Abstract
Immunity to malaria has long been thought to be stage-specific. In this study we show that immunization of BALB/c mice with live erythrocytes infected with nonlethal strains of Plasmodium yoelii under curative chloroquine cover conferred protection not only against challenge by blood stage parasites but also against sporozoite challenge. This cross-stage protection was dose-dependent and long lasting. CD4+ and CD8+ T cells inhibited malaria liver but not blood stage. Their effect was mediated partially by IFN-γ, and was completely dependent of NO. Abs against both pre-erythrocytic and blood parasites were elicited and were essential for protection against blood stage and liver stage parasites. Our results suggest that Ags shared by liver and blood stage parasites can be the foundation for a malaria vaccine that would provide effective protection against both pre-erythrocytic and erythrocytic asexual parasites found in the mammalian host.
Malaria infection is initiated by the inoculation of sporozoites by an infected anopheline female mosquito. Sporozoites are injected into the dermis of the mammalian host where the majority takes several hours before entering the bloodstream (1). From the blood circulation, sporozoites are arrested in the liver where they invade hepatocytes and develop into liver stage parasites. These two parasite forms constitute the pre-erythrocytic stages. The merozoites released from the mature hepatic schizont into the circulation invade erythrocytes and initiate the cyclical asexual erythrocyte cycle responsible for the pathological manifestations of the infection.
The experimental induction of sterile protection has been primarily achieved when whole parasite preparations were used as Ag (2). Immunization with whole parasites has been principally conducted with live sporozoites classically attenuated by irradiation (3, 4) and more recently through genetic modification (5–7). In both cases hepatocytes are invaded by the attenuated sporozoites but none develop to maturity. The sterile protection induced is solely due to the destruction of the pre-erythrocytic stages of the challenge parasites because immunized individuals remain fully susceptible to challenge with blood stage parasites (3–7). These observations, which have been consistently obtained in rodents and humans, helped to establish the stage specificity of acquired immunity as a rule for malaria (8). However, demonstration that the immunity acquired against erythrocytic parasites has no influence on the pre-erythrocytic stages is as yet unavailable. First, until recently it was inherently difficult to demonstrate formally that hosts successfully immunized specifically against erythrocytic stages have not thereby acquired some protection against pre-erythrocytic parasites. Only a few observations were made in this context and these were consistent with stage-specific immunity (9, 10). Second, it had been generally assumed, though not necessarily demonstrated, that the Ags that induce protective immune responses against one parasite stage would not be expressed in the other.
Recent pan-genomic analyses of the proteomes and transcriptomes of the distinct parasite stages observed during the life cycle, have revealed that many Plasmodium proteins are expressed during different stages of the life cycle (11–18), and pertinently that in many cases these included Ags expressed by both pre-erythrocytic and erythrocytic parasites (19–26). This response has raised the possibility that some of these proteins could become the target of parasite-induced immune responses that would then lead to some degree of cross-stage immunity in the mammalian host (24, 25). In the present study, we have investigated the impact of an immunization protocol based on whole erythrocytic parasites on the pre-erythrocytic stages resulting from a sporozoite challenge.
Materials and Methods
Mice and parasites
BALB/cJ female mice were purchased from Harlan Laboratories. Breeding pairs of Ab-deficient homozygous BALB/cJ JhT mice, originally from Taconic Farms, were given by V. Nussenzweig (New York University, New York, NY). All experiments and procedures conformed to the French Ministry of Agriculture 1987 regulations for animal experimentation. Plasmodium yoelii yoelii sporozoites of the uncloned line of the 265BY nonlethal strain (Py265), and of clone 1.1, 17XNL nonlethal strain expressing constitutively GFP (Py17X) (18), were obtained by dissection of the salivary glands of infected Anopheles stephensi mosquitoes 16–21 days after an infected blood meal. Infected RBC (iRBC)3 were obtained from mice infected 8–12 days before with P. yoelii strains 265BY, 17XNL clone 1.1, or P. berghei ANKA (clone BdS) blood stage parasites. Blood samples were collected using heparin (Sigma-ALdrich) as anticoagulant and were then diluted in 10 ml of PBS and washed twice in PBS by centrifugation at 1500 rpm for 10 mn. The percentage of parasitemia was determined by counting the number of iRBC per 1000 RBC on Giemsa-stained blood smears, and the absolute number of iRBC was calculated using total RBC number determined with a Malassez chamber. Py17X parasitemia was determined by cytofluorometry using a previously described protocol (28). Parasitemia between day 8 and 12 was between 10 and 20%. The proportion of gametocytes or of RBC infected with more than one parasite, represented a very minor percentage, <0.1% in each case, of the iRBC used as the immunizing inoculum. It should be noted that 60–80% of the parasites used for immunization were present in reticulocytes.
Immunization schedule and chloroquine treatment
Mice were i.v. injected once or twice with different amounts of P. yoelii 265BY live iRBC (Py-iRBC) or a matching number of normal RBC. Mice were also i.p. injected with 100 μl of 8 mg/ml chloroquine (Sigma-Aldrich) diluted in saline, daily for 10 days starting the day of iRBC injection. In one experiment, chloroquine treatment was extended to 15 days. At the end of the chloroquine treatment, absence of parasites on Giemsa stained blood smears or by cytofluorometry was confirmed in all treated mice.
Challenge and protection assessment
Mice immunized with iRBC and treated with chloroquine were rested for a minimum of 15 days after the last chloroquine injection to allow complete elimination of the drug. The mice were then challenged i.v. with 100 P. yoelii 17X clone 1.1 sporozoites (highly infectious), 4000 P. yoelii 265BY sporozoites, or 105 or 106 Py-iRBC. Blood stage infection was determined by the presence of parasites in Giemsa-stained blood smears or by cytofluorometry on days 4–10 postchallenge. Quantification of parasite load in the liver of animals was performed as previously described (29) with minor modifications. Immunized mice were challenged with 35,000 sporozoites and 42 h later a liver biopsy was performed. At that time, liver parasite maturation is near complete but no merozoites are yet released to initiate a blood stage infection as previously demonstrated using a sensitive nested PCR technique (30). The liver biopsy samples were frozen in liquid nitrogen and crushed in 2 ml of digestion buffer, before incubation overnight at 37°C in the presence of Proteinase K (0.1 mg/ml; Boehringer). For both sample types, DNA to be used for PCR was purified by phenol/chloroform extraction. An initial amplification of β-actin gene fragment was used to verify whether all the samples contained similar amounts of mouse DNA. The DNA was then used as a template in duplicate PCR using oligonucleotides specific for the small subunit ribosomal RNA gene of the parasite. A standard curve corresponding to DNA extracted from equal quantity of erythrocytes in samples containing 10-fold dilutions of a known number of P. yoelii parasite nuclei was made. The PCR products were analyzed on a 2% agarose gel and quantified by scanning densitometry. The standard curve was linear only when the number parasite nuclei per milligram of liver DNA were log transformed. Thus, liver parasite load log units correspond to the log number of parasite nuclei per milligram of liver DNA.
Ab assays
Blood from immunized mice was collected the day before sporozoite or iRBC challenge by retro-orbital puncture. Ab titers in individual mouse serum or pooled serums were determined by an indirect immunofluorescence assay, as previously described (31) on methanol-fixed slides containing air-dried sporozoites, cultured liver stage parasites or air-dried iRBC. Titers were expressed as the reciprocal of the highest positive serum dilution and presented as the mean ± SD for sporozoites and blood stages parasites. Inhibition of sporozoite and liver stage development assay was performed as previously described (32). Briefly, sera (1/10 dilution) from P. yoelii iRBC-immunized mice were added to hepatocyte cultures at the time of sporozoite inoculation and removed 3 h later. Sera from control mice immunized with adjuvant alone were used as control. Schizont numbers were assessed in triplicate cultures by immunofluorescence assay using hyperimmune sera recognizing P. yoelii liver stages as previously described (31). The percentage of inhibition was calculated by comparing the number of parasites in the experimental cultures with the number of parasites in control wells.
In vivo Ab treatment
Purified control rat Abs were purchased from Sigma-Aldrich. Rat IgG2a anti-CD4 mAb (clone GK1.5; American Type Culture Collection (ATCC) TIB 207), and rat IgG2b anti-CD8 mAb (clone 2.43; ATCC TIB 210) were used. The rat IgG2a anti-IL-12 mAb (clone C17.8.20) cell line was provided by Dr. G. Trinchieri (National Cancer Institute, Frederick, MD) (33). The rat IgG1 anti-IL-6 mAb (clone MP5-20F3) and the rat IgG1 anti-IFN-γ (clone XMG-1) (34) cell lines were provided by Dr. P. Minoprio (Institut Pasteur, Paris, France). Abs were purified from culture supernatant by ammonium sulfate precipitation. Immunized mice were injected i.p. with 500 μg of anti-CD8 or anti-CD4 mAb or control rat IgG on days −1 or 0 before challenge. The >98% of blood CD8+ or CD4+ T cells were depleted by this procedure as verified by cytofluorometry (FACScan; BD Biosciences) using anti-CD4 (clone H129-19; Sigma-Aldrich) and anti-CD8 (clone 53-6.7; BD Pharmingen) mAbs that recognized epitopes different from those recognized by the depleting mAbs. IFN-γ neutralization on days −2, −1, 0, +1, and +2 after challenge, mice received a single i.p. dose of 2 mg of the anti-IFN-γ mAb XMG-1. IL-12 neutralization was achieved by i.p. administration of 1 mg of the anti-IL-12 mAb 12 h before and 3 h after challenge. Efficacy of the batches of Abs used in this study was verified previously (35). Because rat IgG1 and IgG2a have a maximal half-life of around 2 and 4 days (36), respectively, the treatment would only effectively neutralize IL-12, IL-6, or IFN-γ at a time when liver stage parasites are present in the animals. Inhibition of NO production was achieved by i.p. injection of a single dose of 100 μg of S-methylisothiourea (SMT; Sigma-Aldrich) in 0.1 ml of PBS, a potent inhibitor of inducible NOS (37), on days 0, +1, and +2 after sporozoites challenge.
IFN-γ ELISA
The 96-well plates (Nunc) coated with anti-IFN-γ (clone R4-6A2; BD Pharmingen) mAb were incubated overnight at 4°C, then the sera were added and the plates incubated overnight at 4°C. Detection was made after adding of second biotinylated Ab (clone XMG1.2; BD Pharmingen) followed by incubation for 45 min at room temperature. Extravidin-phosphatase alkaline was then added and plates were incubated for 1 h at room temperature. The phosphatase activity was measured by adding 4-methylumbelliferyl phosphate (Sigma-Aldrich) as substrate, and reading fluorescence at 360/460 nm using a spectrophotometer. Dilutions of known quantity of IFN-γ were used for the standard curve. The detection limit was 30 pg/ml.
Results
Natural infection induces sterile immunity against blood but not pre-erythrocytic stages
BALB/c mice infected with P. yoelii 265BY sporozoite developed a patent blood infection 3–4 days postinoculation. They controlled the infection and eliminated blood parasites by day 20 (data not shown). Mice were rested for 15 days and then challenged with homologous sporozoites. All the mice that had self-cured after the initial infection were completely protected, whereas naive control mice were not (Fig. 1A). When parasite loads were assessed, using quantitative PCR targeting the parasite’s small subunit ribosomal RNA genes (29), in the livers of mice previously infected with sporozoites and then challenged with homologous sporozoites after self-cure, the development of hepatic parasites was not significantly different from that of control mice (Fig. 1B). This demonstrated that normal sporozoite-induced infection in mice conferred protection against blood but not against liver stage parasites.
FIGURE 1.
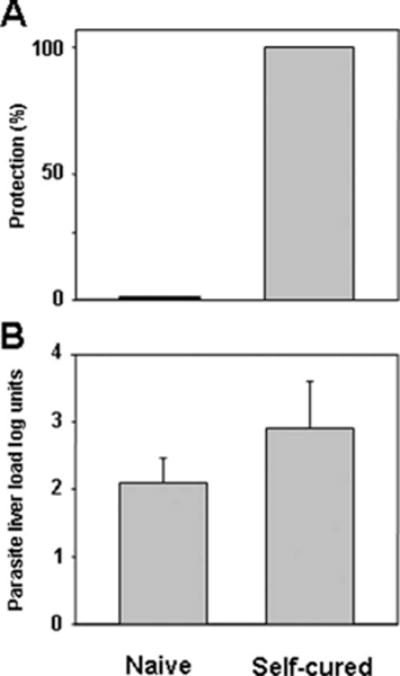
Self-cured infection induced a protective immunity against blood stages but not against liver parasites. Groups of BALB/c mice were i.v. injected with 4000 P. yoelii 265BY sporozoite. All mice develop patent self-limiting blood parasitemia that cured by day 20. The mice were then rested for 15 days before challenge. A, Sterile protection in self-cured mice. Self-cured naive mice were challenged with 4000 homologous sporozoites. All naive mice (n = 5) developed patent blood stage parasitemia after sporozoite challenge. A mouse was considered protected if it did not develop patent blood stage parasitemia by day 10 after sporozoite challenge. Significant difference (p < 0.01) between self-cured naive mice was determined by Fisher’s exact test. B, Groups of naive BALB/c mice were infected allowed to self-cure as in A before challenge with 35,000 live sporozoites, so that the extent of hepatic parasite development could be assessed (29). Results were expressed as mean liver parasite load in log units ± SEM of n = 5 mice.
Immunization with live iRBC under chloroquine cover induces sterile immunity
It has been shown that that patent blood infection could have a suppressive effect on the immunity directed against pre-erythrocytic parasites (38, 39), in which case the development of cross-stage immunity would be hampered. To ascertain whether cross-stage immune responses could be induced, we treated mice immunized with live iRBC with chloroquine, a potent malaria schizonticide. In this manner, the development of blood stage parasitemia was prevented and any suppressive effect minimized. Mice were immunized with Py-iRBC under chloroquine treatment before challenge with homologous sporozoites. It had been previously established that 10 days of chloroquine treatment eliminated all erythrocytic parasites in normal mice (40). Challenge experiments were initiated at least 15 days after the last chloroquine dose at a time when residual drug levels would be so low (36) as to have no measurable effect on parasitemia. This immunization protocol led to dose-dependent protective immunity (Table I), where parasites were not detected following sporozoite challenge by microscopic examination of Giemsa-stained blood smears. Protective immunity was similarly induced when another P. yoelii line, clone 1.1 from the 17XNL strain, was used for immunization and challenge (Table II). One month following the first challenge, the mice immunized with a single dose of 106 Py-iRBC were still completely protected from infection by a sporozoite challenge (5/5 in the immunized group vs 0/5 in the control mice). However, sterile immunity against a similar challenge was not observed in all the mice immunized with a single dose of 105 Py-iRBC (Table I); nonetheless, in the mice where parasitemia was observed, it was of shorter duration and parasite levels were lower than in control animals (Fig. 2A). However, because full protection was also obtained in the mice immunized with 106 Py-iRBC and challenged with Py-iRBC (Table I), it was not possible to ascertain whether a component against the pre-erythrocytic stages contributed to the protective immunity observed.
Table I.
Protection in BALB/c mice after immunization with Py-iRBC
| Inoculationsa
|
Challengeb
|
||||
|---|---|---|---|---|---|
| Immunogen | Dose | No. of Doses | Parasite Form | Infected/Injected | Protection (%) |
| iRBC | 106 | 1 | Spz | 0/10 | 100c |
| iRBC | 105 | 1 | Spz | 7/12 | 41,7c |
| nRBC | 106 | 1 | Spz | 10/10 | 0 |
| iRBC | 105 | 2 | Spz | 0/5 | 100c |
| nRBC | 105 | 2 | Spz | 5/5 | 0 |
| None | None | None | Spz | 10/10 | 0 |
| iRBC | 106 | 1 | iRBC (105) | 0/5 | 100c |
| nRBC | 106 | 1 | iRBC (105) | 5/5 | 0 |
Mice were i.v. injected with live Py-iRBC or normal RBC (nRBC) treated with daily doses of 0.8 mg of chloroquine from day 0 to 10. Data represent pooled results from two experiments using n = 5 mice per group in each experiment.
Mice were challenged i.v. 15 days after the end of the chloroquine treatment with 4000 sporozoites (Spz) or 106 iRBC of P. yoelii 265BY.
p < 0.05 between immunized control mice.
Table II.
Role of B cells in the protection conferred by immunization with iRBC under chloroquine cover
| Mouse Strain | Immunogena | Infected/Injectedb | Protection (%) |
|---|---|---|---|
| WT | None | 5/5 | 0 |
| WT | Py17X-iRBC | 0/5 | 100 |
| BKO | None | 5/5 | 0c |
| BKO | Py17X-iRBC | 3/3 | 0c |
Wild-type (WT) BKO BALB/c mice were immunized with 106 P. yoelii 17XNL clone 1.1 iRBC (Py17X-iRBC) treated with daily doses of 0.8 mg of chloroquine from day 0 to 15.
Mice were challenged by i.v. injection with 4000 sporozoites.
p < 0.05 between immunized WT BKO mice determined by Fischer’s exact test at day 10 postchallenge.
FIGURE 2.
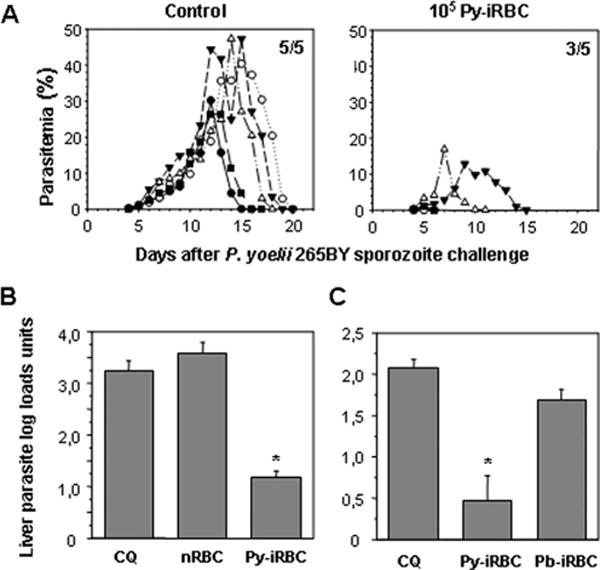
Immunization with iRBC under chloroquine cover induces protective immunity against a sporozoite challenge. A, Blood stage parasitemia in BALB/c mice challenged with 4000 homologous sporozoites 25 days after immunization with 105 Py-iRBC or 105 normal RBC under 10 days chloroquine cover. Parasitemia was monitored in each animal by microscopic examination of Giemsa-stained blood smears. Only points at which parasitemia was patent were plotted. A different symbol is assigned to each animal in the group. The number of mice with patent parasitemia relative to the total number of mice in the group is shown (top right corner). B C, Liver parasite loads in immunized mice challenged with sporozoites. Groups of BALB/c mice were either immunized with matching numbers of normal RBC (nRBC), with 106 Py-iRBC (B) or 106 P. berghei ANKA iRBC (Pb-iRBC) (C) on the day when a 10-day chloroquine treatment was initiated, or treated with chloroquine alone (CQ). Mice were then challenged with 35,000 P. yoelii 265BY sporozoites 25 days or more after immunization, liver parasite development was quantified. Results were expressed as mean liver parasite load log units ± SEM of n = 5 mice. Reduction of parasite load was more than 95% when the arithmetic values were used for calculation. *, p < 0.05, vs control mice using ANOVA followed by Tukey’s test.
Immunity induced with live iRBC inhibits hepatic parasites
We next assessed by quantitative PCR the liver stage parasite loads in the livers of mice immunized with 106 Py-iRBC under chloroquine cover and then challenged with homologous sporozoites. It was clear that in the number or the development of hepatic parasites were drastically curtailed as liver parasite loads in the immunized mice were decreased by >95% as compared with those found in control mice (Fig. 2, B and C). The inhibition of hepatic parasites induced by Py-iRBC immunization was not due to non-specific immune mechanisms because no significant reduction was observed in animals immunized under chloroquine cover with normal RBC (Fig. 2B) or with P. berghei ANKA iRBC (Fig. 2C).
The absence of a full inhibition of liver stage parasites suggested either that those present in the liver of immunized mice were not viable and thus unable to initiate a blood infection, or that viable liver parasites initiated a blood infection that was then very effectively curtailed by anti-blood stage immune responses. Therefore, we subinoculated 100 μl of blood collected 6 days after the sporozoite challenge from the immunized mice into naive recipient mice. One of the recipient mice develop patent parasitemia (Supplemental Table S1),4 demonstrating that some of the remaining liver parasites were viable and capable of initiating a very low grade parasitemia in the immunized mice. When 100 μl of blood were collected at day 7 after sporozoite challenge, patent parasitemia was not observed in the recipient mice, indicating that emerging blood stage parasites were rapidly eliminated in the immunized mice (data not shown).
Effect of Abs in mice immunized with iRBC
Abs present in the sera of mice immunized with 106 Py-iRBC under chloroquine cover reacted against air dried P. yoelii 265BY sporozoites (mean IFA titer of 880 ± 440, n = 5 mice), liver stage parasites (pooled sera IFA titer of 3200), and blood stage parasites (mean IFA titer of 2560 ± 870, n = 5 mice). Equal amounts of IgG1 and IgG2a Abs specific for sporozoites or for blood stage parasites were detected (data not shown). However, the sera from the immunized animals had no detectable effect on in vitro liver stage development when they were added along with P. yoelii 265BY sporozoites to primary hepatocyte cultures (data not shown). This result indicated that the anti-sporozoite and anti-liver stage Abs in the sera were not functional in vitro. BALB/c B cell-deficient (BKO) mice devoid of Abs were then used to assess whether Abs played a role in cross-stage protection in vivo. As expected, BKO that were not treated with chloroquine were unable to control an infection initiated by the blood stages of the nonlethal P. yoelii 17X clone 1.1 (Py17X-iRBC) and mice died with high parasitemia between days 43 and 50 (5 of 5 BKO mice vs 5 of 5 wild-type mice). This confirmed that B cells were crucial for anti-P. yoelii blood stage immunity (41). Furthermore, chloroquine cover for 10 days in BKO mice was not sufficient to suppress the immunizing 106 Py17X-iRBC because parasites were found in the blood of 4 of 5 BKO mice 7 days after the last drug injection. When chloroquine cover was extended for 15 days, the immunizing parasites were cleared in 3 of 5 BKO animals. Upon challenge with sporozoites 15 days after the last chloroquine injection, these three immunized BKO mice, as well as five control BKO mice that received chloroquine for 15 days, developed patent blood stage parasitemia (Table II). Altogether, these observations demonstrated that Abs to erythrocytic but not to pre-erythrocytic parasite forms were essential for sterile protection.
CD4+ or CD8+ T cells are involved in protection induced by Py-iRBC against liver but not blood stage
Treatment of BALB/c mice immunized with 106 Py-iRBC under chloroquine cover with anti-CD4+ T cell, anti-CD8+ T cell Abs fully reversed liver stage inhibition, which was not the case when the mice were treated with control rat IgG (Fig. 3A). Nonetheless, the immunized mice depleted of CD4+ or CD8+ T cells were still protected because parasite could not be detected by microscopy in the blood after challenge (Fig. 3B). These mice were also completely protected against a challenge with blood stage parasites (Fig. 3C). This result indicated that our immunization protocol induced two types of protective immunity, one mediated by T cells that were inhibitory to liver stage parasites and the other mediated by Abs inhibitory to blood stage parasites.
FIGURE 3.
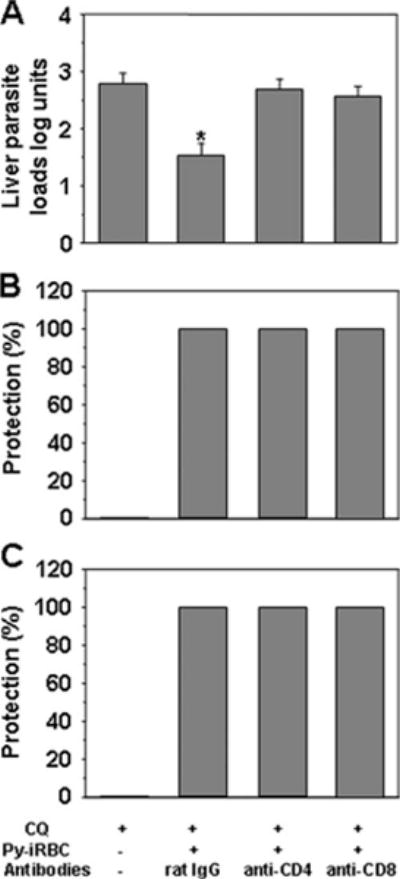
Immunization with iRBC under chloroquine cover induce a protective T cell response against liver but not blood stage parasites. A, Groups of BALB/c mice were immunized with 106 iRBC under chloroquine cover, then i.v. challenged with 35,000 sporozoites 15 days after the last immunizing dose so that the extent of hepatic parasite development could be assessed (29). On day 1 day 0 before the challenge, immunized mice were injected with control rat IgG, rat anti-CD8, or rat anti-CD4. Results were expressed as mean liver parasite load log units ± SEM of n = 5 mice. *, p < 0.05 vs control mice using ANOVA followed by Tukey’s test corresponding to a reduction in parasite load of >95% when the arithmetic values were used for calculation. B, Groups of BALB/c mice were immunized with 106 Py-iRBC under chloroquine cover as in A. Control groups received chloroquine alone. Immunized mice were injected with the control IgG, anti-CD8 or anti-CD4 Abs as described. Mice were challenged with 4000 live sporozoites infection was determined on Giemsa-stained blood smears over a 10-day period. All control mice treated with chloroquine mice (n = 10) developed patent blood stage parasitemia after sporozoite challenge. This experiment was performed twice. C, Groups of BALB/c mice were immunized with 106 iRBC under chloroquine cover as in A. Control groups received chloroquine alone. Immunized mice were injected with the control IgG, anti-CD8 or anti-CD4 Abs as described. Mce were challenged with 105 P. yoelii 265BY iRBC. These mice were completely protected against a challenge with blood stage parasites.
Role of cytokines and NO in the in vivo inhibition of the pre-erythrocytic stages
High levels of circulating IFN-γ were first detected in mice immunized with 106 Py-iRBC under chloroquine treatment 42 h after challenge with homologous sporozoites (Fig. 4A), at a time where blood stage parasites were yet to emerge from the liver. In Py-iRBC-immunized mice, treatment with anti-IFN-γ mAb on the days before and after sporozoite challenge reduced the proportion of fully protected animals (35%) as compared with that obtained in immunized animals (85%) treated with a control Ab (Fig. 3B). The incomplete abrogation of sterile protection by IFN-γ neutralization suggested that other cytokines were involved in the protection. However, Ab neutralization of IL-12 or IL-6, which were previously shown to have anti-liver stage activity (42–45), did not abrogate sterile protection in immunized mice (5 protected of 5 mice in each group).
FIGURE 4.
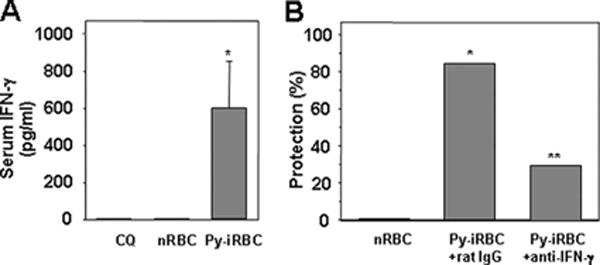
Protection induced by vaccination with Py-iRBC followed by chloroquine treatment is mediated by IFN-γ. A, Concentrations of IFN-γ in the sera collected 42 h after challenge with 2500 sporozoites, from control mice or mice immunized with 106 Py-iRBC or normal RBC under 10-day chloroquine cover starting on the day of immunization. Sera were collected 42 h after challenge with 35,000 sporozoites. Results are expressed as mean ± SEM of n = 5 mice. *, p < 0.05 vs control mice using ANOVA followed by Tukey’s test. B, Groups of BALB/c mice were injected with 106 Py-iRBC treated with chloroquine from day 0 to 10 starting the day of Py-iRBC inoculation. They were challenged i.v. with 4000 sporozoites at least 15 days after the last chloroquine injection. Immunized mice were injected with the rat Abs control rat IgG or anti-IFN-γ. Data are cumulative results of two experiments with n = 4–5 mice per experiment. All control mice treated with chloroquine alone (n = 10) developed patent blood stage parasitemia after sporozoite challenge. Protection was defined as the percentage of the mice that did not develop patent blood stage over 10 days postchallenge in the group that were challenged by sporozoites. **, p <0.05 between immunized mice treated with rat IgG immunized mice treated with anti-IFN—γ Abs, for values on day 10, as calculated by Fisher’s exact test. Number in brackets represents the percentage of protection.
To evaluate the extent with which a known terminal effector of hepatic stage inhibition, NO, contributed to the sterile protection observed, immunized mice were treated with SMT, a potent and selective inhibitor of inducible NO synthase (37). SMT treatment fully abrogated protection after sporozoite but not after blood stage parasites challenge (Table III).
Table III.
Role of NO in the protection conferred by immunization with iRBC during treatment with chloroquine
| Immunogena | Treatmentb | Challengec
|
||
|---|---|---|---|---|
| Parasite Form | Infected/Injected | Protection (%) | ||
| nRBC | None | Spz | 10/10 | 0 |
| iRBC | None | Spz | 2/9 | 78d |
| iRBC | SMT | Spz | 10/10 | 0 |
| nRBC | None | iRBC | 5/5 | 0 |
| iRBC | None | iRBC | 0/5 | 100d |
| iRBC | SMT | iRBC | 0/5 | 100 |
Mice were immunized with 106 normal RBC (nRBC) or 106 Py-iRBC following daily treatment with 0.8 mg of chloroquine for 10 days. Control immunized groups are the same as in Fig. 3B.
Mice were treated with 100 μg of SMT on days 0, +1, +2 after sporozoite (Spz) challenge.
Mice were challenged by i.v. injection with 4000 homologous sporozoites.
p < 0.05 vs mice immunized with normal RBC.
Discussion
In this study we provide evidence that runs counter to the notion that acquired immune responses to Plasmodium parasites are primarily stage-specific. Using mice that had acquired sterilizing immunity solely through exposure to blood stage parasites, we demonstrated that the immune responses included some that have potent inhibitory effects on the pre-erythrocytic stages of challenge parasites. Immunization simply consisted of administering blood stage parasites under chloroquine cover. The efficiency with which sterile immunity was induced using this protocol was dependent of the number of doses and the quantity of immunizing parasites. It was nonetheless possible to obtain full protection reproducibly after a single inoculation of a million parasitized RBC. Under the optimal immunization protocol, sterile protection was observed irrespective of the parasite form used for the challenge: iRBC or sporozoites. The induction of strong inhibitory responses against the pre-erythrocytic stages was clearly revealed by measurements of parasite loads in the livers of immunized mice after sporozoite challenge. Although a 99% reduction in liver load was obtained, some of the liver parasites were still viable and able to lead to a transient blood stage parasitemia that was undetectable by microscopy because it was rapidly inhibited by the immunity acquired to the blood stages. Thus, the sterile immunity observed results from the combined action of immunity against both liver and blood stage parasites.
Our results provided confirmation that patent blood stage infection suppresses the development of cross-stage immunity (38, 39) because in mice infected with sporozoites and challenged with sporozoites after self-cure of the blood stage infection no effect against the liver stages could be observed (Fig. 1), whereas sterile immunity against blood stage parasites was induced. It is likely that this phenomenon accounts for the lack of acquired protective immunity against natural reinfections in inhabitants of endemic areas. Indeed, depending on the endemic site, 80–100% of adults radically cured of blood stage infections get reinfected within a 3-mo period (46, 47).
When the immune responses induced were investigated, it could be demonstrated that both CD4+ and CD8+ cells were necessary to mediate the immunity to liver stage parasites because depletion of either cell types nearly fully reversed the inhibition of liver stage development in the immune mice. The fact that partial reversion of liver stage inhibition was not observed suggested either that the two T cell subsets acted in synergy to eliminate liver stage parasites, or that one subset was important for the expression of the inhibitory activity of the other, for example by controlling migration of effector T cells to the infected liver or of cytokine production. This intriguing phenomenon deserves further studies. Depletion of either of the two T cell subsets did not reverse the protection acquired against blood parasites, either after sporozoite or blood stage parasite challenge, demonstrating that sterile immunity induced by our protocol against the blood stages is T cell-independent.
When the Ab responses were analyzed, it was clear that they played a central role in the elimination of erythrocytic parasites because sterile protection could not be obtained when the immunization was conducted in BKO mice. These observations confirmed previous reports of a crucial role for B cells in the control of blood stage parasitemia (41) or in enhancing parasite elimination during treatment with antimalarial drugs (48). However, although the Abs induced in immunized mice recognized pre-erythrocytic parasites, in vitro functional tests provided no indication that they had an inhibitory activity on sporozoite invasion, or on parasite development in hepatocytes. Nonetheless, it remains to be tested whether the B cells induced were involved in the quality of the T cell responses, a role already demonstrated for P. chabaudi blood stage malaria (48). In a recent study, the sterile protection obtained by immunization with genetically attenuated sporozoites, whose development is arrested in the liver and are, therefore, incapable of leading to a blood stage infection, was equally observed in BKO mice (49). If the antihepatic parasite immune effector mechanisms induced by genetically attenuated sporozoites and via immunization with live sporozoites under chloroquine are broadly similar, the abrogation of protection we observed in BKO mice would be due to the inability of the immunized mice to clear the erythrocytic parasites that would have emerged from the few liver parasites that escaped the cross-stage protective responses we observed. The problems inherent to interpreting data on protection against hepatic stages in hosts fully immune to the blood stages have also been encountered in another recently reported work in which mice were immunized with blood stage of the otherwise lethal P. yoelii YM that was genetically attenuated to give self-limiting infections (50). Following a primary self-cured infection, the mice were equally fully protected from sporozoite or erythrocytic parasite challenges. In that work, however, hepatic parasite loads in the liver were not assessed, and thus it was not possible to ascertain whether a component against the pre-erythrocytic stages contributed to the protective immunity induced.
Previous work had shown that hepatic parasites were susceptible to radical nitrogen intermediates induced by different cytokines (IFN-γ, IL-6, or TNF-α) (51, 52), or after immunization with irradiated sporozoites (53, 54). We showed that the inhibition of pre-erythrocytic parasites induced by blood stage immunization under chloroquine cover was strictly dependent on NO production. Although IFN-γ was shown to play a role against the hepatic parasites in the immunized mice, the levels of protection were not fully reduced by effective Ab neutralization of this cytokine, suggesting the involvement of another mediator. However, we found that the cytokines IL-12 and IL-6 that have been shown previously to mediate the inhibition of liver stage development (42–45), were not to be implicated in protection. Thus, our results imply that, in our immunization model, other cytokines may lead to NO-mediated killing, and identifying them deserves further studies.
Our demonstration of cross-stage immunity relied on measurements of the parasite load in the livers of sporozoite-challenged animals as well as on experimental depletions of defined immunological effectors. These investigations could not be envisaged in humans on ethical grounds. However, in essence the immunization protocol we adopted, inoculation of low parasite numbers whose growth was checked by drug treatment is akin to the immunization with ultra low-dose blood stage parasites used to induce sterile protection in humans against challenge with P. falciparum blood stage parasites (55). It would be interesting to determine whether protective immunity in the human volunteers might extend to pre-erythrocytic parasites. However, demonstrating such protection would require a massive P. falciparum sporozoite challenge, a pre-requisite for the quantification of the parasites emerging from the liver into the bloodstream.
Our demonstration of effective cross-stage immunity strongly indicates that parasite Ags expressed in both blood stage and pre-erythrocytic stage parasites are implicated, though the humoral and cellular immune responses induced appear to act differentially on the two parasite stages. It is at present unclear what Ag leads to the acquisition of the cross-stage immunity demonstrated in this study. A different repertoire of Ags might be responsible for the sterile protection induced by irradiated or genetically attenuated sporozoites because the resulting hepatic forms are arrested at an early stage of development. Although the nature of the Ags responsible for sterile immunity remains speculative, the possibility to develop a vaccine effective against both blood and hepatic stage parasites justifies the efforts that would be needed to identify and characterize the cross-stage protective Ags.
Footnotes
This work was supported by Institut National de la Santé et de la Recherche Médicale. F.T.M.C. was supported by fellowship BEX 0446/98-0 from Coordination for the Improvement of Higher Education Personnel (CAPES) Foundation Brazil. E.B. was supported by a fellowship from Ministère de la Recherche et de la Technologie France. A.M.V. was supported by Fellowship BD/9255/96 from Junta Nacional de Investigação Científica e Tecnológica Portugal. T.V. was supported by Training Grant T32 AI07180 and P.S. by Grant R01 AI056840 from the National Institutes of Health.
Abbreviations used in this paper: iRBC, infected RBC; Py-iRBC, P. yoelii 265BY iRBC; Py17X-iRBC, P. yoelii 17X iRBC; SMT, S-methylisothiourea; BKO, B cell-deficient mice.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Yamauchi LM, Coppi A, Snounou G, Sinnis P. Plasmodium sporozoites trickle out of the injection site. Cell Microbiol. 2007;9:1215–1222. doi: 10.1111/j.1462-5822.2006.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rénia L, Grüner AC, Mauduit M, Snounou G. Vaccination against malaria with live parasites. Expert Rev Vaccines. 2006;5:473–481. doi: 10.1586/14760584.5.4.473. [DOI] [PubMed] [Google Scholar]
- 3.Nussenzweig RS, Vanderberg JP, Spitalny GL, Rivera-Ortiz C, Orton CG, Most H. Sporozoite induced immunity in mammalian malaria: a review. Am J Trop Med Hyg. 1972;21:722–728. doi: 10.4269/ajtmh.1972.21.722. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, Sacci J, de la Vega P, Dowler M, Paul C, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 5.Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433:164–167. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- 6.Mueller AK, Camargo N, Kaiser K, Andorfer C, Frevert U, Matuschewski K, Kappe SH. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc Natl Acad Sci USA. 2005;102:3022–3027. doi: 10.1073/pnas.0408442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dijk MR, Douradinha B, Franke-Fayard B, Heussler V, van Dooren MW, van Schaijk B, van Gemert GJ, Sauerwein RW, Mota MM, Waters AP, Janse CJ. Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc Natl Acad Sci USA. 2005;102:12194–12199. doi: 10.1073/pnas.0500925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nussenzweig RS, Vanderberg JP, Most H, Orton CG. Specificity of protective immunity produced by X-irradiated Plasmodium berghei sporozoites. Nature. 1969;222:488–489. doi: 10.1038/222488a0. [DOI] [PubMed] [Google Scholar]
- 9.Boyd MF, Kitchen SF. Is the acquired homologous immunity to Plasmodium vivax equally effective against sporozoites and trophozoites? Am J Trop Med. 1936;16:317–322. [Google Scholar]
- 10.Richards WHG, Mitchell GH, Butcher GA, Cohen S. Merozoite vaccination of rhesus monkeys against Plasmodium knowlesi malaria: immunity to sporozoite (mosquito-transmitted) challenge. Parasitology. 1977;74:191–198. [Google Scholar]
- 11.Florens L, Washburn MP, Raine JD, Anthony RM, Grainger M, Haynes JD, Moch JK, Muster N, Sacci JB, Tabb DL, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419:520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 12.Grüner AC, Snounou G, Brahimi K, Letourneur F, Rénia L, Druilhe P. Pre-erythrocytic antigens of Plasmodium falciparum: from rags to riches? Trends Parasitol. 2003;19:74–78. doi: 10.1016/s1471-4922(02)00067-3. [DOI] [PubMed] [Google Scholar]
- 13.Grüner AC, Hez-Deroubaix S, Snounou G, Hall N, Bouchier C, Letourneur F, Landau I, P Druilhe P. Insights into the P. y. yoelii hepatic stage transcriptome reveals complex transcriptional patterns. Mol Biochem Parasitol. 2005;142:184–192. doi: 10.1016/j.molbiopara.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Sacci JB, Jr, Ribeiro JM, Huang F, Alam U, Russell JA, Blair PL, Witney A, Carucci DJ, Azad AF, Aguiar JC. Transcriptional analysis of in vivo Plasmodium yoelii liver stage gene expression. Mol Biochem Parasitol. 2005;142:177–183. doi: 10.1016/j.molbiopara.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Hall N, Karras M, Raine JD, Carlton JM, Kooij TW, Berriman M, Florens L, Janssen CS, Pain A, Christophides GK, et al. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307:82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser K, Matuschewski K, Camargo N, Ross J, Kappe SH. Differential transcriptome profiling identifies Plasmodium genes encoding pre-erythrocytic stage-specific proteins. Mol Microbiol. 2004;51:1221–1232. doi: 10.1046/j.1365-2958.2003.03909.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Brown S, Roos DS, Nussenzweig V, Bhanot P. Transcriptome of axenic liver stages of Plasmodium yoelii. Mol Biochem Parasitol. 2004;137:161–168. doi: 10.1016/j.molbiopara.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Tarun AS, Peng X, Dumpit RF, Ogata Y, Silva-Rivera H, Camargo N, Daly TM, Bergman LW, Kappe SH. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci USA. 2008;105:305–310. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szarfman A, Lyon JA, Walliker D, Quakyi IA, Howard RJ, Sun S, Ballou WR, Esser K, London WT, Wirtz RA, et al. Mature liver stages of cloned Plasmodium falciparum share epitopes with proteins from sporozoites and asexual blood stages. Parasite Immunol. 1988;10:339–351. doi: 10.1111/j.1365-3024.1988.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 20.Rénia L, Mattei DM, Goma J, Pied S, Dubois P, Miltgen F, Nüssler A, Matile H, Menégaux F, Gentilini M, et al. A malaria heat-shock-like determinant expressed on the infected hepatocyte surface is the target of antibody-dependent cell-mediated cytotoxic mechanisms by nonparenchymal liver cells. Eur J Immunol. 1990;20:1445–1449. doi: 10.1002/eji.1830200706. [DOI] [PubMed] [Google Scholar]
- 21.Rénia L, Ling IT, Marussig M, Miltgen F, Holder AA, Mazier D. Immunization with a recombinant C-terminal fragment of Plasmodium yoelii merozoite surface protein 1 protects mice against homologous but not heterologous P. yoelii sporozoite challenge. Infect Immun. 1997;65:4419–4423. doi: 10.1128/iai.65.11.4419-4423.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robson KJ, Hall JR, Jennings MW, Harris TJ, Marsh K, Newbold CI, Tate VE, Weatherall DJ. A highly conserved amino-acid sequence in thrombospondin, properdin and in proteins from sporozoites and blood stages of a human malaria parasite. Nature. 1988;335:79–82. doi: 10.1038/335079a0. [DOI] [PubMed] [Google Scholar]
- 23.Grüner AC, Brahimi K, Eling WMC, Konings RNH, Meis JFGM, Aikawa M, Daubersies P, Guérin-Marchand C, Mellouk S, Snounou G, Druilhe P. The Plasmodium falciparum knob-associated PfEMP3 antigen is also expressed at pre-erythrocytic stages induces antibodies which inhibit sporozoite invasion. Mol Biochem Parasitol. 2001;112:253–261. doi: 10.1016/s0166-6851(00)00373-x. [DOI] [PubMed] [Google Scholar]
- 24.Grüner AC, Brahimi K, Letourneur F, Rénia L, Eling WMC, Snounou G, Druilhe P. Expression of the erythrocyte-binding antigen 175 in sporozoites in liver stages of Plasmodium falciparum. J Infect Dis. 2001;184:892–897. doi: 10.1086/323394. [DOI] [PubMed] [Google Scholar]
- 25.Preiser PR, Rénia L, Singh N, Balu B, Jarra W, Voza T, Kaneko O, Blair P, Torii M, Landau I, Adams JH. Antibodies against MAEBL ligdomains M1 M2 inhibit sporozoite development in vitro. Infect Immun. 2004;72:3604–3608. doi: 10.1128/IAI.72.6.3604-3608.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silvie O, Franetich JF, Charrin S, Mueller MS, Siau A, Bodescot M, Rubinstein E, Hannoun L, Charoenvit Y, Kocken CH, et al. A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. J Biol Chem. 2004;279:9490–9496. doi: 10.1074/jbc.M311331200. [DOI] [PubMed] [Google Scholar]
- 27.Franke-Fayard B, Trueman H, Ramesar J, Mendoza J, van der Keur M, van der Linden R, Sinden RE, Waters AP, Janse CJ. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol Biochem Parasitol. 2004;137:23–33. doi: 10.1016/j.molbiopara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Voza T, Vigario AM, Belnoue E, Grüner AC, Deschemin JC, Kayibanda M, Delmas F, Janse CJ, Franke-Fayard B, Waters AP, et al. Species-specific inhibition of cerebral malaria in mice coinfected with Plasmodium spp. Infect Immun. 2005;73:4777–4786. doi: 10.1128/IAI.73.8.4777-4786.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hulier E, Petour P, Snounou G, Nivez MP, Miltgen F, Mazier D, Rénia L. A method for the quantitative assessment of malaria parasite development in organs of the mammalian host. Mol Biochem Parasitol. 1996;77:127–135. doi: 10.1016/0166-6851(96)02584-4. [DOI] [PubMed] [Google Scholar]
- 30.Preiser PR, Khan SM, Costa FTM, Jarra W, Belnoue E, Ogun S, Holder AA, Voza T, Landau I, Snounou G, Rénia L. Stage-specific transcription of distinct repertoires of a multigene family during Plasmodium life cycle. Science. 2002;295:342–345. doi: 10.1126/science.1064938. [DOI] [PubMed] [Google Scholar]
- 31.Marussig M, Rénia L, Motard A, Miltgen F, Pétour P, Chauhan V, Corradin G, Mazier D. Linear multiple antigen peptides containing defined T B epitopes of the Plasmodium yoelii circumsporozoite protein: antibody-mediated protection boosting by sporozoite infection. Int Immunol. 1997;9:1817–1824. doi: 10.1093/intimm/9.12.1817. [DOI] [PubMed] [Google Scholar]
- 32.Nudelman S, Rénia L, Charoenvit Y, Yuan L, Miltgen F, Beaudoin RL, Mazier D. Dual action of anti-sporozoite antibodies in vitro. J Immunol. 1989;143:996–1000. [PubMed] [Google Scholar]
- 33.Wysocka M, Kubin M, Viera LQ, Ozmen L, Garotta G, Scott P, Trinchieri G. Interleukin-12 is required for interferon-γ production lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- 34.Cherwinski HM, Schumacher JH, Brown KD, Mossmann TR. Two types of mouse T helper T cell clone. III. Further differences in lymphokine synthesis between Th1 Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, monoclonal antibodies. J Exp Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belnoue E, Costa FTM, Frankenberg T, Vigário AM, Voza T, Leroy N, Rodrigues MM, Landau I, Snounou G, Rénia L. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J Immunol. 2004;172:2487–2495. doi: 10.4049/jimmunol.172.4.2487. [DOI] [PubMed] [Google Scholar]
- 36.Skarlatos S, Pardridge WM. Targeting of an anti-CR3 (CD11b/CD18) monoclonal antibody to spleen but not brain in vivo in mice. J Drug Target. 1995;3:9–1. doi: 10.3109/10611869509015927. [DOI] [PubMed] [Google Scholar]
- 37.Szabo C, Southan GJ, Thiemermann C. Attenuation of the induction of nitric oxide synthase by endogenous glucocorticoids accounts for endotoxin tolerance in vivo. Proc Natl Acad Sci USA. 1994;91:12472–12476. doi: 10.1073/pnas.91.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orjih A. Acute malaria prolongs susceptibility of mice to Plasmodium berghei sporozoite. Clin Exp Immunol. 1987;61:67–77. [PMC free article] [PubMed] [Google Scholar]
- 39.Ocagna-Morgner C, Mota MM, Rodriguez A. Malaria blood stage suppression of liver stage immunity by dendritic cells. J Exp Med. 2003;197:143–151. doi: 10.1084/jem.20021072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cambie G, Verdier F, Gaudebout C, Clavier F, Ginsburg H. The pharmacokinetics of chloroquine in healthy Plasmodium chabaudi-infected mice: implications for chronotherapy. Parasite. 1994;1:219–226. doi: 10.1051/parasite/1994013219. [DOI] [PubMed] [Google Scholar]
- 41.van der Heyde HC, Huszar D, Woodhouse C, Manning DD, Weidanz WP. The resolution of acute malaria in a definitive model of B cell deficiency, the JHD mouse. J Immunol. 1994;152:4557–4562. [PubMed] [Google Scholar]
- 42.Pied S, Rénia L, Nüssler AK, Miltgen F, Mazier D. Inhibitory activity of IL-6 on malaria hepatic stages. Parasite Immunol. 1991;13:211–217. doi: 10.1111/j.1365-3024.1991.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 43.Pied S, Civas A, Berliot-Picard F, Rénia L, Miltgen F, Gentilini M, Doly J, Mazier D. IL-6 induced by IL-1 inhibits malaria pre-erythrocytic stages but its secretion is down-regulated by the parasite. J Immunol. 1992;148:197–201. [PubMed] [Google Scholar]
- 44.Sedegah M, Finkelman F, Hoffman SL. Interleukin 12 induction of interferon γ-dependent protection against malaria. Proc Natl Acad Sci USA. 1994;91:10700–10702. doi: 10.1073/pnas.91.22.10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nüssler AK, Pied S, Goma J, Rénia L, Miltgen F, Grau GE, Mazier D. TNF inhibits malaria hepatic stages in vitro via IL-6 liver synthesis. Int Immunol. 1991;3:317–321. doi: 10.1093/intimm/3.4.317. [DOI] [PubMed] [Google Scholar]
- 46.Owusu-Agyei S, Koram KA, Baird JK, Utz GC, Binka FN, Nkrumah FK, Fryauff DJ, Hoffman SL. Incidence of symptomatic asymptomatic Plasmodium falciparum infection following curative therapy in adult residents of Northern Ghana. Am J Trop Med Hyg. 2001;65:197–203. doi: 10.4269/ajtmh.2001.65.197. [DOI] [PubMed] [Google Scholar]
- 47.Sagara I, Sangaré D, Dolo G, Guindo A, Sissoko M, Sogoba M, Niambélé MB, Yalcoué D, Kaslow DC, Dicko A, et al. A high malaria reinfection rate in children young adults living under a low entomological inoculation rate in a periurban area of Bamako, Mali. Am J Trop Med Hyg. 2002;66:310–313. doi: 10.4269/ajtmh.2002.66.310. [DOI] [PubMed] [Google Scholar]
- 48.Langhorne J, Cross CE, Seixas EM, Li C, von der Weid T. A role for B cells in the development of T cell helper function in a malaria infection in mice. Proc Natl Acad Sci USA. 1998;95:1730–1734. doi: 10.1073/pnas.95.4.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mueller AK, Deckert M, Heissw K, Goetz K, Matuschewski K, Schlüter D. Genetically attenuated Plasmodium berghei liver stages persist elicit sterile protection primarily via CD8 T cells. Am J Pathol. 2007;171:107–115. doi: 10.2353/ajpath.2007.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ting LM, Gissot M, Coppi A, Sinnis P, Kim K. Attenuated Plasmodium yoelii lacking purine nucleoside phosphorylase confer protective immunity. Nat Med. 2008;14:954–958. doi: 10.1038/nm.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nüssler AK, Drapier JC, Rénia L, Pied S, Miltgen F, Gentilini M, Mazier D. L-arginine dependent destruction of intrahepatic malaria parasite in response to tumor necrosis factor and/or interleukin 6 stimulation. Eur J Immunol. 1991;21:227–230. doi: 10.1002/eji.1830210134. [DOI] [PubMed] [Google Scholar]
- 52.Mellouk S, Green SJ, Nacy CA, Hoffman SL. IFN-γ inhibits development of Plasmodium berghei exoerythrocytic stages in hepatocytes by an L-arginine-dependent effector mechanism. J Immunol. 1991;146:3971–3976. [PubMed] [Google Scholar]
- 53.Nüssler AK, Rénia L, Pasquetto V, Miltgen F, Matile H, Mazier D. In vivo induction of the nitric oxide pathway in hepatocytes after injection with irradiated malaria sporozoites, malaria blood parasites or adjuvants. Eur J Immunol. 1993;23:882–887. doi: 10.1002/eji.1830230417. [DOI] [PubMed] [Google Scholar]
- 54.Seguin MC, Klotz FW, Schneider I, Weir JP, Goodbary M, Slayter M, Raney JJ, Aniagolu JU, Green SJ. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon-y CD8+ T cells. J Exp Med. 1994;180:353–358. doi: 10.1084/jem.180.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pombo DJ, Lawrence G, Hirunpetcharat C, Rzepczyk CM, Bryden M, Cloonan N, Anderson K, Mahakunkijcharoen Y, Martin LB, Wilson D, et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet. 2002;360:610–617. doi: 10.1016/S0140-6736(02)09784-2. [DOI] [PubMed] [Google Scholar]


