Abstract
Purpose
The accessory lacrimal glands (ALG) are an understudied component of the tear functional unit, even though they are important in the development of dry eye syndrome (DES). To advance our understanding of aging changes, regenerative potential and histologic correlates to human characteristics, we investigated human ALG tissue from surgical samples to determine the presence or absence of progenitor cell markers and lacrimal epithelial markers and to correlate marker expression to relevant patient characteristics.
Materials and Methods
ALG tissues obtained from Muller’s Muscle Conjunctival Resection (MMCR) specimens were created using tissue microarrays (TMAs). Immunofluorescence staining of MMCR sections was performed using primary antibodies specific to cell protein markers. Cell marker localization in TMAs was then assessed by two blinded observers using a standardized scoring system. Patient characteristics including age, race, and status of ocular surface health were then compared against expression of stem cell markers.
Results
Human ALG expressed a number of epithelial markers, and in particular, histatin-1 was well correlated with the expression of epithelial markers and was present in most acini. In addition, we noted the presence of precursor cell markers nestin, ABCG2 and CD90 in ALG tissue. There was a decrease in precursor cell marker expression with increasing age. Finally, we noted that a negative association was present between histatin-1 expression and DES.
Conclusions
Thus, we report for the first time that human ALG tissues contain precursor marker positive cells and that this marker expression may decrease with increasing age. Moreover, histatin-1 expression may be decreased in DES. Future studies will be performed to use these cell markers to isolate and culture lacrimal epithelial cells from heterogeneous tissues, determine the relevance of histatin-1 expression to DES and isolate candidate precursor cells from ALG tissue.
Keywords: Accessory lacrimal gland, ocular surface infection, histatin, stem cell, dry eye syndrome
Introduction
Dry eye syndrome (DES) is a multifactorial, chronic and disabling disease often caused by decreased function of the lacrimal gland1. The global prevalence of dry eye is estimated to be 11% to 22%2. The first line of treatment for DES is lubricating eye drops or other palliative measures3. These treatment modalities provide only temporary relief4. In cases of severe dry eye, advanced therapies such as anti-inflammatory medications, and surgical interventions like punctal occlusion and salivary gland transplantation have had limited success3. It would be desirable for the treatment of severe DES to employ strategies that would increase the natural production of tears, maintain ocular surface integrity and reduce or eliminate the levels of existing inflammation and prevent infection5. With these objectives in mind, the option of cell based therapy is being increasingly explored for restoring the function of damaged lacrimal gland. Investigation into the potential of using in vitro cultured cells for regenerative therapy may have promising outcomes. Similar to other exocrine tissues, main lacrimal glands (MLG) contain precursor cells6. These precursor cells proliferate in response to inflammation and injury, but are present in the uninjured gland as well7. Cultures of human lacrimal gland have been shown to contain a sub-population of progenitor cells which persist in culture6.
The accessory lacrimal gland (ALG) is a critical contributor to baseline tear production8, 9, and limited data exist regarding ALG function and biology10. In addition, ALG is more readily accessible to surgical intervention than main lacrimal gland (MLG) and represents an understudied component of the tear production apparatus9. Numerous limitations exist in the development of a better understanding of ALG biology. One, human tissue is not frequently accessible and, when available, is limited in quantity11. Two, ALG and MLG cell surface markers are frequently present in other surrounding tissues, like conjunctiva12, 13.
The lacrimal gland is a compound tubuloacinar gland consisting of acini, ducts, nerves, plasma cells and myoepithelial cells14. E-Cadherin is a pan-epithelial cell marker6, 15. Lacrimal tissue markers10 include lactoferrin16, lacritin17 and aquaporin-518–20. Lactoferrin is known to be produced by other ocular surface tissues, including conjunctiva21,22. Lacritin is a secreted protein found in tears and saliva and is mainly produced by the lacrimal gland23. Aquaporin-5 is a water channel protein also involved in tear generation process24. We recently demonstrated the utility of histatin-1 as a potential lacrimal epithelial marker, but a gold-standard lacrimal epithelial marker should be determined through the use of multiple studies and we utilize multiple markers to phenotype lacrimal epithelial cells25.
The existence of the stem/progenitor cells in tissue may imply regenerative potential, and characterization of these cells is important for development of regenerative therapies. The murine lacrimal gland has been noted to contain a population of cells which are upregulated in response to injury and inflammation that express nestin, a multi-lineage precursor cell marker for neuronal, lacrimal tissue, salivary tissue and pancreas tissue26, 27. PDX-1 is a pancreatic stem cell marker28. CD9, CD29, CD 44, CD81, CD 90 and CD105 are markers of salivary and pancreatic stem cells27. ABCG-2 is a marker of murine lacrimal stem cells5, 29.
Various surgical approaches have been utilized to obtain human tissue for evaluation30–32. Muller Muscle Conjunctival Resection (MMCR) surgery is a procedure commonly used to treat blepharoptosis30. Both MMCR surgery and the Fasanella-Servat ptosis procedure involve the resection of the posterior layers of the eyelid in the area of the ALG31, 32. ALG tissue is noted in the surgical specimen in MMCR procedures approximately 50–60% of the time31. These surgical specimens contain conjunctiva, stromal tissue, Muller’s muscle (smooth muscle), blood vessels, nerve tissue and ALG tissue. ALG tissue contains myoepithelial cells, acinar epithelium and ductal cells6, 33–35.
In order to develop a better understanding of ALG biology, we utilized MMCR specimens to determine the localization of markers of lacrimal epithelium, the presence or absence of stem cell markers in these tissues and related their presence to relevant patient characteristics.
This study aimed to examine and assess the expression of markers for lacrimal cells and the presence of precursor marker positive cells in human ALG from MMCR specimens. In addition, we investigated whether or not histological findings combined with patient characteristics could serve as predictors for DES.
Materials and Methods
Written informed consent was obtained from patients using a consent form specifically approved for this study by the Institutional Review Board (IRB) and processed by The University of Illinois at Chicago. Completed, signed consent forms were maintained according to the university guidelines following an IRB approved protocol specific for this study.
Patient Record Evaluation
Patient samples were obtained prospectively and consented in an IRB approved study. Inclusion criteria included age greater than or equal to 18 years old, and undergoing blepharoptosis repair via Muller’s Muscle Conjunctival Resection. Seventeen patients and twenty four eyelid specimens were included. Patient records were obtained and examined after IRB approval, and DES status was determined by retrospective chart review to find documentation of an existing diagnosis of DES by a practicing ophthalmologist (VKA).
Tissue Microarray Construction
A TMA was constructed from 24 MMCR patient specimens. Paraffin embedded H&E sections were analyzed to define representative ALG. The TMA block was constructed using a manual tissue array (K7 Biosystem Inc., Chicago, IL). A single 2.0 diameter cylindrical core of paraffin embedded tissue was retrieved from each paraffin block donor. Tissue cylinders were then inserted into a blank recipient paraffin block. After the array was completed and tempered in an incubator, the recipient block was cut into 3–5um sections using an HM340E paraffin microtome (Microm International Gmbh, Walldorf, Germany). Each sample section was chosen and marked to indicate position of ALG by an ocular pathologist.
Immunofluorescence Staining and Confocal Microscopy
Single and double immunofluorescence staining of MMCR sections was performed using primary antibodies specific to stem cell protein markers: ABCG2, CD90 and nestin; a myoepithelial cell marker: α-Smooth Muscle Actin; and lacrimal epithelial markers: aquaporin-5, histatin-1, e-cadherin, lactoferrin and lacritin, which were counter stained with respective secondary antibodies. Images of stained ALG were captured with 10× and 20× microscope objectives, and analyzed using the Zeiss LSM 710 Confocal Microscope.
Immunoreactivity Evaluation and Statistical Analysis
Immunoreactivity was visually assessed by two independent observers in all 24 MMCR cores by scanning the cores with 20× microscope objective. The evaluation was performed semi-quantitatively by assigning a score based on the proportion of positively stained acini and their immediately surrounding myoepithelial cells over total number of acini (percent positivity) ranging from 0 to 100. Furthermore, the percent positivity scores were grouped into 0–30%, 31–70%, and 71–100% for data analysis. The scoring method has been found to be reproducible 36,24. When analyzing intensity of expression of the different lacrimal and stem cell markers (stain positive acini:total acini), we categorized intensity into three categories (low, medium and high). Low was 0–30%, medium was 31–70% and high was 71–100%. Following this categorization we ran a Chi-Square test to test for significance between these categories and DED (DED positive and DED negative).” Salivary, pancreas, prostate, breast, brain and placental tissues were used as positive and negative control. Primary antibody omission was used for the negative control. The collected data were statistically analyzed and interpreted using standard methods. Patient characteristics such as age, gender, race, and status of ocular surface health were compared against semi-quantitative localization of markers for lacrimal cells, aging changes, and stem cells.
Statistical analysis was performed by analyzing scores from both observers. Pearson’s correlation coefficients, linear regression analysis and standard statistical methods were determined using SAS. Associations between patient characteristics and individual antibody staining were determined by comparing the ratio of positive acini divided by total number of acini counted by each observer.
Patients were analyzed as subgroups for age and percent positivity for localization of protein markers. With regards to age, 0–40 was one group and 41 and above was a second group. Forty years of age was used as a cut off to be consistent with aging related eye disease studies37. This grouping takes into account the decreasing efficacy of stem cells in middle aged and above patients, and the increasing prevalence of dry eye disease in older patients38.
Results
Seventeen patients and twenty four MMCR cores from twenty four eyelids were included in the study. Each core contained a robust sample of ALG with appropriate histological appearance on H and E staining. Patient characteristics are noted in (Table 1). The mean age of patients was 57.7 years, 52.9% were male and 47.1% were female. 41.2% had dry eye disease and 58.8% did not have dry eye disease. H & E image of tissue microarray containing human MMCR specimen of accessory lacrimal glands is noted in Figure 1.
Table 1.
Patient Characteristics (n=17)
| Variable | Mean± SD/ n(%) |
|---|---|
| Age | 57.7 ± 18.3 |
|
Gender Female Male |
8 (47.1%) 9 (52.9%) |
|
Race White African American Hispanic Asian Other |
5 (29.4%) 5 (29.4%) 5 (29.4%) 1 (5.9%) 1 (5.9%) |
|
Dry Eye Yes No |
7 (41.2%) 10 (58.8%) |
Figure 1.
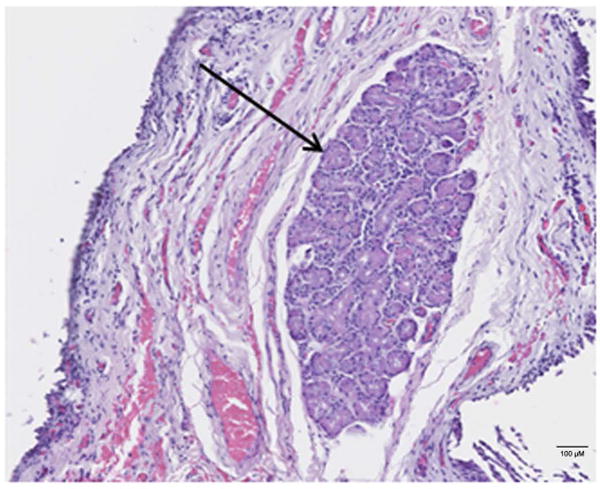
H & E image of formalin fixed paraffin embedded MMCR specimen. Black arrow is indicating ALG location in heterogeneous tissue from MMCR. Scale bar is 100 μM.
Localization of lacrimal and epithelial markers (e-cadherin, aquaporin-5, lacritin, lactoferrin and histatin-1) to ALG epithelial cells is shown in Figure 2. E-Cadherin localizes well to lateral and apical membranes of lacrimal epithelium, aquaporin-5 localizes to apical membranes of lacrimal epithelium, histatin-1 localizes to lacrimal epithelial cytoplasm as do lactoferrin and lacritin. Alpha smooth muscle actin localizes to peri-epithelial myo-epithelial cells.
Figure 2.
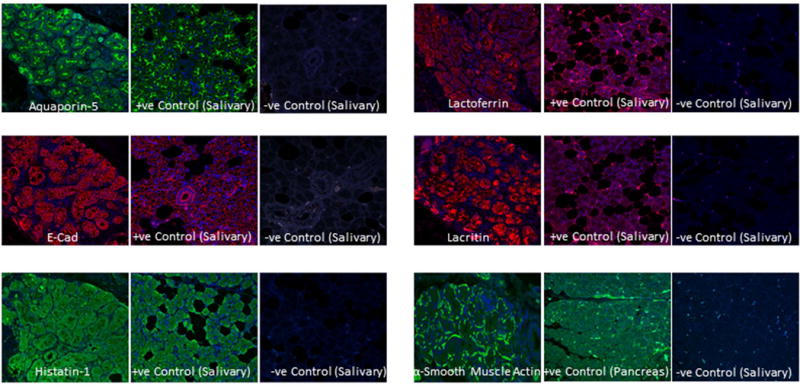
Expression of lacrimal markers in ALG. Immunofluorescence imaging shows the localization of aquaporin-5, e-cad, histatin-1, lactoferrin, lacritin and α-smooth muscle actin in ALG. Sections of salivary glands were used as positive control and without primary antibodies respectively as negative control.
Similarly, markers for precursor cells were localized to subpopulations of cells in ALG and immunofluorescence images are shown in Figure 3. ABCG-2 localized to apical and lateral epithelial cell membranes, as well as the cytoplasm while CD90 and nestin localized to acinar epithelial cells and periacinar cells. ABCG-2 was almost exclusively found inside acini. Immuno-localization for other precursor cell markers (PDX1, CD9, CD14, CD29, CD81 and CD105) was non-specific (data not shown).
Figure 3.
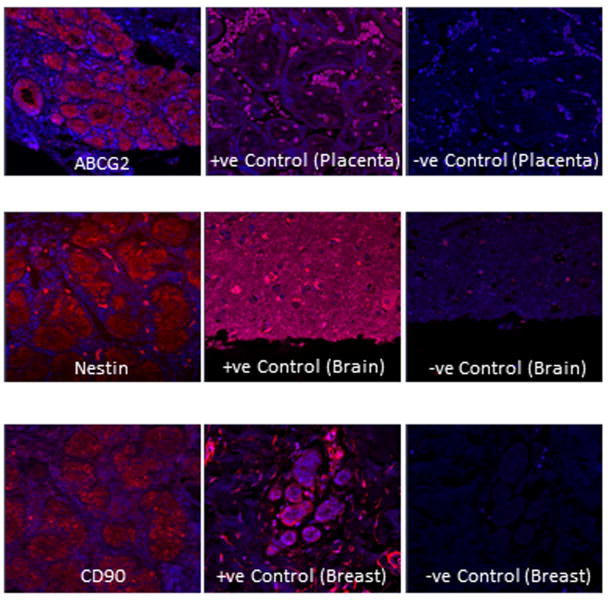
Identification of stem cell markers in ALG. Immunofluorescence imaging shows the localization of the precursor cell markers ABCG2, nestin, and CD90 in ALG. Sections of placenta, brain and breasts were imaged after exposure to respective antibodies as positive control and negative control (without respective primary antibodies).
We then examined associations among lacrimal markers (Table 2,Tables 3a and b). In particular, we found that all lacrimal markers show significant correlation with total acini, implying consistent localization in most acini of each marker. Table 3a demonstrates the correlation between aquaporin-5 lactoferrin and lacritin. Table 3b demonstrates the correlation between histatin-1 lactoferrin and lacritin. We also noted a detectable but not-strong correlation between histatin-1 and aquaporin-5 (0.35).
Table 2.
Lacrimal vs Acini
| Lacrimal Cell Marker |
Total Acini Number r-values (p< 0.001) |
|---|---|
| E-Cadherin | 0.99 |
| Aquaporin-5 | 0.93 |
| Lacritin | 0.78 |
| Lactoferrin | 0.51 |
| Histatin-1 | 0.78 |
Table 3a.
Correlation Coefficients for Aquaporin-5
| Histatin-1 | Lactoferrin | Lacritin | |
|---|---|---|---|
| Aquaporin-5 | 0.35 (P=0.07) |
0.44 (P=0.02) |
0.68 (P<0.001) |
Table 3b.
Correlation Coefficients for Histatin-1
| Aquaporin-5 | Lactoferrin | Lacritin | |
|---|---|---|---|
| Histatin-1 | 0.35 (P=0.07) |
0.55 (P=0.002) |
0.55 (P=0.003) |
Linear regression analysis between total acini and aquaporin-5 revealed a beta coefficient of 1.00 after controlling for scorer, age, gender, race and dry eye syndrome (P<0.001). This indicates a strong, consistent localization of aquaporin-5 in most lacrimal acini. Linear regression between total acini and e-cadherin revealed a beta coefficient of 0.95 after controlling for scorer, age, gender, race and dry eye disease (P<0.001). These data indicate a strong, consistent localization of e-cadherin in most lacrimal acini. Thus, immuno-localization analysis of TMAs demonstrated that there is a significant and strong correlation among lacrimal cell acinar count and e-cadherin, aquaporin-5, histatin-1, lactoferrin and lacritin.
Table 4 demonstrates the cross-tabulation between the categorized ratio of histatin-1 positive to total acini staining and patient DES status. Patients with DES were less likely (76.1%) to have a high (>71%) ratio of acini with histatin-1 localization than those without DES (83.7%) (p=0.04). We did not find a significant association among other lacrimal markers and patient age, gender or DES.
Table 4.
Categorized Ratio of Histatin-1 Positive Acini to Total Acini and Patient DES Status
| Positivity (%) |
Dry Eye (+) |
Dry Eye (−) |
|---|---|---|
| Low | 11.1 % | 10.0 % |
| Medium | 12.8 % | 6.3 % |
| High | 76.1 % | 83.7 % |
Analysis was run by Chi-Square (P=0.04).
Finally, we compared the association between precursor cell markers and patient characteristics. Table 5 demonstrates the correlation coefficients among precursor cell markers and age. Noted is a significant and negative association between age and ABCG-2. No significant associations between DES status or gender were found with precursor cell markers. Linear regression between age and ABCG-2 reveals a beta value of −0.47 after controlling for scorer, gender, race and dry eye in a significant manner (P=0.04).
Table 5.
Correlation of Precursor Cell Marker Positive Acini to Total Acini and Patient Age
| Precursor Cell Marker | Age |
|---|---|
| ABCG2 | −0.34 (P = 0.03) |
| Nestin | −0.24 (P = 0.11) |
| CD90 | −0.13 (P = 0.43) |
Discussion
This study evaluated human ALG tissue for the presence or absence of precursor marker positive cells, and the presence or absence of lacrimal cell markers. In addition, we studied the localization of histatin-1, a protein previously thought to be primarily expressed in salivary tissues39. In a recent study, we demonstrated that histatin-1 is a useful marker for lacrimal epithelium25. We also attempted to correlate immuno-localization results to relevant patient characteristics.
Our findings in Table 2 demonstrate that the markers utilized all correlated well and significantly with the number of acini, suggesting that these markers could be useful in assessing the amount of lacrimal acini in heterogenous tissue samples. Table 3a demonstrates the significant correlation between aquaporin-5 and histatin-1, lactoferrin and lacritin. Similarly Table 3b demonstrates the significant correlation between histatin-1 and aquaporin-5, lactoferrin and lacritin. Taken together these data suggest that co-localization of lacritin and lactoferrin with either aquaporin-5 or histatin-1 could be utilized identify lacrimal epithelial cells. However, given that this study does not address all possible markers, and the presence of epithelial markers in multiple tissue types, caution should be taken with relying simply on immuno-localization. For true identification of lacrimal epithelial cells in the context of a heterogeneous sample we would recommend multiple corroborating assays.
Interestingly, we noted that the ratio of histatin-1 positive acini decreases with dry eye syndrome. We only noted a difference between DES and non-DES patients when comparing the high (>71%) percent positive ratio patients. Histatin-1 proteins have been implicated in wound healing in the mouth and also in the protection of the oral mucosa from microbial invasion, and inflammation associated with LPS40–42. Presence of histatin-1 in the lacrimal tissue may have implications for ocular infections, given the anti-microbial properties of histatin peptides. DES patients may have diminished ability to heal epithelial wounds, demonstrate greater ocular surface inflammation and have increased infection risk43. However, previous studies44 have demonstrated only limited expression of histatin-1 on the ocular surface45–47, and previous tear proteomic experiments have not demonstrated histatin-1 family members in the tear film 48. These results may indicate that histatin-1 proteins are not secreted onto the ocular surface, or they may be undetected due to the limitations of body fluid proteomic analysis48. It may also be the case that histatins are secreted in response to environmental factors or at different times of day49–51. These issues underline the importance of further, larger scale studies. Furthermore, a comparison of sub-types and severity of DES and histatin-1 expression would be a useful future study.
Adult tissue stem cells have been found in numerous exocrine tissues, such as adult human pancreas52 and salivary glands27 and may have potential therapeutic applications. In addition, stem cells in various human glandular tissues share a similar expression profile27. Recently, mouse lacrimal gland tissue has been found to contain proliferating, nestin expressing cells after induction of inflammation26. Nestin upregulation has been observed after injury, and is thought to be a marker of multi-lineage precursor cells26, 27.
Recent studies have shown that rat main lacrimal gland contains myoepithelial cells, which express α -smooth muscle actin, nestin, and ABCG-2, and may represent progenitor cells7. Other authors6 have noted similar findings in human main lacrimal gland cultures, but have emphasized the presence of an ABCG-2 positive population of cells as representing precursors. Localization of α -smooth muscle actin was similar in human ALG compared with other studies7, but we did find presence of ABCG-2, CD90 and nestin in acinar cells, as opposed to solely peri-acinar cells.
We found a negative correlation between precursor cell marker ABCG2 present in ALG and age. The relationship between ABCG-2 and age was statistically significant, but the relationship between nestin and age and CD90 and age, was not statistically significant. This suggests that an ABCG-2 positive cell population is present in ALG and decreases with increasing age, consistent with findings in other cell types7, 52.
Although we chose to focus on nestin26,27, ABCG229,53 and CD9054,55 as epithelial progenitor cell markers based on our evaluation of the available literature and negative results with (CD9, CD29, CD81, CD105) there are a number of other potential markers CD4427 and PDX-128 which could be investigated further. This study is the first to demonstrate the presence of a population of cells in adult ALG tissue expressing precursor markers. In a recent study, we showed the expression of a number of precursor and stem cell markers in an Affymetrix® gene array analysis of ALG56. However, future studies are necessary to further characterize and isolate this cell population for analysis.
Limitations of this study include small sample size, limited dry eye status assessment, semi-quantitative assessment of marker expression and staining. Future studies should be performed on a prospective basis, with detailed assessments of etiology and severity of dry eye syndrome and with more quantitative assessment of immuno-localization. In addition, larger studies with quantitative grading schema and corroborative data using protein assays such as western blotting on larger tissue samples should be performed to further characterize the role of age and other patient characteristics in cell marker expression. However, gene expression analysis performed on accessory lacrimal gland does support the expression of stem cell genes in adult accessory lacrimal gland.
Conclusion
Thus, we report that ALG tissue can be accessed and analyzed in tissue microarrays, these ALG tissues contain precursor marker positive cells, and that histatin-1 may be associated with dry eye syndrome. However, subsequent studies will be necessary to determine if these cell markers can be effectively utilized to separate and culture lacrimal epithelial cells from heterogeneous tissues, determine the relevance of histatin-1 expression to dry eye syndrome and isolate candidate precursor cells from ALG tissue.
Acknowledgments
Sources of Funding: This work was supported by NIH-NEI Grant (K08EY024339), a seed grant from Illinois Society to Prevent Blindness, a Research Grant from Midwest Eye Banks, a Grant-in-Aid from Fight for Sight and Departmental Support through an NIH-NEI core grant (2P30EY001792-36A1) and an unrestricted grant from Research to Prevent Blindness (NY, NY).
Footnotes
There is no conflicting commercial relationship in the form of financial support or personal financial interest to report.
Declaration of Interest
The authors report that they have no conflicts of interest.
References
- 1.The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Abelson MB, Ousler GW, Maffei C. Dry eye in 2008. Curr Opin Opthalmo. 2009;20(4):282–6. doi: 10.1097/ICU.0b013e32832b7578. [DOI] [PubMed] [Google Scholar]
- 3.Calonge M. The treatment of dry eye. Surv Ophthalmol. 2001;45(Suppl 2):S227–39. doi: 10.1016/s0039-6257(00)00205-8. [DOI] [PubMed] [Google Scholar]
- 4.Perry HD. Dry eye disease: pathophysiology, classification, and diagnosis. Am J Manag Care. 2008;14(3 Suppl):S79–87. [PubMed] [Google Scholar]
- 5.Tiwari S, Ali MJ, Vemuganti GK. Human lacrimal gland regeneration: Perspectives and review of literature. Saudi J Ophthalmol. 2014;28(1):12–8. doi: 10.1016/j.sjopt.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiwari S, Ali MJ, Balla MM, Naik MN, Honavar SG, Reddy VA, et al. Establishing human lacrimal gland cultures with secretory function. PLoS One. 2012;7(1):e29458. doi: 10.1371/journal.pone.0029458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shatos MA, Haugaard-Kedstrom L, Hodges RR, Dartt DA. Isolation and characterization of progenitor cells in uninjured, adult rat lacrimal gland. Invest Ophthalmol Vis Sci. 2012;53(6):2749–59. doi: 10.1167/iovs.11-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt S, Spitznas M, Seifert P, Rauwolf M. Organ culture of human main and acessary lacrimal glands and their secretory behaviour. Exp Eye Res. 1996;62(5):541–54. doi: 10.1006/exer.1996.0064. [DOI] [PubMed] [Google Scholar]
- 9.Seifert P, Spitznas M. Demonstration of nerve fibers in human accessory lacrimal glands. Graefes Arch Clin Exp Ophthalmol. 1994;232(2):107–14. doi: 10.1007/BF00171672. [DOI] [PubMed] [Google Scholar]
- 10.Ubels JL, Gipson IK, Spurr-Michaud SJ, Tisdale AS, Van Dyken RE, Hatton MP. Gene expression in human accessory lacrimal glands of Wolfring. Invest Ophthalmol Vis Sci. 2012;53(11):6738–47. doi: 10.1167/iovs.12-10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshino K, Tseng SC, Pflugfelder SC. Substrate modulation of morphology, growth, and tear protein production by cultured human lacrimal gland epithelial cells. Exp Cell Res. 1995;220(1):138–51. doi: 10.1006/excr.1995.1300. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi Y, Watanabe A, Matsuda H, Nakamura Y, Nakano T, Asamoto K, et al. Anatomy of secretory glands in the eyelid and conjunctiva: a photographic review. Ophthal Plast Reconstr Surg. 2013;29(3):215–9. doi: 10.1097/IOP.0b013e3182833dee. [DOI] [PubMed] [Google Scholar]
- 13.Toshida H, Ohta T, Suto C, Murakami A. Effect of subconjunctival lacrimal gland transplantation in a rabbit dry eye model. Cornea. 2013;32(Suppl 1):S46–51. doi: 10.1097/ICO.0b013e3182a1bb21. [DOI] [PubMed] [Google Scholar]
- 14.Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–77. doi: 10.1016/j.preteyeres.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirayama M, Ogawa M, Oshima M, Sekine Y, Ishida K, Yamashita K, et al. Functional lacrimal gland regeneration by transplantation of a bioengineered organ germ. Nat Commun. 2013;4:2497. doi: 10.1038/ncomms3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vigneswaran N, Wilk CM, Heese A, Hornstein OP, Naumann GO. Immunohistochemical characterization of epithelial cells in human lacrimal glands. Normal major and accessory lacrimal glands. Graefes Arch Clin Exp Ophthalmol. 1990;228(1):58–64. doi: 10.1007/BF02764293. [DOI] [PubMed] [Google Scholar]
- 17.McKown RL, Wang N, Raab RW, Karnati R, Zhang Y, Williams PB, et al. Lacritin and other new proteins of the lacrimal functional unit. Exp Eye Res. 2009;88(5):848–58. doi: 10.1016/j.exer.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding C, Nandoskar P, Lu M, Thomas P, Trousdale MD, Wang Y. Changes of aquaporins in the lacrimal glands of a rabbit model of Sjögren’s syndrome. Curr Eye Res. 2011;36(6):571–8. doi: 10.3109/02713683.2011.574330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki Y, Tsubota K, Kawedia JD, Menon AG, Yasui M. The difference of aquaporin distribution in acinar and ductal cells in lacrimal and parotid glands. Curr Eye Res. 2007;32(11):923–9. doi: 10.1080/02713680701733076. [DOI] [PubMed] [Google Scholar]
- 20.Ohashi Y, Tsuzaka K, Takeuchi T, Sasaki Y, Tsubota K. Altered distribution of aquaporin 5 and its C-terminal binding protein in the lacrimal glands of a mouse model for Sjögren’s syndrome. Curr Eye Res. 2008;33(8):621–9. doi: 10.1080/02713680802262819. [DOI] [PubMed] [Google Scholar]
- 21.Flanagan JL, Willcox MD. Role of lactoferrin in the tear film. Biochimie. 2009;91(1):35–43. doi: 10.1016/j.biochi.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Santagati MG, La Terra Mulè S, Amico C, Pistone M, Rusciano D, Enea V. Lactoferrin expression by bovine ocular surface epithelia: primary cell culture model to study lactoferrin gene promoter activity. Ophthalmic Res. 2005;37(5):270–8. doi: 10.1159/000087372. [DOI] [PubMed] [Google Scholar]
- 23.Sanghi S, Kumar R, Lumsden A, Dickinson D, Klepeis V, Trinkaus-Randall V, et al. cDNA and genomic cloning of lacritin, a novel secretion enhancing factor from the human lacrimal gland. Journal of Molecular Biology. 2001;310(1):127–39. doi: 10.1006/jmbi.2001.4748. [DOI] [PubMed] [Google Scholar]
- 24.Verkman AS. Role of aquaporin water channels in eye function. Exp Eye Res. 2003;76(2):137–43. doi: 10.1016/s0014-4835(02)00303-2. [DOI] [PubMed] [Google Scholar]
- 25.Shah D, Ali M, Pasha Z, Jaboori AJ, Jassim SH, Jain S, et al. Histatin-1 is a marker for human lacrimal epithelium. PLoS One. 2016 Jan 29;11(1):e0148018. doi: 10.1371/journal.pone.0148018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zoukhri D, Fix A, Alroy J, Kublin CL. Mechanisms of murine lacrimal gland repair after experimentally induced inflammation. Invest Ophthalmol Vis Sci. 2008t;49(10):4399–406. doi: 10.1167/iovs.08-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorjup E, Danner S, Rotter N, Habermann J, Brassat U, Brummendorf TH, et al. Glandular tissue from human pancreas and salivary gland yields similar stem cell populations. Eur J Cell Biol. 2009;88(7):409–21. doi: 10.1016/j.ejcb.2009.02.187. [DOI] [PubMed] [Google Scholar]
- 28.Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5(4):258–65. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- 29.You S, Kublin CL, Avidan O, Miyasaki D, Zoukhri D. Isolation and propagation of mesenchymal stem cells from the lacrimal gland. Invest Ophthalmol Vis Sci. 2011;52(5):2087–94. doi: 10.1167/iovs.10-5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Putterman AM, Urist MJ. Müller’s muscle-conjunctival resection ptosis procedure. Opthalmic Surg. 1978;9(3):27–32. [PubMed] [Google Scholar]
- 31.Kulchaiyawat V, Aakalu VK, Sajja K, Gupta S, Hallak J, Setabutr P. A clinicopathological correlation between müller’s muscleconjunctival resection and corneal staining pattern. Invest Ophthalmol Vis Sci. 2010;51(13):1472. [Google Scholar]
- 32.Buckman G, Jakobiec FA, Hyde K, Lisman RD, Hornblass A, Harrison W. Success of the Fasanella-Servat operation independent of Müller’s smooth muscle excision. Ophthalmology. 1989;96(4):413–8. doi: 10.1016/s0161-6420(89)32876-4. [DOI] [PubMed] [Google Scholar]
- 33.Rocha EM, Alves M, Rios JD, Dartt DA. The aging lacrimal gland: changes in structure and function. Ocul Surf. 2008;6(4):162–74. doi: 10.1016/s1542-0124(12)70177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obata H, Yamamoto S, Horiuchi H, Machinami R. Histopathologic study of human lacrimal gland. Statistical analysis with special reference to aging. Ophthalmology. 1995;102(4):678–86. doi: 10.1016/s0161-6420(95)30971-2. [DOI] [PubMed] [Google Scholar]
- 35.Obata H. Anatomy and histopathology of the human lacrimal gland. Cornea. 2006;25(10 Suppl 1):S82–9. doi: 10.1097/01.ico.0000247220.18295.d3. [DOI] [PubMed] [Google Scholar]
- 36.Zlobec I, Terracciano L, Jass JR, Lugli A. Value of staining intensity in the interpretation of immunohistochemistry for tumor markers in colorectal cancer. Virchows Arch. 2007;451(4):763–9. doi: 10.1007/s00428-007-0466-8. [DOI] [PubMed] [Google Scholar]
- 37.McCarty CA, Bansal AK, Livingston PM, Stanislavsky YL, Taylor HR. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology. 1998;105:1114–9. doi: 10.1016/S0161-6420(98)96016-X. [DOI] [PubMed] [Google Scholar]
- 38.Gipson IK. Age related changes and diseases of the ocular surface and cornea. Invest Ophthalmol Vis Sci. 2013 Dec 13;54(14):ORSF48–53. doi: 10.1167/iovs.13-12840. [DOI] [PubMed] [Google Scholar]
- 39.Melino S, Santone C, Di Nardo P, Sarkar B. Histatins: salivary peptides with copper(II)- and zinc(II)-binding motifs: perspectives for biomedical applications. FEBS J. 2014;281(3):657–72. doi: 10.1111/febs.12612. [DOI] [PubMed] [Google Scholar]
- 40.Oudhoff MJ, Kroeze KL, Nazmi K, van den Keijbus PA, van’t Hof W, Fernandez-Borja M, et al. Structure-activity analysis of histatin, a potent wound healing peptide from human saliva: cyclization of histatinpotentiates molar activity 1,000-fold. FASEB J. 2009;23(11):3928–35. doi: 10.1096/fj.09-137588. [DOI] [PubMed] [Google Scholar]
- 41.Kavanagh K, Dowd S. Histatins: antimicrobial peptides with therapeutic potential. J Pharm Pharmacol. 2004;56(3):285–9. doi: 10.1211/0022357022971. [DOI] [PubMed] [Google Scholar]
- 42.Oudhoff MJ, Blaauboer ME, Nazmi K, Scheres N, Bolscher JG, Veerman EC. The role of salivary histatin and the human cathelicidin LL-37 in wound healing and innate immunity. Biol Chem. 2010;391(5):541–8. doi: 10.1515/BC.2010.057. [DOI] [PubMed] [Google Scholar]
- 43.Daneshvar R. Cataract Surgery and Dry Eye. Mashhad University of Medical Sciences; Iran: Book chapter 23. [DOI] [Google Scholar]
- 44.Huang LC, Jean D, Proske RJ, Reins RY, McDermott AM. Ocular surface expression and in vitro activity of antimicrobial peptides. Curr Eye Res. 2007;32(7–8):595–609. doi: 10.1080/02713680701446653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jumblatt MM, Imbert Y, Young WW, Jr, Foulks GN, Steele PS, Demuth DR. Glycoprotein 340 in normal human ocular surface tissues and tear film. Infect Immun. 2006;74(7):4058–63. doi: 10.1128/IAI.01951-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steele PS, Jumblatt MM, Smith NB, Pierce WM. Detection of Histatin 5 in Normal Human Schirmer Strip Samples by Mass Spectroscopy. Invest Ophthalmol Vis Sci. 2002;43(13):98. [Google Scholar]
- 47.Steele PS, Jumblatt MM. Defense Proteins of the Ocular Surface. Invest Ophthalmol Vis Sci. 2004;45(13):3792. [Google Scholar]
- 48.Perumal N, Funke S, Wolters D, Pfeiffer N, Grus FH. Characterization of human reflex tear proteome reveals high expression of lacrimal proline-rich protein 4 (PRR4) Proteomics. 2015;15(19):3370–81. doi: 10.1002/pmic.201400239. [DOI] [PubMed] [Google Scholar]
- 49.Gusman H, Leone C, Helmerhorst EJ, Nunn M, Flora B, Troxler RF, et al. Human salivary gland specific daily variations in histatin concentrations determined by a novel quantitationtechnique. Arch Oral Biol. 2004;49(1):11–22. doi: 10.1016/s0003-9969(03)00182-1. [DOI] [PubMed] [Google Scholar]
- 50.Campese M, Sun X, Bosch JA, Oppenheim FG, Helmerhorst EJ. Concentration and fate of histatins and acidic proline-rich proteins in the oral environment. Arch Oral Biol. 2009;54(4):345–53. doi: 10.1016/j.archoralbio.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manconi B, Cabras T, Pisano E, Sanna MT, Olianas A, Fanos V, et al. Modifications of the acidic soluble salivary proteome in human children from birth to the age of 48monthsinvestigated by a top-down HPLC-ESI-MS platform. J Proteomics. 2013;91:536–43. doi: 10.1016/j.jprot.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 52.Lardon J, Corbeil D, Huttner WB, Ling Z, Bouwens L. Stem cell marker prominin-1/AC133 is expressed in duct cells of the adult human pancreas. Pancreas. 2008;36(1):e1–6. doi: 10.1097/mpa.0b013e318149f2dc. [DOI] [PubMed] [Google Scholar]
- 53.Ding XW, Wu JH, Jiang CP. ABCG2: a potential marker of stem cells and novel target in stem cell and cancer therapy. Life Sci. 2010 Apr 24;86(17–18):631–7. doi: 10.1016/j.lfs.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Buishand FO, Arkesteijn GJ, Feenstra LR, Oorsprong CW, Mestemaker M, Starke A, et al. Identification of CD90 as Putative Cancer Stem Cell Marker and Therapeutic Target in Insulinomas. Stem Cells Dev. 2016 May 5; doi: 10.1089/scd.2016.0032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pringle S, Van Os R, Coppes RP. Concise review: Adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells. 2013 Apr;31(4):613–9. doi: 10.1002/stem.1327. [DOI] [PubMed] [Google Scholar]
- 56.Jassim SA, Lin A, Setabutr P, Jaboor AH, Jain S, Aakalu V. A Tissue Microarray Analysis of Stem cell markers positive cells in Human Accessory Lacrimal Glands (ALG) from Muller Muscle Conjunctival Resection (MMCR) Specimens. Invest Ophthalmol Vis Sci. 2014;55(13):3116. [Google Scholar]


