Abstract
Failure to convert computer-identified possible kidney paired donation (KPD) exchanges into transplants has prohibited KPD from reaching its full potential. This study analyzes the progress of exchanges in moving from “offers” to completed transplants. Offers were divided into individual segments called 1-way transplants in order to calculate success rates. From 2007 to 2014, the Alliance for Paired Donation performed 243 transplants, 31 in collaboration with other KPD registries and 194 independently. Sixty-one of 194 independent transplants (31.4%) occurred via cycles, while the remaining 133 (68.6%) resulted from nonsimultaneous extended altruistic donor (NEAD) chains. Thirteen of 35 (37.1%) NEAD chains with at least three NEAD segments accounted for 68% of chain transplants (8.6 tx/chain). The “offer” and 1-way success rates were 21.9 and 15.5%, respectively. Three reasons for failure were found that could be prospectively prevented by changes in protocol or software: positive laboratory crossmatch (28%), transplant center declined donor (17%) and pair transplanted outside APD (14%). Performing a root cause analysis on failures in moving from offer to transplant has allowed the APD to improve protocols and software. These changes have improved the success rate and the number of transplants performed per year.
Introduction
Kidney paired donation (KPD) is an effective means of overcoming immunological barriers to living donor transplantation (1–12). The competition engendered by various paired donation registries has led to unique strategies and innovations (13). However, in order for KPD to reach its full potential in the United States, it has been suggested that larger pools, perhaps under a single national registry, would be beneficial (9,13–15). If a strong national registry is to be created, the best strategies from each registry should be adopted. This poses a challenge, however, as variations in outcome reporting make direct comparisons difficult.
Though progress has been made, matching algorithms continue to generate potential exchanges that ultimately fail to produce transplants. KPD registries have adopted one of two systems to deal with this problem: either attempt to rapidly move through offers until a success is found, or spend time creating more robust offers that have a higher likelihood of succeeding. Few programs have reported on this aspect of KPD (9). KPD registries that operate within a single center/system have fewer communication and financial barriers so that less time and effort are wasted in failed offers (16,17). For those registries that oversee multiple independent centers, sharing information requires the coordinated efforts of multiple individuals, and more time and energy are expended on failed offers in multi-institutional KPD programs than in those that operate as a single center.
Nondirected donors (NDD) have been utilized to start chains of KPD transplants that can be either simultaneous, called domino paired donation (DPD), or nonsimultaneous, called nonsimultaneous extended altruistic donor (NEAD) chains. In NEAD chains, a NDD starts a segment of a nonsimultaneous series of transplants that ends with a bridge donor, who can start another segment to continue the NEAD chain at a later date (18). DPD eliminates the possibility of a bridge donor reneging by creating simultaneous KPD chains that end with a donation to a patient on the waiting list (8,19). The pros and cons of these approaches have been evaluated using simulated pools with real patient data, but actual experience is lacking (20–23).
Since its inception, the Alliance for Paired Donation (APD) has allowed simple cycles and NEAD chains to freely compete within its optimization algorithm and in converting computer-identified possible transplants, or “offers,” into completed transplants. In this study, we reviewed the efficacy of the APD matching system in converting computer-identified cycle and chain offers into completed transplants. Recognizing the observational nature of the evolution of the APD’s approach, the goals of the study were to: (i) determine the organization’s success rate over time; (ii) discern the reasons for failure to progress from an offer to successful transplants within different historical eras; (iii) compare the utility of cycles and chains of varying lengths; and (iv) correlate success rates with changes to matching software and procedures. We have also endeavored to delineate factors other than the matching algorithm, such as tissue typing standardization and communication between the APD and centers, that have impacted the matching process. The identified reasons for failure and the strategies employed to overcome these failures raise important practical and philosophical questions for discussion regarding future policy decisions for KPD in the United States.
Materials and Methods
The efficacy of the APD in converting proposed computer-identified kidney transplants into completed kidney transplants was analyzed from January 2007 through August 2014 (IRB approved, #104347). Data was collected in Excel, and statistical analysis was performed with IBM SPSS Statistics version 19.0 (IBM Corp., Armonk, NY). p-values were determined using x2 tests.
Utilizing an optimization algorithm developed by Roth, Sonmez, and Unver, the APD generates solutions of cycles and NEAD segments restricted to a length of 2-, 3-, 4-, or unrestricted exchanges to optimize the quantity and quality of possible transplants (24). See Table 1 for a list of terms.
Table 1.
List of key terminology
| Exchange | A proposed kidney transplant between a donor of one incompatible pair with a recipient of a different incompatible pair. |
| 1-way | Synonymous with exchange. Full term would be 1-way exchange. |
| Cycle | A set of N exchanges in a closed loop found by the matching algorithm in which each recipient receives a kidney from a donor within the cycle. |
| NEAD segment | A set of N exchanges started by a nondirected donor (NDD) or bridge donor (BD) that ends either in a bridge donor or in donation to the deceased donor waiting list. |
| NEAD chain | The accumulation of multiple NEAD segments linked together through BD’s and initiated by a NDD. |
| DPD | A set of N exchanges started by an NDD that occur simultaneously and ends with donation to the deceased donor waiting list. |
| Offer | A NEAD segment or cycle that has been presented to transplant centers for consideration. |
| 1-way success rate | The number of exchanges completed divided by the total number of exchanges presented in offers. |
| Offer success rate | The number of offers containing at least 1 successful transplant divided by the total number of offers. |
The process of converting computerized match runs into transplants involves four steps. First, the APD software identifies all immunologically feasible 1-way exchanges among the enrolled incompatible pairs, creating a compatibility matrix of possible 1-way exchanges. This compatibility matrix was initially based on ABO compatibility and a virtual HLA crossmatch based on center-reported specificities, but was later updated to include discretionary exclusion factors. The second step involves the APD software assigning a point score to the compatible 1-way exchanges using a scoring rubric (Table S1). The third step optimizes these 1-way exchanges into an overall solution comprised of cycles and NEAD segments according to the pre-determined maximum number of exchanges allowed per cycle or segment. At this point a centralized tissue-typing laboratory performs a screening crossmatch to eliminate false negative virtual crossmatch results. Center reported calculated PRA (cPRA) was introduced in October 2009 in accordance with UNOS specifications. The fourth step involves offering out the proposed exchanges (referred to subsequently as offers) in the optimized solution to be reviewed by transplant centers. Centers are then required to perform a confirmatory crossmatch to ensure HLA compatibility before the transplant proceeds.
When NDDs are entered into the pool, they initiate NEAD chain segments (NEADseg) that end in bridge donors. These bridge donors are re-entered into the pool individually in an equivalent fashion to NDDs to create another NEADseg. These segments build upon one another to create full NEAD chains. A NEAD chain ends when a bridge donor donates to the deceased donor waiting list or is withdrawn from the pool.
In order to evaluate the efficiency of converting computer-identified matches into completed transplants, each offer was divided into individual (1-way) exchanges. For instance, a 3-way cycle is composed of three 1-way exchanges, and a NEADseg-3 chain is composed of three 1-way exchanges with an overhanging bridge donor. The success rate was calculated by evaluating the conversion rate of cycles and chain segment offers generated from the optimized solution (offer success rate), and by evaluating the conversion rate of the 1-way exchanges that comprised these offers (1-way success rate). Identifying the successful conversion of both 1-ways and entire cycles/NEADsegs was necessary because, in the case of NEADsegs, an offer has the potential to generate some transplants but fail further down the chain. These partially failed segments still result in a bridge donor, so the chain can be continued at a later date. If any transplants were performed as a result of a NEADseg offer, the offer was considered successful even though some 1-ways within the offer may have been unsuccessful.
Next, reasons for failure were evaluated to determine the most common causes of an offer failing to result in transplants. It is important to note that a 1-way could fail to culminate in a transplant due to a specific reason, or it could fail due to dependency on a preceding 1-way being completed. We identified these as “failures of dependency,” which were excluded from the analysis of reasons for failure so that we could determine the relative incidence of specific reasons for failure. The 1-way and offer success rate calculations did incorporate failures of dependency. Reasons for failure were not available for 2007, so this analysis consists only of offers made from January 2008 to August 2014.
Results
Offers, transplants, and success rates of cycles and NEAD chains
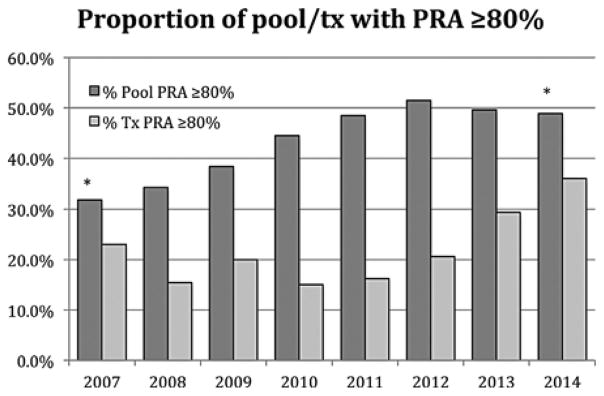
Seventy-six transplant centers across 27 states entered incompatible pairs in the APD registry. Forty-three centers in 24 states performed at least one APD transplant. The pool size increased steadily from 81 in January 2007 to 145 in January 2009, and then remained between 150 and 185 from 2009–present. The percent of the pool with a PRA 80% increased from 34% in 2007 to 49% in 2014 (Figure 1, p < 0.001).
Figure 1.
Proportion of pool recipients with a PRA 2:80% (left series), and proportion of transplanted recipients with a PRA 2:80% (right series) stratified by year. * p-value between pool PRA in 2007 and 2014 <0.001.
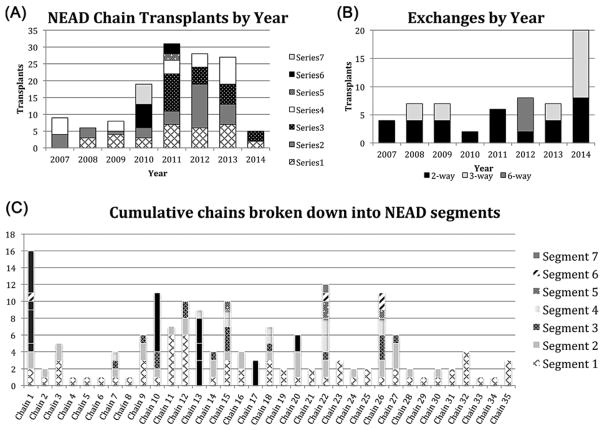
As of September 2014, the APD performed 243 transplants, both independently and in conjunction with several other KPD registries. This analysis consists of the 194 transplants performed from 98 offers made exclusively by the APD from January 2007 through August 2014. Sixty-one of 194 (31.4%) transplants were achieved through cycles (Figure 2A). NEAD chains accounted for 133 of 194 (68.6%) transplants (Figure 2b). The percentage of transplanted patients with a PRA 80% increased from 15.4% in 2007 to 36.0% in 2014 (Figure 1). Each chain was composed of various length NEAD segments (NEADseg-N); successful segments varied between one and seven transplants (NEADseg-1 to NEADseg-7). These NEAD segments, linked together through bridge donors, were part of 35 cumulative chains ranging from 1 to 16 transplants (Figure 2C). This study provides failure analysis only for 133 NEAD chain transplants facilitated solely by the APD. However, the APD worked with other KPD registries to achieve an additional 39 transplants so that the NEAD chains discussed achieved 164 transplants overall, leading to an average NEAD chain length of 4.6 transplants/chain. It is noteworthy that 13 of these 35 NEAD chains were composed of three or more NEADsegs (8.6 transplants/chain average), and these very long chains were responsible for 112 of 164 (68.3%) transplants through chains.
Figure 2.
Transplants performed by APD. (A) Number of transplants performed through cycles stratified by cycle length and year. (B) Number of transplants performed through NEAD segments stratified by intended chain length and year. (C) Cumulative chains divided into NEAD chain segments. Black bars indicate portions of chains completed by other centers in conjunction with the APD.
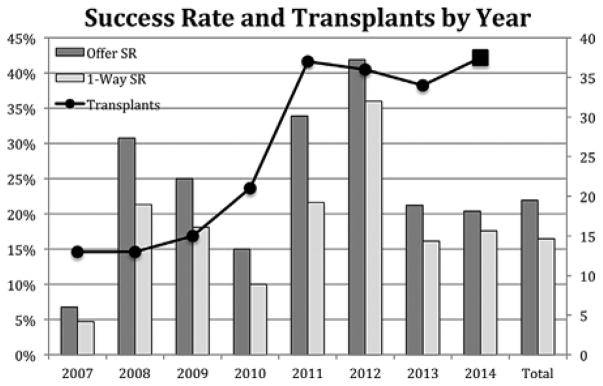
A total of 447 offers were made exclusively by the APD and contained 1255 1-ways. The offer success rate was 21.9%, and the 1-way success rate was 15.5% (Figure 3). The optimization algorithm allowed cycles and NEADsegs to freely compete in a “natural selection” process that sought only to maximize the quantity or quality of transplants offered. Of the 447 computer-identified offers, 173 (38.7%) offers were cycles and 256 (61.3%) were NEADsegs (Table S2). Though many were offered, only one cycle of length four or greater was completed (6-way). The majority (115) of the 133 transplants from NEAD chains were produced from NEADseg -1, −2, −3, and −4, while 18 were produced from NEADseg-5, −6, and −7. Several NEADsegs of length 8, 10 and 11 were offered, but no segment longer than a NEADseg-7 was successful. The offer and 1-way success rates of cycles was 14.5 and 12.4%, compared to 26.6 and 17.4% for NEAD segments. This represents a second round of natural selection that, overall, led to NEAD segments producing over twice as many transplants as cycles (133 vs. 61), despite the fact that segments were not actively pursued over cycles.
Figure 3.
Offers, transplants, and success rates. One-way and offer success rate by year graphed with number of transplants performed by year. &: Projected 2014 transplants based on 25 completed transplants as of August 30, 2014.
Partially completed NEAD segments
To better quantify the number of transplants performed through partially completed NEAD segments, the originally intended NEADseg lengths were compared to the final completed lengths (Table 2). A total of 101 transplants were performed from 58 NEADseg offers that were completed to their originally intended length. An additional 32 transplants were performed from 15 NEADseg offers that did not reach their originally intended length 70 NEADseg-4, −5, −6, and −7 were offered, of which only 13 resulted in transplants. Although only four of these thirteen segments reached their intended length (three NEADseg-4, one NEADseg-6), the remaining nine generated an additional 24 transplants of final lengths 1, 2, 3, and 6. In contrast, when one transplant failed in simultaneous cycles, the entire offer was always retracted.
Table 2.
Intended versus final NEAD chain segment lengths
| Offers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Final | Failed | NEADseg-1 | NEADseg-2 | NEADseg-3 | NEADseg-4 | NEAOseg-5 | NEADseg-6 | NEADseg-7 | Successful offers |
| Intended | |||||||||
|
|
|||||||||
| NEADseg-1 | 31 | 32 | 32 | ||||||
| NEADseg-2 | 53 | 4 | 15 | 19 | |||||
| NEADseg-3 | 56 | 0 | 2 | 7 | 9 | ||||
| NEADseg-4 | 33 | 0 | 3 | 2 | 3 | 8 | |||
| NEADseg-5 | 15 | 0 | 1 | 0 | 0 | 0 | 1 | ||
| NEADseg-6 | 7 | 1 | 0 | 1 | 0 | 0 | 1 | 3 | |
| NEADseg-7 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
|
|
|||||||||
| Total | 197 | 37 | 21 | 10 | 3 | 0 | 2 | 0 | 73 |
|
| |||||||||
| 1-way transplants | |||||||||
|
| |||||||||
| Final | Failed | NEADseg-1 | NEADseg-2 | NEADseg-3 | NEADseg-4 | NEAOseg-5 | NEADseg-6 | NEADseg-7 | Completed transplants |
| Intended | |||||||||
|
| |||||||||
| NEADseg-1 | 31 | 32 | 32 | ||||||
| NEADseg-2 | 106 | 4 | 30 | 34 | |||||
| NEADseg-3 | 168 | 0 | 4 | 21 | 25 | ||||
| NEADseg-4 | 132 | 0 | 6 | 6 | 12 | 24 | |||
| NEADseg-5 | 75 | 0 | 2 | 0 | 0 | 0 | 2 | ||
| NEADseg-6 | 42 | 1 | 0 | 3 | 0 | 0 | 6 | 10 | |
| NEADseg-7 | 14 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 6 |
|
|
|||||||||
| Total | 568 | 37 | 42 | 30 | 12 | 0 | 12 | 32 | 133 |
Rows show the intended lengths, while columns show the final completed lengths. Top: number of offers. Bottom: number of transplants that resulted.
The reasons for failure in simultaneous cycles and NEAD segments
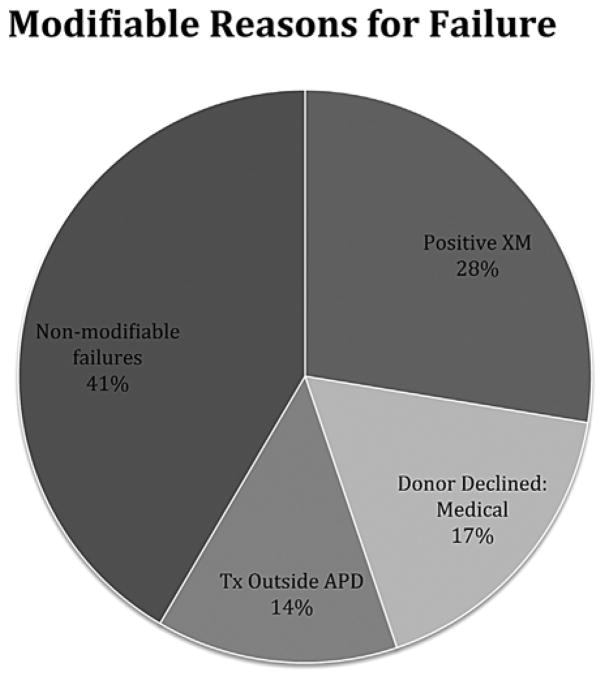
The reasons for failure were documented from 2008 through 2014. During this period, the total number of offers was 358, which included 978 1-ways resulting in 181 completed transplants. Of the 358 offers made during this period, 266 offers (74%) failed to result in any transplants. Out of 978 1-ways, 797 failed (81.5%), both from the 266 completely failed offers as well as from partially completed NEADsegs. Of the failed 1-ways, 308 failed for a specific identifiable reason (Table S3), and 489 failed due to dependency on preceding 1-ways (failures of dependency). Three reasons for failure have occurred predictably, and were thus potentially preventable: (i) positive crossmatch; (ii) transplant center declined donor for medical reasons; and (iii) transplant center failed to deactivate donor transplanted outside the APD. These three MRFs accounted for 59% of all reasons for failure (Figure 4).
Figure 4.
Reasons for failure classified into 3 modifiable categories and 1 nonmodifiable category.
Eras of the APD
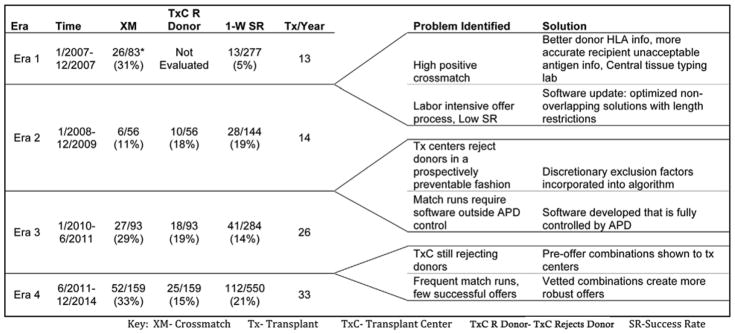
Modifications to software and protocols over time can be divided into four distinct “eras” of the APD. Each era is characterized by a new strategy, software update and/or protocol modification in an attempt to improve efficacy and minimize the impact of MRFs without increasing the workload for transplant centers. Figure 5 shows the era, MRFs identified from the previous era, modifications and how these modifications impacted the success rate and reasons for failure.
Figure 5.
Eras of the APD showing problems identified, solutions, and the impact of modifications on the reasons for failure, 1-way success rate, and transplants/year. *Upon reviewing laboratory data, 26 positive crossmatches were found in 2007 that accounted for 31% of the 83 failed offers. However, because all failure data was not systematically collected in 2007, these 26 XM failures were not incorporated into the overall failure analysis.
Though the reasons for failure were not systematically collected, the predominant reasons for failure in 2007 (Era 1) were positive crossmatch, software that was limited to identifying only 2-way exchanges, and inefficiencies in coordination between the APD and participating transplant centers. Starting in 2008 (Era 2), when failure data began to be systematically collected, a centralized tissue-typing laboratory was initiated, and the matching software was modified to collect more complete donor HLA information (e.g. Cw and DQ β antigen identification became mandatory), and more specific recipient unacceptable HLA information. Additionally in Era 2, an improved matching algorithm allowed the identification of an optimized, nonoverlapping solution where the total number of potential transplants per chain could be limited to a length of 2, 3, 4, or unrestricted. While we did not prospectively evaluate an alternative, employing these strategies in Era 2 reduced positive crossmatch as a reason for failure (31–11%, p 0.007). An improvement in success rate (5–19%, p < 0.001) was also observed, though the number of transplants/year did not increase significantly 13–14tx/yr.
By the end of Era 2, it became apparent that offers were failing because transplant centers were consistently rejecting potential donors for reasons that could have been specified and accounted for prospectively. Era 3 saw the creation of discretionary exclusion factors that, once incorporated into the software, precluded exchanges containing the prospective donors that transplant centers would have eventually rejected. The APD also incorporated the optimization software developed by Roth, Sonmez, and Unver, directly into the web-based application, allowing the execution of match runs by APD staff as frequently as necessary (24). The ability to rapidly repeat failed match runs resulted in a higher offer turnover (31–56 offers/year), leading to a decreased success rate overall, but we observed an increased number of transplants/year by nearly 85% 14–26tx/yr. Failures due to transplant centers rejecting donors remained relatively unchanged (18–19%), while positive crossmatch failures increased significantly (11–29%, p = 0.014).
In Era 4 the APD began to show transplant centers multiple combinations of donor-recipient exchanges prior to making formal offers. These potential combinations, which could have been arranged into a large number of overlapping optimized solutions, were then vetted by the clinical teams, allowing them to decline combinations prospectively, thus improving the certitude of the compatibility matrix that was then used to generate the single best optimized non-overlapping solution. Following these modifications we observed a decrease in the 1-way failures from transplant centers rejecting donors from 19 to 15%, though this change was not statistically significant (p 0.12). The success rate improved (14–21%, p 0.014), and the number of transplants/year increased by an additional 37% (26–33tx/yr).
Discussion
To be successful, KPD programs must overcome different barriers. While large donor/recipient pools increase the probability of finding a matching donor for each patient, especially for highly sensitized patients, large pools require efficient cooperation between often geographically and philosophically diverse transplant centers. When trying to coordinate exchanges between multiple transplant centers, each with its own policies and agendas, failures in the matching process lead to frustration and mistrust of the KPD search process. It is not surprising that the KPD program with the highest volume of transplants in the United States operates as a single center, in which coordinated efforts with a unified goal allows failures to be corrected swiftly and efficiently (16).
A KPD program overseeing multiple diverse transplant centers requires tolerance, transparency and muted expectations, accepting that many attempts may be necessary until a successful solution is found. The organization overseeing a multicenter KPD program should develop efficient software and policies that mimic the best practices of a single center approach, recognizing that a single center approach limits overall patient access to KPD (25). Indeed, while being cognizant of the fact that there are real barriers that must be accommodated when working with diverse transplant centers, the search for matches needs to be timely and fair. In reviewing the APD process, it has become clear that factors other than the matching algorithm play a role in generating successful offers. The APD has not only developed and improved its software, but also adjusted its policies on HLA reporting, specifying acceptable donor criteria and incorporating recipient preferences to minimize efforts from participating transplant centers, while maximizing opportunities for patients to find a matching donor.
Evaluation of Era 1 revealed that many offers failed due to positive crossmatches, which was substantially decreased in Era 2. In Era 3, the failures due to positive crossmatch increased from 11 to 29%. We hypothesize that this reflected an attempt to perform Luminex-based bead crossmatching with solubilized donor cells. Due to the clear lack of efficacy for detecting HLA class II-specific antibodies, bead crossmatching was replaced by flow crossmatching in Era 4. Era 4 also saw the initiation of desensitization attempts by transplants centers, which led to more frequent positive crossmatches, as well as the use of endothelial crossmatch by some centers. These three changes may have accounted for the increased positive crossmatch failures. Another contributing factor may be the increasing prevalence of highly sensitized recipients in the pool, which has been predicted and described by other registries and also holds true for the APD (26). Indeed, the number of pool recipients with a PRA 80% has increased from 31.7% in 2007 to 48.9% in 2014 (Figure 1). In an effort to generate transplants for highly sensitized patients, we inevitably had to crossmatch more highly sensitized candidates, which has likely led to an increase in positive crossmatches. While the number of transplanted patients is not large enough to prove causality, the percent of transplanted patients with a PRA 80% has increased from 15.4% in 2007 to 36.0% in 2014.
The APD developed a centralized tissue-typing lab and standardized the required data elements for HLA typing and unacceptable antigens. The centralized lab freezes donor lymphocytes and candidate serum so that, when feasible transplants are identified, crossmatches can be run immediately without requiring the shipment of blood. National KPD programs in the Netherlands, Canada and Australia also utilize centralized tissue-typing laboratories (7,27,28). Though amelioration of positive crossmatch failures has not been consistent, developing standardized tissue typing protocols and retaining recipient and donor blood samples for future crossmatches has improved the efficient, simultaneous evaluation of easy-to-match donors with multiple highly sensitized candidates.
A 2012 KPD consensus conference provided recommended guidelines for histocompatibility testing, and suggested that, by correlating antibody assays with transplant center risk criteria, the goal for KPD virtual crossmatching is to achieve 95% accuracy in laboratory crossmatch prediction (13). A preliminary analysis shows that the APD virtual crossmatch false negative rate was 19% in 2009 and displayed a downward trend each year, ending with 7% in 2014 (data not shown), consistent with recent literature (29–31). With more extensive HLA typing, such as adding DQα and DPβ, it may be possible to reduce false negative virtual crossmatches, but this may come at the expense of eliminating possible transplant opportunities due to positive virtual crossmatches that would not have negatively impacted the clinical outcome. Gombos et al note the challenge of false positive virtual crossmatches limiting viable transplant opportunities; the APD uses the concept of amenable antigens to allow individual centers to specify which DSA are not clinically relevant (32). This balance between reducing both false negative and false positive virtual crossmatches remains an area of active research interest.
Software modifications have been geared toward two distinct endpoints: improving the quality of information collected and processed to improve the compatibility matrix, and minimizing the time required to progress from a match run output to successful transplants. By the end of Era 2, it was apparent that transplant centers were rejecting donors for reasons that were not consistent across centers. For instance, one center would not accept a donor if there was more than a six-inch height discrepancy with the recipient, which was not the case with any other center. The development of discretionary exclusion factors allowed center-specific information to be incorporated into the algorithm.
However, not unexpectedly, transplant centers have sometimes relaxed their exclusion criteria to avoid the possibility of denying a donor they might actually accept. For example, a center might normally exclude any donors over the age of 65, but would accept a 65-year-old donor who presented a zero-antigen mismatch and excellent renal function. Consequently, that transplant center might relax their exclusion criterion in all cases, to avoid unknowingly excluding one acceptable exception. The introduction of combinations in Era 3 allowed transplant centers to accept or reject potential donors in advance of a formal offer, thus allowing the APD the opportunity to develop a more accurate compatibility matrix.
In comparing cycles versus NEAD chains, it is important to note that the algorithm did not actively choose chains over cycles, but only sought to optimize either the quantity or quality of transplants. The predominance of NEAD chains over simple cycles represents two rounds of natural selection. First, chains and cycles freely competed in the optimization process, wherein the algorithm optimized quantity or quality by utilizing chains in 61% of offers. In the second round, the ability of chains to generate transplants in partially completed offers, whereas cycles were required to be fully completed, resulted in chains being responsible for 69% of the total transplants. Thus, chains offer a distinct advantage over cycles.
As can be seen in Table S2, cycles/chain segments fewer than four exchanges in length have had a much higher success rate. Though several longer chain segments were successful, Table 2 shows that a majority of long segments failed before completion, such that 83% of “final” segments were 1, 2, or 3 transplants in length. The APD has never completed an entire set of offers comprising a single optimized solution as first suggested by the computer output. That being said, the APD has been able to successfully build long chains not from a single optimized match run, but by taking advantage of the ability of BDs to extend NEAD chains over time. Had chain segments been immediately directed to the deceased donor waitlist, only 59 transplants with a maximum chain length six would have been achieved (Figure 2c, segment 1). While some of the excluded recipients would have been transplanted through other offers, our results suggest that nonsimultaneous chains achieve more transplants than an approach that relies on a single match run with no BDs.
If KPD registries could build optimal solutions from a perfect compatibility matrix, the 1-way and offer success rates would be 100%. Until then, KPD registries have focused on one or both of two strategies: attempt to minimize the time from offer to failure so that many offers can be extended in as little time as possible until a successful offer is identified, or focus on building systems that improve the accuracy of the compatibility matrix before an offer is made, so that fewer offers fail. The first strategy requires rapid-fire offers, and the second strategy requires time to build a better compatibility matrix. In the United States, competing kidney exchange programs employ both of these strategies. In countries such as the UK, Canada, the Netherlands and Australia where single national KPD programs exist, only the second strategy is used (7,27,28,33). As long as the United States has competing registries that utilize the first approach, and patients enroll in more than one registry, efforts to improve the second approach will not be possible because 1-way failures due to competing offers from systems that employ the first strategy will prevent the second strategy from reaching its fullest potential. While transplants in competing registries benefit the patient transplanted, the other recipients involved in the failed offer will have experienced a delay in finding a donor because time was spent creating an offer with pairs that are not truly available. This inefficiency overall may reduce the total number of transplants achieved nationally.
On the whole, the diversity in strategies employed by the different KPD registries has been instrumental in the evolution of KPD in the United States. However, it is clear that the next phase in the process is the creation of a national registry (9,13–15). Lessons learned through analyzing 7 years of APD efforts raise important philosophical questions with respect to a national registry. While the U.S. performed 588 KPD transplants in 2013, a recent article predicts that 1600 KPD transplants are possible per year in the U.S. (34,35). In order to achieve this end, the strategy adopted by a national registry should incorporate the best aspects of current KPD programs. The process of generating the compatibility matrix used should incorporate as much information as possible so that it will be an accurate reflection of reality, and time should be spent creating the most robust offers possible. Tissue typing should be standardized and streamlined. Offers should be reviewed as expediently as possible, and transplant centers should be transparent and forgiving in their dealings with the KPD registry.
Finally, perhaps the biggest barrier to KPD success in the U.S. is not an issue that can be resolved by software or process changes. A recent article by Massie et al, suggests that an additional 1000 KPD transplants could be performed per year, and in agreement with a recent national consensus conference, identified financial barriers as one of the biggest obstacles to expanding KPD (13,34). Overcoming financial barriers would allow more centers to participate and more incompatible pairs to be enrolled, thus increasing the available pool size and allowing more patients to receive KPD transplants. To address this issue, several authors have suggested a national KPD “standard acquisition charge” approach, and the Alliance for Paired Donation recently received an Agency for Healthcare Research and Quality (AHRQ) grant to pilot such a project (13,15,36–38).
Supplementary Material
Table S1: Variables used in scoring rubric and the values associated with each. This rubric has been amended periodically by the APD’s Scientific Operations Committee, comprised of physicians from participating transplant centers. Last amended 02/2014. *Y Years since recipient was registered. N Number of recipients currently in pool. R Rank in the list of recipients when they are put in order from the person with the most recent registration date to the person with the earliest registration date.
Table S2: Offers, transplants, and success rates by length of cycle or NEAD segment.
Table S3: Major categories of reasons for failure (row 1) and subcategories (rows 2–9).
Acknowledgments
MAR, SMS, and JEK were supported in part by NIAID grants R21 AI-111579 and R01-AI090244. MAR, LJR, SER, and ABL were supported in part by AHRQ grant R18 HS-020610. MAR was funded in part by NIDDK grant R01 DK-093513. AER was funded in part by NSF grant no. 1061932.
Abbreviations
- AHRQ
Agency for Healthcare Research and Quality
- APD
Alliance for Paired Donation
- DPD
domino paired donation
- KPD
kidney paired donation
- MRF
modifiable reason for failure
- NEAD chain
nonsimultaneous extended altruistic donor chain
- NEADseg
nonsimultaneous extended altruistic donor segment
- NDD
nondirected donor
- SR
success rate
- Tx
transplant
- XM
crossmatch
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. The Alliance for Paired Donation has received grant support from Sanofi, Genentech, Novartis, Astellas, Pfizer, and Optum.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Rapaport FT. The case for a living emotionally related international kidney donor exchange registry. Transplant Proc. 1986;18(3) Suppl 2:5–9. [PubMed] [Google Scholar]
- 2.Kwak JY, Kwon OJ, Lee KS, Kang CM, Park HY, Kim JH. Exchange-donor program in renal transplantation: A single-center experience. Transplant Proc. 1999;31:344–345. doi: 10.1016/s0041-1345(98)01655-8. [DOI] [PubMed] [Google Scholar]
- 3.Delmonico FL, Morrissey PE, Lipkowitz GS, et al. Donor kidney exchanges. Am J Transplant. 2004;4:1628–1634. doi: 10.1111/j.1600-6143.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 4.Roth AE, Sonmez T, Unver MU. Kidney exchange. Quarterly Journal of Economics. 2004;119:32. [Google Scholar]
- 5.Montgomery RA, Zachary AA, Ratner LE, et al. Clinical results from transplanting incompatible live kidney donor/recipient pairs using kidney paired donation. JAMA. 2005;294:1655–1663. doi: 10.1001/jama.294.13.1655. [DOI] [PubMed] [Google Scholar]
- 6.Roth AE, Unver MU, Sonmez T. A kidney exchange clearinghouse in New England. Am Econo Rev Pap Proc. 2005;95:376–380. doi: 10.1257/000282805774669989. [DOI] [PubMed] [Google Scholar]
- 7.de Klerk M, Keizer KM, Claas FH, Witvliet M, Haase-Kromwijk BJ, Weimar W. The Dutch national living donor kidney exchange program. Am J Transplant. 2005;5:2302–2305. doi: 10.1111/j.1600-6143.2005.01024.x. [DOI] [PubMed] [Google Scholar]
- 8.Roth AE, Sonmez T, Unver MU, Delmonico FL, Saidman SL. Utilizing list exchange and nondirected donation through “chain” paired kidney donations. Am J Transplant. 2006;6:2694–2705. doi: 10.1111/j.1600-6143.2006.01515.x. [DOI] [PubMed] [Google Scholar]
- 9.Hanto RL, Reitsma W, Delmonico FL. The development of a successful multiregional kidney paired donation program. Transplantation. 2008;86:1744–1748. doi: 10.1097/TP.0b013e3181909035. [DOI] [PubMed] [Google Scholar]
- 10.Wallis CB, Samy KP, Roth AE, Rees MA. Kidney paired donation. Nephrol Dial Transplant. 2011;26:2091–2099. doi: 10.1093/ndt/gfr155. [DOI] [PubMed] [Google Scholar]
- 11.Melcher ML, Leeser DB, Gritsch HA, et al. Chain transplantation: Initial experience of a large multicenter program. Am J Transplant. 2012;12:2429–2436. doi: 10.1111/j.1600-6143.2012.04156.x. [DOI] [PubMed] [Google Scholar]
- 12.Melcher ML, Veale JL, Javaid B, et al. Kidney transplant chains amplify benefit of nondirected donors. JAMA Surg. 2013;148:165–169. doi: 10.1001/2013.jamasurg.25. [DOI] [PubMed] [Google Scholar]
- 13.Melcher ML, Blosser CD, Baxter-Lowe LA, et al. Dynamic challenges inhibiting optimal adoption of kidney paired donation: Findings of a consensus conference. Am J Transplant. 2013;13:851–860. doi: 10.1111/ajt.12140. [DOI] [PubMed] [Google Scholar]
- 14.Segev DL, Kucirka LM, Gentry SE, Montgomery RA. Utilization and outcomes of kidney paired donation in the United States. Transplantation. 2008;86:502–510. doi: 10.1097/TP.0b013e3181812f85. [DOI] [PubMed] [Google Scholar]
- 15.Irwin FD, Bonagura AF, Crawford SW, Foote M. Kidney paired donation: a payer perspective. Am J Transplant. 2012;12:1388–1391. doi: 10.1111/j.1600-6143.2012.03998.x. [DOI] [PubMed] [Google Scholar]
- 16.Bingaman AW, Wright FH, Jr, Kapturczak M, Shen L, Vick S, Murphey CL. Single-center kidney paired donation: The methodist San Antonio experience. Am J Transplant. 2012;12:2125–2132. doi: 10.1111/j.1600-6143.2012.04070.x. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Stegall MD, Dean PG, et al. Assessing the efficacy of kidney paired donation-performance of an integrated three-site program. Transplantation. 2014;98:300–305. doi: 10.1097/TP.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 18.Rees MA, Kopke JE, Pelletier RP, et al. A nonsimultaneous, extended, altruistic-donor chain. N Engl J Med. 2009;360:1096–1101. doi: 10.1056/NEJMoa0803645. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery RA, Gentry SE, Marks WH, et al. Domino paired kidney donation: A strategy to make best use of live non-directed donation. Lancet. 2006;368:419–421. doi: 10.1016/S0140-6736(06)69115-0. [DOI] [PubMed] [Google Scholar]
- 20.Ashlagi I, Gilchrist DS, Roth AE, Rees MA. Nonsimultaneous chains and dominos in kidney-paired donation-revisited. Am J Transplant. 2011;11:984–994. doi: 10.1111/j.1600-6143.2011.03481.x. [DOI] [PubMed] [Google Scholar]
- 21.Gentry SE, Montgomery RA, Swihart BJ, Segev DL. The roles of dominos and nonsimultaneous chains in kidney paired donation. Am J Transplant. 2009;9:1330–1336. doi: 10.1111/j.1600-6143.2009.02622.x. [DOI] [PubMed] [Google Scholar]
- 22.Gentry SE, Segev DL. The honeymoon phase and studies of nonsimultaneous chains in kidney-paired donation. Am J Transplant. 2011;11:2778–2779. doi: 10.1111/j.1600-6143.2011.03802.x. author reply 80–1. [DOI] [PubMed] [Google Scholar]
- 23.Ashlagi I, Gilchrist DS, Roth AE, Rees M. NEAD Chains in transplantation. Am J Transplant. 2011;11:2. doi: 10.1111/j.1600-6143.2011.03481.x. [DOI] [PubMed] [Google Scholar]
- 24.Roth AE, Sonmez T, Unver MU. Efficient kidney exchange: Coincidence of wants in markets with compatibility-based preferences. Am Eco Rev. 2007;97:828–841. doi: 10.1257/aer.97.3.828. [DOI] [PubMed] [Google Scholar]
- 25.Ashlagi I, Roth AE. Free riding and participation in large scale, multi-hospital kidney exchange. Theoretical Economics. 2014;9:817–823. [Google Scholar]
- 26.Li H, Stegall MD, Dean PG, et al. Assessing the efficacy of kidney paired donation–performance of an integrated three-site program. Transplantation. 2014;98:300–305. doi: 10.1097/TP.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 27.Ferrari P, Fidler S, Wright J, et al. Virtual crossmatch approach to maximize matching in paired kidney donation. Am J Transplant. 2011;11:272–278. doi: 10.1111/j.1600-6143.2010.03313.x. [DOI] [PubMed] [Google Scholar]
- 28.Cole E, Malik S. Foundations and principles of the Canadian living donor paired exchange program. Can J Kidney Health Dis. 2014;1:6–12. doi: 10.1186/2054-3581-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis TM, Schiller JJ, Roza AM, Cronin DC, Shames BD, Johnson CP. Diagnostic accuracy of solid phase HLA antibody assays for prediction of crossmatch strength. Hum Immunol. 2012;73:706–710. doi: 10.1016/j.humimm.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Nikaein A, Cherikh W, Nelson K, et al. Organ procurement and transplantation network/united network for organ sharing histocompatibility committee collaborative study to evaluate prediction of crossmatch results in highly sensitized patients. Transplantation. 2009;87:557–562. doi: 10.1097/TP.0b013e3181943c76. [DOI] [PubMed] [Google Scholar]
- 31.Piazza A, Ozzella G, Poggi E, Caputo D, Manfreda A, Adorno D. Virtual crossmatch in kidney transplantation. Transplant Proc. 2014;46:2195–2198. doi: 10.1016/j.transproceed.2014.07.053. [DOI] [PubMed] [Google Scholar]
- 32.Gombos P, Opelz G, Scherer S, et al. Influence of test technique on sensitization status of patients on the kidney transplant waiting list. Am J Transplant. 2013;13:2075–2082. doi: 10.1111/ajt.12332. [DOI] [PubMed] [Google Scholar]
- 33.Hunter J, Heetun M, Sinha S. Finding the perfect match: The living donor paired exchange system. Br J Hosp Med (Lond) 2014;75:202–206. doi: 10.12968/hmed.2014.75.4.202. [DOI] [PubMed] [Google Scholar]
- 34.Massie AB, Gentry SE, Montgomery RA, Bingaman AA, Segev DL. Center-level utilization of kidney paired donation. Am J Transplant. 2013;13:1317–1322. doi: 10.1111/ajt.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Transplant: Donor Relation by Transplant Year (2012–2013) [Internet] U.S. Department of Health & Human Resources; 2013. [cited 2014 Dec 29]. Available from: http://optn.transplant.hrsa.gov/latestData/rptData.asp. [Google Scholar]
- 36.Rees MA, Schnitzler MA, Zavala EY, et al. Call to develop a standard acquisition charge model for kidney paired donation. Am J Transplant. 2012;12:1392–1397. doi: 10.1111/j.1600-6143.2012.04034.x. [DOI] [PubMed] [Google Scholar]
- 37.Tracking Accountability in Government Grants System [Internet] Health Resources & Services Administration; 2013. [cited 2013 Sept 3]. Available from: https://taggs.hhs.gov/RecipInfo.CFM?SelEin=LCYqSys%2BTF5MQzxLVlM6OEsK. [Google Scholar]
- 38.Advisory Committee on Organ Transplantation [Internet] Human Resources & Services Administration; 2012. [cited 2012 Aug 28]. Available from: http://organdonor.gov/legislation/acotaugust2012-notes.html. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Variables used in scoring rubric and the values associated with each. This rubric has been amended periodically by the APD’s Scientific Operations Committee, comprised of physicians from participating transplant centers. Last amended 02/2014. *Y Years since recipient was registered. N Number of recipients currently in pool. R Rank in the list of recipients when they are put in order from the person with the most recent registration date to the person with the earliest registration date.
Table S2: Offers, transplants, and success rates by length of cycle or NEAD segment.
Table S3: Major categories of reasons for failure (row 1) and subcategories (rows 2–9).