Abstract
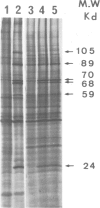
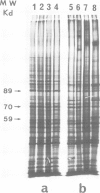
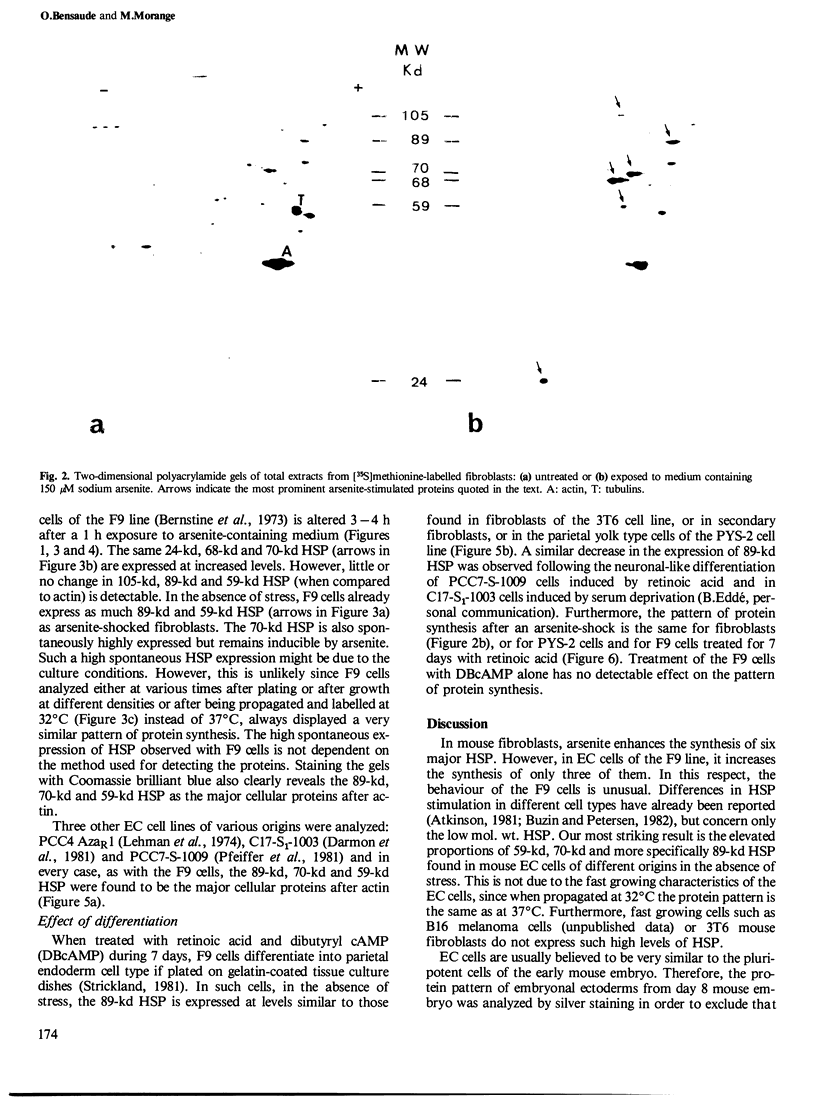
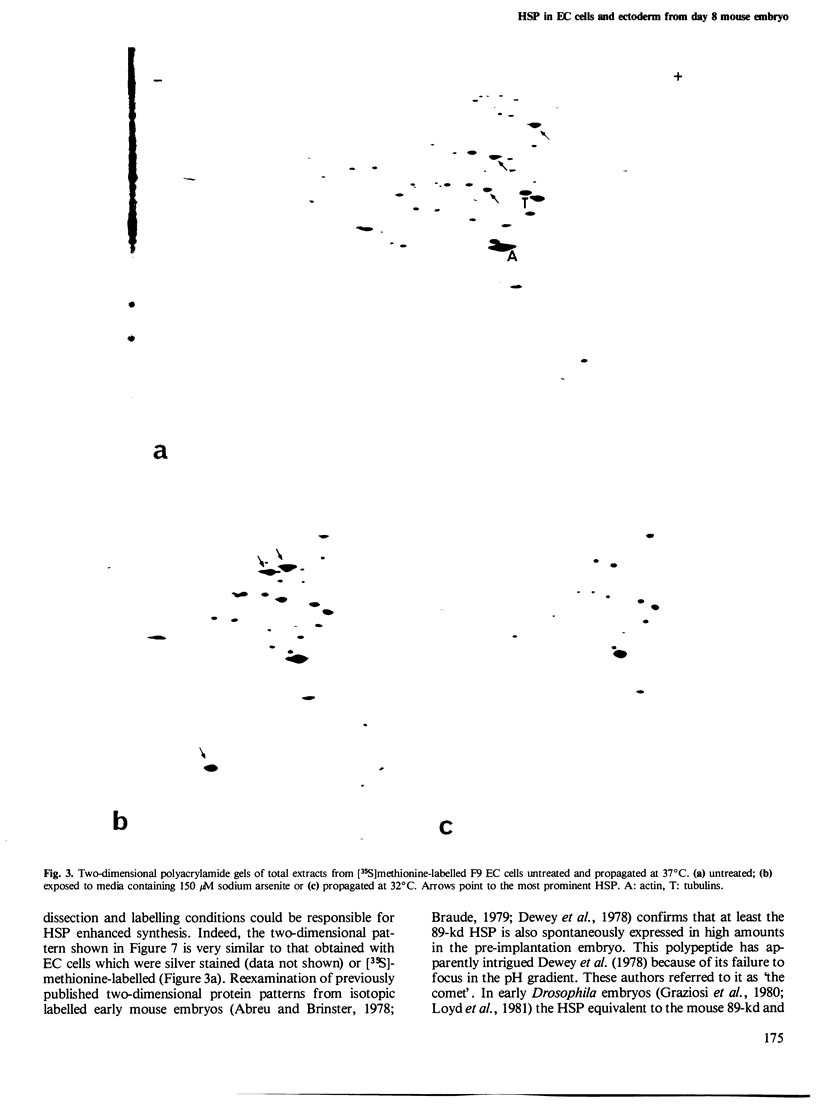
When submitted to a heat-shock, mouse embryonal carcinoma (EC) and fibroblast cells show very different behavior. All the EC cells so far analyzed express very high levels of several heat-shock proteins (HSP) in the absence of stress and independent of their origin and culture conditions. In such cells, the 89-kd, 70-kd and 59-kd HSP are the most prominent proteins after actin. In addition, the 89-kd and 59-kd HSP are not stimulated by an arsenite shock in contrast to what is observed with fibroblasts or cells of the parietal yolk sac type. Arsenite induces the synthesis of a 105-kd polypeptide in fibroblasts but not in EC cells. In vitro differentiation of F9 cells induced by retinoic acid and dibutyryl cAMP is accompanied by a decrease in the spontaneous relative abundance of HSP and restores the arsenite-induced synthesis of the 105-kd polypeptide. EC cells are usually believed to be similar to inner cell mass cells of mouse blastocyst. Furthermore, data in the literature together with our own results suggest that the same three HSP are also spontaneously expressed in high amounts in the early mouse embryo.
Full text
PDF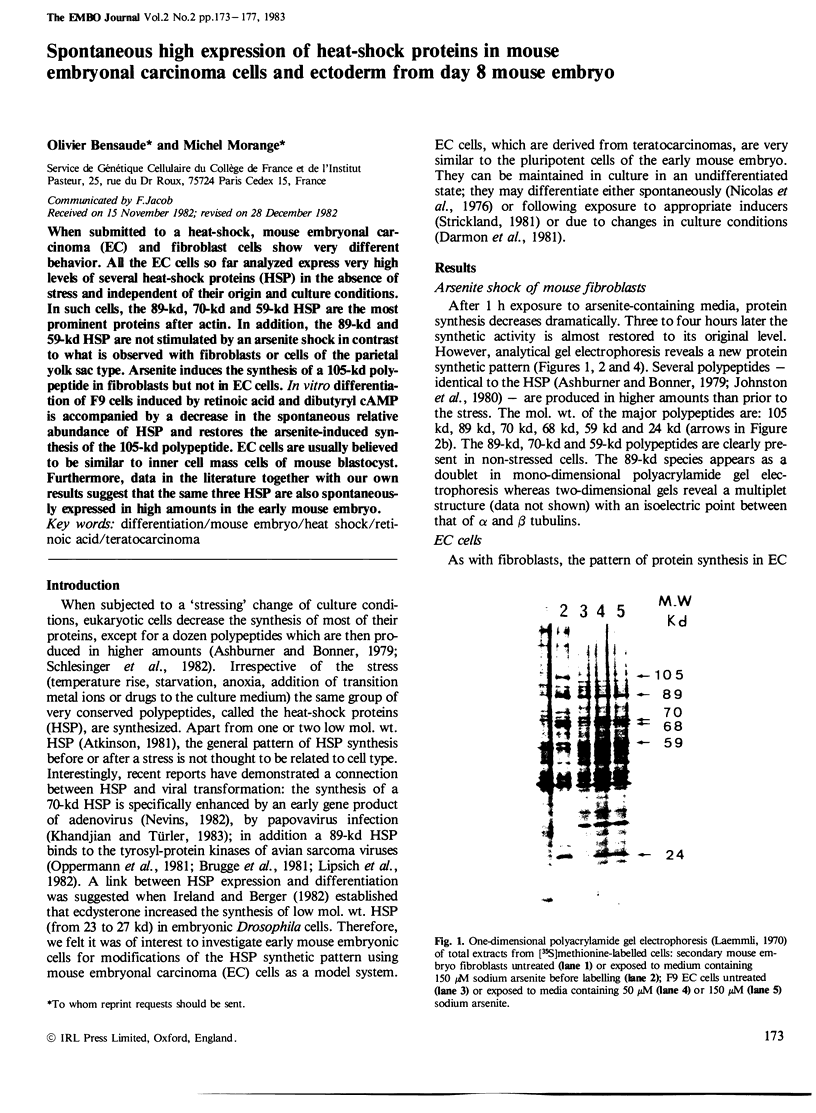

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abreu S. L., Brinster R. L. Synthesis of tubulin and actin during the preimplantation development of the mouse. Exp Cell Res. 1978 Jun;114(1):135–141. doi: 10.1016/0014-4827(78)90045-9. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Atkinson B. G. Synthesis of heat-shock proteins by cells undergoing myogenesis. J Cell Biol. 1981 Jun;89(3):666–673. doi: 10.1083/jcb.89.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berstine E. G., Hooper M. L., Grandchamp S., Ephrussi B. Alkaline phosphatase activity in mouse teratoma. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3899–3903. doi: 10.1073/pnas.70.12.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braude P. R. Control of protein synthesis during blastocyst formation in the mouse. Dev Biol. 1979 Feb;68(2):440–452. doi: 10.1016/0012-1606(79)90216-1. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Buzin C. H., Petersen N. S. A comparison of the multiple Drosophila heat shock proteins in cell lines and larval salivary glands by two-dimensional gel electrophoresis. J Mol Biol. 1982 Jun 25;158(2):181–201. doi: 10.1016/0022-2836(82)90428-4. [DOI] [PubMed] [Google Scholar]
- Darmon M., Bottenstein J., Sato G. Neural differentiation following culture of embryonal carcinoma cells in a serum-free defined medium. Dev Biol. 1981 Jul 30;85(2):463–473. doi: 10.1016/0012-1606(81)90277-3. [DOI] [PubMed] [Google Scholar]
- Dewey M. J., Filler R., Mintz B. Protein patterns of developmentally totipotent mouse teratocarcinoma cells and normal early embryo cells. Dev Biol. 1978 Jul;65(1):171–182. doi: 10.1016/0012-1606(78)90188-4. [DOI] [PubMed] [Google Scholar]
- Ireland R. C., Berger E. M. Synthesis of low molecular weight heat shock peptides stimulated by ecdysterone in a cultured Drosophila cell line. Proc Natl Acad Sci U S A. 1982 Feb;79(3):855–859. doi: 10.1073/pnas.79.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D., Oppermann H., Jackson J., Levinson W. Induction of four proteins in chick embryo cells by sodium arsenite. J Biol Chem. 1980 Jul 25;255(14):6975–6980. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehman J. M., Speers W. C., Swartzendruber D. E., Pierce G. B. Neoplastic differentiation: characteristics of cell lines derived from a murine teratocarcinoma. J Cell Physiol. 1974 Aug;84(1):13–27. doi: 10.1002/jcp.1040840103. [DOI] [PubMed] [Google Scholar]
- Li G. C., Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982 May;79(10):3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsich L. A., Cutt J. R., Brugge J. S. Association of the transforming proteins of Rous, Fujinami, and Y73 avian sarcoma viruses with the same two cellular proteins. Mol Cell Biol. 1982 Jul;2(7):875–880. doi: 10.1128/mcb.2.7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd J. E., Raff E. C., Raff R. A. Site and timing of synthesis of tubulin and other proteins during oogenesis in Drosophila melanogaster. Dev Biol. 1981 Sep;86(2):272–284. doi: 10.1016/0012-1606(81)90185-8. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Nicolas J. F., Avner P., Gaillard J., Guenet J. L., Jakob H., Jacob F. Cell lines derived from teratocarcinomas. Cancer Res. 1976 Nov;36(11 Pt 2):4224–4231. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oppermann H., Levinson W., Bishop J. M. A cellular protein that associates with the transforming protein of Rous sarcoma virus is also a heat-shock protein. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1067–1071. doi: 10.1073/pnas.78.2.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N. S., Mitchell H. K. Recovery of protein synthesis after heat shock: prior heat treatment affects the ability of cells to translate mRNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1708–1711. doi: 10.1073/pnas.78.3.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer S. E., Jakob H., Mikoshiba K., Dubois P., Guenet J. L., Nicolas J. F., Gaillard J., Chevance G., Jacob F. Differentiation of a teratocarcinoma line: preferential development of cholinergic neurons. J Cell Biol. 1981 Jan;88(1):57–66. doi: 10.1083/jcb.88.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccheri M. C., Di Bernardo M. G., Giudice G. Synthesis of heat-shock proteins in developing sea urchins. Dev Biol. 1981 Apr 15;83(1):173–177. doi: 10.1016/s0012-1606(81)80020-6. [DOI] [PubMed] [Google Scholar]
- Strickland S. Mouse teratocarcinoma cells: prospects for the study of embryogenesis and neoplasia. Cell. 1981 May;24(2):277–278. doi: 10.1016/0092-8674(81)90313-5. [DOI] [PubMed] [Google Scholar]