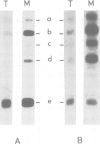
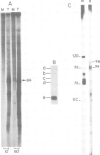
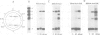Abstract
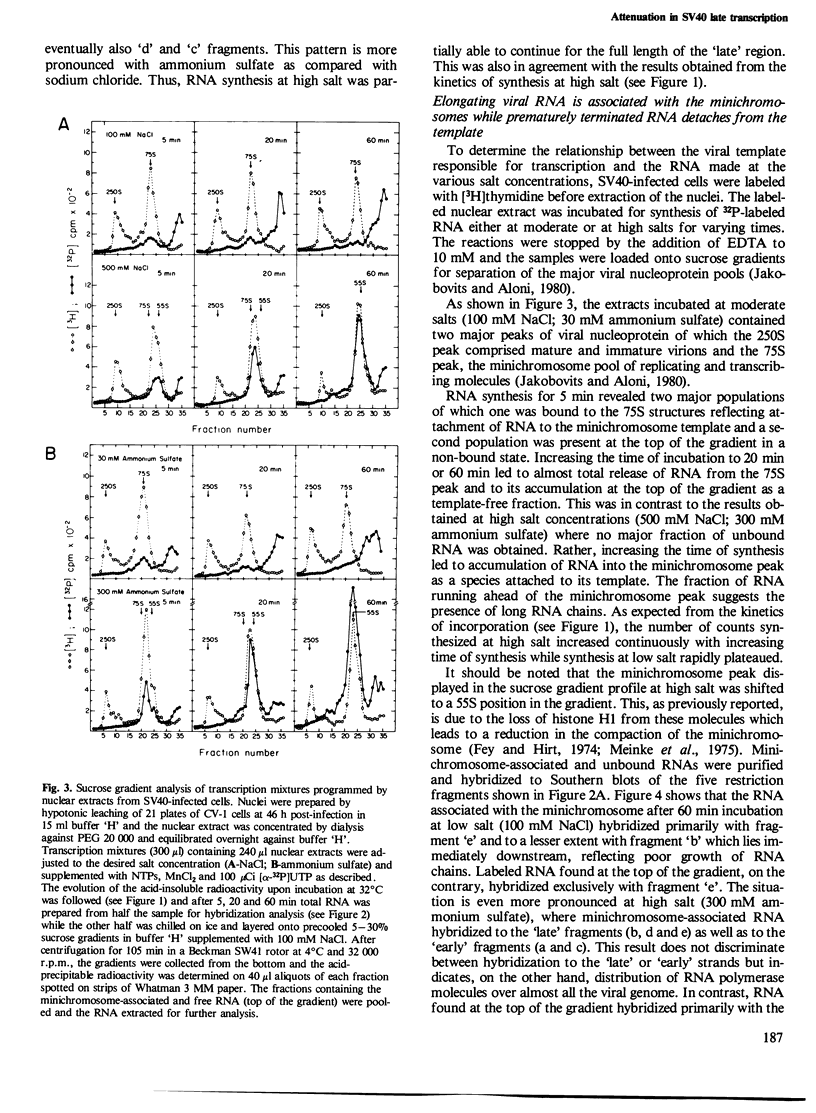
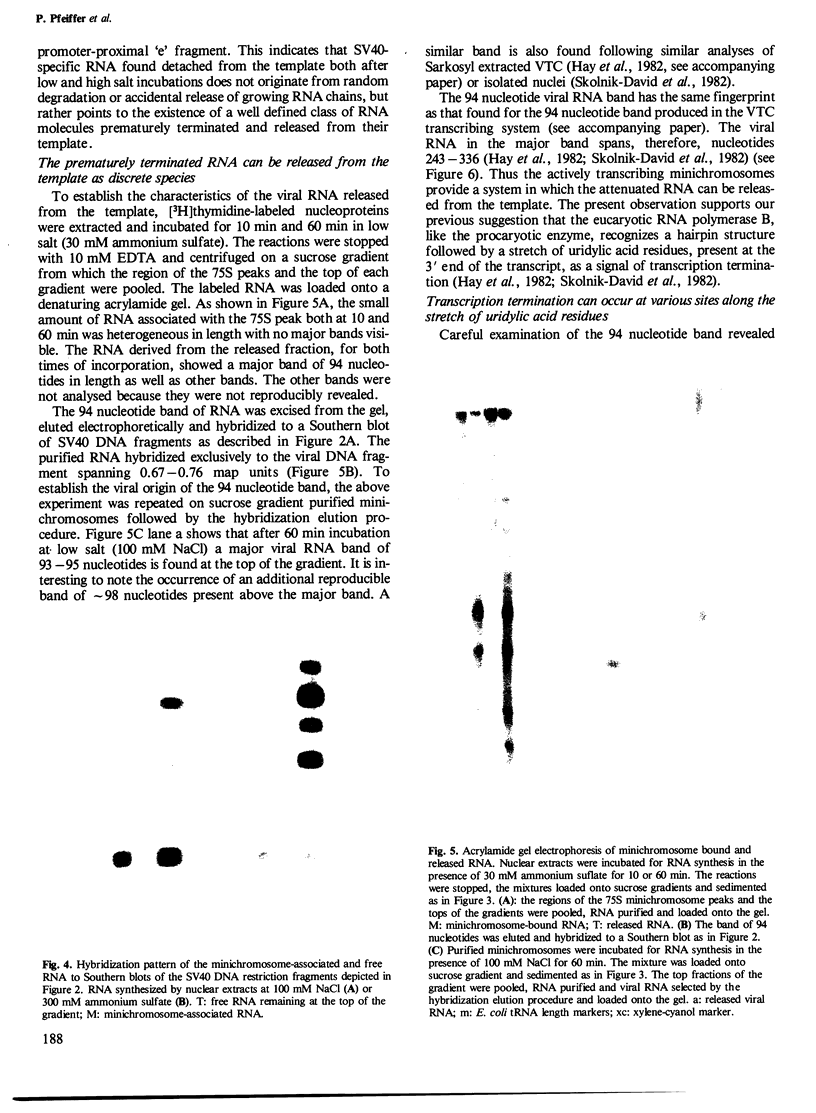
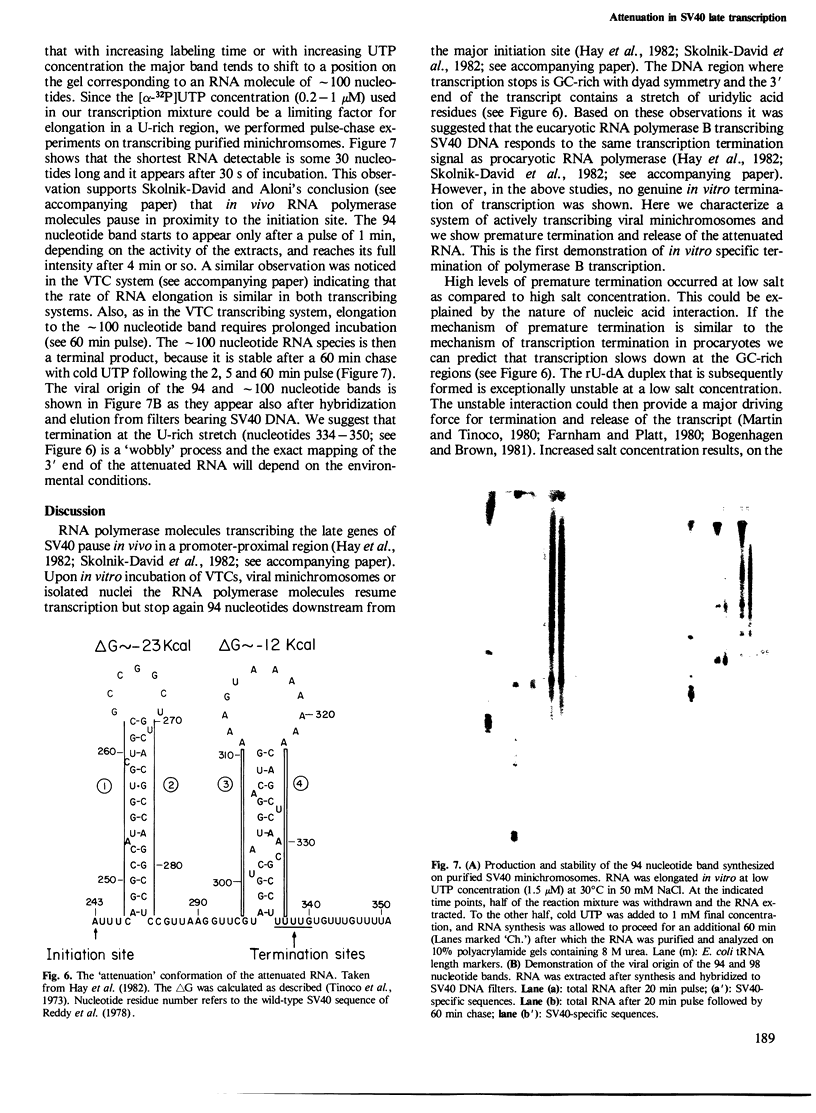
Nuclear extracts and viral transcribing minichromosomes were prepared from SV40-infected cells and incubated in vitro with [alpha-32P]UTP under conditions which allow the elongation of preinitiated RNA chains. Sucrose gradient lysis of the transcription mixtures revealed two populations of SV40-specific RNA: elongating chains that remain associated with the viral minichromosomes, and, at the top of the gradient, small free RNA detached from the template and hybridizing exclusively to the promoter-proximal region of SV40 DNA. This free RNA was shown by polyacrylamide gel electrophoresis to comprise essentially a 94 nucleotide species, which could, however, at high UTP concentration, be elongated a further few nucleotides before terminating. These results thus show that the actively transcribing minichromosomes provide a sytem in which the attenuated RNA can be released from the template. Moreover, this is the first demonstration of specific in vitro termination of polymerase B transcription. The conditions which lead to transcription termination are discussed.
Full text
PDF
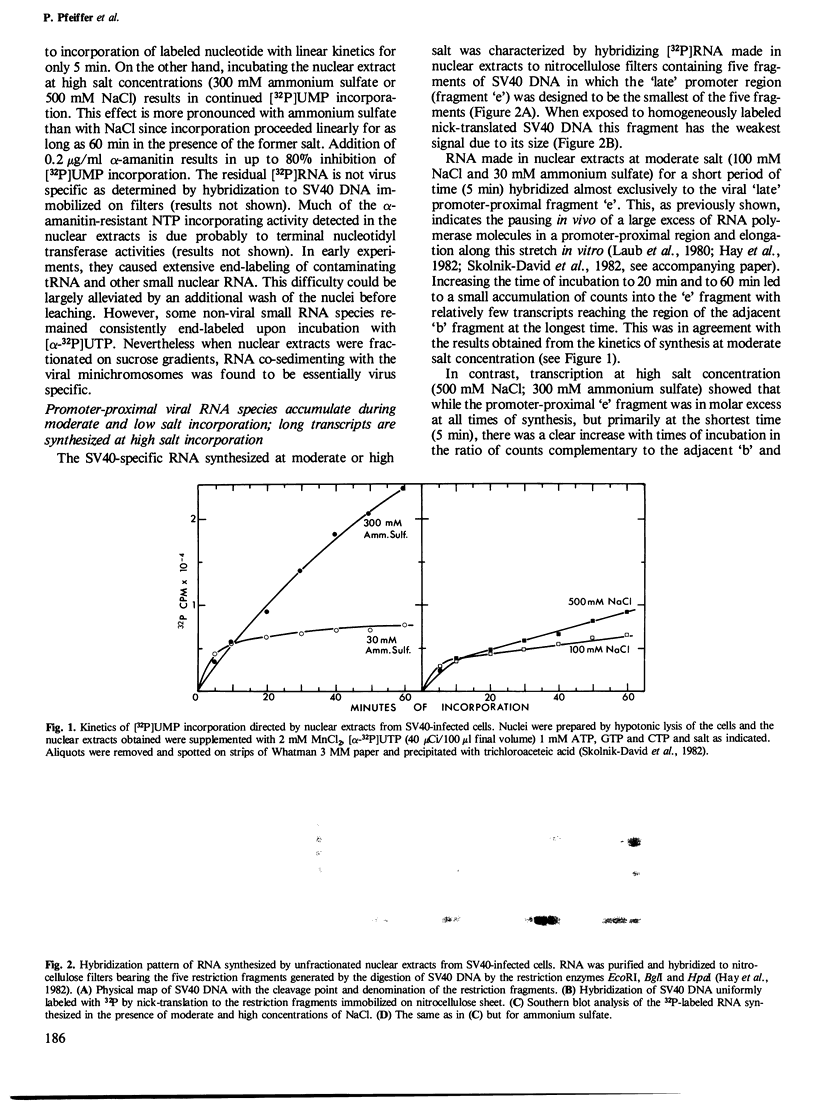


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y. Extensive symmetrical transcription of Simian Virus 40 DNA in virus-yielding cells. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2404–2409. doi: 10.1073/pnas.69.9.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard P., Nyfeler K. Transcription of Simian Virus 40 chromosomes in an extract of HeLa cells. EMBO J. 1982;1(1):9–14. doi: 10.1002/j.1460-2075.1982.tb01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenhagen D. F., Brown D. D. Nucleotide sequences in Xenopus 5S DNA required for transcription termination. Cell. 1981 Apr;24(1):261–270. doi: 10.1016/0092-8674(81)90522-5. [DOI] [PubMed] [Google Scholar]
- Canaani D., Kahana C., Mukamel A., Groner Y. Sequence heterogeneity at the 5' termini of late simian virus 40 19S and 16S mRNAs. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3078–3082. doi: 10.1073/pnas.76.7.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P. Summary: the molecular biology of the eukaryotic genome is coming of age. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1209–1234. doi: 10.1101/sqb.1978.042.01.122. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Stauffer G. V. Regulation of tryptophan biosynthesis. Annu Rev Biochem. 1980;49:163–195. doi: 10.1146/annurev.bi.49.070180.001115. [DOI] [PubMed] [Google Scholar]
- Cremisi C. The appearance of DNase I hypersensitive sites at the 5' end of the late SV40 genes is correlated with the transcriptional switch. Nucleic Acids Res. 1981 Nov 25;9(22):5949–5964. doi: 10.1093/nar/9.22.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Subramanian K. N., Pan J., Weissman S. M. Structure of a large segment of the genome of simian virus 40 that does not encode known proteins. Proc Natl Acad Sci U S A. 1977 Mar;74(3):827–831. doi: 10.1073/pnas.74.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R., Weber J., Ziff E., Darnell J. E. Premature termination during adenovirus transcription. Nature. 1979 Mar 22;278(5702):367–370. doi: 10.1038/278367a0. [DOI] [PubMed] [Google Scholar]
- Farnham P. J., Greenblatt J., Platt T. Effects of NusA protein on transcription termination in the tryptophan operon of Escherichia coli. Cell. 1982 Jul;29(3):945–951. doi: 10.1016/0092-8674(82)90457-3. [DOI] [PubMed] [Google Scholar]
- Farnham P. J., Platt T. A model for transcription termination suggested by studies on the trp attenuator in vitro using base analogs. Cell. 1980 Jul;20(3):739–748. doi: 10.1016/0092-8674(80)90320-7. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Fey G., Hirt B. Fingerprints of polyoma virus proteins and mouse histones. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):235–241. doi: 10.1101/sqb.1974.039.01.030. [DOI] [PubMed] [Google Scholar]
- Fraser N. W., Sehgal P. B., Darnell J. E., Jr Multiple discrete sites for premature RNA chain termination late in adenovirus-2 infection: enhancement by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2571–2575. doi: 10.1073/pnas.76.6.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluppi G. R., Richardson J. P. ATP-induced changes in the binding of RNA synthesis termination protein Rho to RNA. J Mol Biol. 1980 Apr 15;138(3):513–539. doi: 10.1016/s0022-2836(80)80016-7. [DOI] [PubMed] [Google Scholar]
- Gariglio P., Bellard M., Chambon P. Clustering of RNA polymerase B molecules in the 5' moiety of the adult beta-globin gene of hen erythrocytes. Nucleic Acids Res. 1981 Jun 11;9(11):2589–2598. doi: 10.1093/nar/9.11.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariglio P., Llopis R., Oudet P., Chambon P. The template of the isolated native simian virus 40 transcriptional complexes is a minichromosome. J Mol Biol. 1979 Jun 15;131(1):75–105. doi: 10.1016/0022-2836(79)90302-4. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Swinscoe J., Choudary P. V., Lebowitz P., Weissman S. M. The 5'-terminal leader sequence of late 16 S mRNA from cells infected with simian virus 40. J Biol Chem. 1978 May 25;253(10):3643–3647. [PubMed] [Google Scholar]
- Gottesman M. E., Adhya S., Das A. Transcription antitermination by bacteriophage lambda N gene product. J Mol Biol. 1980 Jun 15;140(1):57–75. doi: 10.1016/0022-2836(80)90356-3. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Hay N., Skolnik-David H., Aloni Y. Attenuation in the control of SV40 gene expression. Cell. 1982 May;29(1):183–193. doi: 10.1016/0092-8674(82)90102-7. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Saragosti S., Blangy D., Yaniv M. Fine structure of the origin-proximal DNAase I-hypersensitive region in wild-type and EC mutant polyoma. Cell. 1981 Sep;25(3):651–658. doi: 10.1016/0092-8674(81)90172-0. [DOI] [PubMed] [Google Scholar]
- Horowitz M., Laub O., Bratosin S., Aloni Y. Splicing of SV40 late mRNA is a post-transcriptional process. Nature. 1978 Oct 12;275(5680):558–559. doi: 10.1038/275558a0. [DOI] [PubMed] [Google Scholar]
- Jackson V., Chalkley R. Use of whole-cell fixation to visualize replicating and maturing simian virus 40: identification of new viral gene product. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6081–6085. doi: 10.1073/pnas.78.10.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits E. B., Aloni Y. Isolation and characterization of various forms of simian virus 40 DNA-protein complexes. Virology. 1980 Apr 15;102(1):107–118. doi: 10.1016/0042-6822(80)90074-4. [DOI] [PubMed] [Google Scholar]
- Jakobovits E. B., Bratosin S., Aloni Y. A nucleosome-free region in SV40 minichromosomes. Nature. 1980 May 22;285(5762):263–265. doi: 10.1038/285263a0. [DOI] [PubMed] [Google Scholar]
- Jakobovits E. B., Saragosti S., Yaniv M., Aloni Y. Escherichia coli RNA polymerase in vitro mimics simian virus 40 in vivo transcription when the template is viral nucleoprotein. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6556–6560. doi: 10.1073/pnas.77.11.6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., Nomura S., Anderson C. W., Khoury G. Identification of the SV40 agnogene product: a DNA binding protein. Nature. 1981 May 28;291(5813):346–349. doi: 10.1038/291346a0. [DOI] [PubMed] [Google Scholar]
- Keene M. A., Corces V., Lowenhaupt K., Elgin S. C. DNase I hypersensitive sites in Drosophila chromatin occur at the 5' ends of regions of transcription. Proc Natl Acad Sci U S A. 1981 Jan;78(1):143–146. doi: 10.1073/pnas.78.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R. D. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- Laub O., Aloni Y. Transcription of simian virus 40. V. Regulattion of simian virus 40 gene expression. J Virol. 1975 Nov;16(5):1171–1183. doi: 10.1128/jvi.16.5.1171-1183.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laub O., Aloni Y. Transcription of simian virus 40. VI. SV 40 DNA-RNA polymerase complex isolated from productively infected cells transcribed in vitro. Virology. 1976 Dec;75(2):346–354. doi: 10.1016/0042-6822(76)90033-7. [DOI] [PubMed] [Google Scholar]
- Laub O., Bratosin S., Horowitz M., Aloni Y. The initiation of transcription of SV40 DNA at late time after infection. Virology. 1979 Jan 30;92(2):310–323. doi: 10.1016/0042-6822(79)90136-3. [DOI] [PubMed] [Google Scholar]
- Laub O., Jakobovits E. B., Aloni Y. 5,6-dichloro-1-beta-ribofuranosylbenzimidazole enhances premature termination of late transcription of simian virus 40 DNA. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3297–3301. doi: 10.1073/pnas.77.6.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F. H., Tinoco I., Jr DNA-RNA hybrid duplexes containing oligo(dA:rU) sequences are exceptionally unstable and may facilitate termination of transcription. Nucleic Acids Res. 1980 May 24;8(10):2295–2299. doi: 10.1093/nar/8.10.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D., Oudet P., Chambon P. Structure of transcribing chromatin. Prog Nucleic Acid Res Mol Biol. 1980;24:1–55. doi: 10.1016/s0079-6603(08)60670-4. [DOI] [PubMed] [Google Scholar]
- Meinke W., Hall M. R., Goldstein D. A. Proteins in intracellular simian virus 40 nucleoportein complexes: comparison with simian virus 40 core proteins. J Virol. 1975 Mar;15(3):439–448. doi: 10.1128/jvi.15.3.439-448.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Salditt-Georgieff M., Harpold M., Chen-Kiang S., Darnell J. E., Jr The addition of 5' cap structures occurs early in hnRNA synthesis and prematurely terminated molecules are capped. Cell. 1980 Jan;19(1):69–78. doi: 10.1016/0092-8674(80)90389-x. [DOI] [PubMed] [Google Scholar]
- Saragosti S., Moyne G., Yaniv M. Absence of nucleosomes in a fraction of SV40 chromatin between the origin of replication and the region coding for the late leader RNA. Cell. 1980 May;20(1):65–73. doi: 10.1016/0092-8674(80)90235-4. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Wigmore D. J. Sites in simian virus 40 chromatin which are preferentially cleaved by endonucleases. Cell. 1978 Dec;15(4):1511–1518. doi: 10.1016/0092-8674(78)90073-9. [DOI] [PubMed] [Google Scholar]
- Skolnik-David H., Hay N., Aloni Y. Site of premature termination of late transcription of simian virus 40 DNA: enhancement by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Proc Natl Acad Sci U S A. 1982 May;79(9):2743–2747. doi: 10.1073/pnas.79.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Tamm I., Kikuchi T. Early termination of heterogeneous nuclear RNA transcripts in mammalian cells: accentuation by 5,6-dichloro 1-beta-D-ribofuranosylbenzimidazole. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5750–5754. doi: 10.1073/pnas.76.11.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. Unique mode of transcription in vitro by Vesicular stomatitis virus. Cell. 1980 Aug;21(1):267–275. doi: 10.1016/0092-8674(80)90134-8. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Tweeten K. A., Molloy G. R. Induction of premature termination of transcription of the mouse beta-globin gene by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole (DRB). Nucleic Acids Res. 1981 Jul 24;9(14):3307–3319. doi: 10.1093/nar/9.14.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Sundin O., Bohn M. A stretch of "late" SV40 viral DNA about 400 bp long which includes the origin of replication is specifically exposed in SV40 minichromosomes. Cell. 1979 Feb;16(2):453–466. doi: 10.1016/0092-8674(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Larsen A., Groudine M. Alpha-Globin-gene switching during the development of chicken embryos: expression and chromosome structure. Cell. 1981 May;24(2):333–344. doi: 10.1016/0092-8674(81)90323-8. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Recognition of specific DNA sequences in eukaryotic chromosomes. Nucleic Acids Res. 1980 Oct 24;8(20):4745–4753. doi: 10.1093/nar/8.20.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]