Abstract
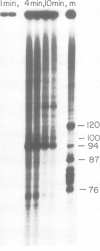
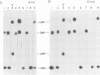
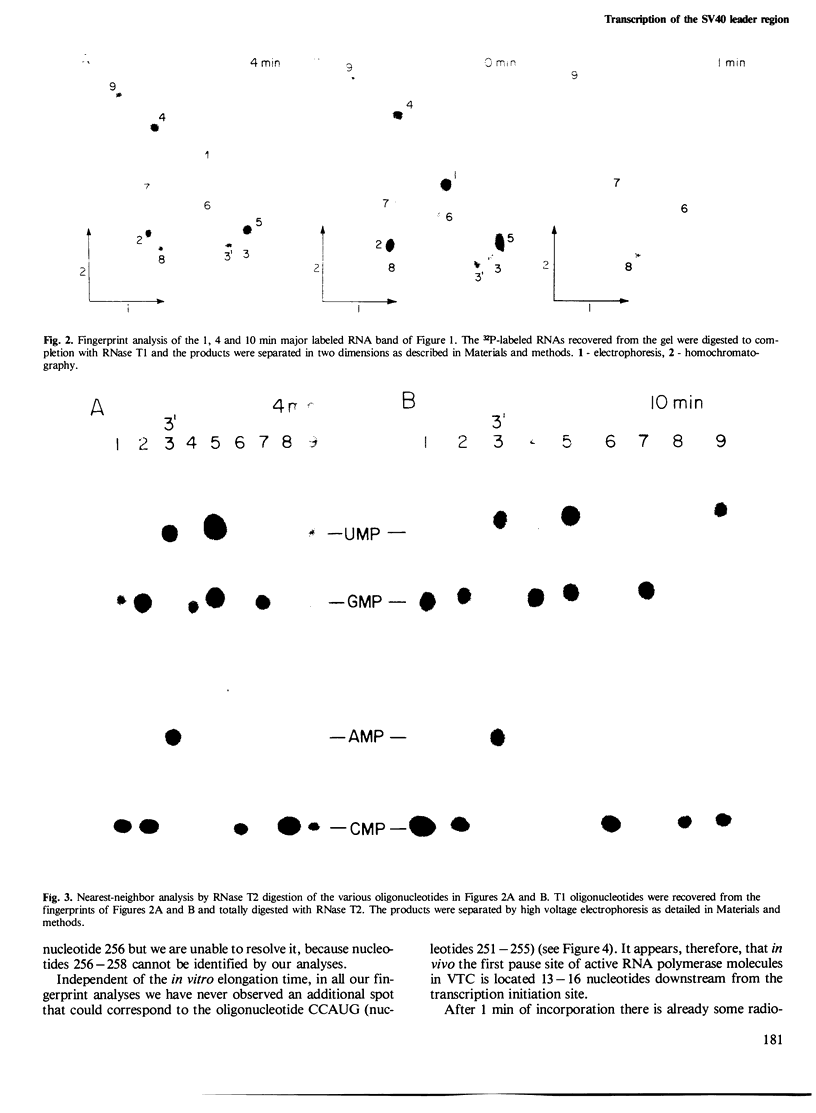
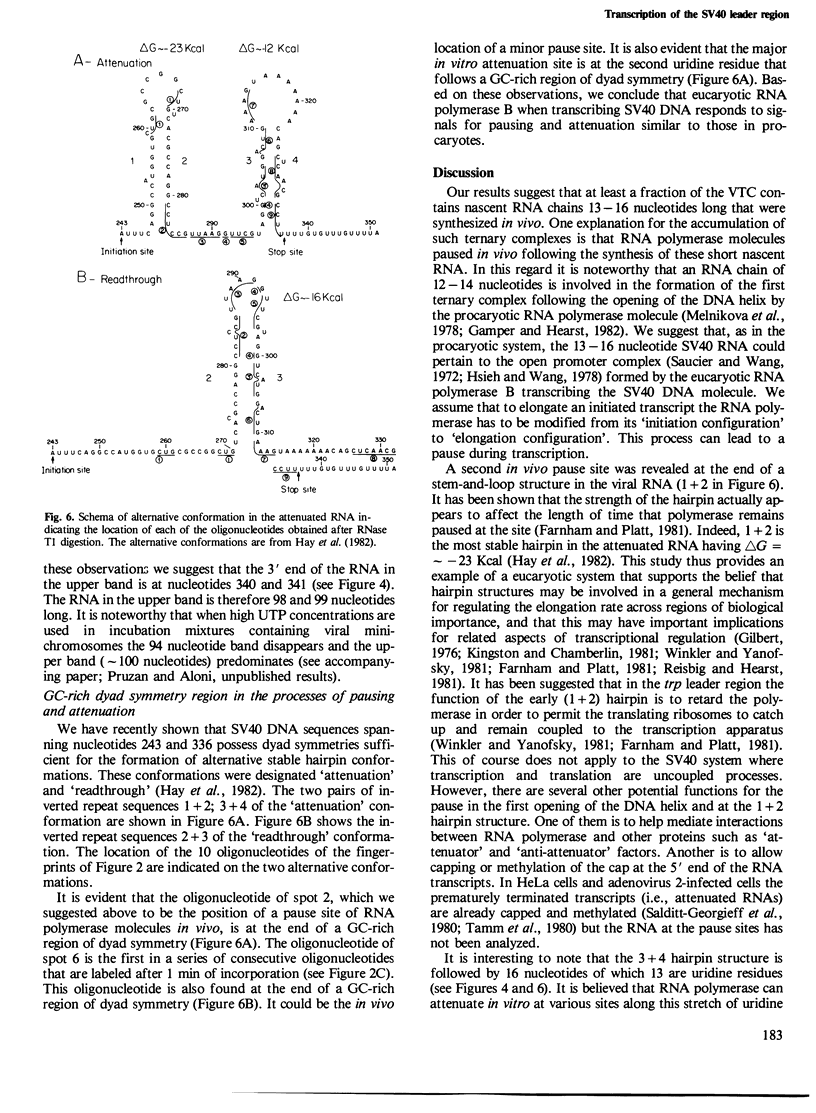
Viral transcription complexes were isolated from SV40-infected cells and incubated in vitro in the presence of [alpha-32P]UTP to allow elongation of the promoter-proximal RNA up to the attenuation sites. The 94 nucleotide attenuated RNA (spanning nucleotides 243-336) was purified, digested with RNase T1 and fingerprinted. The labeled oligonucleotides were then isolated, digested with RNase T2 and their base composition was determined. Based on these analyses 10 consecutive oligonucleotides, spanning residues 259-336, were identified. As the in vivo synthesized oligonucleotides are unlabeled the junctions between labeled and unlabeled oligonucleotides define the in vivo pause sites of RNA polymerase molecules. The characterization of the 10 radioactive spots and their relative intensities allowed the localization of two in vivo pause sites: one at 13-16 nucleotides downstream from the major initiation site presumably at the initial opening of the DNA helix and the second at approximately 40 nucleotides downstream from the major initiation site, just past a GC-rich region of dyad symmetry. It is postulated that pausing of RNA polymerase molecules in the leader region is an essential process in the control of SV40 late transcription.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Stauffer G. V. Regulation of tryptophan biosynthesis. Annu Rev Biochem. 1980;49:163–195. doi: 10.1146/annurev.bi.49.070180.001115. [DOI] [PubMed] [Google Scholar]
- Farnham P. J., Platt T. A model for transcription termination suggested by studies on the trp attenuator in vitro using base analogs. Cell. 1980 Jul;20(3):739–748. doi: 10.1016/0092-8674(80)90320-7. [DOI] [PubMed] [Google Scholar]
- Farnham P. J., Platt T. Rho-independent termination: dyad symmetry in DNA causes RNA polymerase to pause during transcription in vitro. Nucleic Acids Res. 1981 Feb 11;9(3):563–577. doi: 10.1093/nar/9.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinand F. J., Brown M., Khoury G. Synthesis and characterization of late lytic simian virus 40 RNA from transcriptional complexes. Virology. 1977 May 1;78(1):150–161. doi: 10.1016/0042-6822(77)90087-3. [DOI] [PubMed] [Google Scholar]
- Galluppi G. R., Richardson J. P. ATP-induced changes in the binding of RNA synthesis termination protein Rho to RNA. J Mol Biol. 1980 Apr 15;138(3):513–539. doi: 10.1016/s0022-2836(80)80016-7. [DOI] [PubMed] [Google Scholar]
- Gamper H. B., Hearst J. E. A topological model for transcription based on unwinding angle analysis of E. coli RNA polymerase binary, initiation and ternary complexes. Cell. 1982 May;29(1):81–90. doi: 10.1016/0092-8674(82)90092-7. [DOI] [PubMed] [Google Scholar]
- Gottesman M. E., Adhya S., Das A. Transcription antitermination by bacteriophage lambda N gene product. J Mol Biol. 1980 Jun 15;140(1):57–75. doi: 10.1016/0022-2836(80)90356-3. [DOI] [PubMed] [Google Scholar]
- Hartman J. R., Laub O., Aloni Y., Winocour E. Transcription of the cellular DNA sequences in a cloned host-substituted SV40 DNA variant. Virology. 1979 Apr 15;94(1):82–94. doi: 10.1016/0042-6822(79)90440-9. [DOI] [PubMed] [Google Scholar]
- Hay N., Skolnik-David H., Aloni Y. Attenuation in the control of SV40 gene expression. Cell. 1982 May;29(1):183–193. doi: 10.1016/0092-8674(82)90102-7. [DOI] [PubMed] [Google Scholar]
- Hsieh T., Wang J. C. Physiochemical studies on interactions between DNA and RNA polymerase. Ultraviolet absorption measurements. Nucleic Acids Res. 1978 Sep;5(9):3337–3345. doi: 10.1093/nar/5.9.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston R. E., Chamberlin M. J. Pausing and attenuation of in vitro transcription in the rrnB operon of E. coli. Cell. 1981 Dec;27(3 Pt 2):523–531. doi: 10.1016/0092-8674(81)90394-9. [DOI] [PubMed] [Google Scholar]
- Laub O., Aloni Y. Transcription of simian virus 40. V. Regulattion of simian virus 40 gene expression. J Virol. 1975 Nov;16(5):1171–1183. doi: 10.1128/jvi.16.5.1171-1183.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laub O., Aloni Y. Transcription of simian virus 40. VI. SV 40 DNA-RNA polymerase complex isolated from productively infected cells transcribed in vitro. Virology. 1976 Dec;75(2):346–354. doi: 10.1016/0042-6822(76)90033-7. [DOI] [PubMed] [Google Scholar]
- Laub O., Bratosin S., Horowitz M., Aloni Y. The initiation of transcription of SV40 DNA at late time after infection. Virology. 1979 Jan 30;92(2):310–323. doi: 10.1016/0042-6822(79)90136-3. [DOI] [PubMed] [Google Scholar]
- Laub O., Jakobovits E. B., Aloni Y. 5,6-dichloro-1-beta-ribofuranosylbenzimidazole enhances premature termination of late transcription of simian virus 40 DNA. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3297–3301. doi: 10.1073/pnas.77.6.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llopis R., Stark G. R. Two deletions within genes for simian virus 40 structural proteins VP2 and VP3 lead to formation of abnormal transcriptional complexes. J Virol. 1981 Apr;38(1):91–103. doi: 10.1128/jvi.38.1.91-103.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Efstratiadis A. Fractionation of low molecular weight DNA or RNA in polyacrylamide gels containing 98% formamide or 7 M urea. Methods Enzymol. 1980;65(1):299–305. doi: 10.1016/s0076-6879(80)65040-x. [DOI] [PubMed] [Google Scholar]
- Martin F. H., Tinoco I., Jr DNA-RNA hybrid duplexes containing oligo(dA:rU) sequences are exceptionally unstable and may facilitate termination of transcription. Nucleic Acids Res. 1980 May 24;8(10):2295–2299. doi: 10.1093/nar/8.10.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell I. H., Maxwell F., Hahn W. E. Removal of RNase activity from DNase by affinity chromatography on agarose coupled aminophenylphosphoryl-uridine-2' (3')-phosphate. Nucleic Acids Res. 1977 Jan;4(1):241–246. doi: 10.1093/nar/4.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikova A. F., Beabealashvilli R., Mirzabekov A. D. A study of unwinding of DNA and shielding of the DNA grooves by RNA polymerase by using methylation with dimethylsulphate. Eur J Biochem. 1978 Mar;84(1):301–309. doi: 10.1111/j.1432-1033.1978.tb12169.x. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Reisbig R. R., Hearst J. E. Escherichia coli deoxyribonucleic acid dependent ribonucleic acid polymerase transcriptional pause sites on SV40 DNA F1. Biochemistry. 1981 Mar 31;20(7):1907–1918. doi: 10.1021/bi00510a029. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Salditt-Georgieff M., Harpold M., Chen-Kiang S., Darnell J. E., Jr The addition of 5' cap structures occurs early in hnRNA synthesis and prematurely terminated molecules are capped. Cell. 1980 Jan;19(1):69–78. doi: 10.1016/0092-8674(80)90389-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Saucier J. M., Wang J. C. Angular alteration of the DNA helix by E. coli RNA polymerase. Nat New Biol. 1972 Oct 11;239(93):167–170. doi: 10.1038/newbio239167a0. [DOI] [PubMed] [Google Scholar]
- Shani M., Birkenmeier E., May E., Salzman N. P. Properties of simian virus 40 transcriptional intermediates isolated from nuclei of permissive cells. J Virol. 1977 Jul;23(1):20–28. doi: 10.1128/jvi.23.1.20-28.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnik-David H., Hay N., Aloni Y. Site of premature termination of late transcription of simian virus 40 DNA: enhancement by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Proc Natl Acad Sci U S A. 1982 May;79(9):2743–2747. doi: 10.1073/pnas.79.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I., Kikuchi T., Darnell J. E., Jr, Salditt-Georgieff M. Short capped hnRNA precursor chains in HeLa cells: continued synthesis in the presence of 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Biochemistry. 1980 Jun 10;19(12):2743–2748. doi: 10.1021/bi00553a032. [DOI] [PubMed] [Google Scholar]
- Winkler M. E., Yanofsky C. Pausing of RNA polymerase during in vitro transcription of the tryptophan operon leader region. Biochemistry. 1981 Jun 23;20(13):3738–3744. doi: 10.1021/bi00516a011. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]