Abstract
Background
To explore the relationship between binge eating disorder (BED) and obesity in patients with bipolar disorder (BP).
Methods
717 patients participating in the Mayo Clinic Bipolar Biobank completed structured diagnostic interviews and questionnaires for demographic and illness-related variables. They also had weight and height measured to determine body mass index (BMI). The effects of BED and obesity (BMI≥30 kg/m2), as well as their interaction, were assessed on one measure of general medical burden and six proxies of psychiatric illness burden.
Results
9.5% of patients received a clinical diagnosis of BED and 42.8% were obese. BED was associated with a significantly elevated BMI. Both BED and obesity were associated with greater psychiatric and general illness burden, but illness burden profiles differed. After controlling for obesity, BED was associated with suicidality, psychosis, mood instability, anxiety disorder comorbidity, and substance abuse comorbidity. After controlling for BED status, obesity was associated with greater general medical comorbidity, but lower substance abuse comorbidity. There were no significant interaction effects between obesity and BED, or BMI and BED, on any illness burden outcome.
Limitations
There may have been insufficient power to detect interactions between BED and obesity. Conclusions: Among patients with BP, BED and obesity are highly prevalent and correlated, but associated with different profiles of enhanced illness burden. As the association of BED with greater psychiatric illness burden remained significant even after accounting for the effect of obesity, BP with BED may represent a clinically important sub-phenotype.
Keywords: Bipolar disorder, Binge eating disorder, Obesity
1. Introduction
Bipolar disorder (BP) is associated with obesity (McElroy and Keck, 2012; Simon et al., 2006; Taylor et al., 2012) and binge eating disorder (BED) (Hudson et al., 2007; Javaras et al., 2008b; Kessler et al., 2013; McElroy et al., 2005, 2011). BED, the consumption of abnormally large amounts of food accompanied by a sense of lack of control but without inappropriate compensatory behaviors, is associated with obesity (Hudson et al., 2006, 2007; Kessler et al., 2013). All three conditions are associated with psychiatric and medical comorbidity, disability, and show patterns of familial aggregation (Cheung and Mao, 2012; Hudson et al., 2006, 2007; Javaras et al., 2008a; Kessler et al., 2013; Lilenfeld et al., 2008; Schulze et al., 2006). Both obesity and eating disorders are associated with greater psychiatric and general medical burden in BP patients (Bond et al., 2011; Calkin et al., 2009; Fagiolini et al., 2003, 2004; Goldstein et al., 2011; Kemp et al., 2013; McElroy et al., 2005, 2011; McElroy and Keck, 2012; Wildes et al., 2008; Yim et al., 2012). The relationship among obesity, BED, and illness burden among individuals with BP, however, has received little empirical attention. Thus, the association between BED and illness burden in BP could depend on body weight, and discriminating if this association is dependent on BED alone, obesity alone, or an interaction of both is important. Moreover, variability in body mass index (BMI) has been shown to be associated with genetic variation (Yang et al., 2012). Potential clinically important sub-phenotypes of BP have been based on either specific symptomatology or comorbidity (Belmonte Mahon et al., 2011; MacQueen et al., 2005; Saunders et al., 2008). Under this hypothesis, BP with comorbid BED and/or obesity might be clinically important sub-phenotypes of BP.
In this study, we evaluated the relationship between BED status and obesity (defined as BMI≥30 kg/m2) in 719 BP patients participating in a biobank. We assessed the effects of BED and obesity, as well as their interaction, on psychiatric and general medical illness burden, using proxy measures of illness severity based upon previously recognized clinically important BP sub-phenotypes (Belmonte Mahon et al., 2011; MacQueen et al., 2005; Saunders et al., 2008). We hypothesized that: BED would be associated with increased BMI; that both BED and obesity would be associated with elevated but different illness burden patterns; and that BP plus BED and BP plus obesity might each represent overlapping but distinct clinical sub-phenotypes. We also hypothesized that BED and obesity would enhance illness burden in BP patients in an additive or synergistic manner.
2. Methods
The Mayo Clinic Bipolar Biobank is a collaborative network of four sites (Mayo Clinic, Rochester, MN; Mayo Clinic Health System, Austin Medical Center, MN; Lindner Center of HOPE, Mason, OH; and University of Minnesota, Minneapolis, MN) formed to facilitate studies on disease risk and pharmacogenomics in BP. The protocol was approved by an Institutional Review Board at each site. Every participant had to provide written informed consent in order to be included in the study.
This ongoing project consists of a cross-sectional clinical/phenotypical assessment obtained from a convenience sample of consecutive BP patients drawn from academic mood clinics and inpatient units. Individuals were eligible for entry into the biobank if they had BPI or BPII disorder, or schizoaffective disorder, BP type, by DSM-IV criteria and confirmed by structured clinical interview. Exclusion criteria were inability to speak English, inability or unwillingness to provide written informed consent, and the presence of active suicidality or psychosis. This report involved patients who were enrolled in the biobank from July 2009 through December, 2012.
The clinical phenotype of participants was identified with the Structured Clinical Interview for DSM-IV (SCID) (First et al., 2005), the Bipolar Biobank Clinical Questionnaire (BiB-CQ), and the Bipolar Biobank Patient Questionnaire (BiB-PQ). Module D of the SCID was used to establish the diagnosis of BPI or II disorder, or schizoaffective disorder, bipolar type. A structured clinical questionnaire, the BiB-CQ, was administered by a member of the study team and used to determine historical illness variables (e.g., history of suicide attempts, psychotic symptoms, rapid cycling, and cycle acceleration over time), co-occurring psychiatric disorders (including BED), and treatment variables. Completed by the patient, the BiB-PQ assessed other clinical as well as demographic (e.g., age and gender) variables. General medical comorbidity was assessed with the Modified Cumulative Illness Rating Scale (CIRS) (Hudon et al., 2005), which measures patient-reported organ-specific comorbid medical illnesses and their severity.
Weight was obtained with the individual in light clothing but no shoes. Height was measured with a stadiometer. Body mass index (BMI), calculated by dividing weight (in kilograms) by height (in meters) squared, was used to estimate amount of body fat. Obesity was defined as BMI≥30 kg/m2, and extreme obesity as BMI≥40 kg/m2.
Psychiatric illness burden was evaluated with six different proxy measures of illness severity in BP: suicidality, psychosis, mood instability, anxiety disorder comorbidity, and substance abuse comorbidity. Suicidality and psychosis were dichotomous yes-no variables. Suicidality was positive if the patient endorsed ≥1 suicide attempt requiring medical intervention. Psychosis was positive if the patient had a lifetime history of hallucinations or delusions. The mood instability domain was determined by the sum of the lifetime presence of mixed episodes, rapid cycling, ultra rapid/ultradian cycling, cycle acceleration over time, and increased episode severity over time, each coded as no = 0 and yes = 1, and therefore ranged from 0 to 5. The anxiety disorder comorbidity domain (range 0–6) was the sum of the following lifetime comorbid anxiety disorders: post-traumatic stress disorder (PTSD), generalized anxiety disorder (GAD), social anxiety disorder (SAD), obsessive-compulsive disorder (OCD), phobia, and panic disorder. The substance abuse comorbidity domain was the sum of having lifetime alcohol abuse or dependence, drug abuse or dependence, or nicotine dependence, and ranged from 0 to 3. General medical burden was evaluated with CIRS score.
3. Statistical analysis
Patients included in this analysis had BP or schizoaffective disorder, bipolar type, BED status completed on BiB-CQ, and a recorded BMI. First, univariate analyses were used to compare demographic and clinical characteristics of BP patients with and without BED, and BP patients with and without obesity. For the univariate analyses, chi-square tests of association were used to compare distributions of gender, BP type, and each separate clinical feature or comorbid disorder between groups, while Wilcoxon rank sum tests were used to compare distributions of continuous variables.
Multivariate analyses were then performed to evaluate the joint effects of obesity and BED on general medical burden (CIRS scores) and the various psychiatric burden measures. Multivariate logistic regression models were used to study the effects of obesity, BED, and their interaction on psychiatric burden defined as history of a suicide attempt or psychosis. Ordinal logistic regression was used to examine obesity, BED, and their interaction as the predictors of substance abuse comorbidity (sum 0–3). Multivariate linear regression was used to study the effects of obesity, BED, and their interaction on sums of mood instability (sum 0–5), anxiety disorder comorbidity (sum 0–6), and general medical comorbidity (CIRS scores).
In addition, similar models were constructed using the quantitative BMI measure instead of obesity as a predictor. Finally, Poisson regression models of mood instability and anxiety disorder comorbidity sums were also examined and gave similar results to the linear regression analyses, but did not provide superior fit. Thus, the linear regression results are reported.
4. Results
Of 717 patients identified, 76.3% had BPI disorder, 9.5% had BED, 42.8% were obese, and 10.0% had extreme obesity (Table 1). 47 (6.6%) patients had BED and obesity. Univariate analyses demonstrated that BP patients with BED had a significantly higher BMI (by an average of 4.2 kg/m2); significantly higher degrees of suicidality, psychosis, mood instability, and anxiety disorder comorbidity; significantly higher CIRS total scores; and were also more likely to be women, compared to those without BED (Table 1). BP patients with obesity were more likely to have BED (15.3% versus 5.1% of non-obese patients; p < 0.0001), had higher degrees of suicidality, and had higher CIRS scores than non-obese patients. They were also significantly older, more likely to be women, and less likely to have nicotine dependence. Neither BED nor obesity were otherwise associated with substance abuse comorbidity.
Table 1. Univariate analyses comparing demographic and clinical characteristics of BP patients with and without BED, and with and without obesity.
| Demographic | N | % or mean ± SD | No BED (N = 649) | BED (N = 68) | p-value | Non-obese (N = 409) | Obese (N = 308) | p-value |
|---|---|---|---|---|---|---|---|---|
| Age | 714 | 42.1 ± 15.4 | 42.0 ± 15.6 | 42.4 ± 14.0 | 0.650 | 41.2 ± 15.9 | 43.2 ± 14.8 | 0.047 |
| Male | 301 | 43.6 | 45.8 | 22.7 | < 0.001 | 47.5 | 38.4 | 0.018 |
| BP I | 547 | 76.3 | 75.3 | 85.3 | 0.160 | 76.1 | 76.6 | 0.741 |
| BP II | 163 | 22.7 | 23.7 | 14.7 | 22.7 | 22.7 | ||
| Schizoaffective | 7 | 1.0 | 1.1 | 0.0 | 1.2 | 0.7 | ||
| BED | 68 | 9.5 | - | - | - | 5.1 | 15.3 | < 0.001 |
| BMI | 717 | 30.1 ± 7.1 | 29.7 ± 6.9 | 33.9 ± 8.3 | < 0.001 | 25.3 ± 3.0 | 36.5 ± 5.8 | - |
| < 25 | 175 | 24.4 | 25.6 | 13.2 | 42.8 | 0 | ||
| 25–29.9 | 236 | 32.8 | 34.2 | 17.7 | 57.2 | 0 | ||
| 30–39.9 | 236 | 32.8 | 31.6 | 45.6 | 0 | 76.6 | ||
| 40+ | 72 | 10.0 | 8.6 | 23.5 | 0 | 23.4 | ||
| ≥ 1 Suicide attempt | 226 | 31.7 | 29.9 | 50.0 | < 0.001 | 28.2 | 36.5 | 0.019 |
| Psychosis | 325 | 45.7 | 44.3 | 60.3 | 0.012 | 47.5 | 43.6 | 0.300 |
| Mixed episodes | 94 | 16.4 | 16.3 | 17.5 | 0.807 | 16.9 | 15.8 | 0.705 |
| Rapid cycling | 378 | 52.9 | 52.4 | 57.4 | 0.437 | 50.7 | 55.7 | 0.18 |
| Ultra rapid/cycling | 169 | 23.7 | 22.9 | 32.4 | 0.080 | 25.2 | 21.9 | 0.308 |
| Cycle acceleration | 177 | 24.8 | 23.5 | 38.8 | 0.006 | 24.2 | 25.7 | 0.684 |
| Increased severity | 234 | 32.7 | 31.9 | 40.3 | 0.162 | 32.4 | 33.0 | 0.871 |
| Mood instability sum (0–5) | 561 | 1.5 ± 1. 4 | 1.5 ± 1.4 | 1. 9 ± 1. 4 | 0. 018 | 1.5 ± 1.5 | 1. 6 ± 1.4 | 0.253 |
| PTSD | 179 | 25.1 | 23.1 | 44.8 | < 0.001 | 22.9 | 28.2 | 0.108 |
| GAD | 343 | 48.6 | 47.6 | 59.1 | 0.075 | 48.9 | 48.3 | 0.881 |
| SAD | 192 | 27.2 | 25.2 | 47.7 | < 0.001 | 25.7 | 29.3 | 0.281 |
| OCD | 115 | 16.2 | 15.1 | 26.9 | 0.013 | 17.9 | 13.9 | 0.145 |
| Phobia | 69 | 9.8 | 8.6 | 21.2 | 0.001 | 8.2 | 11.9 | 0.097 |
| Panic | 230 | 32.3 | 31.2 | 43.3 | 0.044 | 29.7 | 36.0 | 0.075 |
| Anxiety disorder comorbidity sum (0–6) | 715 | 1.6 ± 1. 5 | 1. 5 ± 1.4 | 2.4 ± 1. 6 | < 0.001 | 1.5 ± 1.5 | 1. 7 ± 1.5 | 0.293 |
| Alcohol dep/abuse | 272 | 38.6 | 38.2 | 43.9 | 0.358 | 39.4 | 37.8 | 0.674 |
| Nicotine dep | 225 | 35.9 | 34.8 | 46.2 | 0.068 | 39.0 | 31.6 | 0.042 |
| Drug dep/abuse | 176 | 26.2 | 25.9 | 29.2 | 0.563 | 28.0 | 23.8 | 0.233 |
| Substance abuse comorbidity sum (0–3) | 716 | 1.0 ± 1. 0 | 1.0 ± 1.0 | 1. 2 ± 1.0 | 0.095 | 1.0 ±1.0 | 0.9 ± 1.0 | 0.056 |
| CIRS score | 636 | 4.0 ± 3.6 | 3.9 ± 3.6 | 4.9 ± 3.6 | 0.010 | 3.5 ± 3.3 | 4.6 ± 3.8 | < 0.001 |
Table 2 shows the mean scores on the general medical burden (CIRS) and various psychiatric burden measures by presence of BED and obesity. Multivariate analyses showed that, after controlling for the effect of obesity, the presence of BED remained positively associated with suicidality, psychosis, mood instability, and anxiety disorder comorbidity, but not with general medical comorbidity (Table 2). Also, the presence of BED became positively associated with substance abuse comorbidity. In contrast, after accounting for the effect of BED, obesity remained positively associated with general medical comorbidity, but was negatively associated with substance abuse comorbidity. There was no evidence of significant interaction of BED and obesity on any of the illness burden outcomes. However, as shown in Fig. 1, BP patients with BED and obesity had the numerically greatest mean CIRS, suicidality, and anxiety disorder comorbidity scores, while non-obese BP patients with BED had the highest psychosis, mood instability, and substance abuse comorbidity scores.
Table 2. Multivariate analysis of obesity and BED effectsa on BP illness burden.
| N | Effect | OR | 95% CI | p-Value | ||
|---|---|---|---|---|---|---|
| Binary outcomesb | 711 | Obesity | 1.348 | 0.975 | 1.865 | 0.0709 |
| ≥1 Suicide attempt (Y/N) | BED | 2.165 | 1.296 | 3.613 | 0.0032 | |
| Psychosis (Y/N) | 709 | Obesity | 0.792 | 0.583 | 1.075 | 0.1346 |
| BED | 2.046 | 1.217 | 3.440 | 0.0069 | ||
| Ordinal outcomec | ||||||
| Substance abuse comorbidity sum | 716 | Obesity | 0.733 | 0.556 | 0.967 | 0.0279 |
| BED | 1.589 | 1.001 | 2.524 | 0.0497 | ||
| Estimate | St. Err. | t-Value | p-Value | |||
|
| ||||||
| Continuous outcomesd | 561 | Obesity | 0.053 | 0.124 | 0.43 | 0.6671 |
| Mood instability sum | BED | 0.433 | 0.205 | 2.11 | 0.0353 | |
| Anxiety disorder comorbidity sum | 715 | Obesity | 0.038 | 0.111 | 0.34 | 0.7321 |
| BED | 0.890 | 0.189 | 4.77 | < 0.0001 | ||
| CIRS | 636 | Obesity | 1.018 | 0.287 | 3.54 | 0.0004 |
| BED | 0.743 | 0.486 | 1.53 | 0.1266 | ||
Interactions between obesity and BED were also explored for each outcome, but none were included in the above models due to non-significance (p-values > 0.05).
Logistic regression.
Ordinal logistic regression.
Linear regression.
Fig. 1.
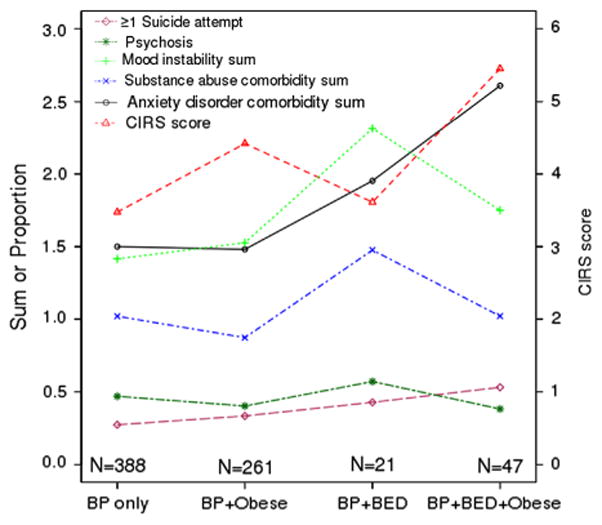
Means and proportions of illnesses burdens by BED and obesity phenotypes. For each psychiatric burden measure, the proportion (suicide, psychosis) or mean score (mood instability, anxiety disorder comorbidity, substance abuse comorbidity) is plotted using the left-side y-axis, for patients in the four groups defined along the x-axis: BP without BED or obesity, BP with obesity only, BP with BED only, and BP with both BED and obesity. To demonstrate general medical burden in the four groups, the mean CIRS score is plotted for each group using the right-side y-axis.
For most comparisons, similar results were obtained when the quantitative BMI variable was considered in the multivariable models rather than the dichotomous obesity variable (Supplementary Table 1). However, when BMI was considered as the predictor rather than obesity, a positive association of BMI with suicidality and a negative association with psychosis became significant, while the association with substance abuse comorbidity was not significant.
5. Conclusions
Our findings confirm the association of BED with obesity in patients with BP reported in earlier studies, and provide further evidence that BED and obesity are associated with greater illness burden in BP. However, the burden profiles differed, especially after accounting for the presence of the other comorbid factor: BED was associated with suicidality, psychosis, mood instability, anxiety disorder comorbidity, and substance abuse comorbidity but not general medical comorbidity, whereas obesity was associated with general medical comorbidity, but negatively with substance abuse comorbidity. Moreover, contrary to our hypothesis, we did not detect a statistically significant interaction between BED and obesity on illness burden: the estimated effects of BED on psychiatric illness burden did not depend on obesity, and conversely, the estimated effect of obesity on illness burden was the same regardless of BED status. In other words, the BP plus BED phenotype was associated with greater suicidality, psychosis, mood instability, anxiety disorder comorbidity, and substance abuse comorbidity than BP without BED, regardless of obesity. The BP plus obesity phenotype was associated with greater general medical comorbidity, but with reduced substance abuse comorbidity, irrespective of BED status.
Family history studies showing links between BED in probands and BP in relatives (Hudson et al., 2008), and between obesity in probands and BP in relatives (Black et al., 1992), suggest these phenotypes may have some shared genetic risk factors. However, the familial aggregation of BP, obesity, and BED as individual disorders, along with our findings suggesting that BP with comorbid BED and BP with obesity are related clinical sub-phenotypes that have some degree of differentiation, indicate that they are also distinct phenotypes with unique genetic contributors. Thus, future studies should consider searching for genetic factors for these sub-phenotypes (BP with obesity, and BP with BED) separately. As the biobank expands, we plan to explore the genetic architecture underlying these overlapping yet distinct clinical sub-phenotypes.
Our findings also suggest that although BED and obesity overlap in BP, the association of BED with psychiatric illness burden is larger than that for obesity. This is consistent with other studies showing binge eating, but not obesity, is associated with psychopathology in mood disorder patients (Telch and Agras, 1994) and findings that obese individuals with BED have greater psychopathology than their comparably obese counterparts without BED (Wonderlich et al., 2009). It is therefore possible that some of the psychiatric illness burden associated with obesity in BP found in other studies might instead be due to binge eating (Calkin et al., 2009; Goldstein et al., 2011; Yim et al., 2012). Conversely, our finding that obesity remained associated with general medical burden after accounting for BED is consistent with data showing obesity in BP may be more strongly associated with general medical than psychiatric burden (Goldstein et al., 2013). The general trends observed for the effects of BED and obesity on general medical and psychiatric burden in BP patients are displayed in Fig. 2.
Fig. 2.
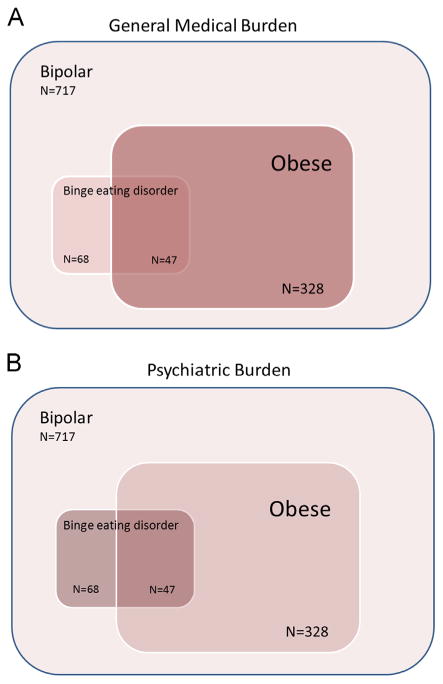
Schematic diagram representing the effects of obesity and BED on (A) general medical burden and (B) psychiatric burden. The sizes of the boxes roughly represent the sizes of the overlapping groups, while the shades of red represent the severity of burden in the subgroup. Panel A demonstrates that BED on its own is associated with little increase in general medical burden, while obesity is associated with a large increase in general medical burden, with the group with both BED and obesity having the highest CIRS scores. Panel B demonstrates that BED is associated with greater psychiatric burden than obesity, but again the group with both BED and obesity has possibly the greatest psychiatric burden (at least regarding suicidality and anxiety disorder comorbidity). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Of interest, obesity was associated negatively with substance abuse comorbidity after accounting for the effect of BED. This finding is consistent with other reports of obesity and substance abuse being inversely correlated in BP (McIntyre et al., 2007). The association of obesity with decreased prevalence of nicotine dependence in the univariate analysis is consistent with a large literature showing an inverse relationship between obesity and smoking (Tweed et al., 2012). Similarly, the positive association of BMI with suicidality has been previously reported (Fagiolini et al., 2004). However, the association of increasing BMI with less psychosis was unexpected, but may be consistent with studies suggesting that high fat diets protect against the effects of social stress (Finger et al., 2011) or that favorable antipsychotic response is associated with long-term weight gain (Bai et al., 2006).
One potential explanation of this differential effect of obesity and BED on illness burden could be inflammation. Inflammatory disturbances have been found in both obesity and BP (Goldstein et al., 2009), but have not yet been reported in eating disorders (Monteleone et al., 1999). Additionally, links between macronutrient intake and inflammatory processes could be operating (Esser et al., 2013). Obese BP patients could have deranged inflammatory mechanisms and, subsequently, could develop medical illnesses associated with inflammatory dysregulation, such as diabetes, hypertension, and cardiovascular disease, in higher rates as compared to non-obese BP patients. This hypothesis is supported in part by the increased CIRS scores found in our obese patients, but not in BED patients after accounting for the effect of obesity. It is not supported, however, by findings of metabolic abnormalities in BED after controlling for obesity (Hudson et al., 2010). On the other hand, if BED is one of the drivers of more severe psychopathology in BP patients after accounting for the effect of obesity, non-inflammatory biological mechanisms might underlie the association between BED and greater psychiatric burden.
This study has a number of strengths. The sample size was adequate to have sufficient statistical power to detect the differences that were found. The clinical phenotype of BP was carefully assessed using structured interviews and research-oriented questionnaires. The use of dimensional proxy measures to assess psychiatric illness burden, even though novel, delivered a richer granularity to the analyses and overrode the limitations of the exclusive use of dichotomous definitions and categorical variables.
Several limitations should be considered. BED was diagnosed clinically and not with a structured interview. It is thus possible that the “true” number of patients with BED was different from that determined by clinical questionnaire. A measure of depressive burden was not evaluated, and obesity in BP patients has been associated with degree of depressive symptomalotogy (Fagiolini et al., 2003; Goldstein et al., 2011; Kemp et al., 2013); an association between obesity and depressive burden may have existed that was not detected. Some proxy measures of illness burden were novel, especially dimensional measures of mood instability, anxiety disorder comorbidity, and substance abuse comorbidity, and thus of unclear validity. However, several of the proxies have been shown to be clinically relevant and to have familial association in BP, and thus hypothesized to reflect clinically important sub-phenotypes of BP (Belmonte Mahon et al., 2011; MacQueen et al., 2005; Saunders et al., 2008). These include suicidality, psychosis, rapid cycling, comorbid panic disorder or anxiety (Belmonte Mahon et al., 2011; MacQueen et al., 2005; Saunders et al., 2008; Schulze et al., 2006), and comorbid substance abuse (Saunders et al., 2008). Other proposed sub-phenotypes of BP shown to have familial segregation were not assessed (such as age of onset or lithium responsiveness (Belmonte Mahon et al., 2011)).
Another limitation is that there may have not been enough statistical power to detect interactions between BED and obesity on illness burden in BP due to low sample size in some of the groups. While the overall sample size was large, the BP plus BED and obesity and the BP plus BED without obesity groups contained only 47 and 21 patients, respectively. Indeed, some of the trends visible in Fig. 1 could be indicative of interaction effects that were undetectable with the current sample size. For instance, obesity seemed to be related to a very slight increase in mood instability in the group with no BED, but among those with BED, degree of mood instability was considerably higher in non-obese as compared to obese individuals. If this trend can be demonstrated in a larger sample, it would indicate a different association of obesity with mood instability depending on the presence of BED (i.e., a BED-obesity interaction effect). A further limitation is that potentially relevant variables were not accounted for, such as family history of BED or obesity, familial BMI, cognitive function, and past and current medication exposure. Finally, nothing can be concluded about the treatment of BP when it co-occurs with BED, obesity, or both conditions.
Further research into the relationship between obesity and binge eating behavior in BP is needed at many levels. Studies of the neurobiology and neurocognition of BP should account for both weight and comorbid eating disorder status. Family history, genetic, and controlled treatment studies of BP with BED and other obesity-related comorbidities, such as cardiovascular disease and type 2 diabetes, are needed. Both the metabolic and anti-binge eating effects of new medications for BP, and the thymoleptic and anti-binge eating properties of new anti-obesity agents, need to be determined in BP patients with obesity or BED. Moreover, as more is learned about the neuroscience and biology of obesity and binge eating behavior, these findings need to be applied to patients with BP.
Supplementary Material
Acknowledgments
We thank Ms. Genie Groff, who assisted with the preparation of the manuscript.
Role of funding source: The funding source had no role in study design, collection or interpretation of data, or drafting of the manuscript.
Footnotes
Conflict of interest: Dr. McElroy is a consultant to or member of the scientific advisory boards of Alkermes, Bracket, Corcept, MedAvante, Shire, and Teva. She is a principal or co-investigator on studies sponsored by the Agency for Healthcare Research and Quality (AHRQ), Alkermes, AstraZeneca, Cephalon, Eli Lilly and Company, Forest, Marriott Foundation, National Institute of Mental Health, Orexigen Therapeutics, Inc., Pfizer, Shire, Takeda Pharmaceutical Company Ltd., and Transcept Pharmaceutical, Inc. She is also an inventor on United States Patent no. 6,323,236 B2, Use of Sulfamate Derivatives for Treating Impulse Control Disorders, and along with the patent's assignee, University of Cincinnati, Cincinnati, Ohio, has received payments from Johnson & Johnson, which has exclusive rights under the patent.
Dr. Crow has received research grants from Shire, Alkermes, and Phillips Respironics.
Dr. Prieto has received honoraria for speaker activities and development of educational presentations from GlaxoSmithKline, has received travel support from GlaxoSmithKline, Lilly, Lundbeck and Pharmavita, and has received scholarships from the Government of Chile.
Dr. Frye has had grant support from Pfizer, Myriad, National Institute of Mental Health (NIMH), National Institute of Alcohol Abuse and Alcoholism (NIAAA), Mayo Foundation, and has served as an unpaid consultant for Allergan, Myriad, Sunovion, and Teva Pharmaceuticals.
Dr. Biernacka, Dr. Winham, Ms. Geske, Dr. Cuellar Barboza, Dr. Chauhan, Ms. Seymour and Ms. Mori have no conflicts to declare.
Appendix A. Supporting information: Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.jad.2013.05.024.
References
- Bai YM, Lin CC, Chen JY, Lin CY, Su TP, Chou P. Association of initial antipsychotic response to clozapine and long-term weight gain. American Journal of Psychiatry. 2006;163:1276–1279. doi: 10.1176/ajp.2006.163.7.1276. [DOI] [PubMed] [Google Scholar]
- Belmonte Mahon P, Pirooznia M, Goes FS, Seifuddin F, Steele J, Lee PH, Huang J, Hamshere ML, Depaulo JR, Jr, Kelsoe JR, Rietschel M, Nothen M, Cichon S, Gurling H, Purcell S, Smoller JW, Craddock N, Schulze TG, McMahon FJ, Potash JB, Zandi PP. Genome-wide association analysis of age at onset and psychotic symptoms in bipolar disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2011;156B:370–378. doi: 10.1002/ajmg.b.31172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DW, Goldstein RB, Mason EE, Bell SE, Blum N. Depression and other mental disorders in the relatives of morbidly obese patients. Journal of Affective Disorders. 1992;25:91–95. doi: 10.1016/0165-0327(92)90071-d. [DOI] [PubMed] [Google Scholar]
- Bond DJ, Lang DJ, Noronha MM, Kunz M, Torres IJ, Su W, Honer WG, Lam RW, Yatham LN. The association of elevated body mass index with reduced brain volumes in first-episode mania. Biological Psychiatry. 2011;70:381–387. doi: 10.1016/j.biopsych.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Calkin C, van de Velde C, Ruzickova M, Slaney C, Garnham J, Hajek T, O'Donovan C, Alda M. Can body mass index help predict outcome in patients with bipolar disorder? Bipolar Disorders. 2009;11:650–656. doi: 10.1111/j.1399-5618.2009.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung WW, Mao P. Recent advances in obesity: genetics and beyond. ISRN Endocrinology. 2012;2012:536905. doi: 10.5402/2012/536905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser D, Oosterink E, Op 't Roodt J, Henry RM, Stehouwer CD, Muller M, Afman LA. Vascular and inflammatory high fat meal responses in young healthy men; a discriminative role of IL-8 observed in a randomized trial. PLoS One. 2013;8:e53474. doi: 10.1371/journal.pone.0053474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini A, Kupfer DJ, Houck PR, Novick DM, Frank E. Obesity as a correlate of outcome in patients with bipolar I disorder. American Journal of Psychiatry. 2003;160:112–117. doi: 10.1176/appi.ajp.160.1.112. [DOI] [PubMed] [Google Scholar]
- Fagiolini A, Kupfer DJ, Rucci P, Scott JA, Novick DM, Frank E. Suicide attempts and ideation in patients with bipolar I disorder. Journal of Clinical Psychiatry. 2004;65:509–514. doi: 10.4088/jcp.v65n0409. [DOI] [PubMed] [Google Scholar]
- Finger BC, Dinan TG, Cryan JF. High-fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience. 2011;192:351–360. doi: 10.1016/j.neuroscience.2011.06.072. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. New York State Psychiatric Institute; New York: 2005. Structured Clinical Interview for DSM-IV-TR Axis I Disorders: Patient Edition (SCID-I/P) [Google Scholar]
- Goldstein BI, Kemp DE, Soczynska JK, McIntyre RS. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. Journal of Clinical Psychiatry. 2009;70:1078–1090. doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Liu SM, Schaffer A, Sala R, Blanco C. Obesity and the three-year longitudinal course of bipolar disorder. Bipolar Disorders. 2013 doi: 10.1111/bdi.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Liu SM, Zivkovic N, Schaffer A, Chien LC, Blanco C. The burden of obesity among adults with bipolar disorder in the United States. Bipolar Disorders. 2011;13:387–395. doi: 10.1111/j.1399-5618.2011.00932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudon C, Fortin M, Vanasse A. Cumulative Illness Rating Scale was a reliable and valid index in a family practice context. Journal of Clinical Epidemiology. 2005;58:603–608. doi: 10.1016/j.jclinepi.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Javaras KN, Laird NM, VanderWeele TJ, Pope HG, Jr, Hernan MA. A structural approach to the familial coaggregation of disorders. Epidemiology. 2008;19:431–439. doi: 10.1097/EDE.0b013e31816a9de7. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Lalonde JK, Berry JM, Pindyck LJ, Bulik CM, Crow SJ, McElroy SL, Laird NM, Tsuang MT, Walsh BT, Rosenthal NR, Pope HG., Jr Binge-eating disorder as a distinct familial phenotype in obese individuals. Archives of General Psychiatry. 2006;63:313–319. doi: 10.1001/archpsyc.63.3.313. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Lalonde JK, Coit CE, Tsuang MT, McElroy SL, Crow SJ, Bulik CM, Hudson MS, Yanovski JA, Rosenthal NE, Pope HG. Longitudinal study of the diagnosis of components of the metabolic syndrome in individuals with binge eating disorder. American Journal of Clinical Nutrition. 2010;91:1568–1573. doi: 10.3945/ajcn.2010.29203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaras KN, Laird NM, Reichborn-Kjennerud T, Bulik CM, Pope HG, Jr, Hudson JI. Familiality and heritability of binge eating disorder: results of a case-control family study and a twin study. International Journal of Eating Disorders. 2008a;41:174–179. doi: 10.1002/eat.20484. [DOI] [PubMed] [Google Scholar]
- Javaras KN, Pope HG, Lalonde JK, Roberts JL, Nillni YI, Laird NM, Bulik CM, Crow SJ, McElroy SL, Walsh BT, Tsuang MT, Rosenthal NR, Hudson JI. Co-occurrence of binge eating disorder with psychiatric and medical disorders. Journal of Clinical Psychiatry. 2008b;69:266–273. doi: 10.4088/jcp.v69n0213. [DOI] [PubMed] [Google Scholar]
- Kemp DE, Sylvia LG, Calabrese JR, Nierenberg AA, Thase ME, Reilly-Harrington NA, Ostacher MJ, Leon AC, Ketter TA, Friedman ES, Bowden CL, Rabideau DJ, Pencina M, Iosifescu DV. General medical burden in bipolar disorder: findings from the LiTMUS comparative effectiveness trial. Acta Psychiatrica Scandinavica. 2013 doi: 10.1111/acps.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Chiu WT, Deitz AC, Hudson JI, Shahly V, Aguilar-Gaxiola S, Alonso J, Angermeyer MC, Benjet C, Bruffaerts R, de Girolamo G, de Graaf R, Maria Haro J, Kovess-Masfety V, O'Neill S, Posada-Villa J, Sasu C, Scott K, Viana MC, Xavier M. The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilenfeld LR, Ringham R, Kalarchian MA, Marcus MD. A family history study of binge-eating disorder. Comprehensive Psychiatry. 2008;49:247–254. doi: 10.1016/j.comppsych.2007.10.001. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Hajek T, Alda M. The phenotypes of bipolar disorder: relevance for genetic investigations. Molecular Psychiatry. 2005;10:811–826. doi: 10.1038/sj.mp.4001701. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Frye MA, Hellemann G, Altshuler L, Leverich GS, Suppes T, Keck PE, Nolen WA, Kupka R, Post RM. Prevalence and correlates of eating disorders in 875 patients with bipolar disorder. Journal of Affective Disorders. 2011;128:191–198. doi: 10.1016/j.jad.2010.06.037. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Keck PE., Jr Obesity in bipolar disorder: an overview. Current Psychiatry Reports. 2012;14:650–658. doi: 10.1007/s11920-012-0313-8. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Kotwal R, Keck PE, Jr, Akiskal HS. Comorbidity of bipolar and eating disorders: distinct or related disorders with shared dysregulations? Journal of Affective Disorders. 2005;86:107–127. doi: 10.1016/j.jad.2004.11.008. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, McElroy SL, Konarski JZ, Soczynska JK, Bottas A, Castel S, Wilkins K, Kennedy SH. Substance use disorders and overweight/ obesity in bipolar I disorder: preliminary evidence for competing addictions. Journal of Clinical Psychiatry. 2007;68:1352–1357. doi: 10.4088/jcp.v68n0905. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Maes M, Fabrazzo M, Tortorella A, Lin A, Bosmans E, Kenis G, Maj M. Immunoendocrine findings in patients with eating disorders. Neuropsychobiology. 1999;40:115–120. doi: 10.1159/000026606. [DOI] [PubMed] [Google Scholar]
- Saunders EH, Scott LJ, McInnis MG, Burmeister M. Familiality and diagnostic patterns of subphenotypes in the National Institutes of Mental Health bipolar sample. American Journal of Medical Genetics Part B: Neuro-psychiatric Genetics. 2008;147B:18–26. doi: 10.1002/ajmg.b.30558. [DOI] [PubMed] [Google Scholar]
- Schulze TG, Hedeker D, Zandi P, Rietschel M, McMahon FJ. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Archives of General Psychiatry. 2006;63:1368–1376. doi: 10.1001/archpsyc.63.12.1368. [DOI] [PubMed] [Google Scholar]
- Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Archives of General Psychiatry. 2006;63:824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VH, McIntyre RS, Remington G, Levitan RD, Stonehocker B, Sharma AM. Beyond pharmacotherapy: understanding the links between obesity and chronic mental illness. Canadian Journal of Psychiatry. 2012;57:5–12. doi: 10.1177/070674371205700103. [DOI] [PubMed] [Google Scholar]
- Telch CF, Agras WS. Obesity, binge eating and psychopathology: are they related? International Journal of Eating Disorders. 1994;15:53–61. doi: 10.1002/1098-108x(199401)15:1<53::aid-eat2260150107>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Tweed JO, Hsia SH, Lutfy K, Friedman TC. The endocrine effects of nicotine and cigarette smoke. Trends in Endocrinology and Metabolism. 2012;23:334–342. doi: 10.1016/j.tem.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildes JE, Marcus MD, Fagiolini A. Prevalence and correlates of eating disorder co-morbidity in patients with bipolar disorder. Psychiatry Research. 2008;161:51–58. doi: 10.1016/j.psychres.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonderlich SA, Gordon KH, Mitchell JE, Crosby RD, Engel SG. The validity and clinical utility of binge eating disorder. International Journal of Eating Disorders. 2009;42:687–705. doi: 10.1002/eat.20719. [DOI] [PubMed] [Google Scholar]
- Yang J, Loos RJ, Powell JE, Medland SE, Speliotes EK, Chasman DI, Rose LM, Thorleifsson G, Steinthorsdottir V, Magi R, Waite L, Smith AV, Yerges-Armstrong LM, Monda KL, Hadley D, Mahajan A, Li G, Kapur K, Vitart V, Huffman JE, Wang SR, Palmer C, Esko T, Fischer K, Zhao JH, Demirkan A, Isaacs A, Feitosa MF, Luan J, Heard-Costa NL, White C, Jackson AU, Preuss M, Ziegler A, Eriksson J, Kutalik Z, Frau F, Nolte IM, Van Vliet-Ostaptchouk JV, Hottenga JJ, Jacobs KB, Verweij N, Goel A, Medina-Gomez C, Estrada K, Bragg-Gresham JL, Sanna S, Sidore C, Tyrer J, Teumer A, Prokopenko I, Mangino M, Lindgren CM, Assimes TL, Shuldiner AR, Hui J, Beilby JP, McArdle WL, Hall P, Haritunians T, Zgaga L, Kolcic I, Polasek O, Zemunik T, Oostra BA, Junttila MJ, Gronberg H, Schreiber S, Peters A, Hicks AA, Stephens J, Foad NS, Laitinen J, Pouta A, Kaakinen M, Willemsen G, Vink JM, Wild SH, Navis G, Asselbergs FW, Homuth G, John U, Iribarren C, Harris T, Launer L, Gudnason V, O'Connell JR, Boerwinkle E, Cadby G, Palmer LJ, James AL, Musk AW, Ingelsson E, Psaty BM, Beckmann JS, Waeber G, Vollenweider P, Hayward C, Wright AF, Rudan I, Groop LC, Metspalu A, Khaw KT, van Duijn CM, Borecki IB, Province MA, Wareham NJ, Tardif JC, Huikuri HV, Cupples LA, Atwood LD, Fox CS, Boehnke M, Collins FS, Mohlke KL, Erdmann J, Schunkert H, Hengstenberg C, Stark K, Lorentzon M, Ohlsson C, Cusi D, Staessen JA, Van der Klauw MM, Pramstaller PP, Kathiresan S, Jolley JD, Ripatti S, Jarvelin MR, de Geus EJ, Boomsma DI, Penninx B, Wilson JF, Campbell H, Chanock SJ, van der Harst P, Hamsten A, Watkins H, Hofman A, Witteman JC, Zillikens MC, Uitterlin-den AG, Rivadeneira F, Kiemeney LA, Vermeulen SH, Abecasis GR, Schlessinger D, Schipf S, Stumvoll M, Tonjes A, Spector TD, North KE, Lettre G, McCarthy MI, Berndt SI, Heath AC, Madden PA, Nyholt DR, Montgomery GW, Martin NG, McKnight B, Strachan DP, Hill WG, Snieder H, Ridker PM, Thorsteinsdottir U, Stefansson K, Frayling TM, Hirschhorn JN, Goddard ME, Visscher PM. FTO genotype is associated with phenotypic variability of body mass index. Nature. 2012;490:267–272. doi: 10.1038/nature11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim CY, Soczynska JK, Kennedy SH, Woldeyohannes HO, Brietzke E, McIntyre RS. The effect of overweight/obesity on cognitive function in euthymic individuals with bipolar disorder. European Psychiatry. 2012;27:223–228. doi: 10.1016/j.eurpsy.2011.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


