Abstract
Objective
Combined inhibition of PI3K and PARP has been shown to be effective in the treatment of preclinical models of breast cancer and prostate cancer independent of BRCA or PIK3CA mutational status. However, the knowledge about this combination treatment in ovarian cancer is limited. The aim of this study was to evaluate the therapeutic effect of PI3K inhibitor BKM120 and PARP inhibitor Olaparib on ovarian cancer cell lines bearing wild-type PIK3CA genes.
Methods
We exposed three wild-type PIK3CA ovarian cancer cell lines to a PI3K inhibitor BKM120 and /or a PARP inhibitor Olaparib. The effect of BKM120 as a single-agent or in combination with Olaparib was evaluated by Cell Count Kit (CCK8) assay, immunoblotting, comet assay, flow cytometry and immunofluorescence staining assay. The combination indexes for synergistic effect on cell viability were calculated with the Chou-Talalay method. Ex vivo cultured ovarian cancer tissues from patients were analyzed by histological and immunohistochemical analyses.
Results
Combined inhibition of PI3K and PARP effectively synergized to block the growth of three wild-type PIK3CA ovarian cancer cell lines and explants of a primary ovarian tumor specimen. Mechanistically, dual blockade of PI3K and PARP in these ovarian cancer cell lines resulted in substantially attenuated PI3K/AKT/mTOR signaling, impaired DNA damage response and deficient homologous recombination repair, with remarkable BRCA downregulation.
Conclusions
The combined use of PI3K inhibitor BKM120 and PARP inhibitor Olaparib may be effective in ovarian cancers with a broader spectrum of cancer-associated genetic alterations but not limited to those with mutant PIK3CA or BRCA genes. BRCA downregulation may be a potential biomarker for the effective response to the proposed combination treatment.
Keywords: Ovarian cancer, BKM120, Olaparib, BRCA, combination therapy
Introduction
The phosphoinositide 3-kinase (PI3K) pathway is an important signaling network that regulates a wide range of cellular processes [1, 2]. Accumulating evidence revealed that the PI3K/AKT signaling was deregulated in a significant fraction of ovarian cancers, justifying clinical trials of PI3K-directed therapeutics for this challenging disease [3, 4]. A number of PI3K inhibitors have shown significant anti-tumor activities either as single-agents or when used in combination with cytotoxic anti-cancer agents in in vitro and in vivo models of ovarian cancers [5, 6]. While PI3K inhibitors demonstrated a high response rate in patients with mutant PIK3CA, recent studies revealed that some tumors with wild-type PIK3CA gene also appear to benefit from PI3K inhibition [7,8]. BKM120, a pan-class I PI3K inhibitor currently has exhibited antitumor activity in Phase I/II clinical trials, and in a variety of cell lines and xenograft models of cancers with or without aberrant PI3K pathway activation [9–12]. In addition, recent studies showed that PI3K blockade with BKM120 resulted in impaired DNA homologous recombination repair and sensitization to poly (ADP-ribose) polymerase (PARP) inhibitor Olaparib in TNBCs with proficient or deficient BRCA genes [13,14], providing a rationale for clinical trials of dual PI3K and PARP inhibition in cancers with a broader spectrum of cancer-associated alterations.
Homologous recombination is defective in approximately half of all high-grade serous ovarian cancer cases [3], which shed new light on clinical trials of PARP inhibitors targeting this subtype of ovarian tumors. It has been reported that PARP inhibitors show response rates of 30–45% in BRCA1/2 mutation carriers with platinum-sensitive relapsed high-grade serous ovarian cancer [15, 16]. These clinical investigations have led to the FDA approval of the PARP inhibitor Olaparib as the first “personalized therapy” for advanced ovarian cancer with BRCA deficiency [17]. Thus, while some BRCA deficient ovarian cancers responded to PARP inhibitors [18], there is still a substantial fraction of BRCA deficient ovarian cancers do not respond to PARP inhibition [19]. These discrepancies in clinical observations raise the question of how to increase the response rate of ovarian cancer to PARP inhibitors. To address this question, we explored the effect of combined use of PI3K and PARP inhibitors on ovarian cancer cell lines with mutant PIK3CA [20] and those with wild-type PIK3CA. In the current study, we showed that combined use of BKM120 and Olaparib effectively treated wild-type PIK3CA ovarian cancer cells and that concomitant downregulation of BRCA expression is likely a biomarker to predict the effective response to combined inhibition of PI3K and PARP in ovarian cancer. These studies suggest PI3K inhibition as a plausible strategy for expanding the utility of PARP inhibitors to a broader spectrum of ovarian cancer.
Materials and methods
Cell culture
The human ovarian cancer OVCAR8, OVCAR5 and OVCA433 cell lines were obtained from Dana-Farber/Harvard Cancer Center. The culture condition of OVCAR8, OVCAR5 and OVCA433 cells have been described earlier [21]. All these cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS). All cell cultures were conducted at 37 °C in 5% CO2. In cell line OVCAR8, BRCA1 was methylated and there was a corresponding decrease in gene expression [21]. The cell line OVCA433 has a deleterious BRCA2 heterozygous mutation which does not show loss of heterozygosity, and the cell line OVCAR5 has wild-type BRCA1 and BRCA2 [21].
Clonogenic assay
The effects of the given drugs on cell proliferation were evaluated as previously described [22]. Briefly, cells were seeded into six-well plates at 5000 cells/well density and then treated with indicated concentration of BKM120, combination (BKM120 plus Olaparib at the respective concentrations) or dimethyl sulfoxide (DMSO) control. Cell culture medium containing drug or vehicle control was changed every 3 days. The cells were incubated for about 10 days. Cells were fixed with cold methanol, stained with crystal violet (Sigma–Aldrich) and subsequently extracted with 10% glacial acetic acid. The optical density (OD) was measured at 570 nm by EnSpire® Multimode Plate Readers (PerkinElmer).
Growth inhibition assay and drug combination analysis
Cell viability was evaluated by the Cell Counting Kit-8 assay (Dojindo Molecular Technologies), according to the manufacturer’s guidelines and as previously described [23]. The half-maximal inhibitory concentration (IC50) values were calculated from dose-response curves utilizing Prism. The combination effect was determined by the combination index (CI) method [24] using the Calcusyn software program (Biosoft). Data from cell viability assays were expressed as the fraction of growth inhibition by the individual drugs or the combination in drug-treated cells. Synergism was indicated by a CI values less than 1 and antagonism by a CI value more than 1 at 50% effect (Fraction Affected = 0.5).
Protein isolation and immunoblotting
Cells were exposed to BKM120 and/or Olaparib for the indicated times and concentrations. The cells were then lysed in RIPA buffer supplemented with protease and phosphatase inhibitors as described [14]. We used the following primary antibodies: phosphorylated AKT (pAKT) (Ser473), phosphorylated ERK1/2 (pERK1/2) (Thr202/Tyr204), cleaved-PARP, phosphorylated ribosomal protein S6 (pS6RP) (Ser235/Ser236) (Cell Signaling Technology), BRCA1 (Proteintech), and vinculin (Sigma-Aldrich).
Apoptosis analysis
Apoptosis in ovarian cancer cells were analyzed with Annexin V-FITC Apoptosis Detection Kit (Dojindo Molecular Technologies) according to manufacturer’s instructions. Briefly, cultured cells were trypsinized with 0.25% trypsin without EDTA, and stained with Annexin V-FITC and Propidium iodide (PI) solution. Stained cells were then subjected to flow cytometry analysis on a BD Accuri™ C6 (BD Biosciences, NJ).
Comet assay
Cells were treated and harvested 48h after drugs treatment, and subjected to the neutral comet assay as described [25]. Following electrophoresis, the cells were stained with ethidium bromide. We analyzed about 200 individual cell images from each group using Comet Assay Software Pect (CaspLab) software. Tail intensity (percentage DNA in the tail) was defined and served as a quantitative measure of DNA damage.
Immunofluorescence staining analysis of γH2AX and RAD51
OVCA433, OVCAR5, and OVCAR8 cells were cultured on coverslips in 24-well plates in respective medium containing inhibitors for 48 hours. The cells were incubated with rabbit anti-RAD51 polyclonal antibody (Santa Cruz Biotechnology) or rabbit anti-γH2AX (Ser139) polyclonal antibody (Cell Signaling Technology), and then incubated with secondary antibodies and DAPI. Images were acquired and quantified using an immunofluorescence microscope (Leica). The dynamics of phosphorylated histone H2AX (γH2AX) and RAD51 foci accumulation, as well as percentage of positive cells (more than 5 foci in one cell) were calculated based on analysis of about 200 cells.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
The relative expression of BRCA1 and BRCA2 mRNAs were detected using qRT-PCR. Briefly, total RNAs were isolated from cultured cells with TRIzol reagent (Life Technologies), according to the manufacturers’ instructions. Reverse transcription reactions were performed using PrimeScript™ RT reagent Kit with gDNA Eraser (Takara). To quantify the amount of transcripts, SYBR Green based qPCR was performed with PrimeScript™ RT Master Mix (Takara) using Real Time PCR System (Stratagene Mx3000p). The human BRCA1 forward primer was 5’-GTCCCATCTGTCTGGAGTTGA-3’, the reverse primer was 5’-AAAGGACACTGTGAAGGCCC-3’. The human BRCA2 forward primer was 5’ -AAAGGACACTGTGAAGGCCC-3’, and the reverse primer was 5’-TTCTTCCTCTCTTTCATTGCG-3’. The specificity of amplicons was verified with melting curve analysis and the messenger levels were normalized using GAPDH, as an internal control.
Patient information, tissue preparation and ex vivo culture of patient tumor tissue
We present the case of a 58-year-old patient with newly diagnosed primary high-grade serous ovarian cancer without prior treatment or previous family history. The wild-type BRCA1/2 genes (no deleterious mutation detected) were documented in the medical record. Tumor tissue acquisition was performed under an Institutional Review Board protocol approved at the Second Hospital of Dalian Medical University. The primary ovarian tumor specimens were obtained from this patient who underwent a bilateral oophorectomy at the Department of Gynecology, the Second Hospital of Dalian Medical University and subjected to RNA isolation and ex vivo culture experiments.
Total RNAs were isolated with TRIzol reagent (Life Technologies) and used as a template in the reverse transcription reaction using PrimeScript™ RT reagent Kit (Takara) to generate cDNAs. PCR amplification for cDNA sequences covering all exons of the PIK3CA gene was conducted using Q5 High-Fidelity DNA Polymerase (New England Biolabs). Sequencing analysis (Sangon Biotech, Shanghai) of the amplified PCR products revealed no somatic mutations in the PIK3CA gene.
For ex vivo culture, 1 cm2 hemostatic gelatin dental sponges were hydrated in explant media in which RPMI 1640 base was supplemented with 10% FBS, Antibiotic/Antimycotic solution, Hydrocortisone (1mg/100ml), and insulin (1mg/100ml) [26]. While the sponges were hydrating, tissues cut into 1 mm3 blocks were transferred to the top surface of gelatin sponges. Tissues were placed near the edges of the sponge to allow for media perfusion in between tissue sections. Each treatment condition was examined with at least three pieces of tissue per sponge. Following plating, tissue blocks were allowed to recover in explant media for 24 hours. Tissue blocks were then treated for 48 hours with PI3K inhibitor BKM120 and PARP inhibitor Olaparib as single-agents or in combination. All tissues were cultured at 37°C and 5% CO2 until harvest. Tissue blocks were removed from culture, fixed with paraformaldehyde and processed for immunohistochemical analyses.
Histology and immunohistochemistry
Tissue blocks were fixed in 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Immunohistochemistry (IHC) was carried out using the antibodies Ki67 (Vector), pAKT (Ser473) (Invitrogen), pS6RP, γH2AX (Cell Signaling Technology), BRCA1 (Proteintech) and RAD51 (Santa Cruz). All IHCs were done as described previously [26]. Staining was evaluated quantitatively by the Image-Pro Plus software (Media Cybernetics). The staining intensity of Ki67, cleaved-caspase3, γH2AX, RAD51 and BRCA1 was quantified as the percentage of staining positive cells per high power field. The pS6RP and pAKT staining were quantified by integrated optical density (IOD) equaled to average optical density by area.
Statistical analysis
Data were expressed as mean ± SD. Quantitative results were analyzed by two-tailed unpaired Student’s t test. P < 0.05 was considered statistically significant. All statistical analyses were performed using the GraphPad Prism 5.0 (San Diego, CA, USA).
Results
Combined use of BKM120 and Olaparib synergistically inhibited the growth of wild-type PIK3CA ovarian cancer cells
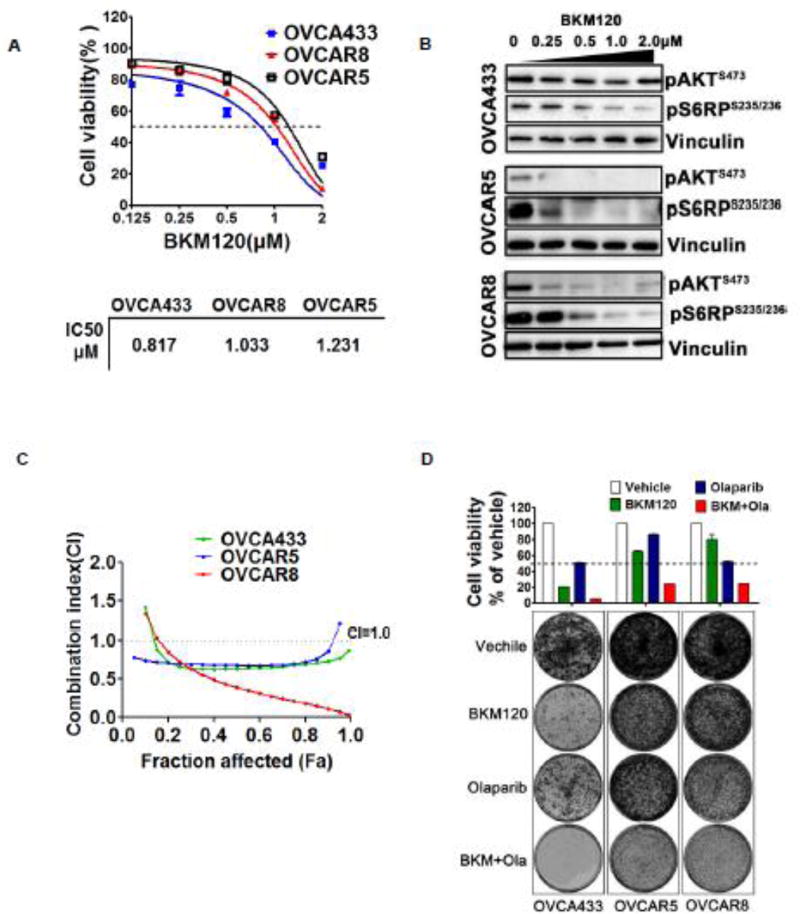
To evaluate the efficacy of PI3K inhibitor BKM120 in wild-type PIK3CA ovarian cancer cells, we chose OVCA433, OVCAR5, and OVCAR8 cells. Cell viability assay using Cell Counting Kit-8 (CCK-8) revealed the IC50s of OVCA433, OVCAR5, and OVCAR8 for BKM120 are 0.817µM, 1.033µM, and 1.231µM, respectively (Fig. 1A). Western blot analysis showed that while BKM120 treatment resulted in substantial downregulation of phosphorylated AKT signals in OVCAR5 and OVCAR8 cells but not in OVCA433 cells, phosphorylated S6RP signals were dramatically attenuated in all three cell lines in a dose-dependent manner (Fig. 1B), validating target inhibition of mTOR signaling as a result of PI3K inhibition by BKM120.
Fig. 1. Effects of BKM120 and in combination with Olaparib on growth inhibitory and PI3K/mTOR pathway of wild-type PIK3CA ovarian cancer cells.
(A) IC50s values of three ovarian cancer cell lines (OVCA433, OVCAR5, and OVCAR8) treated with BKM120 for 72 hours were calculated to the data of the CCK8 assay. (B) The given cell lines were treated with the indicated concentration BKM120 for 48 hours. Western blot analysis of proteins (pAKT and pS6RP) in ovarian cancer cells treated with BKM120. Vinculin served as a loading control. Representative images from three independent experiments were shown. (C) The three ovarian cancer cell lines were treated with BKM120 and Olaparib as single-agents or in combination for 72 hours and then subjected to CCK8 assay. The combined drug effect was analyzed using the CI equation and presented with FA combinations. (D) Ovarian cancer cells were treated with inhibitors as indicated for 10 days and then crystal violet stained. Mean ± S.D. for 3 independent experiments are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student’s t test).
We next evaluated the synergistic effect of drug combination on cell viability using CCK-8 assay followed by median-effect analysis. We treated three wild-type PIK3CA ovarian cancer cells with increasing concentrations of BKM120 and Olaparib, each alone and in combination for 72 hours. Combined treatment with BKM120 and Olaparib resulted in a synergistic increase in viability inhibition at 0.5 fractions affected (FA) and combination index (CI) values of less than 1 over the majority of concentrations tested in OVCA433, OVCAR5 and OVCAR8 cells (Fig. 1C), suggesting a synergistic effect of the combination treatment on all three wild-type PIK3CA ovarian cancer cell lines.
To determine the long-term effect of drug combination on wild-type PIK3CA ovarian cancer cells, we conducted clonogenic assay. PI3K inhibitor BKM120 as single-agent markedly reduced the proliferation of OVCA433, OVCAR5 and OVCAR8 cells, whereas PARP inhibitor Olaparib exhibited only moderate growth inhibitory effect (Fig. 1D). Furthermore, combined use of BKM120 and Olaparib nearly completely suppressed the growth of OVCA433, and strongly blocked the growth of OVCAR5 and OVCAR8 cells (Fig. 1D). Thus, in addition to PIK3CA mutant ovarian cancer cells [20], combined use of BKM120 and Olaparib may also have the potential to effectively treat wild-type PIK3CA ovarian cancer cells.
Combined use of BKM120 and Olaparib induced apoptosis in wild-type PIK3CA ovarian cancer cells
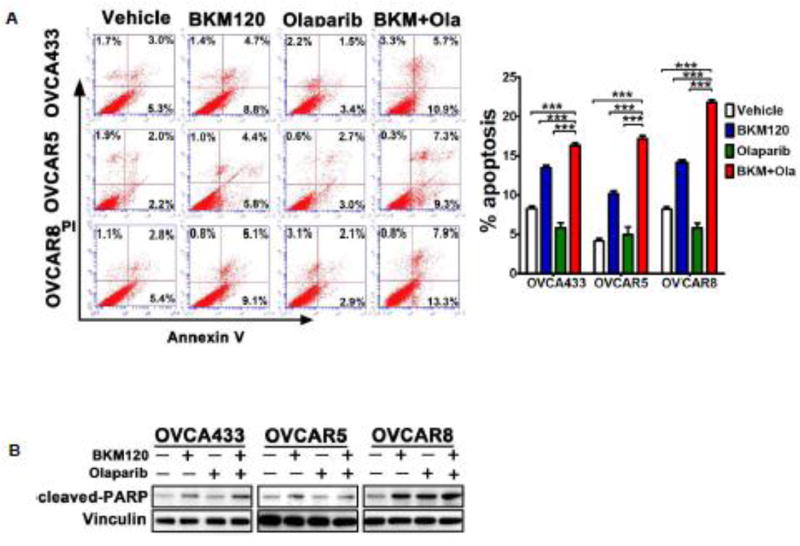
To determine the effect of dual inhibition of PI3K and PARP on cell death, we next conducted apoptosis assay using Annexin-V by FACS analysis in the three ovarian cancer cell lines. While inhibition of PI3K or PARP monotherapy each yielded an increase in apoptotic cell population (Annexin-V positive) to some degree, combined treatment led to significantly increased apoptosis in all three ovarian cancer cell lines examined (Fig. 2A). Consistently, combination treatment also induced a markedly increased level of cleaved PARP, a marker for active apoptosis, in these ovarian cancer cells (Fig. 2B). Together, these results indicated that dual inhibition of PI3K and PARP has potential therapeutic efficacy in ovarian cancer.
Fig. 2. Effects of BKM120 and Olaparib as single-agents and in combination on the survival of ovarian cancer cells.
(A) Ovarian cancer cells were treated by BKM120 (1µM) and Olaparib (2µM), each alone or in combination, for 48 hours. The percentage of apoptotic cells was determined by Annexin V and PI staining. Mean ± S.D. for three independent experiments are shown. ***, P <0.001 (Student’s t test) (B) Western blot analysis of cleaved-PARP in ovarian cancer cells treated as indicated for 48 hours. Vinculin was used as a loading control.
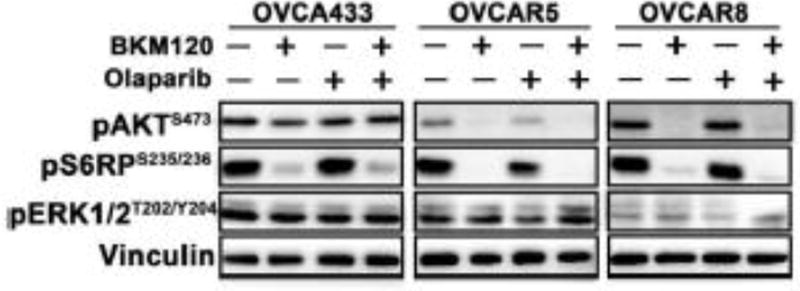
BKM120 as single-agent or in combination with PARP inhibitor Olaparib sufficiently attenuated mTOR signaling downstream of PI3K
BKM120 is a pan-PI3K inhibitor that specifically targets all class I PI3K and inhibits PI3K downstream effector signaling [11]. Western blot analysis revealed that BKM120 as single-agent or in combination with PARP inhibitor Olaparib resulted in substantially reduced phosphorylated S6RP signal, a downstream effector of PI3K/AKT/mTOR signaling, in all three ovarian cancer cell lines examined (Fig. 3). Of note, while BKM120 as single-agent or in combination with Olaparib markedly reduced phosphorylated AKT signals in OVCAR5 and OVCAR8 cells, little effect was seen with AKT phosphorylation in OVCA433 cells (Fig. 3). We next further examined the effect of the PI3K inhibitor BKM120 on OVCA433 cells (PIK3CA wild-type) in a time-dependent manner. Treatment with BKM120 inhibited phosphorylation of AKT by 2 hours after drug was added (Supplementary Fig. S1). At 4 hour, AKT phosphorylation began to rise but remained inhibited at 8 hour. It is thus likely that alterations in the signaling nodes (but not PIK3CA encoding p110α) of the PI3K pathway may contribute to the oncogenic rewiring and rebounded AKT phosphorylation in OVCA433 cells exposed to PI3K inhibition (Fig. 1B).
Fig. 3. Effects of BKM120 and Olaparib as single-agents and in combination on PI3K/mTOR and MEK pathway of ovarian cancer cells.
Western blot analysis of proteins (pAKT, pERK and pS6RP) as indicated in ovarian cancer cells treated with BKM120 and Olaparib as single agents or in combination for 48 hours. Vinculin was used as a loading control.
It has been recently reported that inhibition of S6RP phosphorylation by mTOR inhibitor rapamycin restores the sensitivity to PARP inhibitor Olaparib [28]. We next examined if reduced S6RP phosphorylation as a result of PI3K inhibition by BKM120 may sensitize OVCA433 cells to PARP inhibition by Olaparib (Fig. 1D). For this, we used RAD001, an mTOR inhibitor, to downregulate S6RP phosphorylation. The combined use of RAD001 and Olaparib synergize to inhibit the growth of OVCA433 cells and induce DNA double-strand breaks (Supplementary Fig. S2). Together, our results suggested that phosphorylated S6RP signals may be a potential predictive biomarker for the response to the combination treatment in these ovarian cancer cells.
Combined use of BKM120 and Olaparib results in DNA damage with deficient homologous recombination repair
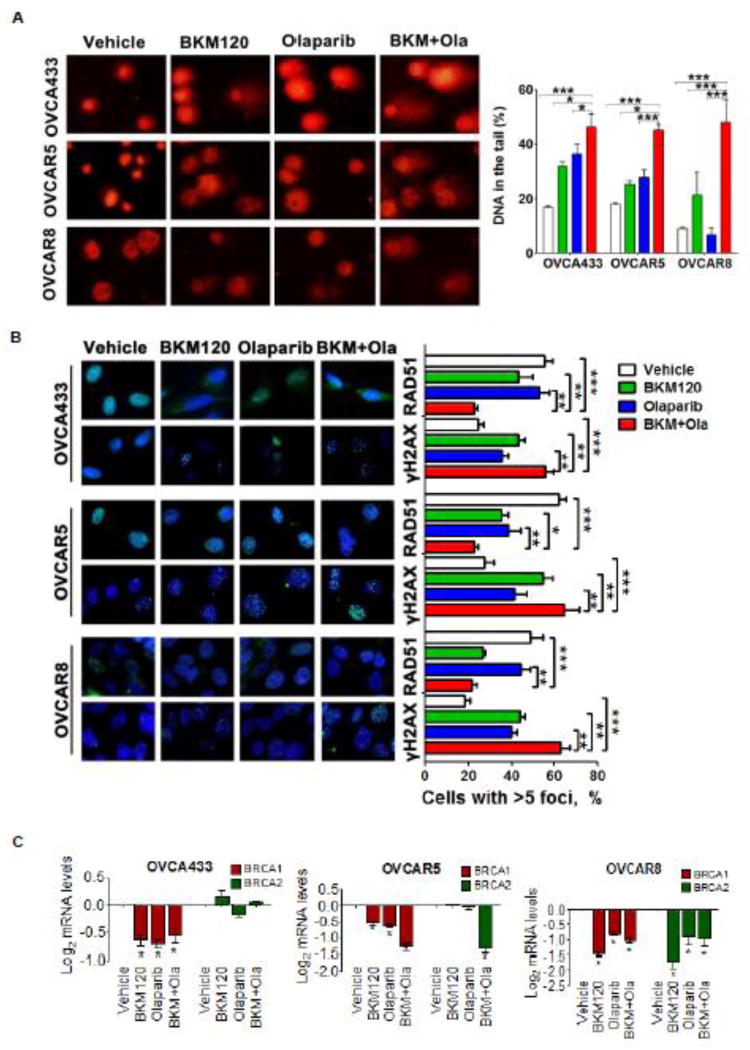
To understand the therapeutic effect of the combination treatment as shown above, we exposed ovarian cancer cells, OVCA433, OVCAR5 and OVCAR8, to drug treatment and then subjected them to a comet assay to evaluate the extent of DNA damage. Compared to the single-agent treatments, the dual treatment with BKM120 and Olaparib generated the highest tail intensity in all three cell lines examined, indicating the production of large numbers of DNA double-strand breaks as a result of dual inhibition of PI3K and PARP (Fig. 4A).
Fig. 4. Effects of BKM120 and Olaparib as single-agents and in combination on DNA damage response in ovarian cancer cells.
(A) DNA damage in ovarian cancer cells was determined by comet assay. The ovarian cancer cells as indicated were treated with BKM120 and Olaparib as single-agents or in combination for 48 hours. Comet images ×200 taken by fluorescent microscope were shown. Tail intensity was used to quantify DNA damage and evaluated by CASP software (CaspLab). Means ± S.D. of three independent experiments are shown. (B) Representative images of immunofluorescence staining of γH2AX and RAD51 in ovarian cancer cells treated as indicated for 48 hours (left panel). Cell nuclei were stained with DAPI. The percentage of cells with γH2AX and RAD51 foci was shown (right panel). Means ± S.D. of three independent experiments are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student’s t test). (C) Quantitative reverse transcription PCR analysis of BRCA1 and BRCA2 expression in ovarian cancer cells treated with BKM120 and Olaparib as single-agents and in combination. Gene expression was normalized to GAPDH. Mean ± S.D. for three independent experiments are shown. *, P < 0.05 (Student’s t test).
We next determined the effect of drug treatment on the repair of double-stranded DNA breaks in ovarian cancer cells with wild-type PIK3CA. For this, we examined nuclear foci of γH2AX, a biological marker for DNA double-stranded breaks by immunofluorescence staining. For all three ovarian cancer cell lines examined, combined use of BKM120 and Olaparib led to significantly increased number of γH2AX foci (Fig. 4B). The failure to repair DSBs by homologous recombination underlies the sensitivity of cancer cells to PARP inhibitor [13,14,29]. To examine the impact of drug treatment on the ability of ovarian cancer cells to repair double-strand DNA breaks, we conducted immunofluorescence staining analysis of RAD51 protein, a marker for the competency of homologous recombination repair. We observed significantly reduced RAD51 foci formation in drug combination-treated cells compared to those in single-agent or vehicle treated cells (Fig. 4B), indicating impaired homologous recombination repair as a result of dual inhibition of PI3K and PARP.
Recent studies reported that BRCA1/2 downregulation may represent a potential biomarker for the treatment response to dual inhibition of PI3K and PARP in PIK3CA mutant ovarian cancer cells [20] and BRCA proficient triple-negative breast cancer [13]. In line with these previous findings, wild-type PIK3CA ovarian cancer cells, OVCA433, OVCAR5 and OVCAR8, also exhibited concomitant downregulation of BRCA1/2 expression at both mRNA and protein levels in response to inhibition by BKM120 as single agents or in combination with Olaparib (Fig. 4C and Supplementary Fig. S3). It has been shown that PI3K inhibition may render BRCA-proficient breast tumor cells more responsive to PARP inhibition through ERK signaling-mediated downregulation of BRCA1/2 expression [13]. Nevertheless, as PI3K inhibitor BKM120 did not affect the status of ERK activation in the ovarian cancer cell lines examined (Fig. 3), it is likely that other mechanisms may account for BRCA downregulation in the current study.
Combined use of PI3K and PARP inhibition has potent activity in ovarian cancer explants
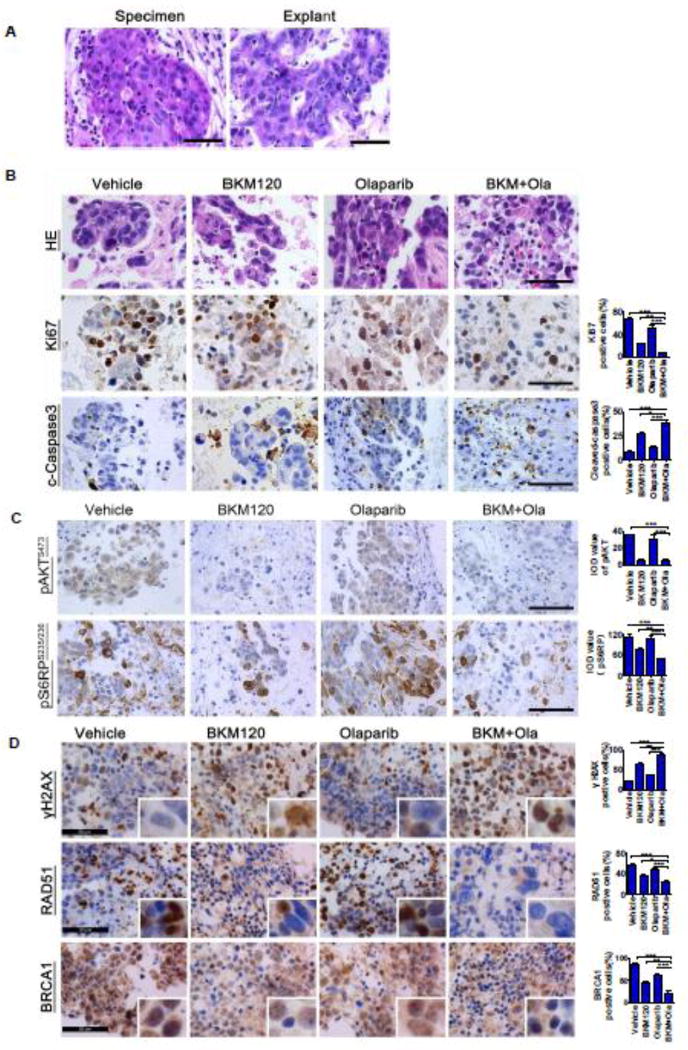
We next used an ex vivo culture model of ovarian cancer to evaluate the therapeutic effect of the combination treatment. For this, fresh surgical specimen of a primary serous ovarian carcinoma (PIK3CA, wild-type; BRCA1/2, wild-type) was dissected into approximately 1mm blocks. These blocks were cultured on an absorbable gelatin sponge for 24 hours and then exposed to BKM120 and Olaparib as single-agents or in combination for 48 hours. By histological examination, we found that vehicle-treated explants retained architecture and cellularity comparable to that of primary tumor (Fig. 5A). When treated with BKM120 and Olaparib, the explant displayed remarkably disrupted cellular integrity compared to that in single agent treatment group (Fig. 5B), indicating a strong therapeutic effect exerted by dual inhibition of PI3K and PARP. Consistently, immunohistochemical analysis revealed that the combination treatment resulted in remarkably reduced proliferation and significantly enhanced apoptotic cell death as determined by Ki67 and cleaved caspase 3 staining, respectively (Fig. 5B). Together, these data validated the potential use of BKM120 and Olaparib in combination to treat ovarian cancer carrying wild-type PIK3CA gene.
Fig. 5. Response of primary tumor explants to BKM120 and Olaparib as single-agents and in combination.
(A) Representative hematoxylin/eosin staining between surgical specimens and tumor explants. (B, C, D) Representative images for H&E staining and immunohistochemical staining analyses of Ki67, cleaved-caspase 3, pAKT, pS6RP, γH2AX, RAD51 and BRCA1 on tumor explants treated with either single-agents or the combination. Scale bars, 50µm.
To understand the molecular mechanisms underlying the potential therapeutic efficacy of the combination treatment, we assessed the effect of drug treatment on in vivo signaling pathways in the explants by immunohistochemical analysis. As expected, levels of pAKT and pS6RP, effectors of the PI3K/AKT/mTOR signaling pathway, were substantially reduced in explants treated with PI3K inhibitor BKM120 as single-agent or in combination with PARP inhibitor Olaparib (Fig. 5C). Strikingly, a substantial increase in the formation of γH2AX foci was found in ex vivo cultured tissues treated with BKM120 as single-agent and in combined with Olaparib (Fig. 5D), indicating exacerbated DNA damage. In contrast, the recruitment of homologous recombination repair proteins RAD51 and BRCA1 to DNA damage sites was significantly attenuated in cells with the same treatment (Fig. 5D). Of note, the immunohistochemical analysis revealed a remarkably reduced BRCA1 expression in the nucleus, although to a lesser extent, in the cytoplasm. This result is consistent with induced DNA double-strand breaks (DSBs) as shown by γH2AX staining and compromised homologous recombination repair as shown by RAD51 staining (Fig. 5D). Together, the markedly attenuated PI3K/AKT/mTOR signaling and the failure to repair DSBs by homologous recombination repair as a result of PI3K inhibitor BKM120 may underlie the tumor response to PARP inhibitor Olaparib.
Discussion
Here we reported that combined inhibition of PI3K and PARP effectively blocked cell proliferation and survival in three in vitro ovarian cancer cell line models and one patient-derived ex vivo culture model of ovarian cancer with wild-type PIK3CA genes. In accordance with previous findings from preclinical studies of breast cancer [13, 14] and prostate cancer [30], our study revealed that PI3K inhibitor BKM120 treatment led to induced DNA double-strand breaks and impaired homologous recombination repair in wild-type PIK3CA ovarian cancer cells. Of note, similar observations were reported in our recent study with PIK3CA mutant ovarian cancer [20].
It has been shown recently that phosphorylation of S6RP confers PARP inhibitor resistance in BRCA-deficient cancers [28]. In line with this, the current study with wild-type PIK3CA ovarian cancer cells and our recent study with PIK3CA mutant ovarian cancer cells [20] revealed that irrespective of BRCA or PIK3CA mutational status, ovarian cancer cells with concomitant downregulation of phosphorylated S6RP following PI3K inhibitor BKM120 or mTOR inhibitor RAD001 treatment exhibited impaired DNA damage response and compromised homologous recombination repair, and were thus responsive to PARP inhibition. Nevertheless, we did not observe a direct correlation between the status of AKT activation and cellular response to PARP inhibition in one of the three wild-type PIK3CA ovarian cancer cell lines, e.g. OVCA433.
ERK-dependent BRCA1/2 downregulation has been shown to account for the sensitivity to PARP inhibition in BRCA proficient triple negative breast cancer [14]. However, as PI3K inhibition by BKM120 did not lead to an increase in phosphorylated ERK signals in wild-type PIK3CA ovarian cancer cells in our study, it is less likely that ERK activation may contribute to the observed BRCA1/2 downregulation following PI3K inhibitor BKM120 treatment. Future studies understanding molecular mechanisms by which PI3K inhibition leads to BRCA downregulation may help define rational combination strategy with Olaparib in the treatment of ovarian cancer.
Preclinical and clinical studies have demonstrated synthetic lethality by inhibiting PARP in tumor cells harboring BRCA deficiency caused by germline mutation, somatic mutation or promoter methylation [21,31,32]. In our study, we found that the three wild-type PIK3CA ovarian cancer cell line models responded well to PI3K inhibitor BKM120 with compromised homologous recombination repair (HRR) and concomitant BRCA1/2 downregulation, consistent with the concept of synthetic lethal interaction between HRR deficiency and PARP inhibition [13, 33]. Of note, among the three wild-type PIK3CA ovarian cancer cell lines examined in our study, OVCAR5 cells harbor wild-type BRCA genes whereas OVCAR8 and OVCA433 cells carry defective BRCA1 and BRCA2 genes, respectively. Thus our data implicate that PI3K inhibition could be exploited to induce HR deficiency and BRCA downregulation in ovarian cancer cells irrespective of BRCA mutational status.
In this study, we also employed an ex vivo culture model of surgically resected primary ovarian cancer specimen to evaluate drug efficacy. As the cultured tissue explants retain the tissue architecture and degrees of cellularity recapitulating those observed in the primary tumor, this approach offers an amenable platform to test acute therapeutic response of ovarian cancer. Indeed, combined use of BKM120 and Olaparib also effectively treated tumor explants. Together, data from our in vitro and ex vivo models of wild-type PIK3CA ovarian cancer suggested that the impaired homologous recombination repair induced by PI3K inhibition contributes to the sensitivity to PARP inhibition, and that BRCA downregulation may serve as a potential biomarker for treatment response to combined use of PI3K and PARP inhibitors. Despite the limited number of ovarian cancer cell line models and ex vivo model investigated in this study, our data suggested that a combination of PI3K and PARP inhibitors may benefit ovarian cancer patients with a broader spectrum of cancer-associated genetic alterations, but not limited to those with BRCA and/or PIK3CA alterations.
Supplementary Material
Highlights.
PI3K inhibition induces BRCA downregulation in wild-type PIK3CA ovarian cancer.
PI3K and PARP inhibitors synergistically suppress ovarian cancer cell growth.
BRCA downregulation following PI3K inhibition may predict PARP inhibitor response.
Acknowledgments
Acknowledgements and grant support
This work was supported by the National Natural Science Foundation of China (No. 81472447 to H. Cheng; No. 81372853 and No. 81572586 to P. Liu), Liaoning Provincial Climbing Scholars Supporting Program of China (2012 to P. Liu, 2014 to H. Cheng), Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13049 to P. Liu), and Provincial Natural Science Foundation of Liaoning (No. 2014023002 to P. Liu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare that they have no conflict of interest.
References
- 1.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Cancer Genome Atlas Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, et al. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin. Cancer Res. 2005;11:2875–2878. doi: 10.1158/1078-0432.CCR-04-2142. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee S, Kaye SB. New Strategies in the Treatment of Ovarian Cancer: Current Clinical Perspectives and Future Potential. Clin. Cancer Res. 2013;19:961–968. doi: 10.1158/1078-0432.CCR-12-2243. [DOI] [PubMed] [Google Scholar]
- 6.Carden CP, Stewart A, Thavasu P, Kipps E, Pope L, Crespo M, et al. The Association of PI3 Kinase Signaling and Chemoresistance in Advanced Ovarian Cancer. Mol. Cancer Ther. 2012;11:1609–1617. doi: 10.1158/1535-7163.MCT-11-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rexer BN, Chanthaphaychith S, Dahlman K, Arteaga CL. Direct inhibition of PI3K in combination with dual HER2 inhibitors is required for optimal antitumor activity in HER2+ breast cancer cells. Breast Cancer Res. 2014;16:R9. doi: 10.1186/bcr3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roper J, Sinnamon MJ, Coffee EM, Belmont P, Keung L, Georgeon-Richard L, et al. Combination PI3K/MEK inhibition promotes tumor apoptosis and regression in PIK3CA wild-type, KRAS mutant colorectal cancer. Cancer Lett. 2014;347:204–211. doi: 10.1016/j.canlet.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodon J, Brana I, Siu LL, De Jonge MJ, Homji N, Mills D, et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2014;32:670–681. doi: 10.1007/s10637-014-0082-9. [DOI] [PubMed] [Google Scholar]
- 10.Bedard PL, Tabernero J, Janku F, Wainberg ZA, Paz-Ares L, Vansteenkiste J, et al. A Phase Ib Dose-Escalation Study of the Oral Pan-PI3K Inhibitor Buparlisib (BKM120) in Combination with the Oral MEK1/2 Inhibitor Trametinib (GSK1120212) in Patients with Selected Advanced Solid Tumors. Clin. Cancer Res. 2014:730–738. doi: 10.1158/1078-0432.CCR-14-1814. [DOI] [PubMed] [Google Scholar]
- 11.Burger MT, Pecchi S, Wagman A, Ni Z, Knapp M, Hendrickson T, et al. Identification of NVP-BKM120 as a Potent, Selective, Orally Bioavailable Class I PI3 Kinase Inhibitor for Treating Cancer. Acs Med. Chem. Lett. 2011;2:774–779. doi: 10.1021/ml200156t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brachmann SM, Kleylein-Sohn J, Gaulis S, Kauffmann A, Blommers MJJ, Kazic-Legueux M, et al. Characterization of the Mechanism of Action of the Pan Class I PI3K Inhibitor NVP-BKM120 across a Broad Range of Concentrations. Mol. Cancer Ther. 2012;11:1747–1757. doi: 10.1158/1535-7163.MCT-11-1021. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim YH, Garcia-Garcia C, Serra V, He L, Torres-Lockhart K, Prat A, et al. PI3K Inhibition Impairs BRCA1/2 Expression and Sensitizes BRCA-Proficient Triple-Negative Breast Cancer to PARP Inhibition. Cancer Discov. 2012;2:1036–1047. doi: 10.1158/2159-8290.CD-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juvekar A, Burga LN, Hu H, Lunsford EP, Ibrahim YH, Balmana J, et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2012;2:1048–1063. doi: 10.1158/2159-8290.CD-11-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolton KL, Chenevix-Trench G, Goh C, Sadetzki S, Ramus SJ, Karlan BY, Lambrechts D, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307:382–390. doi: 10.1001/jama.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 17.Kim G, Ison G, McKee AE, Zhang H, Tang S, Gwise T, et al. FDA Approval Summary: Olaparib Monotherapy in Patients with Deleterious Germline BRCA-Mutated Advanced Ovarian Cancer Treated with Three or More Lines of Chemotherapy. Clin. Cancer Res. 2015;21:4257–4261. doi: 10.1158/1078-0432.CCR-15-0887. [DOI] [PubMed] [Google Scholar]
- 18.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 19.Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Wang M, Jiang N, Zhang Y, Bian X, Wang X, et al. Effective use of PI3K inhibitor BKM120 and PARP inhibitor Olaparib to treat PIK3CA mutant ovarian cancer. Oncotarget. 2016;7:13153–13166. doi: 10.18632/oncotarget.7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stordal B, Timms K, Farrelly A, Gallagher D, Busschots S, Renaud M, et al. BRCA1/2 mutation analysis in 41 ovarian cell lines reveals only one functionally deleterious BRCA1 mutation. Mol. Oncol. 2013;7:567–579. doi: 10.1016/j.molonc.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Chang W, Yang G, Ren C, Park S, Karantanos T, et al. Targeting Poly(ADP-Ribose) Polymerase and the c-Myb-Regulated DNA Damage Response Pathway in Castration-Resistant Prostate Cancer. Sci. Signal. 2014;7:ra47–ra47. doi: 10.1126/scisignal.2005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Junttila TT, Akita RW, Parsons K, Fields C, Lewis PG, Friedman LS, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Chou TC. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 25.Du Y, Yamaguchi H, Wei Y, Hsu JL, Wang HL, Hsu YH, et al. Blocking c-Met-mediated PARP1 phosphorylation enhances anti-tumor effects of PARP inhibitors. Nat. Med. 2016;22:194–201. doi: 10.1038/nm.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dean JL, McClendon AK, Hickey TE, Butler LM, Tilley WD, Witkiewicz AK, et al. Therapeutic response to CDK4/6 inhibition in breast cancer defined by ex vivo analyses of human tumors. Cell Cycle. 2012;11:2756–2761. doi: 10.4161/cc.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun CK, Zhang F, Xiang T, Chen Q, Pandita TK, Huang Y, et al. Phosphorylation of ribosomal protein S6 confers PARP inhibitor resistance in BRCA1-deficient cancers. Oncotarget. 2014;5:3375–3385. doi: 10.18632/oncotarget.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukhopadhyay A, Elattar A, Cerbinskaite A, Wilkinson SJ, Drew Y, Kyle S, et al. Development of a Functional Assay for Homologous Recombination Status in Primary Cultures of Epithelial Ovarian Tumor and Correlation with Sensitivity to Poly(ADP-Ribose) Polymerase Inhibitors. Clin. Cancer Res. 2010;16:2344–2351. doi: 10.1158/1078-0432.CCR-09-2758. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Billalabeitia E, Seitzer N, Song SJ, Song MS, Patnaik A, Liu XS, et al. Vulnerabilities of PTEN-TP53-Deficient Prostate Cancers to Compound PARP-PI3K Inhibition. Cancer Discov. 2014;4:896–904. doi: 10.1158/2159-8290.CD-13-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 32.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. T. Helleday, Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 33.Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.