2. Suppression of endothelial cells via small molecule inhibition of VEGFR does not affect specification of lung progenitors
The observation that endothelial cells are present in and around the presumptive respiratory field before the lung bud emerges, along with evidence from studies on the liver and pancreas suggesting that endothelial cells are required for bud initiation of those organs, led us to hypothesize that endothelial cells may also be required for early lung specification and bud initiation. To investigate the role of endothelial cells during early lung development, we first utilized a foregut culture system that supports specification and initiation of early lung cells (Chen et al., 2007; Desai et al., 2004), and treated these explants with the small molecule VEGFR inhibitors Ki8751 and SU5416. These inhibitors effectively block VEGF signaling (Fong et al., 1999; Kubo et al., 2005), which is necessary for endothelial cell proliferation, differentiation, and survival (Dvorak et al., 1995; Ferrara et al., 2003), and we have previously shown that they inhibit embryonic lung endothelial cells (Havrilak and Shannon, 2015b). Based on studies in the pancreas (Lammert et al., 2001) and liver (Matsumoto et al., 2001), we anticipated that pharmacological inhibition of VEGF signaling would cause a loss of endothelial cells and that this loss of endothelial cells would inhibit lung bud formation.
For these experiments we cultured the foregut region from E8.5 (4–10ss) embryos, because at this stage there was no detectable NKX2-1 expression in the ventral endoderm of the prospective respiratory field (Fig. 1), suggesting that respiratory progenitors have not yet been specified. Given its close proximity to the respiratory field, the heart was left intact in these explants to avoid damage to the endoderm during dissection. Furthermore, the heart may be a source of signaling molecules necessary for lung specification (Serls et al., 2005).
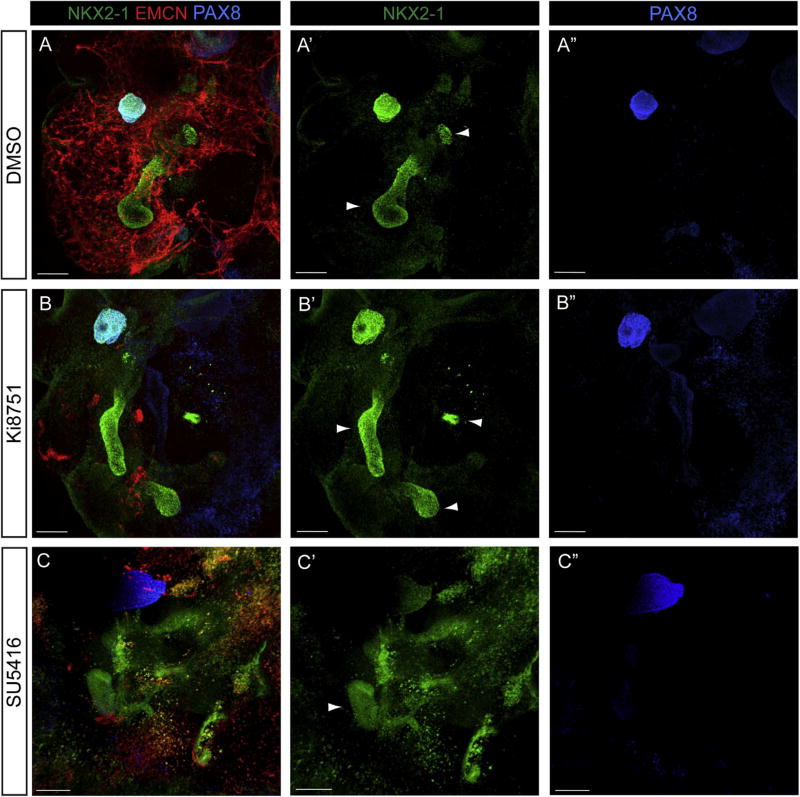
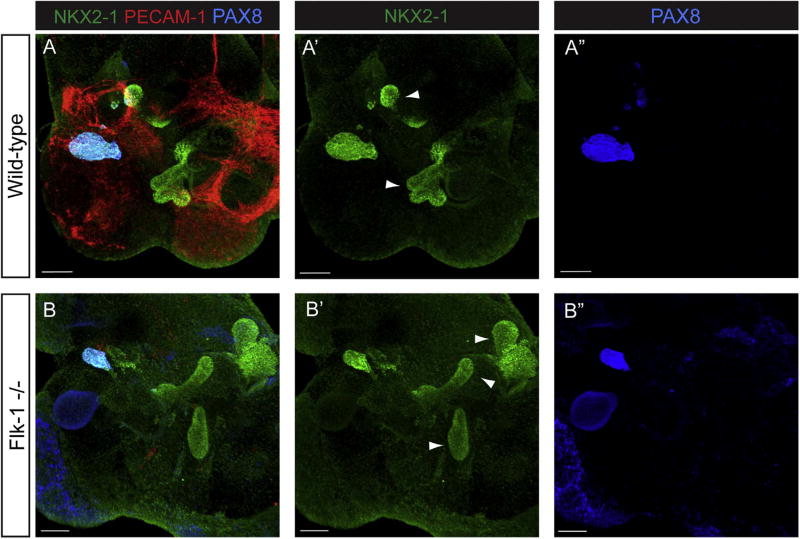
Explants cultured with DMSO for four days showed regions where buds with robust NKX2-1 expression had emerged from the endoderm, suggesting respiratory progenitor specification had occurred (Fig. 2A–A’, arrowheads). EMCN staining confirmed that an extensive vascular network was present throughout the explant (Fig. 2A). However, since NKX2-1 is also expressed in the developing thyroid, we needed to confirm that lung specification was occurring. To do this, we co-stained explants with PAX8 (Fig. 2A, A”), a thyroid marker, and found that PAX8 was only present in a discrete subset of cells and not in the more distal lung buds. We therefore concluded that the PAX8-negative, NKX2-1-positive buds represented lung. Our results on lung and thyroid formation in cultured foreguts recapitulates exactly what has been observed by others (Chen et al., 2010; Desai et al., 2004).
Fig. 2.
VEGFR inhibition does not impede specification or bud initiation in cultured E8.5 foregut endoderm. E8.5 (4–10ss) foregut explants were cultured for 4 days in the presence of either DMSO or the VEGFR inhibitors Ki8751 and SU5416. EMCN staining (red) shows that an extensive vascular network is present in explants cultured in the presence of DMSO (A). NKX2-1 (green) is expressed in two discrete areas of the explants (A’, arrowheads). Staining for the transcription factor PAX8 (blue), which is expressed in the thyroid but not the lung, reveals that one of the NKX2-1 positive regions is thyroid (A”). Foregut explants cultured with Ki8751 or SU5416 show a marked reduction in the number of vascular cells (B, C), but no change in the specification of either the lung (arrowheads) or thyroid fields (B’, B”, C’, C”). Scale bars =100 µm. Th, Thyroid; Lg, Lung. N ≥6 individual explants.
As expected, when foreguts were cultured with the VEGFR inhibitors Ki8751 or SU5416, EMCN positive endothelial cells were virtually eliminated (Fig. 2B, C). Somewhat surprisingly, explants treated with the inhibitor were still clearly able to specify respiratory progenitors as visualized by NKX2-1 expression, and to initiate morphogenesis of a lung bud (Fig. 2B, B’, 2C, 2C’, arrowheads). As in DMSO treated cultures, discrete areas of PAX8 positive cells were seen in Ki8751 treated explants (Fig. 2B”) and SU5461 treated explants (Fig. 2C”), and these PAX8-positive regions co-stained with only one of the NKX2-1 positive fields (Fig. 2B, C). Contrary to our expectations, these data suggested that endothelial cells were dispensable for lung specification and bud initiation.
2.3. Genetic loss of VEGF signaling does not affect specification of lung progenitors
One potential problem with the approach of using small molecule inhibitors of VEGFR in the experiments described above is that all of the endothelial cells may not have been ablated, even though expression levels of endothelial markers were significantly decreased after treatment with the inhibitors. As can be seen in Fig. 2B and C, a few EMCN-positive endothelial cells appear to remain after treatment with the inhibitors, indicating that they were not 100% effective at depleting the system of endothelial cells. This raised the possibility that the few remaining endothelial cells might be sufficient to drive lung specification. A second potential problem is that the VEGFR inhibitor might possibly have off-target effects on other signaling pathways affecting lung morphogenesis. Lastly, even though NKX2-1 expression was not yet detectable when we initiated our cultures, it is possible that the respiratory field was already patterned prior to treatment with the inhibitors and subsequent loss of the endothelial cells. To exclude these possibilities, we took a genetic approach utilizing a mouse model that does not develop any endothelial cells.
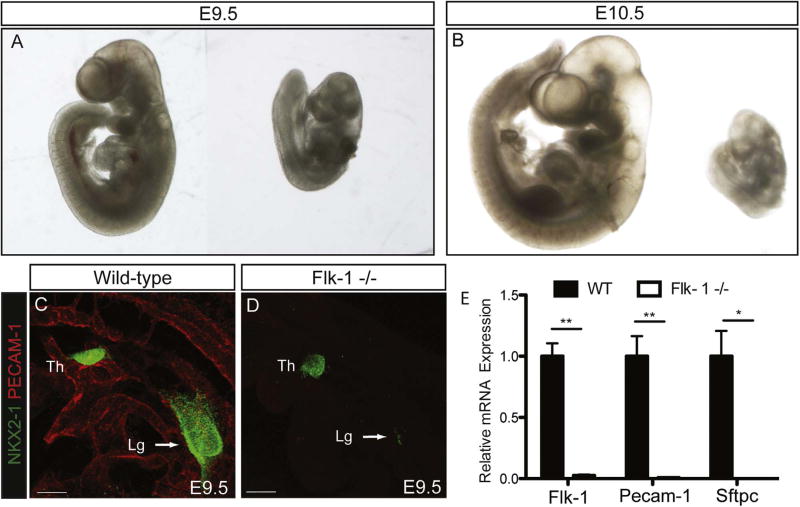
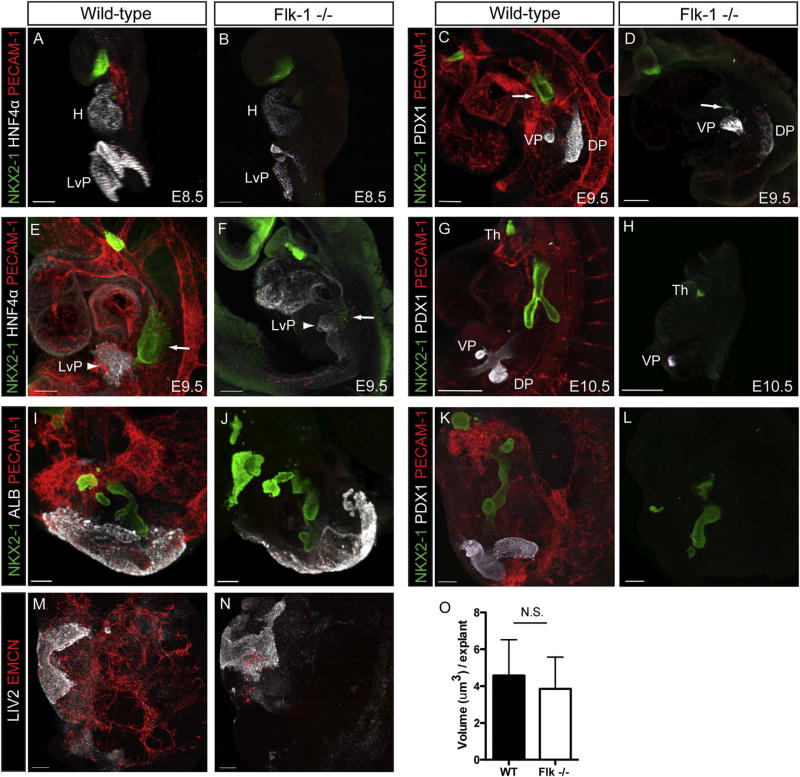
We obtained orvieto mutant mice (Sandell et al., 2011), which have a point mutation in the Flk-1 coding sequence at bp 1311, resulting in the conversion of TYR to STOP and a subsequent loss of Flk-1 expression. Flk-1+/− heterozygote embryos were viable and developed normally. Flk-1−/− mutants, however, showed an embryonic lethal phenotype, with few embryos surviving to E10.5. Mutants had severe developmental defects and were about half the size of Flk-1+/− and wild-type littermates at E9.5 (Fig. 3A), a size differential that was even more apparent in the embryos that survived to E10.5 (Fig. 3B). Immunostaining for PECAM-1 showed no endothelial cells in Flk-1−/−mutant embryos at E9.5 (Fig. 3D), whereas control littermates had widespread endothelial cell staining (Fig. 3C). The absence of endothelial cells in Flk-1−/− mutant embryos was further confirmed by qPCR analysis demonstrating the lack of Flk-1 and Pecam-1 expression (Fig. 3E).
Fig. 3.
The prospective lung field of Flk-1−/− mutant embryos contains few NKX2-1 positive progenitors. Comparison of wild-type (WT) embryos (left) with those containing a mutation in the Flk-1 gene (right) on E9.5 (A) and E10.5 (B). Note that the mutant E9.5 embryo is significantly smaller than its wild-type littermate, and that this size disparity is even more pronounced on E10.5. Immunofluorescent staining of E9.5 embryos for PECAM-1 shows the developing vasculature in wild-type embryos (C), but reveals a complete lack of vascular cells in the Flk-1−/− mutants (D). The lack of endothelial cells in Flk-1−/− mutants is confirmed by qPCR analysis for Flk-1 and Pecam-1 (E; **= p < 0.001, N ≥5). Immunostaining for NKX2-1 shows that many respiratory progenitors have emerged in the region of the foregut endoderm destined to become the lung in wild-type embryos (C, arrow), while Flk-1−/− embryos contain only a few cells expressing NKX2-1 in the prospective lung field (D, arrow). Analysis of E10.5 embryos by qPCR (E) shows that Sftpc is detected in wild-type embryos, but not in Flk-1−/− mutants (*= p < 0.01, N ≥4 samples). Scale bars =100 µm.
To determine if embryos lacking endothelial cells could form a lung bud, we examined the embryos at E9.5 and E10.5 for expression of NKX2-1. Both wild-type (Figs. 3C, 6C, 6E, 6G) and Flk-1−/− (Figs. 3D, 6D, 6F) embryos showed NKX2-1 expression by immunofluorescence. In E9-9.5 Flk-1−/− mutant embryos, however, NKX2-1 expression in the region where respiratory progenitors are found was limited to a small number of cells (Figs. 3D, 6D, 6F) in 81% (17/21) of the embyros examined. Furthermore, respiratory NKX2-1 expression was not seen in E10.5 mutant embryos (Fig. 6H). The lack of lung development in E10.5 mutant versus wild-type embryos was confirmed by the lack of expression of the lung epithelial marker Sftpc in Flk-1−/− mutant embryos (Fig. 3E). These observations stood in contrast to our cultured foregut data and suggest that endothelial cells are indeed required for respiratory specification and expansion.
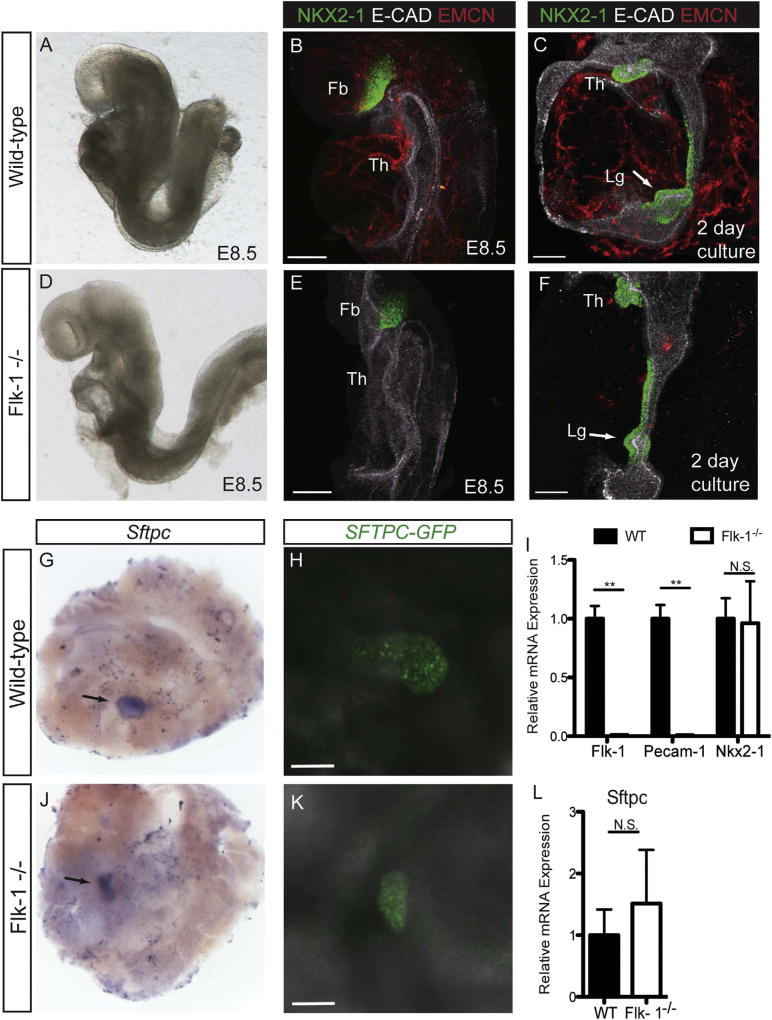
The paucity of NKX2-1 positive cells in the respiratory field of E9.5 Flk-1−/− mutants suggested that the respiratory field was not normally specified in the absence of endothelial cells (Fig. 3), which was in contrast to our in vitro data using VEGFR inhibitors (Fig. 2). Significantly, however, the Flk-1−/− mutant embryos were about half the size of their E9.5 wild-type and heterozygous littermates (Fig. 3A) (Sandell et al., 2011). This raised the question of whether endothelial cells were necessary for lung specification, or whether these embryos were simply developmentally delayed due to the lack of the vascular system necessary for the delivery of sufficient oxygen and nutrients to the developing embryo. Since the lack of vascular development leads to embryonic death on day E10.5 in this and other Flk-1−/− mutant mouse strains (Sandell et al., 2011; Shalaby et al., 1995), we could not examine mutant embryos later in development for delayed lung specification. We therefore utilized the foregut explant system to determine if Flk-1−/− mutant embryos could initiate lung development in vitro. To do this we cultured foreguts from E8.5 embryos, when Flk-1−/− embryos were morphologically indistinguishable from wild-type and Flk-1+/− littermates (Fig. 4A, D). Importantly, E8.5 Flk-1−/− mutant embryos had no endothelial cells when the cultures were initiated, as determined by the lack of EMCN expression (Fig. 4E), whereas EMCN was robustly expressed in wild-type littermate controls (Fig. 4B). When we examined these cultures for specification of NKX2-1 positive lung progenitors we found that foreguts from both E8.5 wild-type and Flk-1−/− mutant embryos specified respiratory progenitors after two days of culture (Fig. 4C, F). Furthermore, the intensity of NKX2-1 expression and its distribution in the lung field of cultured E8.5 Flk-1−/− mutant foreguts (Fig. 4F) was no different from that of wild-type littermates (Fig. 4C). The lack of EMCN staining (Fig. 4F) and any detectable expression of Flk-1 or Pecam-1 by qPCR (Fig. 4I) confirmed the absence of endothelial cells in the mutant embryos. Notably, the regions of NKX2-1 expression at the distal end of the lung field in both control and mutant embryos appeared to have an emerging lung bud (Fig. 4C, F, arrows). Since NKX2-1 is also expressed in both the developing thyroid and forebrain, we wanted confirm that Flk-1−/−embryos were in fact inducing a lung bud. We therefore performed in situ hybridization on cultured foreguts for the lung-specific marker Sftpc, which is detectable as soon as lung buds emerge from the ventral foregut endoderm on E9.5 (Wert et al., 1993). We found discrete areas of Sftpc mRNA present in both wild-type and mutant embryo cultures (Fig. 4G, J), confirming the identity of the induced bud, and supporting the conclusion that endothelial cells are not required for the induction of the early lung bud. For further confirmation we bred a mouse strain bearing a transgene in which GFP expression is driven by the 3.7 kb human SFTPC promoter onto the Flk-1−/− mutant background; this allowed us to visualize the induction of bud morphogenesis in real time. In both wild-type and Flk-1−/− mutant foregut cultures we observed GFP expression localized to lung buds (Fig. 4H, K). Finally, when we measured levels of Sftpc expression by qPCR analysis, we found no significant difference in the amount of Sftpc mRNA in cultured wild-type versus Flk-1−/− mutant foreguts (Fig. 4L). Our observations in this series of experiments indicated that lung specification and bud initiation could occur in Flk-1−/− mutant foreguts lacking endothelial cells if cultures are initiated on E8.5, before the onset of growth delay or embryo death in vivo.
Fig. 4.
E8.5 Flk-1−/− mutants specify lung progenitors in vitro. Wild-type (A) and Flk-1−/− mutant (D) embryos are grossly indistinguishable at E8.5 (8–10ss). EMCN-positive endothelial cells are detectable in wild-type (B), but not Flk-1−/− mutant (E) embryos at E8.5. Immunofluorescent staining for NKX2-1 shows that after 2 days in culture, both wild-type (C) and Flk-1−/− (F) foreguts contain NKX2-1-positive progenitors in the lung field (arrows). qPCR analysis of Flk-1 and Pecam-1 expression in cultured explants (I) confirms the absence of endothelial cells in Flk-1−/− mutants, and shows no difference in the levels of Nkx2-1 mRNA expression between wild-type and Flk-1−/− mutants (**= p < 0.001, N ≥10 individual explants). The presence of a lung bud is demonstrated in both wild-type (G) and Flk-1−/− mutant (J) cultured foregut explants by whole-mount in situ hybridization for Sftpc mRNA; cultures were maintained for 5 days. Live confocal imaging demonstrates the presence of SFTPC/GFP expression in both wild-type (H) and Flk-1−/− (K) foregut explants. qPCR analysis shows no significant difference in Sftpc mRNA levels in wild-type verses Flk-1−/− foregut cultures (L; N ≥10 individual explants from at least 4 independent experiments). Th = Thyroid; Lg = Lung. Scale bars =100 µm.
2.4. Lung specification occurs in foreguts cultured in the absence of serum
Since we cultured foreguts in the presence of 20% FBS, we wished to determine what influence, if any, factors present in serum might have on the ability to induce lung formation in vitro. We tested this by using a defined culture medium in which we replaced FBS with KnockOut Serum Replacement (KOSR), a defined serum substitute developed for culturing stem cells. We observed NKX2-1 staining in both wild-type and Flk-1−/− mutant foregut explants cultured for up to five days in medium plus 20% KOSR (Fig. 5A, A’, B, B’). We additionally co-stained these cultures for PAX8, and again observed that NKX2-1 and PAX8 co-localized in a discrete subset of cells (Fig. 5A, A”, B, B”). Since we removed the forebrain from these explants prior to placing them in culture, the remaining NKX2-1-positive/PAX8-negative region caudal to the thyroid represented the developing lung and trachea. Considered together with our data using VEGFR inhibitors, our results demonstrate that endothelial cells are not necessary for respiratory cell specification or lung bud initiation in vitro.
Fig. 5.
Lung bud initiation occurs in serum-free culture. E8.5 wild-type and Flk-1−/− mutant foreguts were cultured in medium with 20% KnockOut Serum Replacement for 4–5 days. NKX2-1 staining is detected in the lung buds (arrowheads) of both wild-type (A, A’) and Flk-1−/− (B, B’) explants cultured in the KnockOut serum replacement. Endothelial cells are marked by PECAM-1 expression (A, B). NKX2-1-positive cells representing thyroid progenitor cells are distinguished by their co-staining with PAX8 (A, A”, B, B”). Since neural tissue was removed from the embryos prior to their being placed in culture, NKX2-1-positive, PAX8-negative cells represent lung progenitors. Scale bars =100 µm. N >5 individual explants from 2 independent experiments.
2.5. Embryos lacking endothelial cells initiate liver formation in vitro
As noted above, previous studies have indicated that endothelial cells are required for expansion of liver progenitors into the septum transversum (Matsumoto et al., 2001). When we examined E8.5 embryos for expression of HNF4α, which is required for hepatocyte differentiation and liver morphogenesis (Li et al., 2000; Parviz et al., 2003), we found that it was strongly expressed in the exposed ventral endoderm at the anterior intestinal portal (AIP), as well as in the heart, of both controls and Flk-1−/− mutants (Fig. 6A, B). At E9.5 progenitors were seen in the prospective liver field in both wild-type and Flk-1−/−mutants, but the field size was clearly smaller in the mutants (Fig. 6E, F). This was not surprising, given that mutant embyros are approximately half the size of wild-type littermates (Fig. 3A). These findings confirmed the previous observation (Matsumoto et al., 2001) that specification of liver progenitors does not require the presence of endothelial cells. We next cultured E8.5 wild-type and Flk-1−/− mutant foreguts to determine the extent of liver development when nutrition and oxygen availability were equivalent. In these cultures we assayed albumin (ALB) expression as a marker of liver development instead of HNF4α, which circumvented potentially confounding results due to the presence of the heart. We observed strong ALB expression in cultures of both wild-type and Flk-1−/− mutant foreguts, and the extent of expression appeared to be similar between both groups (Fig. 6I, J). We confirmed this by measuring the volume of ALB-positive tissue in confocal z-stacks, where we found no significant difference between wild-type and Flk-1−/− mutants (Fig. 6O). We also observed equivalent expression of LIV2, a marker of immature hepatocytes (Nierhoff et al., 2005; Watanabe et al., 2002) in cultured wild-type and Flk-1−/− mutant foreguts (Fig. 6M, N).
Fig. 6.
Cultured E8.5 Flk-1−/− mutant foreguts maintain liver, but not pancreas, progenitors. Immunostaining for HNF4α at E8.5 shows strong expression in the ventral endodermal lip of the anterior intestinal portal (AIP) in both wild-type (A) and Flk-1−/− mutant (B) embryos. HNF4α is also expressed in the heart (H). On E9.5 the liver field (LvP) is clearly demarcated by HNF4α staining (E, F, arrowheads). The size of the field in wild-type embryos (E) is noticeably larger than that of the mutants (F). Respiratory progenitors (arrows) are present in both wild-type and mutant embryos, but their number is greatly reduced in the mutants. After 5 days of culture, explants of wild-type (I, M) and Flk-1−/− mutant (J, N) foreguts show a similar expansion of liver precursors, which are identified by the expression of ALB (I, J) and LIV2 (M, N). Volumetric quantitation of cells expressing ALB in confocal z-stacks shows no statistical difference between wild-type and Flk-1−/− mutant explants (O; p > 0.05, N =11 (wild-type) and 8 (Flk-1−/− mutant)). Immunostaining of E9.5 wild-type embryos (C) for PDX1 shows strong expression in both the ventral (VP) and dorsal (DP) pancreatic rudiments. NKX2-1 staining identifies respiratory progenitors in the lung field (arrow). E9.5 Flk-1−/− mutant embryos stained for PDX1 also show expression in the pancreatic field (D); the number of NKX2-1 respiratory progenitors (arrow) is substantially less than that seen in wild-type embryos. Note that PDX1 expression in the mutant VP is equivalent to that seen in wild-type embryos, while PDX1 expression in the DP is considerably less than in littermate controls. On E10.5, both dorsal and ventral pancreatic buds have formed in wild-type embryos (G), but only a ventral pancreatic bud is seen in Flk-1−/− mutants (H). Note the complete loss of NKX2-1-positive respiratory progenitors in E10.5 mutants. Explants of E8.5 wild-type foreguts cultured for 5 days show maintenance of PDX1 expression (K), but PDX1 expression in cultured Flk-1−/− mutant explants was never observed (L). Scale bars: A, B, C, D, E, F, I, J, K, L, M, N =100 µm, G and H =300 µm.
2.6. Embryos lacking endothelial cells do not maintain pancreas formation in vitro
Examination of Flk-1−/− mutant embryos for expression of the pancreas marker PDX1 on E9.5 revealed the presence of PDX1-positive cells in both the ventral and dorsal pancreas regions (Fig. 6C, D). Although the boundaries of the dorsal and ventral pancreas fields appeared similar in wild-type and Flk-1−/− mutants, the intensity of PDX1 staining in the dorsal pancreas field of mutants was substantially less than controls (Fig. 6D). We observed no dorsal PDX1 staining in mutants on E10.5, although ventral PDX1 staining was still evident (Fig. 6H). These data confirm the results of earlier studies (Yoshitomi and Zaret, 2004). Since our results indicated that lung and liver development were compromised in Flk-1−/− mutants due to overall embryonic deterioration, we speculated that the lack of pancreas development might also be the result of a developmental delay or embryo deterioration. To assess this possibility, we examined PDX1 expression in cultured E8.5 wild-type and Flk-1−/− mutant foreguts. Whereas we found robust expression of PDX1 in wild-type foregut cultures (Fig. 6K), we saw no detectable expression in Flk-1−/− mutant cultures (Fig. 6L), confirming that endothelial cells are required for pancreatic development in vitro.
3. Discussion
Epithelial-mesenchymal crosstalk is essential for early lung orga-nogenesis (Masters, 1976; McCulley et al., 2015; Rudnick, 1933; Shannon, 1994). Lung mesenchyme is not a homogeneous tissue, but instead comprises many different cell types that exist in discrete niches (Kumar et al., 2014). Furthermore, the role of any given cell type in influencing lung development may also vary temporally. Because of this complexity, the definition of which cell types are critical for branching and differentiation of the lung epithelium has remained elusive. Several lines of investigation have suggested a role for endothelial cells in lung development. Embryonic lung explants treated with antisense oligodeoxynucleotides targeting Vegf (van Tuyl et al., 2005) or Flk-1 (Del Moral et al., 2006) showed decreased branching in culture. Transgenic embryos expressing a dominant-negative soluble VEGFR1 driven by the SFTPC promoter showed a normal rate of lung branching, but had disrupted stereotypy (Lazarus et al., 2011). Similarly, we have recently demonstrated that embryonic lung epithelium branches normally when associated with mesenchyme depleted of endothelial cells (Havrilak and Shannon, 2015b), demonstrating that the ability of lung epithelium to branch per se does not depend on endothelial cells. Our current observations confirm and extend these results.
Although endothelial cells are not required for early branching of the lung epithelium, epithelial-endothelial interactions are critical for later aspects of normal lung development. The differentiation of the gas exchanging alveoli in the lung occurs late in gestation and carries over into postnatal life (LeCras and Rabinovitch, 2016). Alveolarization requires a great expansion of the pulmonary vasculature and the intimate spatial association of epithelial and endothelial cells. It is therefore not surprising that later perturbations in vascular development, such as selective ablation of Vegfa (Yamamoto et al., 2007), loss of PECAM-1 function (DeLisser et al., 2006), or inhibition of VEGFR (Jakkula et al., 2000) significantly impair alveolus formation. Similarly, the alveologenesis that constitutes much of lung regeneration following partial pneumonectomy is mediated by MMP14 produced by activated endothelial cells in the remaining lung tissue (Ding et al., 2011). Additional insight into the mechanism by which endothelial cells affect alveolarization comes from recent work using distal lung stem cells (BASCs) co-cultured with endothelial cells. These studies have shown that a BMP4-regulated NFATc1-TSP1 axis in endothelial cells controls BASC differentiation into the alveolar cells (Lee et al., 2014).
Although there is much evidence detailing the involvement of endothelial cells in later stages of lung development, no previous information exists regarding the potential involvement of endothelial cells in specification of respiratory progenitors and lung bud initiation. Past studies, however, have implicated endothelial cells as playing a role in the early development of other endoderm-derived organs, such as the liver (Matsumoto et al., 2001) and pancreas (Lammert et al., 2003). We therefore investigated the role of endothelial cells during early lung specification and bud initiation.
As a first approach, we examined whether lung development proceeded in E8.5 foregut explants exposed to VEGFR inhibitors in a culture system that supports lung bud initiation (Desai et al., 2004). As a prelude to these experiments, however, we first needed to define precisely when we could detect NKX2-1, the earliest known lung epithelial marker, in the prospective respiratory field. Knowing this would allow us to conduct our experiments using embryos in which respiratory specification had not yet occurred. Using whole mount immunostaining and high-resolution confocal microscopy, we determined that NKX2-1 expressing cells emerged in the respiratory field between 14 and 16 somites (Fig. 1), a stage when embryo turning has just ended. This localization of NKX2-1 positive cells in the respiratory field at 16ss is earlier than what has previously been reported using whole mount in situ hybridization (Cardoso and Kotton, 2008; Kimura et al., 1996; Minoo et al., 1999). These data formed the basis of our decision to use foreguts from embryos no older than 12ss.
We found that inhibiting VEGF signaling, and hence endothelial cell survival and proliferation, had no effect on specification of the lung field as gauged by the expression of NKX2-1 in a subset of endodermal cells that were not thyroid, which could be distinguished by its co-expression of PAX8 (Fig. 2). These data clearly suggested that endothelial cells were dispensable for lung specification. A caveat in these experiments, however, is that the embryos contained endothelial cells in the period between gastrulation and the initiation of culture on E8.5; it is therefore possible that critical endothelial-endodermal crosstalk could have occurred during this period. A second consideration is that the small molecule inhibitors may not have been 100% effective in eliminating endothelial cells. Finally, these reagents might also have potential off-target effects. To circumvent these limitations, we designed experiments using embryos in which endothelial cells had been genetically ablated.
In a previous study, Sandell et al. (2011) used a phenotype-driven ENU mutagenesis screen to identify new genes critical for craniofacial development. One of the mutants generated, named orvieto, showed a total lack of vascular development, which was subsequently determined to be due to a point mutation in the Flk-1 gene that generated a premature STOP codon. Most of these embryos die by E10.5, a day after lung bud initiation occurs. When we examined mutant embryos for NKX2-1 expressing cells in the area of the ventral foregut endoderm where the lung normally arises, we observed only a few on E9.5 and none on E10.5 (Figs. 3 and 6). These observations were in direct contrast to our experiments using VEGFR inhibitors, apparently supporting the possibility that endothelial cell-endoderm interactions are critical for lung specification. These interactions would necessarily occur prior to 12ss, since inhibiting VEGF signaling after that point had no effect on respiratory specification (Fig. 2). Tempering this interpretation, however, was the fact that the Flk-1−/− mutant embryos were substantially smaller than their wild type littermates, being approximately one-half their size on E9.5, a size discrepancy that was even more pronounced on E10.5 (Fig. 3A, B). We could not discount the possibility that the compromised overall growth and development of the mutant embryos had a significant impact on lung organogenesis. If true, this would suggest that the requirement for endothelial cells in lung development is indirect.
To test the possibility that E9.5–10.5 mutant embryos were developmentally compromised due to yolk sac defects, we cultured foreguts from Flk-1−/− mutant embryos on E8.5, when there was no size differential and the embryos appeared outwardly normal (Fig. 4A, D). Importantly, we found that mutant embryos in these cultures specified a lung field, with the location and intensity of NKX2-1 staining equivalent to that seen in wild-type controls. Unlike our culture experiments using VEGFR inhibitors, the endoderm in these experiments was never exposed to endothelial cells, yet they still initiated lung development, including the formation of lung buds that expressed Sftpc. These data are consistent with our results using VEGFR inhibitors, and demonstrate that endothelial cells are not required for respiratory specification and lung bud initiation. While we often observed lung bud formation in foregut explants, the extent of branching morphogenesis we observed was limited, as has been reported by other investigators (Chen et al., 2007, 2010; Desai et al., 2004). This may be due to the fact that the foregut explants spread out on the filter, which could disrupt spatial arrangements necessary for further branching morphogenesis.
We believe the reason that our Flk-1−/− mutant embryos showed few lung progenitors on E9.5 and none on E10.5 was insufficient vascularization of the yolk sac (Ren et al., 2014). This would result in an inadequate supply of blood, oxygen, nutrients and other factors to the embryo, leading to the delay or cessation of normal developmental processes. The yolk sac is essential for the survival of the embryo, as it breaks down and transfers maternally derived molecules to the embryo proper, synthesizes serum proteins, and removes waste products (reviewed in Garcia and Larina (2014), Zohn and Sarkar (2010)). These processes are taken over later in development by the definitive embryo, specifically by the endodermal gut and liver. Flk-1 positive cells are first seen associated with blood islands in the proximal extraembryonic yolk sac on E7.5 (Shalaby et al., 1995; Yamaguchi et al., 1993), after which they proliferate, differentiate, and migrate distally to form a simple plexus that encompasses the entire yolk sac on E8.5 (Coultas et al., 2005; Drake and Fleming, 2000). This primitive plexus then remodels via angiogenesis, increasing the number and size of the vessels to meet the demands of the growing embryo and expanding yolk sac (Udan et al., 2013). Not surprisingly, yolk sac defects result in perturbed embryo growth and development, and numerous factors known to affect vascular development have been shown to be critical for yolk sac angiogenesis and the formation of a functional yolk sac. These include Flk-1 (Shalaby et al., 1995), Flt-1 (Fong et al., 1995), Vegf (Carmeliet et al., 1996), Ang-1 (Suri et al., 1996), Tie-1 (Puri et al., 1995; Sato et al., 1995), Tie-2 (Dumont et al., 1994; Sato et al., 1995), Np1/Np2 (Takashima et al., 2002), Etv6 (Wang et al., 1997) Ihh and Smo (Byrd et al., 2002) and Tgfbr2 (Oshima et al., 1996). Embryos in all of these models exhibit severe growth defects leading to mortality by E10.5, and the authors often suggest this is likely due to secondary effects of deficient yolk sac vascularization. This is consistent with our view that the requirement for endothelial cells in lung development is indirect, and that the defects in lung development and overall growth we observed in Flk-1−/−mutants are secondary to deficits in yolk sac vascularization. While endothelial cells themselves do not provide signals required for lung specification, they are necessary to form the vessels in the developing yolk sac that function to both supply the embryo with blood, oxygen, and nutrients as well as remove waste products. All of these factors are essential for embryonic development and survival.
One possible concern in our studies using explanted E8.5 foreguts was that they were conducted in medium containing 20% FBS. Since FBS contains significant levels of growth factors and hormones, one could argue that these may be compensating for the lack of endothelial cells and allowing the Flk-1−/− endoderm to specify and induce a lung. Indeed, Zheng et al. (2006) conducted a proteomic analysis on FBS and found it contained some growth factors that are known to have roles during lung development, such as FGF2 (Lebeche et al., 1999; Matsui et al., 1999), TGFβ (Alejandre-Alcázar et al., 2008; Bartram and Speer, 2004) and IGF (Epaud et al., 2012; Silva et al., 2006). We therefore examined lung specification in Flk-1−/− mutant foreguts cultured in a medium containing KnockOut Serum Replacement (KOSR), which is a defined, serum-free growth supplement commonly used to replace FBS in cultured embryonic stem cells. KOSR contains a mixture of amino acids, vitamins, and proteins such as transferrin, albumin, and insulin (Garcia-Gonzalo and Izpisúa Belmonte, 2008). Importantly, KOSR contains no growth factors known to be critical for specification of the lung field and early bud development. Therefore any signals affecting lung specification and bud initiation originate within the embryonic tissue itself. We found that Flk-1−/− mutant foreguts cultured with KOSR specified a respiratory field that was able to produce lung buds, the earliest morphological manifestation of lung organogenesis. These data demonstrated that the FBS did not compensate for the absence of endothelial cells by providing inductive factors to the endoderm, and underscores our belief that the inductive cues originate within the embryo proper in the absence of endothelial cells.
Our data indicate that endothelial cells are not required for specification of the respiratory field and early lung development. However, previous studies have concluded that endothelial cells are necessary for the early development of other endoderm-derived organs, such as the liver (Matsumoto et al., 2001) and pancreas (Lammert et al., 2001; Ranjan et al., 2009). We therefore examined early hepatic and pancreatic gene expression in intact and cultured Flk-1−/− mutant embryos. We saw no difference in expression of the liver marker HNF4α between wild-type and Flk-1−/− embryos on E8.5, which was in keeping with the observations of Matsumoto et al. (2001). They found that the hepatic endoderm thickened normally in Flk-1−/− mutants on E9, and that expression of the early hepatic genes Alb, Hex, and Ttr appeared normal, leading them to conclude that normal specification had occurred. They also observed, however, that subsequent delamination and migration of liver cells into the septum transversum on E9.5 did not occur. Furthermore, explant cultures of E9.5 wild-type and Flk-1−/− liver buds showed only limited expansion of Alb positive cells in the mutant cultures, even though the relative expansion of tissue area was the same in the two groups. Our in vitro results stand in contrast to theirs, as we observed no apparent differences in the expression of ALB and LIV2 in cultured whole E8.5 foreguts. We confirmed the equivalence of liver expansion by quantitating the volume of ALB-positive cells in the liver field of cultured foreguts (Fig. 6). One explanation for the discrepancy between our results and those of Matsumoto et al. is that they cultured only the liver bud from E9.5 embryos, whereas we cultured entire E8.5 foreguts. It is therefore possible that factors required for liver cell migration are being produced by another, non-endothelial cell type outside the liver field in our cultures. We believe a more likely possibility, however, is that the entire Flk-1−/− mutant embryo is compromised by E9.5, as evidenced by its significantly reduced size (Fig. 3), and that the number of liver progenitors is reduced in mutants on E9.5. Indeed, our data show that the size of the prospective liver field is noticeably reduced in E9.5 Flk-1−/− mutants (Fig. 6E, F). This observation is consonant with our observations in the lung, where lung progenitors are seen in E9.5 Flk-1−/− mutants, but are far fewer in number than in their wild-type littermates (Fig. 3); furthermore, these lung progenitors are completely lost as embryo viability deteriorates further over the next 24 h (Fig. 6H). Proliferation and differentiation of lung progenitors occurred equally well when we explanted E8.5 Flk-1−/− and wild-type foreguts, however, which suggests that the size of the cohort of cells giving rise to lung rudiments is likely the same at that time point, when no evidence of embryo deterioration is apparent. Our data indicate that a similar situation exists for the liver: that is, the number of liver progenitors is the same in wild-type and Flk-1−/− embryos on E8.5, but some are lost over the next 24 h in the mutants, when a lack of the yolk sac vasculature necessary to support the growing embryo leads to developmental arrest and eventual embryonic death. Placing mutant foreguts in culture on E8.5 circumvents the deficiencies created by a defective yolk sac, which allows liver development to proceed equally in both wild-type and mutants. Finally, our conclusion that specification and early expansion of liver progenitors does not require endothelial cells is consistent with the observations of Field et al. (2003), who demonstrated that zebrafish lacking the clo gene, which are avascular due to disrupted Flk-1−/−expression (Liao et al., 1997), exhibit normal liver budding and differentiation.
A previous study has shown that although initial induction of PDX1 in the dorsal pancreas occurs in the absence of endothelial cells, maintenance of PDX1 expression and subsequent induction of the critical pancreas transcription factor Ptf1a in the dorsal endoderm requires the presence of endothelial cells, specifically those of the dorsal aorta (Yoshitomi and Zaret, 2004). Consistent with these data, we found that Flk-1−/− mutants showed PDX1 expression in both the ventral and dorsal endoderm on E9.5, but only ventral expression persisted to E10.5 (Fig. 6H). When we examined PDX1 expression in cultured E8.5 Flk-1−/− mutant foreguts, however, we found that no rescue of pancreas development occurred. These results stood in sharp contrast to our observations on lung and liver, and support the conclusion that endothelial cells play a critical role(s) in pancreas development.
The effects of endothelial cells on pancreas development are diverse. Beyond their role in initial pancreatic specification (Lammert et al., 2001; Yoshitomi and Zaret, 2004), endothelial cells also influence pancreatic development by supporting critical Isl-1-positive mesenchymal cells (Ahlgren et al., 1997; Jacquemin et al., 2006). Identification of the signals that endothelial cells are providing to prospective pancreatic endoderm has been elusive. The development of embryonic stem cell (ESC) and induced pluripotent stem cell (iPSC) strategies for producing functional cells from many tissues (Huang et al., 2014; Kurmann et al., 2015; Longmire et al., 2012; McCracken et al., 2014; Spence et al., 2011; Takebe et al., 2013), including the pancreas (for reviews see Grapin-Botton (2016), Schiesser and Wells (2014)), has provided new opportunities for elucidating both the factors required for specification and the temporal sequence in which they act. For example, Kao et al. have recently used co-cultures of pancreatic progenitors derived from human ESCs plus endothelial cells to show that EGFL7, which is produced and secreted by endothelial cells, promotes proliferation of pancreatic progenitors while suppressing their differentiation into endocrine cells (Kao et al., 2015).
In summary, we have demonstrated that both specification of respiratory progenitors and subsequent lung bud initiation occur in the absence of endothelial cells in vitro. We have found that the nascent liver behaves much like the lung, with its specification and early expansion being independent of endothelial cells. Finally, we have also confirmed the critical need for endothelial cells in early pancreatic organogenesis.
4. Materials and methods
4.1. Transgenic mice
All animal procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Children's Hospital Research Foundation. SFTPC/GFP mice, obtained from Dr. Brigid Hogan (Duke University Medical Center), were originally generated by John Heath (Lo et al., 2008). Wild-type FVB/ N mice were purchased from Harlan Laboratories. Flk-1−/− mutant mice were originally generated by Sandell et al. (orvieto mice, (Sandell et al., 2011)). These mice contain a C > A mutation at bp 1311 of the Flk-1 coding sequence, which results in translation change of TYR to STOP. Since heterozygous Flk-1+/− mutant embryos and their wild-type littermates exhibited a normal phenotype, both heterozygous and wild-type embryos were used as controls. Males were housed with females overnight; females positive for a copulating plug were considered at 0.5 days of gestation.
4.2. Foregut culture
Timed pregnant mice were sacrificed on gestational day E8.5, and individual embryos were staged according to somite number (ss). Experiments were performed using embryos between 4–12ss. For E8.5 foregut cultures, the neural headfolds and forebrain were usually removed, as well as tissue caudal to the anterior intestinal portal (AIP). For foregut cultures in which liver and pancreas formation were studied, embryos were cut 4 somites caudal to the AIP to ensure that dorsal pancreas progenitors were captured within the region being cultured (Angelo et al., 2012). Regions containing the foregut were dissected in Hank's balanced salt solution (HBSS), explanted onto 8 µm pore Whatman Nucleopore Track-Etch Membranes (Millipore) or Transwell Collagen Filters (Costar) and cultured for 2–5 days in DMEM (Gibco) +20% fetal bovine serum (FBS; Sigma). In some experiments 20% FBS was replaced with 20% KnockOut Serum Replacement (KOSR; Gibco). The VEGF receptor inhibitors Ki8751 and SU5416 (both from Calbiochem) were used at 10 µM. These compounds have been shown to effectively inhibit VEGF signaling at this concentration (Fong et al., 1999; Havrilak and Shannon, 2015b; Kubo et al., 2005). In all experiments DMSO served as a vehicle control.
4.3. Quantitative real-time PCR (qPCR)
Total RNA was isolated using RNeasy Plus Micro kit (Qiagen) followed by reverse transcription using iScript cDNA synthesis kit (BioRad). A 7300 Real-Time PCR system or StepOnePlus Real-Time PCR system were used for qPCR reactions and relative quantifications, with TaqMan primer/probes (Applied Biosystems). The Taqman primers used were: Flk-1 (Mm00440111_m1), Nkx2.1 (Mm00447558_m1), Pecam-1 (Mm00476702_m1), Rn18s (Mm03928990_g1) and Sftpc (Mm00488144_m1).
4.4. Whole-mount immunohistochemistry
Whole-mount immunohistochemistry was performed using a modification of the protocol from Ahnfelt-Rønne et al. (2007) as previously described (Havrilak and Shannon, 2015b). Optical sections were captured on a Nikon AiRsi inverted laser microscope, and 3D images were created by a composite of z-stacks using Bitplane Imaris software to process the fluorescent images. To calculate the volume of tissue stained for ALB, we created isosurfaces using smoothing thresholds and intensity values that allowed filtering out antibody aggregates or any non-specific staining.
The primary antibodies used were: rabbit anti-NKX2-1 (1:3000, Seven Hills Bioreagents), guinea pig anti-NKX2-1 (1:500, Seven Hills Bioreagents), goat anti-endomucin (1:500, R & D Systems), rat anti-E-cadherin (1:2000, R & D Systems), rabbit anti-PAX8 (1:500, Protein Tech), rat anti-PECAM-1 (1:500, BD Pharmigen), goat anti-HNF4α (1:200, Santa Cruz), goat anti-PDX1 (1:5000, Abcam), goat anti-ALB (1:1000, Bethyl Laboratories), goat anti-FOXA2 (1:500, Santa Cruz), and rat anti-LIV2 (1:200, MBL). Secondary antibodies used were: Alexa Fluor 647 donkey anti-rabbit IgG, Alexa Fluor 568 anti-goat IgG, Alexa Fluor 488 donkey anti-rat IgG, Alexa Fluor 488 donkey antimouse IgG (1:500, all from Life Technologies).
4.5. Whole-mount in situ hybridization
Samples were fixed in 4% paraformaldehyde at 4 °C, followed by serial dehydration to 100% methanol. Mouse Sftpc cDNA cloned into the vector pGEM7b was used to prepare digoxigenin-labeled RNA probes. Whole-mount in situ hybridization was done according to Wilkinson (1992) with slight modifications as previously described (Hyatt et al., 2004).
Acknowledgments
The authors thank Xiaofei Shangguan and Kalpana Srivastava for their excellent technical assistance, and Dr. Matthew Kofron for assistance with confocal microscopy. We also thank Dr. Brigid Hogan for the SFTPC/GFP animals. This work was supported by the National Heart, Lung, and Blood Institute (grant HL098319 to J.M. Shannon).
References
- Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- Ahnfelt-Rønne J, Jørgensen MC, Hald J, Madsen OD, Serup P, Hecksher-Sørensen J. An improved method for three-dimensional reconstruction of protein expression patterns in intact mouse and chicken embryos and organs. J. Histochem. Cytochem. 2007;55:925–930. doi: 10.1369/jhc.7A7226.2007. [DOI] [PubMed] [Google Scholar]
- Alejandre-Alcázar MA, Michiels-Corsten M, Vicencio AG, Reiss I, Ryu J, de Krijger RR, Haddad GG, Tibboel D, Seeger W, Eickelberg O, Morty RE. TGF-beta signaling is dynamically regulated during the alveolarization of rodent and human lungs. Dev. Dyn. 2008;237:259–269. doi: 10.1002/dvdy.21403. [DOI] [PubMed] [Google Scholar]
- Angelo JR, Guerrero-Zayas MI, Tremblay KD. A fate map of the murine pancreas buds reveals a multipotent ventral foregut organ progenitor. PLoS One. 2012;7:e40707. doi: 10.1371/journal.pone.0040707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest. 2004;125:754–765. doi: 10.1378/chest.125.2.754. [DOI] [PubMed] [Google Scholar]
- Brachtendorf G, Kuhn A, Samulowitz U, Knorr R, Gustafsson E, Potocnik AJ, Fässler R, Vestweber D. Early expression of endomucin on endothelium of the mouse embryo and on putative hematopoietic clusters in the dorsal aorta. Dev. Dyn. 2001;222:410–419. doi: 10.1002/dvdy.1199. [DOI] [PubMed] [Google Scholar]
- Byrd N, Becker S, Maye P, Narasimhaiah R, St-Jacques B, Zhang X, McMahon J, McMahon A, Grabel L. Hedgehog is required for murine yolk sac angiogenesis. Development. 2002;129:361–372. doi: 10.1242/dev.129.2.361. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Kotton DN. Specification and patterning of the respiratory system. StemBook. 2008 [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Chen F, Cao Y, Qian J, Shao F, Niederreither K, Cardoso WV. A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium. J. Clin. Investig. 2010;120:2040–2048. doi: 10.1172/JCI40253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Desai TJ, Qian J, Niederreither K, Lü J, Cardoso WV. Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development. 2007;134:2969–2979. doi: 10.1242/dev.006221. [DOI] [PubMed] [Google Scholar]
- Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- Del Moral PM, Sala FG, Tefft D, Shi W, Keshet E, Bellusci S, Warburton D. VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev. Biol. 2006;290:177–188. doi: 10.1016/j.ydbio.2005.11.022. [DOI] [PubMed] [Google Scholar]
- DeLisser HM, Helmke BP, Cao G, Egan PM, Taichman D, Fehrenbach M, Zaman A, Cui Z, Mohan GS, Baldwin HS, Davies PF, Savani RC. Loss of PECAM-1 function impairs alveolarization. J. Biol. Chem. 2006;281:8724–8731. doi: 10.1074/jbc.M511798200. [DOI] [PubMed] [Google Scholar]
- Desai TJ, Malpel S, Flentke GR, Smith SM, Cardoso WV. Retinoic acid selectively regulates Fgf10 expression and maintains cell identity in the prospective lung field of the developing foregut. Dev. Biol. 2004;273:402–415. doi: 10.1016/j.ydbio.2004.04.039. [DOI] [PubMed] [Google Scholar]
- Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, Crystal RG, Simons M, Sato TN, Worgall S, Shido K, Rabbany SY, Rafii S. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood. 2000;95:1671–1679. [PubMed] [Google Scholar]
- Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Epaud R, Aubey F, Xu J, Chaker Z, Clemessy M, Dautin A, Ahamed K, Bonora M, Hoyeau N, Fléjou JF, Mailleux A, Clement A, Henrion-Caude A, Holzenberger M. Knockout of insulin-like growth factor-1 receptor impairs distal lung morphogenesis. PLoS One. 2012;7:e48071. doi: 10.1371/journal.pone.0048071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Field HA, Ober EA, Roeser T, Stainier DY. Formation of the digestive system in zebrafish. I. Liver morphogenesis. Dev. Biol. 2003;253:279–290. doi: 10.1016/s0012-1606(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Fong G-H, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/ KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99–106. [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, Izpisúa Belmonte JC. Albumin-associated lipids regulate human embryonic stem cell self-renewal. PLoS One. 2008;3:e1384. doi: 10.1371/journal.pone.0001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MD, Larina IV. Vascular development and hemodynamic force in the mouse yolk sac. Front. Physiol. 2014;5:308. doi: 10.3389/fphys.2014.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebb SA, Shannon JM. Tissue interactions mediate early events in pulmonary vasculogenesis. Dev. Dyn. 2000;217:159–169. doi: 10.1002/(SICI)1097-0177(200002)217:2<159::AID-DVDY3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Grapin-Botton A. Three-dimensional pancreas organogenesis models. Diabetes Obes. Metab. 2016;18(Suppl. 1):S33–S40. doi: 10.1111/dom.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havrilak JA, Shannon JM. Lung Development. John Wiley & Sons; Chichester: 2015a. [Google Scholar]
- Havrilak JA, Shannon JM. Branching of lung epithelium in vitro occurs in the absence of endothelial cells. Dev. Dyn. 2015b;244:553–563. doi: 10.1002/dvdy.24251. [DOI] [PubMed] [Google Scholar]
- Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141:502–513. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines EA, Sun X. Tissue crosstalk in lung development. J. Cell Biochem. 2014;115:1469–1477. doi: 10.1002/jcb.24811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, Snoeck HW. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 2014;32:84–91. doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt BA, Shangguan X, Shannon JM. FGF-10 induces SP-C and Bmp4 and regulates proximal-distal patterning in embryonic tracheal epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L1116–L1126. doi: 10.1152/ajplung.00033.2004. [DOI] [PubMed] [Google Scholar]
- Jacquemin P, Yoshitomi H, Kashima Y, Rousseau GG, Lemaigre FP, Zaret KS. An endothelial-mesenchymal relay pathway regulates early phases of pancreas development. Dev. Biol. 2006;290:189–199. doi: 10.1016/j.ydbio.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- Kao DI, Lacko LA, Ding BS, Huang C, Phung K, Gu G, Rafii S, Stuhlmann H, Chen S. Endothelial cells control pancreatic cell fate at defined stages through EGFL7 signaling. Stem Cell Rep. 2015;4:181–189. doi: 10.1016/j.stemcr.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- Kubo K, Shimizu T, Ohyama S, Murooka H, Iwai A, Nakamura K, Hasegawa K, Kobayashi Y, Takahashi N, Takahashi K, Kato S, Izawa T, Isoe T. Novel potent orally active selective VEGFR-2 tyrosine kinase inhibitors: synthesis, structure-activity relationships, and antitumor activities of N-phenyl-N’-{4-(4-quinolyloxy)phenyl}ureas. J. Med. Chem. 2005;48:1359–1366. doi: 10.1021/jm030427r. [DOI] [PubMed] [Google Scholar]
- Kumar ME, Bogard PE, Espinoza FH, Menke DB, Kingsley DM, Krasnow MA. Mesenchymal cells. Defining a mesenchymal progenitor niche at single-cell resolution. Science. 2014;346:1258810. doi: 10.1126/science.1258810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurmann AA, Serra M, Hawkins F, Rankin SA, Mori M, Astapova I, Ullas S, Lin S, Bilodeau M, Rossant J, Jean JC, Ikonomou L, Deterding RR, Shannon JM, Zorn AM, Hollenberg AN, Kotton DN. Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells. Cell Stem Cell. 2015;17:527–542. doi: 10.1016/j.stem.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Role of endothelial cells in early pancreas and liver development. Mech. Dev. 2003;120:59–64. doi: 10.1016/s0925-4773(02)00332-5. [DOI] [PubMed] [Google Scholar]
- Lazarus A, Del-Moral PM, Ilovich O, Mishani E, Warburton D, Keshet E. A perfusion-independent role of blood vessels in determining branching stereotypy of lung airways. Development. 2011;138:2359–2368. doi: 10.1242/dev.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeche D, Malpel S, Cardoso WV. Fibroblast growth factor interactions in the developing lung. Mech. Dev. 1999;86:125–136. doi: 10.1016/s0925-4773(99)00124-0. [DOI] [PubMed] [Google Scholar]
- LeCras TD, Rabinovitch M. Pulmonary vascular development. In: Jobe AH, Whitsett JA, Abman SH, editors. Fetal and Neonatal Lung Development: Clinical Correlates and Technologies for the Future. Cambridge University Press; New York: 2016. pp. 34–57. [Google Scholar]
- Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S, Kim CF. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ning G, Duncan SA. Mammalian hepatocyte differentiation requires the transcription factor HNF-4alpha. Genes Dev. 2000;14:464–474. [PMC free article] [PubMed] [Google Scholar]
- Liao W, Bisgrove BW, Sawyer H, Hug B, Bell B, Peters K, Grunwald DJ, Stainier DY. The zebrafish gene cloche acts upstream of a flk-1 homologue to regulate endothelial cell differentiation. Development. 1997;124:381–389. doi: 10.1242/dev.124.2.381. [DOI] [PubMed] [Google Scholar]
- Lo B, Hansen S, Evans K, Heath JK, Wright JR. Alveolar epithelial type II cells induce T cell tolerance to specific antigen. J. Immunol. 2008;180:881–888. doi: 10.4049/jimmunol.180.2.881. [DOI] [PubMed] [Google Scholar]
- Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, Kwok LW, Mou H, Rajagopal J, Shen SS, Dowton AA, Serra M, Weiss DJ, Green MD, Snoeck HW, Ramirez MI, Kotton DN. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters JRW. Epithelial-mesenchymal interaction during lung development: the effect of mesenchymal mass. Dev. Biol. 1976;51:98–108. doi: 10.1016/0012-1606(76)90125-1. [DOI] [PubMed] [Google Scholar]
- Matsui R, Brody JS, Yu Q. FGF-2 induces surfactant protein gene expression in foetal rat lung epithelial cells through a MAPK-independent pathway. Cell Signal. 1999;11:221–228. doi: 10.1016/s0898-6568(98)00070-9. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- McCracken KW, Catá EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai YH, Mayhew CN, Spence JR, Zavros Y, Wells JM. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:400–404. doi: 10.1038/nature13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulley D, Wienhold M, Sun X. The pulmonary mesenchyme directs lung development. Curr. Opin. Genet. Dev. 2015;32:98–105. doi: 10.1016/j.gde.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453:745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(−/−) mouse embryos. Dev. Biol. 1999;209:60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- Nierhoff D, Ogawa A, Oertel M, Chen YQ, Shafritz DA. Purification and characterization of mouse fetal liver epithelial cells with high in vivo repopulation capacity. Hepatology. 2005;42:130–139. doi: 10.1002/hep.20735. [DOI] [PubMed] [Google Scholar]
- Oshima M, Oshima H, Taketo MM. TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev. Biol. 1996;179:297–302. doi: 10.1006/dbio.1996.0259. [DOI] [PubMed] [Google Scholar]
- Parviz F, Matullo C, Garrison WD, Savatski L, Adamson JW, Ning G, Kaestner KH, Rossi JM, Zaret KS, Duncan SA. Hepatocyte nuclear factor 4alpha controls the development of a hepatic epithelium and liver morphogenesis. Nat. Genet. 2003;34:292–296. doi: 10.1038/ng1175. [DOI] [PubMed] [Google Scholar]
- Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 1995;14:5884–5891. doi: 10.1002/j.1460-2075.1995.tb00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan AK, Joglekar MV, Hardikar AA. Endothelial cells in pancreatic islet development and function. Islets. 2009;1:2–9. doi: 10.4161/isl.1.1.9054. [DOI] [PubMed] [Google Scholar]
- Rankin SA, Han L, McCracken KW, Kenny AP, Anglin CT, Grigg EA, Crawford CM, Wells JM, Shannon JM, Zorn AM. A retinoic acid-hedgehog cascade coordinates mesoderm-inducing signals and endoderm competence during lung specification. Cell Rep. 2016;16:66–78. doi: 10.1016/j.celrep.2016.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin SA, Zorn AM. Gene regulatory networks governing lung specification. J. Cell Biochem. 2014;115:1343–1350. doi: 10.1002/jcb.24810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Ustiyan V, Pradhan A, Cai Y, Havrilak JA, Bolte CS, Shannon JM, Kalin TV, Kalinichenko VV. FOXF1 transcription factor is required for formation of embryonic vasculature by regulating VEGF signaling in endothelial cells. Circ. Res. 2014;115:709–720. doi: 10.1161/CIRCRESAHA.115.304382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick D. Developmental capacities of the chick lung in chorioallantoic grafts. J. Exp. Zool. 1933;66:125–154. [Google Scholar]
- Sandell LL, Iulianella A, Melton KR, Lynn M, Walker M, Inman KE, Bhatt S, Leroux-Berger M, Crawford M, Jones NC, Dennis JF, Trainor PA. A phenotype-driven ENU mutagenesis screen identifies novel alleles with functional roles in early mouse craniofacial development. Genesis. 2011;49:342–359. doi: 10.1002/dvg.20727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Schachtner SK, Wang Y, Scott Baldwin H. Qualitative and quantitative analysis of embryonic pulmonary vessel formation. Am. J. Respir. Cell Mol. Biol. 2000;22:157–165. doi: 10.1165/ajrcmb.22.2.3766. [DOI] [PubMed] [Google Scholar]
- Schiesser JV, Wells JM. Generation of β cells from human pluripotent stem cells: are we there yet. Ann. N. Y. Acad. Sci. 2014;1311:124–137. doi: 10.1111/nyas.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132:35–47. doi: 10.1242/dev.01570. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu X-F, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev. Dyn. 1998;212:482–494. doi: 10.1002/(SICI)1097-0177(199808)212:4<482::AID-AJA2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Shannon JM. Induction of alveolar type II cell differentiation in fetal tracheal epithelium by grafted distal lung mesenchyme. Dev. Biol. 1994;166:600–614. doi: 10.1006/dbio.1994.1340. [DOI] [PubMed] [Google Scholar]
- Silva D, Venihaki M, Guo WH, Lopez MF. Igf2 deficiency results in delayed lung development at the end of gestation. Endocrinology. 2006;147:5584–5591. doi: 10.1210/en.2006-0498. [DOI] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki Ji J, Hirota S, Kitamura Y, Kitsukawa T, Fujisawa H, Klagsbrun M, Hori M. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc. Natl. Acad. Sci. USA. 2002;99:3657–3662. doi: 10.1073/pnas.022017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- Udan RS, Culver JC, Dickinson ME. Understanding vascular development. Wiley Interdiscip. Rev. Dev. Biol. 2013;2:327–346. doi: 10.1002/wdev.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tuyl M, Liu J, Wang J, Kuliszewski M, Tibboel D, Post M. Role of oxygen and vascular development in epithelial branching morphogenesis of the developing mouse lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;288:L167–L178. doi: 10.1152/ajplung.00185.2004. [DOI] [PubMed] [Google Scholar]
- Wang LC, Kuo F, Fujiwara Y, Gilliland DG, Golub TR, Orkin SH. Yolk sac angiogenic defect and intra-embryonic apoptosis in mice lacking the Ets-related factor TEL. EMBO J. 1997;16:4374–4383. doi: 10.1093/emboj/16.14.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Nakagawa K, Ohata S, Kitagawa D, Nishitai G, Seo J, Tanemura S, Shimizu N, Kishimoto H, Wada T, Aoki J, Arai H, Iwatsubo T, Mochita M, Watanabe T, Satake M, Ito Y, Matsuyama T, Mak TW, Penninger JM, Nishina H, Katada T. SEK1/MKK4-mediated SAPK/JNK signaling participates in embryonic hepatoblast proliferation via a pathway different from NF-kappaB-induced anti-apoptosis. Dev. Biol. 2002;250:332–347. [PubMed] [Google Scholar]
- Weaver M, Batts L, Hogan BL. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev. Biol. 2003;258:169–184. doi: 10.1016/s0012-1606(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Wert SE, Glasser SW, Korfhagen TR, Whitsett JA. Transcriptional elements from the human SP-C gene direct expression in the primordial respiratory epithelium of transgenic mice. Dev. Biol. 1993;156:426–443. doi: 10.1006/dbio.1993.1090. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Whole mount in situ hybridization of vertebrate embryos. In: Wilkinson DG, editor. In Situ Hybridization, A Practical Approach. IRL Press; New York: 1992. pp. 75–83. [Google Scholar]
- Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Yun EJ, Gerber HP, Ferrara N, Whitsett JA, Vu TH. Epithelial-vascular cross talk mediated by VEGF-A and HGF signaling directs primary septae formation during distal lung morphogenesis. Dev. Biol. 2007;308:44–53. doi: 10.1016/j.ydbio.2007.04.042. [DOI] [PubMed] [Google Scholar]
- Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;131:807–817. doi: 10.1242/dev.00960. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wang K, Ferrara N, Vu TH. Vascular endothelial growth factor coordinates proper development of lung epithelium and vasculature. Mech. Dev. 2005;122:877–886. doi: 10.1016/j.mod.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Zheng X, Baker H, Hancock WS, Fawaz F, McCaman M, Pungor E. Proteomic analysis for the assessment of different lots of fetal bovine serum as a raw material for cell culture. Part IV. Application of proteomics to the manufacture of biological drugs. Biotechnol. Prog. 2006;22:1294–1300. doi: 10.1021/bp060121o. [DOI] [PubMed] [Google Scholar]
- Zohn IE, Sarkar AA. The visceral yolk sac endoderm provides for absorption of nutrients to the embryo during neurulation. Birth Defects Res. A Clin. Mol. Teratol. 2010;88:593–600. doi: 10.1002/bdra.20705. [DOI] [PubMed] [Google Scholar]