Abstract
Deficits in neuronal inhibition via gamma-aminobutyric acid (GABA) type A receptors (GABAA-Rs) are implicated in the pathophysiology of major depressive disorder and the therapeutic effects of current antidepressant treatments, however, the relevant GABAA-R subtype as defined by its alpha subunit is still unknown. We previously reported anxiety- and depressive-like behavior in alpha2+/− and alpha2−/− mice, respectively (Vollenweider, 2011). We sought to determine whether this phenotype could be reversed by chronic antidepressant treatment. Adult male mice received 4 or 8 mg/kg fluoxetine or 53 mg/kg desipramine in their drinking water for four weeks before undergoing behavioral testing. In the novelty suppressed feeding test, desipramine had anxiolytic-like effects reducing the latencies to bite and to eat the pellet in both wild-type and alpha2+/− mice. Surprisingly, 4mg/kg fluoxetine had anxiogenic-like effects in alpha2+/− mice increasing latency to bite and to eat while 8mg/kg fluoxetine increased the latency to eat in both wild-type and alpha2+/− mice. In the forced swim and tail suspension tests, chronic desipramine treatment increased latency to immobility in wild-type and alpha2−/− mice. In contrast, chronic fluoxetine treatment increased immobility in alpha2−/− mice in both tasks while generally having no effect in wild-type mice. These findings suggest that in preclinical paradigms of anxiety and behavioral despair the antidepressant-like effects of desipramine are independent of alpha2-containing GABAA-Rs, while a reduction in alpha2 expression leads to an increased sensitivity to anxiogenic- and prodepressant-like effects with chronic fluoxetine treatment, pointing to a potential role of alpha2-containing GABAA-Rs in the response to serotonin-selective antidepressants.
1. Introduction
1.1. Major depressive disorder and its treatment
Major depressive disorder (MDD) has been identified as a major global health problem, as despite the currently available therapeutics the World Health Organization (WHO) considers depression the leading cause of disability worldwide based on total years lost due to disability [1,2]. Current monoamine-based antidepressants provide long-term relief in only 50% of patients, and chronic treatment (weeks to months) is needed prior to the onset of clinical improvement [3]. While current antidepressant drugs modulate monoamine neurotransmission, it is widely accepted that the molecular mechanisms contributing to depression are more complex and certainly not limited to functional alterations in monoamine systems [4]. The roles of other neurotransmitter systems and signaling molecules in MDD and its treatment have begun to be elucidated to identify novel targets for the treatment of depression.
1.2. GABA and MDD
Clinical studies report decreases in γ-aminobutyric acid (GABA) levels in the cortex of patients with depression, with the greatest deficits found in patients with treatment resistant forms of depression [5,6]. Conversely, antidepressant-induced increases in cortical GABA have been implicated in the therapeutic effects of current antidepressants [7]. Based on both clinical and preclinical evidence, the GABAergic deficit hypothesis of MDD has been proposed [8]. GABA is the major inhibitory neurotransmitter in the central nervous system, and it regulates fast synaptic inhibition in the brain through its ionotropic type A receptor [9–11]. GABAA receptors (GABAARs) are formed by the assembly of five subunits, divided into subclasses based on sequence homology, including α1–6, β1–3, γ1–3, δ, ε, θ, and ρ1–3[12]. Alterations in expression of specific GABAAR subunits are found in the hippocampus and cortex of mice exposed to stress (a factor in the development of depression), as well as in the cortex of depressed patients following suicide [13–16].
Preclinical behavioral studies employing genetically modified mice have begun to delineate the role of specific GABAAR subtypes in depression and its treatment. In particular, global heterozygous knockdown of the GABAAR γ2 subunit (γ2+/−) leads to functional deficits in GABAAR signaling [17], as well as anxiety- and depressive-like behaviors in addition to hyperactivation of the HPA axis [18,19]. While chronic treatment with the noradrenergic-selective reuptake inhibitor desipramine (DMI) and the serotonin-selective reuptake inhibitor fluoxetine (FLX) have anxiolytic-like effects, the depressive-like behaviors and somatic changes are reversed following chronic treatment with DMI, but not FLX in γ2+/− mice [18]. These studies provide further evidence that alterations in GABAAR function may be involved in the development of depression-like behavior, and suggest that alterations in GABAAR function may reduce responsiveness to the antidepressant-like effects of serotonin- but not noradrenergic-selective therapeutics in some behavioral tasks. The potential involvement of a GABAergic mechanism in the antidepressant-like effects of FLX has also been demonstrated in vitro. Therapeutically relevant concentrations of FLX increase excitation of GABAergic interneurons in rat hippocampal slices [20], while the more potent metabolite norfluoextine has been shown to positively modulate recombinant GABAARs at therapeutically relevant concentrations [21]. Furthermore, the antidepressant-like action of FLX in the FST is enhanced by pretreatment with muscimol (a GABAAR agonist) and blocked by pretreatment with the GABAAR antagonist bicuculline [22].
As GABAARs are typically classified by their α subunits, and γ2 subunits are found in 90% of GABAARs, these findings as well as the studies utilizing muscimol and bicuculline did not provide information on which specific GABAAR subtypes (as defined by α subunits) might be involved in the circuitry mediating depression and its treatment. Our laboratory previously reported that similar to γ2+/− mice, global knockout of the GABAAR α2 subunit gene (α2KO) results in anxiety- and depressive-like phenotypes [23,24], suggesting that α2-containing GABAARs may not only be related to anxiety-like, but also depression-like behavior. However, it is important to note that the depressive-like behaviors reported in [23] were not always observed in the current studies, presumably due to different experimental conditions and/or experimenters. As the antidepressant-like effects of chronic DMI but not FLX are detected in γ2+/− mice, we hypothesized that genetic inactivation of α2-containing GABAARs might also alter the action of serotonin- but not noradrenergic-selective antidepressants in preclinical tasks of behavioral despair.
2. Methods
2.1. Animals
All experiments were approved by the McLean Hospital Institutional Animal Care and Use Committee and in accordance with the National Research Council of the National Academies Guide for the Care and Use of Laboratory Animals (Eight edition, 2011). Male wild type (α2+/+), heterozygous (α2+/−) knockout, and homozygous (α2−/−) knockout mice were generated from α2+/− x α2+/− breedings on the 129X1/SvJ background as described previously [23] (origin: RCC Fuellinsdorf, Switzerland). Mice were biopsied for genotyping and ear tagged at 3 weeks of age. Subjects were housed 2–5 per cage (Part#10219ZF-GA, Lab Products, Seaford, DE) with food (Purina Lab Diet 5P76, PMI Nutrition International, Brentwood, MO) and water provided ad libitum. For the behavioral experiments, mice were transferred from the colony to a housing room on a reverse light-dark cycle (12:12h, lights on at 7PM). Mice habituated to the light cycle over the course of 5 weeks (including 4 weeks of drug treatment) before behavioral testing was conducted (during the dark phase). Subjects were 10–12 weeks of age at the time of behavioral testing.
2.2. Drugs
Fluoxetine hydrochloride (FLX, Spectrum: Gardena, CA) and desipramine hydrochloride (DMI, Santa Cruz Biotechnology: Dallas, TX) were administered through the drinking water throughout the course of the study. The drug dosages for FLX and DMI were selected based on previous findings utilizing GABAAR γ2+/− mice raised in the same substrain of mice [18], as research has demonstrated that different strains of mice have varying responses to the same dose of an antidepressant [25]. FLX and DMI doses were calculated based on the average daily liquid intake of cages over one week, and the average weight of the mice in each cage on day 1 of drug dosing. Vehicle treated mice received water. As FLX is light sensitive, all bottles (FLX, DMI, and vehicle) were wrapped in aluminum foil to protect the FLX and ensure that all drugs were delivered in a similar manner. Drinking water was replaced on a weekly basis, or when a leak was detected.
2.3. Behavioral Tests
Behavioral testing began 28 days following chronic drug treatment. Novelty suppressed feeding (NSFT), forced swim test (FST), tail suspension test (TST), and open field were performed on the same cohorts of mice during the dark phase of the daily light cycle following standard protocols previously described in [23]. To limit stress to the mice, tests were separated by 4 days. During the data collection and scoring of all behavioral tests, the experimenters were blinded to the genotype (but not the drug group) of the mice.
Given multiple variables (drug and genotype), it was challenging to examine all three genotypes in each behavioral test (as was done previously in [23]). As such, α2+/+ mice were compared to either α2+/− or α2−/−. However, the data for some comparisons did not meet the standards required to perform 2-way ANOVA’s (normal distribution and variability). While in some cases these issues could be corrected by transforming the data, this was not always possible (NSFT comparisons between α2+/+ and α2−/− mice--FLX measurements and FST/TST latency comparisons between α2+/+ and α2+/− mice—DMI and FLX) even after data transformation. Only the comparisons that met criteria and could be tested are reported.
2.3.1 Novelty Suppressed Feeding Test
Mice were food (but not water) deprived for 24 hours prior to testing. On the day of testing, mice were placed into a clear plexiglass open field arena (42cm × 42cm × 31cm) illuminated to 100 lux. A thin layer of clean bedding was placed on the bottom of the arena before each trial. A food pellet was placed on an inverted petri dish at the center of the chamber, and latencies to bite and to sit and eat the pellet were assessed. Mice were removed from the testing chamber once they began eating, or after a duration of 6 minutes. Following the testing trial, the mouse was returned to their home cage where consumption of a pre-weighed food pellet was monitored for five minutes. Any mouse that did not eat in their home cage was excluded from data analysis.
2.3.2 Forced Swim Test
Mice were placed in a clear plastic cylinder (diameter 20cm) filled with roughly 18cm of 24–27°C water and behavior was recorded (Sony Handycam DCR DVD108, Sony Electronics) over the course of a 6-minute trial. Following the trial, mice were placed in a clean cage filled with paper towels under a heat lamp to warm up and dry off prior to returning to their home cage. Despair-like behavior was manually scored by two different observers using J Watcher [26], and latency to first immobility and percent time spent immobile over the course of the entire 6-minute trial were assessed (the times reported by the two observers were averaged for each data point). Immobility was defined as lack of movement, or only those motions necessary to remain afloat.
2.3.3 Tail Suspension Test
As a second test of behavioral despair, mice were suspended individually by their tails and behavior was recorded during a 6-minute trial (Sony Handycam DCR DVD108, Sony Electronics). Latency to first immobility and percent time spent immobile over the course of the testing period were monitored and manually scored by two different observers using J Watcher [26], with average times between the two observers being reported. Immobility was defined as a lack of movement during suspension.
2.3.4 Familiar Open Field
Mice were habituated to a clear plexiglass open field arena (42cm × 42cm × 31cm) for 30 minutes under red light conditions. 24 hours later, mice were brought back into the same testing room under the same lighting conditions for an additional 30-minute trial in the now familiar arena that was recorded using Ethovision XT (Noldus Information Technology, Netherlands). Drug- and genotype-induced changes in mobility were assessed by measuring the total distance traveled (cm) during the first 6 minutes to correspond to the FST and TST trial length.
2.4. Confirmation of circulating drug levels
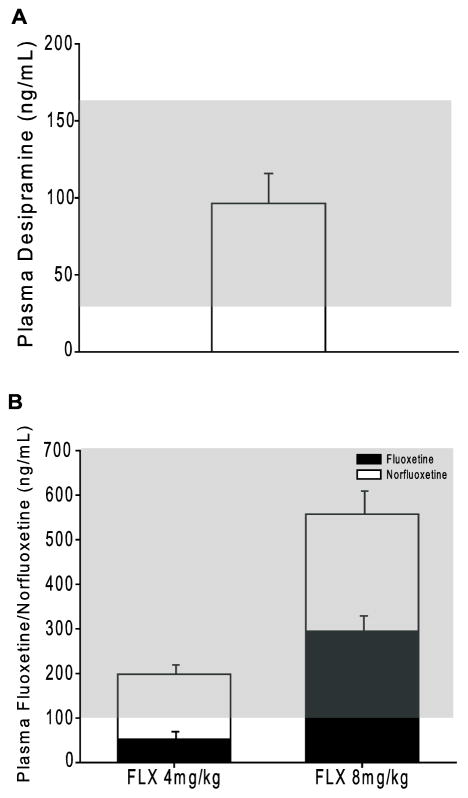
To confirm that the drug doses selected and method of administration (chronic oral administration) led to circulating drug levels that are within the therapeutic range, subsets of mice (from all genotypes) were treated with DMI (53mg/kg) or FLX (4 or 8mg/kg) for 28 days. Following drug administration, trunk blood was collected with a capillary tube following cervical dislocation. Serum was collected following centrifugation (20 minutes at 4°C), and plasma DMI and FLX levels were detected by HPLC (Analytical Psychopharmacology Laboratory, Nathan Kline Institute, Orangeburg, NY).
2.5. Statistics
Statistical analysis was performed using SigmaPlot version 11 (Systat Inc., San Jose, CA). Statistical significance was evaluated using a 95% confidence interval. In all experiments, overall variance was tested using a two-way (genotype x drug) analysis of variance (ANOVA). The effects of FLX and DMI treatment on behavior were examined separately. When statistical significance was detected, Tukey’s HSD was used for post-hoc analysis. NSFT latency to bite (cube root), DMI NSFT latency to sit and eat (square root), FST latency (cube root), and FST/TST percent immobility (square root) data were transformed prior to statistical analysis to correct for skews in the distribution and variance associated with behavioral tasks of this nature. In the case of TST latency, the data did not meet normal distribution despite transformation. As such, planned comparisons were performed within genotype groups to examine drug effects using the appropriate non-parametric test (FLX: Kruskal-Wallis one-way ANOVA based on ranks, DMI: Mann-Whitney Rank Sum Test). Data are graphically expressed as the raw non-transformed means ± the standard error of the mean (SEM).
3. Results
3.1 Effects of chronic desipramine and fluoxetine on anxiety-like behavior
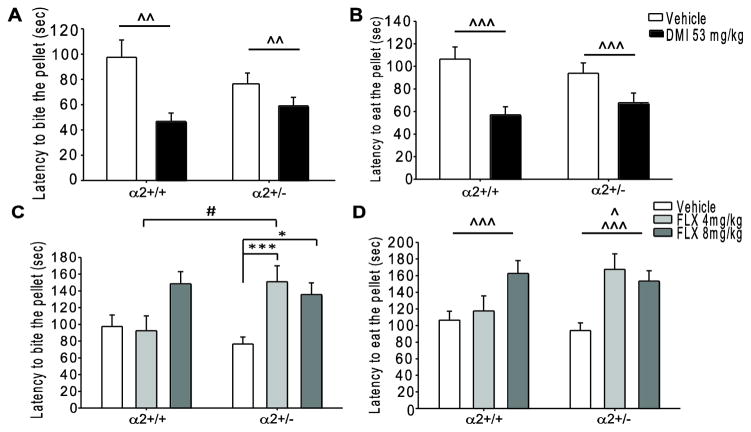
Although the NSFT is a measure of anxiety it is considered to have predictive validity for antidepressant drug screening, as this conflict-based task is sensitive to chronic (but not acute) treatment with monoamine-based antidepressants [27]. As significant increases in anxiety-like behavior were previously reported in α2+/− but not α2−/− mice as compared to α2+/+ mice in the NSFT [23], only α2+/+ and α2+/− mice were analyzed (see Methods 2.3 for further explanation). While the previously reported genotype effect was not detected in the current studies, clear drug effects were observed. There was a main effect of chronic DMI treatment, where DMI significantly decreased latency to bite (Fig. 1A; main effect F1, 53=10.302, p=0.002) and to sit and eat (Fig. 1B; main effect F1, 52=14.389, p<0.001) the pellet in both α2+/+ and α2+/− mice. These findings suggest that chronic DMI treatment has an anxiolytic-like effect, which is not altered by α2KO. It is important to note that chronic DMI treatment also increased home cage food consumption in both α2+/+ and α2+/− mice (Supplementary Fig. 1A; main effect F1, 52=7.225, p=0.01), and this increase in eating may have enhanced the drug-induced decreases in latencies that we observed in the testing chamber. A significant drug x genotype interaction was observed in the latency to bite the pellet following chronic FLX treatment (Fig. 1C; interaction F1, 86=3.679, p=0.029). Post-hoc analysis revealed that α2+/− mice treated with FLX (4mg/kg p<0.001, 8mg/kg p<0.05) took significantly longer to bite the pellet as compared to vehicle treated α2+/− mice as well as 4mg/kg FLX treated α2+/+ mice (p<0.05). FLX had no effect on latency to bite the pellet in α2+/+ mice. Additionally, a main effect of drug was detected in the latency to sit and eat the pellet following chronic FLX treatment (Fig. 1D, main effect F1, 85=7.680, p<0.001), where the 4mg/kg dose increased latency to eat in α2+/− mice and the 8mg/kg dose increased latency in α2+/+ and α2+/− mice. These findings suggest that chronic FLX treatment at a dose of 8mg/kg has an anxiogenic-like effect in both α2+/+ and α2+/− mice. Moreover, the heterozygous α2KO appears to increase sensitivity to the anxiogenic-like effects of FLX as only α2+/− mice exhibit increased latencies in response to chronic FLX treatment at the lower dose of 4mg/kg. Chronic FLX treatment did not influence the amount of food consumed within the home cage (Supplementary Fig. 1B).
Fig. 1.
Chronic administration of desipramine and fluoxetine in the novelty suppressed feeding test. (A) Latency to bite and (B) sit and eat the food pellet following chronic vehicle or DMI (53mg/kg) treatment. (C) Latency to bite and (D) sit and eat the pellet following chronic vehicle or FLX (4 and 8 mg/kg) treatment. *, #, ^ p<0.05, ^^ p<0.01, ***, ^^^ p<0.001. #=within drug difference, *=within genotype difference, ^=main effect of drug. FLX=fluoxetine, DMI=desipramine, α2+/+=wild type, α2+/−= heterozygous α2 knockout. Vehicle α2+/+ n=13, α2+/− n=21; FLX 4mg/kg α2+/+ n=10, α2+/− n=21; FLX 8mg/kg α2+/+ n=11, α2+/− n=16; DMI α2+/+ n=13, α2+/− n=10.
3.2 Effects of chronic desipramine and fluoxetine on despair-like behavior
As greater differences in despair-like behavior were previously reported in α2−/− mice as compared to α2+/+ mice [23], only α2+/+ and α2−/− mice were analyzed in the FST and TST (see Methods 2.3 for further explanation).
3.2.1 Forced Swim Test
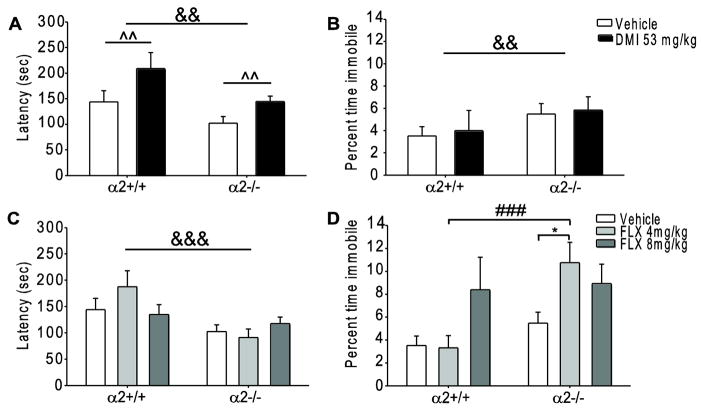
In mice chronically treated with DMI, there were main effects of genotype (main effect F1, 71=8.660, p=0.004) and drug (main effect F1, 71=11.458, p=0.001) on latency to immobility. In line with our previously reported findings [23], α2−/− mice had significantly shorter latencies as compared to α2+/+ mice. Chronic DMI treatment significantly increased latency to immobility in both α2+/+ and α2−/− mice as compared to vehicle treated mice of both genotypes (Fig. 2A). However, only a main effect of genotype (main effect F1, 68=8.613, p=0.004) and not drug (F1, 71=0.0329, p=0.857) was observed in the percent time spent immobile. Although vehicle treated α2−/− spent more time immobile as compared to vehicle treated α2+/+ mice (α2−/−: 21.84 ± 4.03 seconds, α2+/+: 12.68 ± 2.97seconds), chronic DMI treatment did not alter the percent time spent immobile in either genotype (α2−/−: 21.05 ± 4.24 seconds, α2+/+: 14.36 ± 6.53 seconds) (Fig. 2B). Taken together, these results suggest that the antidepressant-like effects of chronic DMI treatment are not altered by α2KO as assessed by certain measurements in the FST.
Fig. 2.
Chronic administration of desipramine and fluoxetine in the forced swim test. (A) Latency to immobility and (B) percent time spent immobile following chronic vehicle or DMI (53mg/kg) treatment. (C) Latency to immobility and (D) percent time spent immobile following chronic vehicle or FLX (4 and 8 mg/kg) treatment. * p<0.05; &&, ^^ p<0.01, ###, &&& p<0.001. #=within drug difference, *=within genotype difference, ^=main effect of drug, &=main effect of genotype. FLX=fluoxetine, DMI=desipramine, α2+/+=wild type, α2−/−= homozygous α2 knockout. Vehicle α2+/+ n=16, α2−/− n=29; FLX 4mg/kg α2+/+ n=13, α2−/− n=18; FLX 8mg/kg α2+/+ n=12, α2−/− n=16. DMI α2+/+ n=13, α2−/− n=18.
In mice chronically treated with FLX, there was a main effect of genotype (main effect F1, 97=14.738, p<0.001) but not drug (F2, 97=0.480, p=0.620) on latency to immobility. While α2−/− mice had shorter latencies as compared to α2+/+ mice, chronic FLX (4 and 8mg/kg) treatment did not affect latency to immobility in either genotype (Fig. 2C). However, a significant drug x genotype interaction was detected in the percent time immobile measurement (interaction F2, 97=3.185, p=0.046). Post-hoc analysis revealed that FLX (4mg/kg) treated α2−/− mice spent significantly more time immobile (38.69 ± 6.41 seconds) as compared to FLX (4mg/kg) treated α2+/+ mice (12.93 ± 4.06 seconds, p<0.001) and vehicle treated α2−/− mice (21.84 ± 4.03 seconds, p<0.05) (Fig. 2D). Fluoxetine treatment had no effect on percent time spent immobile in α2+/+ mice (vehicle: 12.68 ± 2.97 seconds, FLX 4mg/kg: 12.93 ± 4.06 seconds, FLX 8mg/kg: 30.19 ± 10.24 seconds). Such findings are in line with our NSFT results, suggesting that α2KO increases sensitivity to the prodepressant-like effects of FLX as the lower 4mg/kg dose significantly increased immobility in α2−/− but not α2+/+ mice.
3.2.2 Tail Suspension Test
In the TST, the latency data did not meet the normal distribution and equal variance requirements necessary to conduct 2-way ANOVA analyses. Because of this, planned comparisons were carried out within genotype groups to examine the potential antidepressant-like effects of chronic FLX and DMI treatment for this measurement using the appropriate non-parametric statistical test (see Methods 2.6).
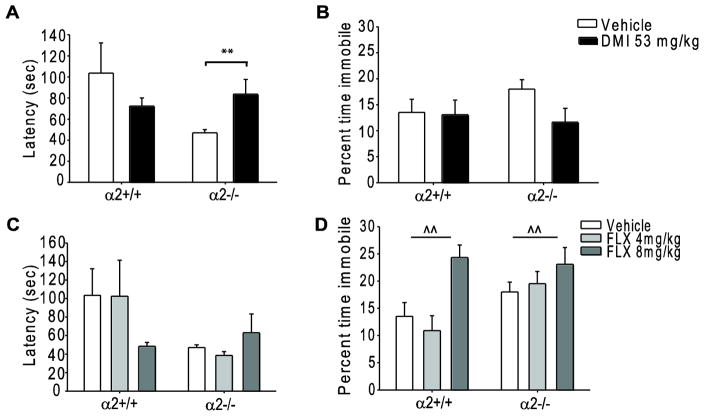
DMI treatment had an antidepressant-like effect in the TST, significantly increasing the latency to immobility in α2−/− mice as compared to vehicle treated α2−/− mice (p=0.005, Fig. 3A) while DMI treatment had no effect on latency in α2+/+ mice (p=0.336). Similar to the FST, DMI treatment did not alter time spent immobile in the TST in either genotype (α2+/+ veh 48.71 ± 9.15 seconds, α2+/+ DMI 47.07 ± 10.12 seconds; α2−/− veh 64.88 ± 6.48 seconds, α2−/− DMI 41.98 ± 9.48 seconds; Fig. 3B) further demonstrating that the antidepressant-like effects of chronic DMI treatment are not altered by α2KO in certain measurements of behavioral despair.
Fig. 3.
Chronic administration of desipramine and fluoxetine in the tail suspension test. (A) Latency to immobility and (B) percent time spent immobile following chronic vehicle or DMI (53mg/kg) treatment. (C) Latency to immobility and (D) percent time spent immobile following chronic vehicle or FLX (4 and 8mg/kg) treatment. **, ^^ p<0.01. *=within genotype difference-planned comparison, ^=main effect of drug. FLX=fluoxetine, DMI=desipramine, α2+/+=wild type, α2−/−= homozygous α2 knockout. Vehicle α2+/+ n=17, α2−/− n=29; FLX 4mg/kg α2+/+ n=11, α2−/− n=18; FLX 8mg/kg α2+/+ n=12, α2−/− n=16. DMI α2+/+ n=13, α2−/− n=18.
Conversely, while chronic FLX did not significantly alter latency to immobility in α2+/+ (p=0.782) or α2−/− (p=0.216) mice as compared to vehicle treated mice of the same genotype (Fig. 3C), a main effect of drug (main effect F2, 97= 6.551, p=0.002) was observed in the percent time immobile measurement. Post-hoc comparisons revealed a pro-depressant-like effect of FLX 8mg/kg (α2+/+ 87.63 ± 8.28 seconds and α2−/− 83.09 ± 11.17 seconds) as compared to vehicle (α2+/+ 48.71 ± 9.15 seconds and α2−/− 64.88 ± 6.48 seconds) and FLX 4mg/kg (α2+/+ 39.27 ± 9.91 and α2−/− 70.28 ± 7.99 seconds, Fig. 3D) treated mice. While not significant, a strong trend towards a main effect of genotype was also observed in the percent time spent immobile measurement (main effect F1, 97=3.620, p=0.06).
3.3 Effects of chronic desipramine and fluoxetine treatment on general behavior
3.3.1 Locomotion
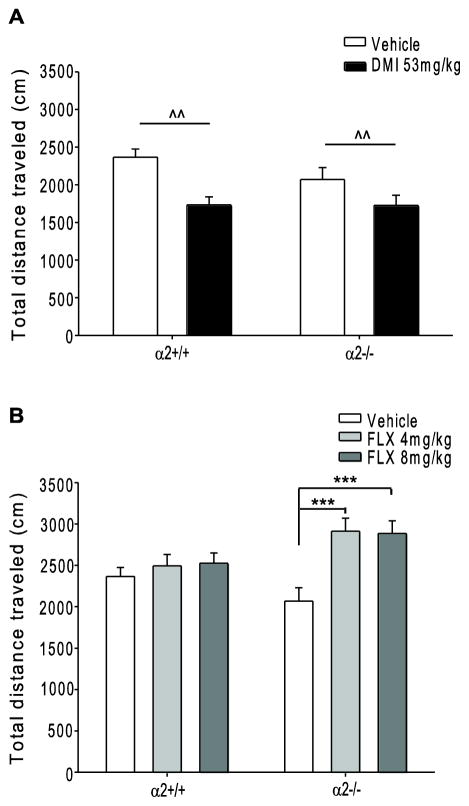
As the FST and TST use immobility as a measurement to assess despair-like behavior, these tests can be confounded by changes in motor activity. Thus, animals were run in a familiar open field and total distance traveled was examined during the first 6 minutes of the session to measure any drug- and genotype-induced changes in baseline activity. In the familiar open field, a measurement of locomotor activity and not anxiety, a main effect of drug was observed following chronic DMI (main effect F1,64=11.564, p=0.001) treatment. Post-hoc analysis revealed that DMI treatment significantly reduced mobility as compared to vehicle treated mice (Fig. 4A). The observed decrease in locomotor activity following DMI treatment in the familiar open field does not confound the antidepressant-like effects of DMI observed in the FST and TST, as if anything, decreased locomotion should lead to reduced mobility in the FST and TST (i.e., the opposite of the antidepressant-like effects we observe following DMI treatment in these tests). A significant drug x genotype interaction was observed following chronic FLX treatment (interaction F1,70=3.463, p=0.037). FLX treatment (4 and 8 mg/kg) increased the total distance traveled in α2−/− as compared to vehicle treated α2−/− (Fig. 4B). FLX treatment had no effect on distance traveled in α2+/+ mice. However, our experiments showed no effect or a prodepressant-like effect of FLX in the FST and TST in α2+/+ and α2−/− mice, which corresponds to reduced mobility. Thus, again, the effects observed following chronic FLX treatment in tests of behavioral despair do not seem to result from nonspecific changes in locomotor activity.
Fig. 4.
Chronic administration of desipramine and fluoxetine in the familiar open field. Overall distance traveled following chronic vehicle or (A) DMI (53mg/kg) or (B) FLX (4 and 8mg/kg) treatment. FLX= fluoxetine, DMI= desipramine, α2+/+= wild type, α2−/−= homozygous α2 knockout. *** p<0.001. *=within genotype difference ^^ p<0.01. ^=main effect of drug. Vehicle α2+/+ n=15, α2−/− n=22; FLX 4mg/kg α2+/+ n=7, α2−/− n=11; FLX 8mg/kg α2+/+ n=10, α2−/− n=11. DMI α2+/+ n=13, α2−/− n=18.
3.3.2 Liquid Consumption
Liquid consumption was monitored throughout the course of the studies to ensure that animals were receiving the proper dose, and to identify leaky bottles. Addition of drug to the drinking water did not alter daily liquid intake, as no differences were detected in average daily liquid consumption (normalized to the number of mice in each cage) across the four drug treatment groups (Supplementary Fig. 2). Genotype differences in liquid intake could not be determined, as mice were group housed in mixed genotypes to avoid the stress of single housing.
3.4 Plasma levels of desipramine and fluoxetine/norfluoxetine are within the therapeutic range
Serum DMI and FLX levels were assessed following 28 days of drug treatment (DMI 53mg/kg, FLX 4mg/kg and 8mg/kg) in the drinking water (Fig. 5). Serum DMI levels were within the therapeutic range of 37–163 ng/mL [28] following chronic drug treatment (Fig. 5A). Average levels of DMI were slightly lower in α2−/− mice as compared to α2+/+ and α2+/− mice (Table 1). However, drug-induced anxiolytic and anti-depressant-like effects were detected in all three genotypes. Additionally, levels of the metabolite 2-hydroxy DMI did not differ between the three genotypes. Thus, we attribute the variation in DMI plasma levels to the low group size, rather than a difference in the metabolism of DMI between genotypes. Similar to previous reports [25,29], a disproportionate increase in plasma FLX was detected following chronic treatment with FLX 8mg/kg as compared to FLX 4mg/kg treatment (Fig. 5B). Norfluoxetine (the major active metabolite of FLX) levels did not increase at a disproportionate rate between doses (Fig. 5B). Importantly, the combined FLX and norfluoxetine plasma levels detected following chronic FLX 4mg/kg and 8 mg/kg treatment were within the therapeutic range of 100–700 ng/mL [25]. Average plasma FLX 4mg/kg levels did not differ between genotypes (Table 1), although levels of the active metabolite norfluoxetine were lower in α2+/− mice as compared to α2+/+ and α2−/− mice. Average plasma FLX 8mg/kg and norfluoxetine levels were also lower in α2+/− mice as compared to α2−/− (Table 1). Despite the lower plasma FLX levels in α2+/− mice, anxiogenic-like effects were observed. Thus, as with DMI treatment, variations in FLX plasma levels are attribute to low group size.
Fig. 5.
Plasma levels of desipramine and fluoxetine following chronic oral administration. Serum (A) DMI or (B) FLX and its active metabolite norfluoxetine measured following 28 days of oral administration in the drinking water. All doses (DMI: 53mg/kg, FLX 4 and 8 mg/kg) lead to measured plasma concentrations within the therapeutic range, which is noted with a gray box for each compound. DMI n=5, FLX 4mg/kg n=9, FLX 8mg/kg n=4.
Table 1.
Average plasma levels of desipramine and fluoxetine and their respective metabolites 2-hydroxy desipramine and norfluoxetine following chronic oral administration, summary of three genotypes. Average plasma concentrations of desipramine and fluoxetine/norfluoxetine between genotypes (while somewhat variable) are all within the therapeutic range (DMI: 37–163ng/mL, FLX/NORFLX: 100–700ng/mL). Despite the variability in FLX levels across genotypes with the 4mg/kg dose, behavioral effects (anxiogenic- and prodepressant-like) were observed in both α2+/− and α2−/− mice suggesting that this variability is due to the low numbers per group and not a difference in drug absorption and metabolism.
| Drug | ||||
|---|---|---|---|---|
| Genotype | DMI 53mg/kg 2-Hydroxy-DMI |
FLX 4mg/kg NORFLX |
FLX 8mg/kg NORFLX |
|
| α2+/+ | 91 ng/mL 5.6 ng/mL (n=2) |
45.8 ng/mL 132.2 ng/mL (n=5) |
||
| α2+/− | 118.5 ng/mL 8.35 ng/mL (n=2) |
66 ng/mL 53 ng/mL (n=2) |
216 ng/mL 189 ng/mL (n=1) |
|
| α2−/− | 63 ng/mL 8 ng/mL (n=1) |
60.5 ng/mL 162.5 ng/mL (n=4) |
321 ng/mL 287 ng/mL (n=3) |
|
4. Discussion
To determine whether deficits in α2-containing GABAARs impact the antidepressant-like action of serotonin (FLX) and noradrenaline (DMI) selective reuptake inhibitors, we examined the effects of chronic drug treatment on anxiety- and despair-like behaviors in global α2KO mice. Our findings indicate that chronic DMI, but not FLX, treatment has an anxiolytic- and antidepressant-like effect in α2+/+ and α2KO (α2+/− and α2−/−) mice. Surprisingly, FLX treatment was not only ineffective, but also increased anxiety- and despair-like behavior in certain measurements. Such effects were observed at lower doses of FLX (4mg/kg) in α2+/− and α2−/− mice as compared to α2+/+ mice (8mg/kg), suggestive of an increased sensitivity to the anxiogenic and pro-depressive-like effects of chronic FLX treatment in the absence of α2-containing GABAARs.
The mechanism by which α2KO contributes to the depressive-like behaviors reported previously [23] and the altered response to chronic FLX treatment are not known. It is possible that a reduction in the number of α2-containing GABAARs directly alters these behaviors, as the α2 subunit gene is expressed at high levels in regions of the brain associated with depression including the nucleus accumbens, amygdala, and hippocampus [12]. Thus, a higher expression level of α2 or signaling through α2-containing GABAARs could potentially have an antidepressant effect. In support of this theory, eszopiclone, a GABAAR positive allosteric modulator with postulated selectivity for α2- and α3-containing GABAARs [30], when given in combination with FLX has a synergistic effect in alleviating symptoms in patients suffering from depression and insomnia [31]. However, it is also possible that alterations in α2 subunit expression may contribute to alterations in general inhibition through compensatory changes in receptor composition, location, or function. Conditional knockdown studies have demonstrated that α2-containing GABAARs are required for the normal development of adult born granule cells in the olfactory bulb [32]. Thus, it is possible that global decreases in α2-containing GABAARs may also alter cellular plasticity, which has been shown to be impaired in response to stress, a critical mediator in the development of depression (for review of pertinent studies see [33]) and enhanced with chronic antidepressant treatment [34]. Future studies could further examine these two potential mechanisms.
One of the drawbacks to preclinical tests such as the FST and TST is that antidepressant-like effects are not always detected using the same drug dose in different strains of mice [25]. As such, the current studies were carried out in the same substrain of mice (129X1/SvJ) as was previously used by others to examine the role of γ2-containing GABAARs in depression and its treatment. Reductions in γ2 subunit expression lead to anxious and depressive-like behaviors as well as an antidepressant-like response to chronic DMI but not FLX treatment [18]. Based on these findings it was suggested that γ2+/− mice serve as a model for melancholic depression, a form of depression where patients exhibit anxious depression that is frequently treatment resistant or more effectively treated with tricyclic antidepressants as compared to SSRIs [8,18]. We previously reported that global knockdown of the α2 subunit gene also leads to anxious and depressive-like behaviors [23,24]. Taken together with our current findings that chronic DMI, but not FLX, treatment has anxiolytic- and antidepressant-like effects in α2KO mice, we posit that α2 knockdown may also have predictive validity in modeling SSRI-resistant depression.
SSRI treatment has been associated with a worsening of depression symptoms and an increased risk of suicide [35]. In one clinical study, patients were three times as likely to attempt suicide after 10 to 29 days of SSRI treatment (mice were treated for a similar length of time before behavioral assessment in the current studies) as compared to patients who had been on antidepressants for over 90 days [36]. As such the FDA issued a public health advisory in 2004, recommending close observation of adult and pediatric patients prescribed SSRIs [35]. In addition, 30–40% of patients do not show a significant therapeutic response to SSRIs even after prolonged treatment [37]. However, SSRIs are still frequently prescribed as many depressed patients do respond well to this class of antidepressants. The variation in patient response to SSRI treatment is a public health concern, and not well understood. One theory that has been developed is the undirected susceptibility to change hypothesis, which posits that chronic SSRI treatment enhances neuronal plasticity in turn enhancing a patient’s response to their environment (either favorable or stressful)[38]. In support of this, chronic FLX treatment was shown to have an antidepressant-like effect in mice raised in an enriched environment, while the same dose had a prodepressant-like effect in mice raised in a stressful environment [38]. Alterations in the human α2 subunit gene (GABRA2) have also been shown to influence responses to the environment, as subjects with polymorphisms in GABRA2 that experienced childhood trauma were reported to be at a greater risk for PTSD in adulthood [39]. Thus, it is feasible that decreases in α2 expression in combination with the mild stress associated with the behavioral tasks performed in the current studies contributed to the anxiogenic and pro-depressant-like response to chronic FLX (4mg/kg) treatment that we observed in α2KO mice but not α2+/+ mice. However, additional studies beyond the scope of the current manuscript are needed to better understand this potential gene x environment interaction.
Previous research in our laboratory demonstrated that global α2KO mice exhibit anxiety- and depressive-like behavior in preclinical conflict and despair-based tasks [23,24]. Due to the variability associated with these behavioral tests, and the greater number of comparisons being made in the current studies (drug x genotype, rather than just genotype), significant genotype effects were not detected in all measurements. However, in most cases where significance was not detected, strong trends were observed. It is also possible that handling during the 4 weeks of chronic drug treatment, which was necessary to monitor potential drug-induced changes in body weight as requested by IACUC, may have contributed to greater variability than was previously observed in certain measured behaviors (as well as the drug-induced changes in locomotor activity reported). Furthermore, certain measurements that were examined previously (such as immobility in the first two minutes of the FST) were not feasible in the current studies (most animals-across all drug treatment and genotype groups-did not exhibit immobility within this early time point). Despite these confounds, clear behavioral effects of chronic DMI and FLX treatment could still be detected in α2+/+ and α2KO mice.
It is important to note that drug effects were detected in α2+/+ mice in only some of our studies. DMI had anxiolytic- (NSFT both measurements) and antidepressant-like (FST latency) effects on α2+/+ mice while only the higher dose of FLX-8mg/kg (a dose which was not examined in anxiety- and depression-related studies with γ2+/− mice raised on the same inbred mouse strain (129X1/SvJ) [18]) had an anxiogenic- (latency to sit and eat) and prodepressant-like (TST percent time immobile) effect in α2+/+ mice. A higher dose of 16mg/kg FLX was also piloted, but this dose was not tolerated in mice from the 129X1/SvJ strain in our hands. Importantly, limited behavioral effects were also reported following chronic drug treatment in wild-type mice in the γ2+/− studies [18]. However, a direct comparison between the γ2+/− studies and our own cannot be made as the γ2+/− studies were conducted in female mice while the current studies were conducted in males. In our previous studies with α2+/− and α2−/− mice, anxiety- and depression-related phenotypes were only detected in males or were stronger in males than in females [23 and unpublished results]. Interestingly, prodepressive-like effects have been reported following higher doses of FLX (14 and 22mg/kg) in C57BL/6J mice [25,40] suggesting that the 129x1/SvJ line of mice may be more sensitive to FLX as anxiogenic- and prodepressive-like effects were observed with the 8mg/kg dose in all genotypes while reduced α2 expression enhances this sensitivity as only α2KO mice responded to the lowest 4mg/kg dose.
4.1.1 Conclusion
Results from our studies support and extend beyond previous findings by others demonstrating that deficits in GABAAR function impact conflict and despair-based tasks and the behavioral response to FLX treatment in these tasks [18]. At a lower concentration of FLX and its active metabolite norfluoxetine (that is in the therapeutic range), we observed anxiogenic- and depressive-like actions in α2KO mice, but not in α2+/+ mice, suggesting that in 129X1/SvJ mice a decrease in α2-containing GABAARs promotes an increased sensitivity to the prodepressant-like effects of chronic FLX treatment. Therefore, it is interesting to speculate that treatment resistance or a negative response to SSRIs observed in some patients may be due in part to a decrease in GABRA2 expression. If this is the case, screening patients for GABRA2 levels (or single nucleotide polymorphisms, which may be markers of decreased expression) prior to the start of AD treatment may help to identify a population of patients that are unlikely to respond favorably to SSRI treatment.
Supplementary Material
Highlights.
Gabra2 knockout (KO) has predictive validity in modeling SSRI-resistant depression.
Desipramine has an anxiolytic and antidepressant-like action in Gabra2 KO mice.
Fluoxetine has an anxiogenic-like action in Gabra2 KO mice.
Fluoxetine has a prodepressive-like (despair-based) action in Gabra2 KO mice.
Acknowledgments
Funding
Research reported in this publication was supported by Award Number R01MH095905 from the National Institute of Mental Health of the National Institutes of Health to UR. RSB was supported by a Rappaport Mental Health Research Scholar Award and a McLean Presidential Fellowship Award. EE was supported by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award, an Eleanor and Miles Shore Harvard Medical School Fellowship, and an Andrew P. Merrill Memorial Research Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. The funding sources had no involvement in the study design, execution, or publication of the work. The study was designed by UR, EE, RSB, and NBH. Data was collected by RSB, NBH, and RFS. The manuscript was written by RSB, with edits from all authors. In the last 3 years, UR has received compensation for professional services from Concert Pharmaceuticals.
Footnotes
Disclosure
The other authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.WHO. The Global Burden of Disease: 2004 update. 2004. pp. 1–160. [Google Scholar]
- 2.Marcus M, Yasamy MT, van Ommeren M, Chisholm D, Saxena S. Depression: a Global Public Health Concern. World Federation of Mental Health; 2012. pp. 6–8. [Google Scholar]
- 3.Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci. 2011;34:1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 5.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 6.Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiat. 2009;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 8.Lüscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macdonald R, Olsen R. Gaba(a) Receptor Channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 10.Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988;11:112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- 11.Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 12.Hörtnagl H, Tasan RO, Wieselthaler A, Kirchmair E, Sieghart W, Sperk G. Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain. Neuroscience. 2013;236:345–372. doi: 10.1016/j.neuroscience.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS ONE. 2009;4:e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu FC, Zhang GJ, Raol YSH, Valentino RJ, Coulter DA, Brooks-Kayal AR. Repeated neonatal handling with maternal separation permanently alters hippocampal GABAA receptors and behavioral stress responses. Proc Natl Acad Sci USa. 2003;100:12213–12218. doi: 10.1073/pnas.2131679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng G, Zhang X, Chen Y, Zhang Y, Luo W, Chen J. Evidence for a role of GABAA receptor in the acute restraint stress-induced enhancement of spatial memory. Brain Res. 2007;1181:61–73. doi: 10.1016/j.brainres.2007.08.077. [DOI] [PubMed] [Google Scholar]
- 17.Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, et al. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- 18.Shen Q, Lal R, Luellen BA, Earnheart JC, Andrews AM, Luscher B. gamma-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol Psychiat. 2010;68:512–520. doi: 10.1016/j.biopsych.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Earnheart JC, Schweizer C, Crestani F, Iwasato T, Itohara S, Möhler H, et al. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J Neurosci. 2007;27:3845–3854. doi: 10.1523/JNEUROSCI.3609-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Méndez P, Pazienti A, Szabó G, Bacci A. Direct alteration of a specific inhibitory circuit of the hippocampus by antidepressants. J Neurosci. 2012;32:16616–16628. doi: 10.1523/JNEUROSCI.1720-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson RT, Drafts BC, Fisher JL. Fluoxetine increases GABA(A) receptor activity through a novel modulatory site. J Pharmacol Exp Ther. 2003;304:978–984. doi: 10.1124/jpet.102.044834. [DOI] [PubMed] [Google Scholar]
- 22.Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice forced swim test. Pharmacol Biochem Be. 2000;67:137–143. doi: 10.1016/s0091-3057(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 23.Vollenweider I, Smith KS, Keist R, Rudolph U. Antidepressant-like properties of α2-containing GABA(A) receptors. Behavioural Brain Research. 2011;217:77–80. doi: 10.1016/j.bbr.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koester C, Rudolph U, Haenggi T, Papilloud A, Fritschy JM, Crestani F. Dissecting the role of diazepam-sensitive γ-aminobutyric acid type A receptors in defensive behavioral reactivity to mild threat. Pharmacol Biochem Be. 2013;103:541–549. doi: 10.1016/j.pbb.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- 26.Blumstein D, Daniel J, Evans C, editors. [accessed February 4, 2016];JWatcher Software. 2012 http://www.jwatcher.ucla.edu/
- 27.Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology. 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- 28.Nelson JC, Jatlow P, Quinlan DM, Bowers MB. Desipramine plasma concentration and antidepressant response. Arch Gen Psychiatry. 1982;39:1419–1422. doi: 10.1001/archpsyc.1982.04290120049010. [DOI] [PubMed] [Google Scholar]
- 29.Koran LM, Cain JW, Dominguez RA, Rush AJ, Thiemann S. Are fluoxetine plasma levels related to outcome in obsessive-compulsive disorder? Am J Psychiatry. 1996;153:1450–1454. doi: 10.1176/ajp.153.11.1450. [DOI] [PubMed] [Google Scholar]
- 30.Nutt DJ, Stahl SM. Searching for perfect sleep: the continuing evolution of GABAA receptor modulators as hypnotics. J Psychopharmacol (Oxford) 2010;24:1601–1612. doi: 10.1177/0269881109106927. [DOI] [PubMed] [Google Scholar]
- 31.Fava M, McCall WV, Krystal A, Wessel T, Rubens R, Caron J, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiat. 2006;59:1052–1060. doi: 10.1016/j.biopsych.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Pallotto M, Nissant A, Fritschy JM, Rudolph U, Sassoè-Pognetto M, Panzanelli P, et al. Early formation of GABAergic synapses governs the development of adult-born neurons in the olfactory bulb. J Neurosci. 2012;32:9103–9115. doi: 10.1523/JNEUROSCI.0214-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duman RS. Depression: A case of neuronal life and death? Biol Psychiat. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 34.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.US Food and Drug Administration Public Health Advisory. Subject: Worsening depression and suicidality in patients being treated with antidepressant medications. U.S. Food and Drug Administration; 2004. [accessed November 1, 2004]. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm161696.htm. [Google Scholar]
- 36.Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. Jama. 2004;292:338–343. doi: 10.1001/jama.292.3.338. [DOI] [PubMed] [Google Scholar]
- 37.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 38.Alboni S, van Dijk RM, Poggini S, Milior G, Perrotta M, Drenth T, et al. Fluoxetine effects on molecular, cellular and behavioral endophenotypes of depression are driven by the living environment. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson EC, Agrawal A, Pergadia ML, Lynskey MT, Todorov AA, Wang JC, et al. Association of childhood trauma exposure and GABRA2 polymorphisms with risk of posttraumatic stress disorder in adults. Mol Psychiatry. 2009;14:234–235. doi: 10.1038/mp.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi K, Ikeda Y, Suzuki H. Behavioral destabilization induced by the selective serotonin reuptake inhibitor fluoxetine. Mol Brain. 2011;4:12. doi: 10.1186/1756-6606-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.